L15 Antimicrobial Drugs
一、 Introduction to Bacteria
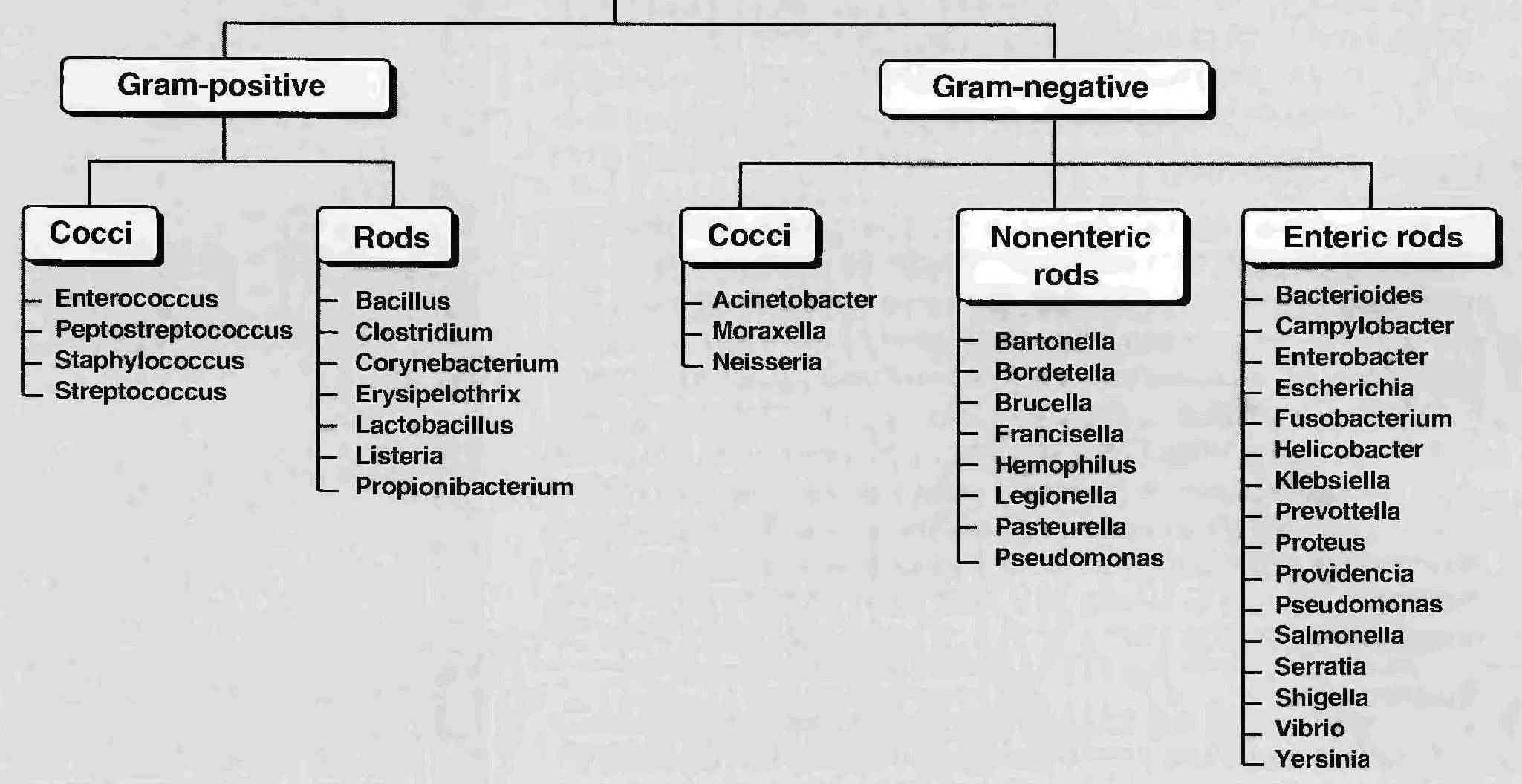
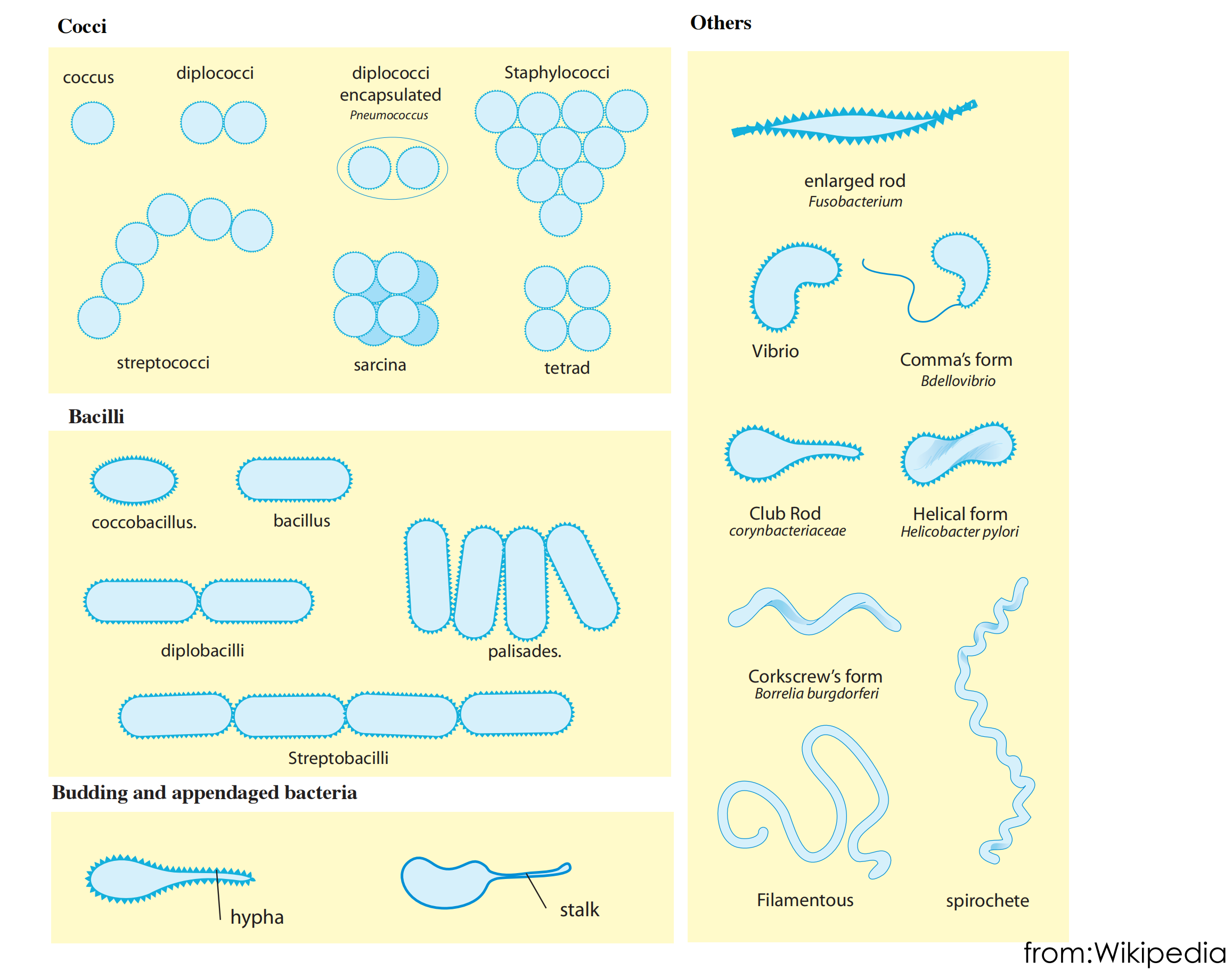
Classification of bacteria according to shape
Bacteria under the electron microscope
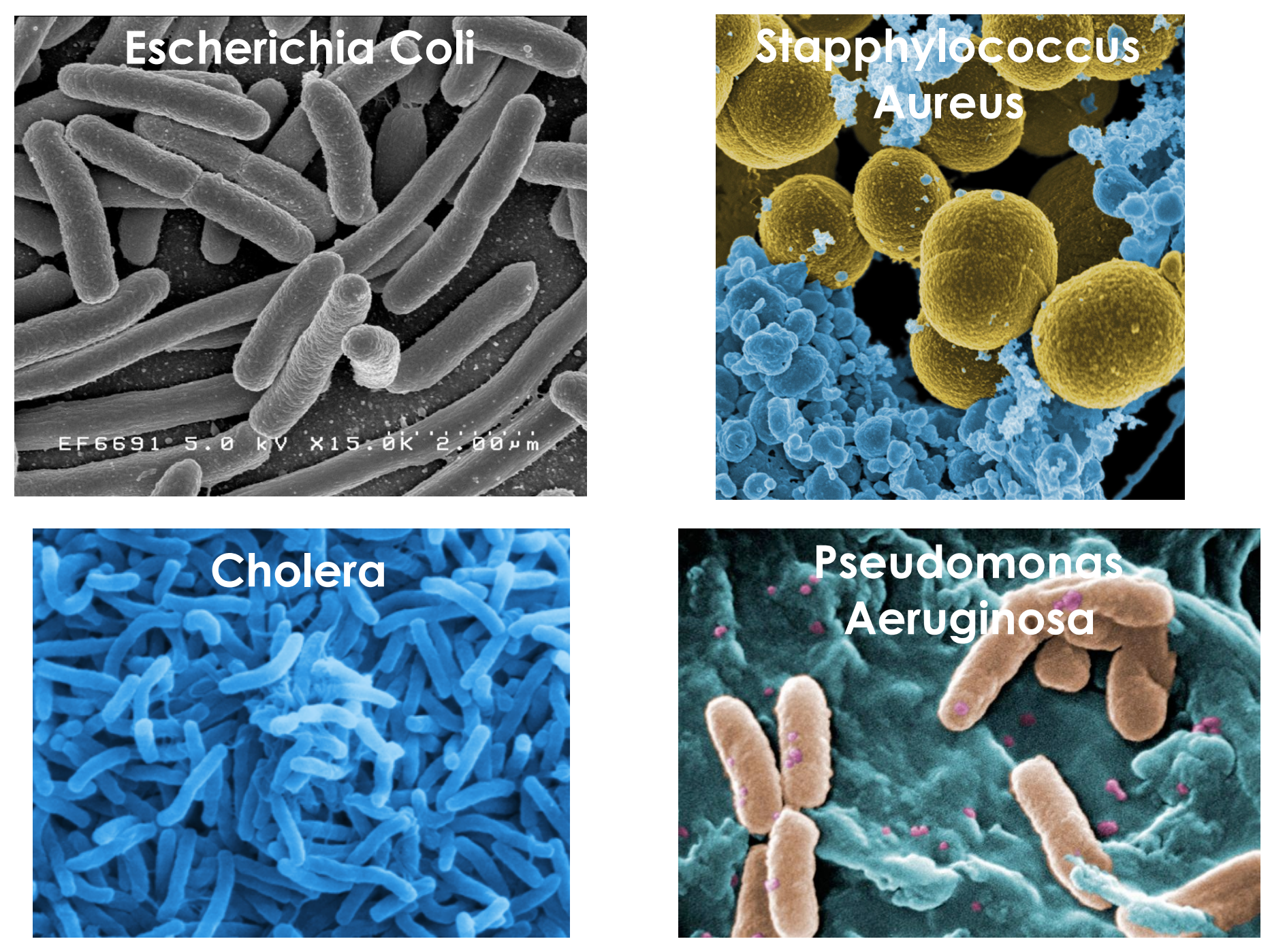
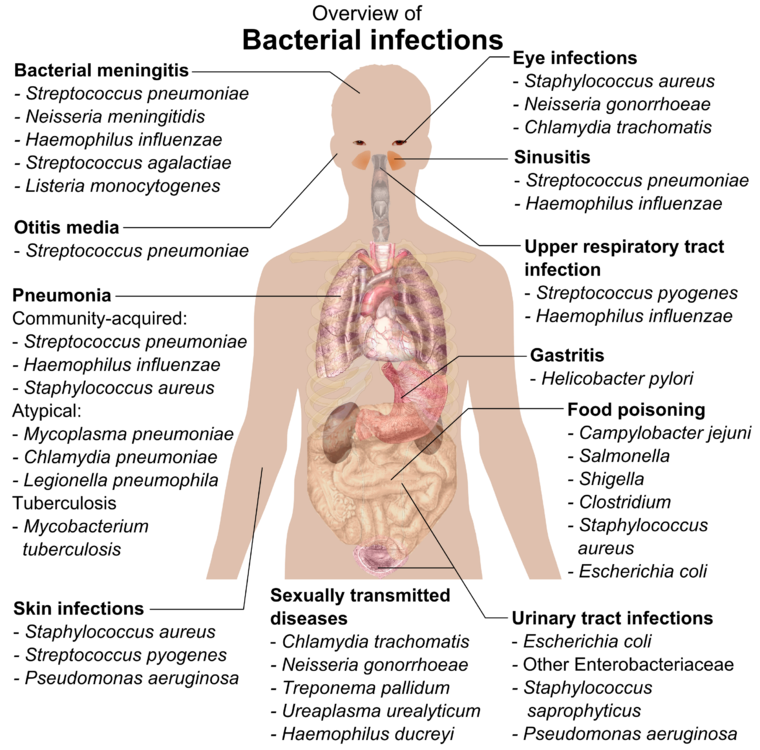
Medically relevant
gram-positive
- Bacillus - degrades complex macromolecules- dust, water, plants, animals fur
- Bacillus anthracis: Common in cattle, Bioterrorism
- Bacillus cereus: food, rice, potatoes, meat
- Clostridium perfringens: progressive, toxins diffuse to healthy tissue, Surgical, compound fractures, sores, septic abortions,gunshot wounds, crushing injuries with dirt
gram-negative cocci
- sexually transmitted disease (Neisseria gonorrhoeae)
- meningitis (Neisseria meningitidis)
- respiratory symptoms (Moraxella catarrhalis).
gram-negative bacilli
- respiratory problems (Hemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Pseudomonas aeruginosa)
- urinary problems (Escherichia coli, Proteus mirabilis, Enterobacter cloacae, Serratia marcescens)
- gastrointestinal problems (Helicobacter pylori, Salmonella enteritidis, Salmonella typhi)
二、The introduction of antibacterials
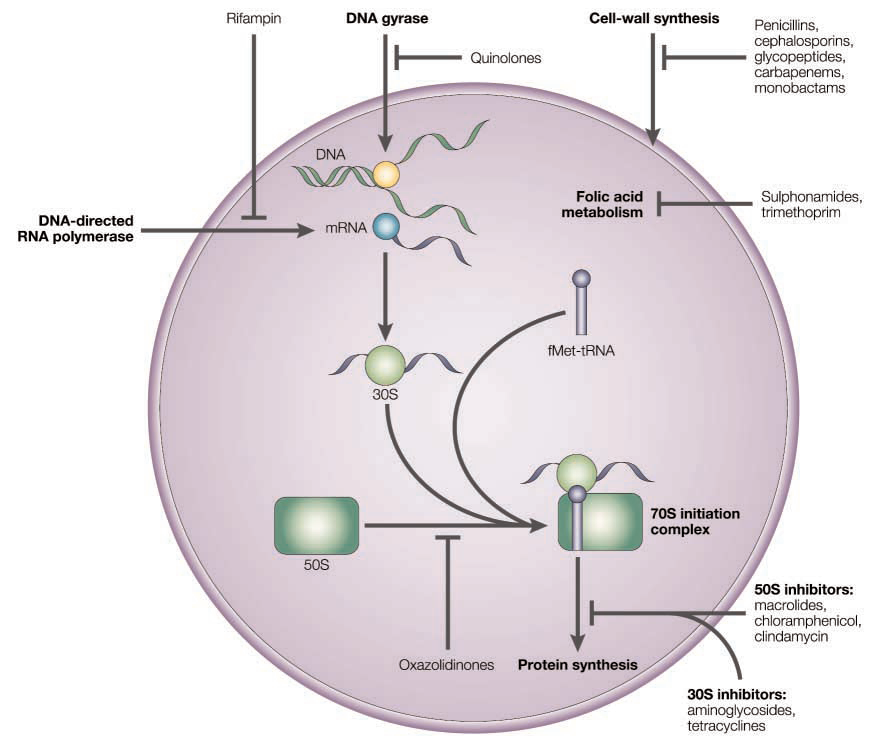
The mode of action of antibacterial compounds
The mode of action of antibacterial compounds
- Inhibition of metabolism (antimetabolites): Sulfonamides
- Inhibition of bacterial cell wall synthesis: Penicillins, Cephalosporins, Vancomycin
- Interaction with the plasma membrane: Polymyxin, Tyrothricin
- Disruption of protein synthesis: Rifamycins, aminoglycosides, tetracyclines, chloramphenicol
- Inhibition of nucleic acid transcription and replication: Nailidixic acid, proflavine
Time line and structure of different antibacterials
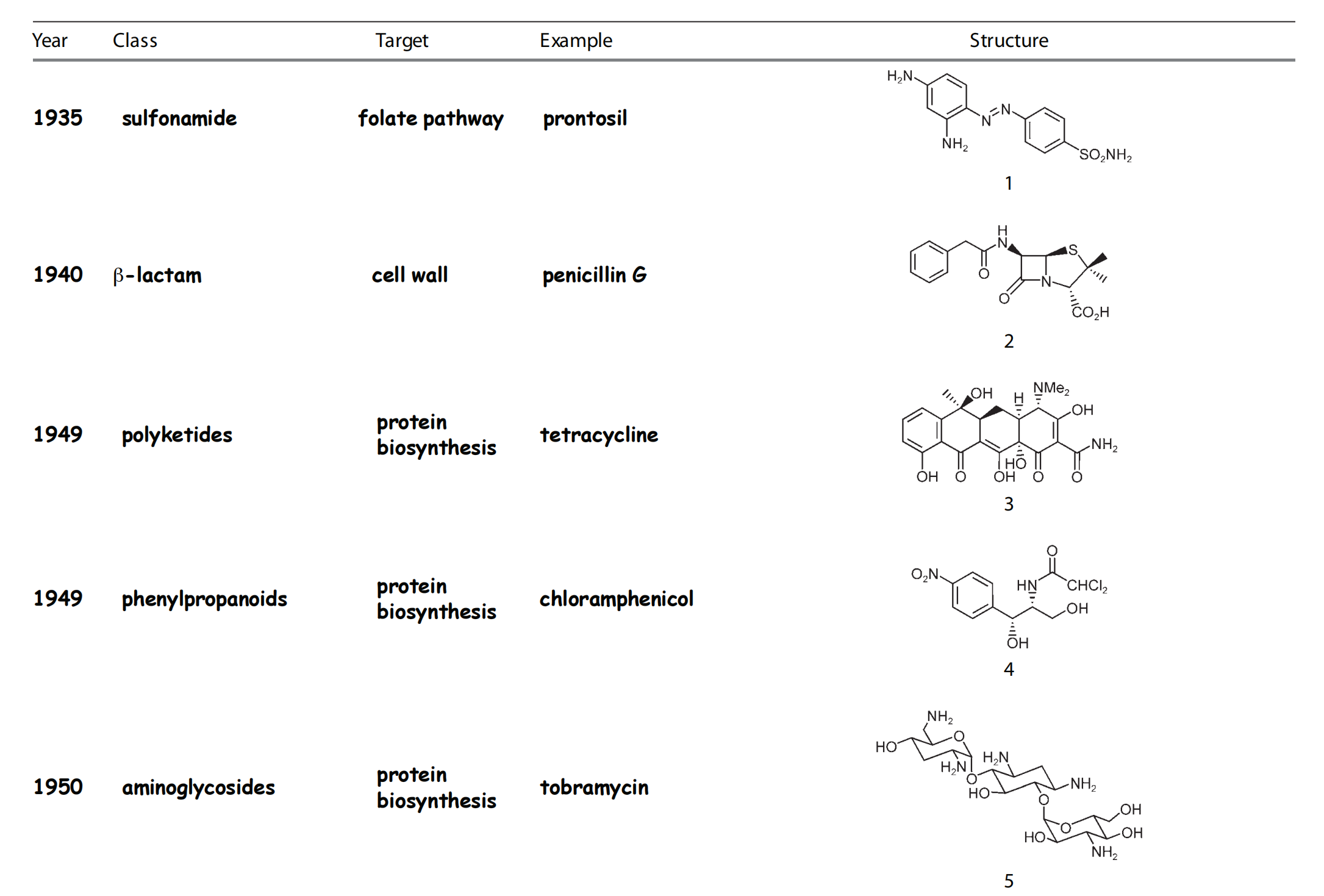
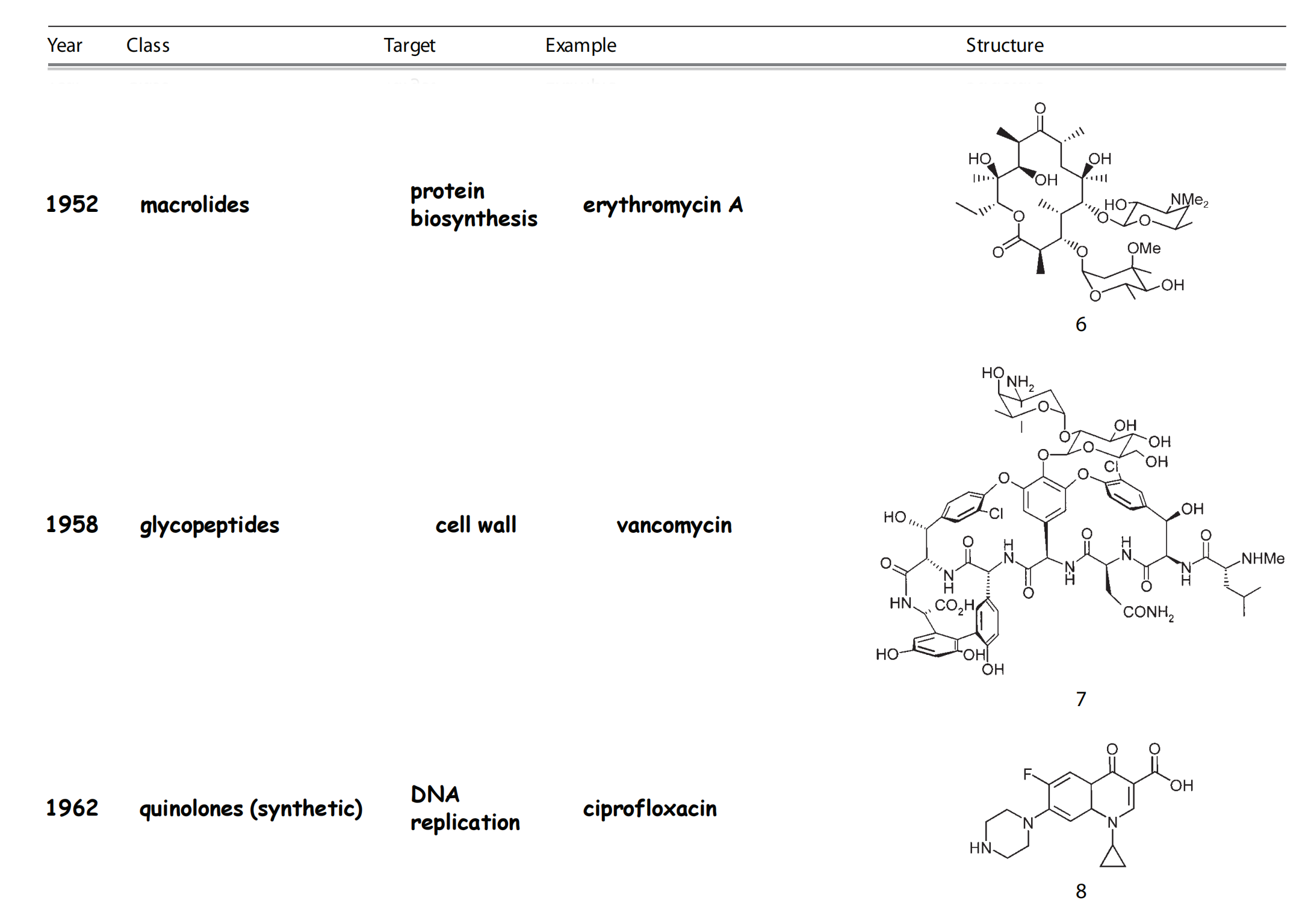
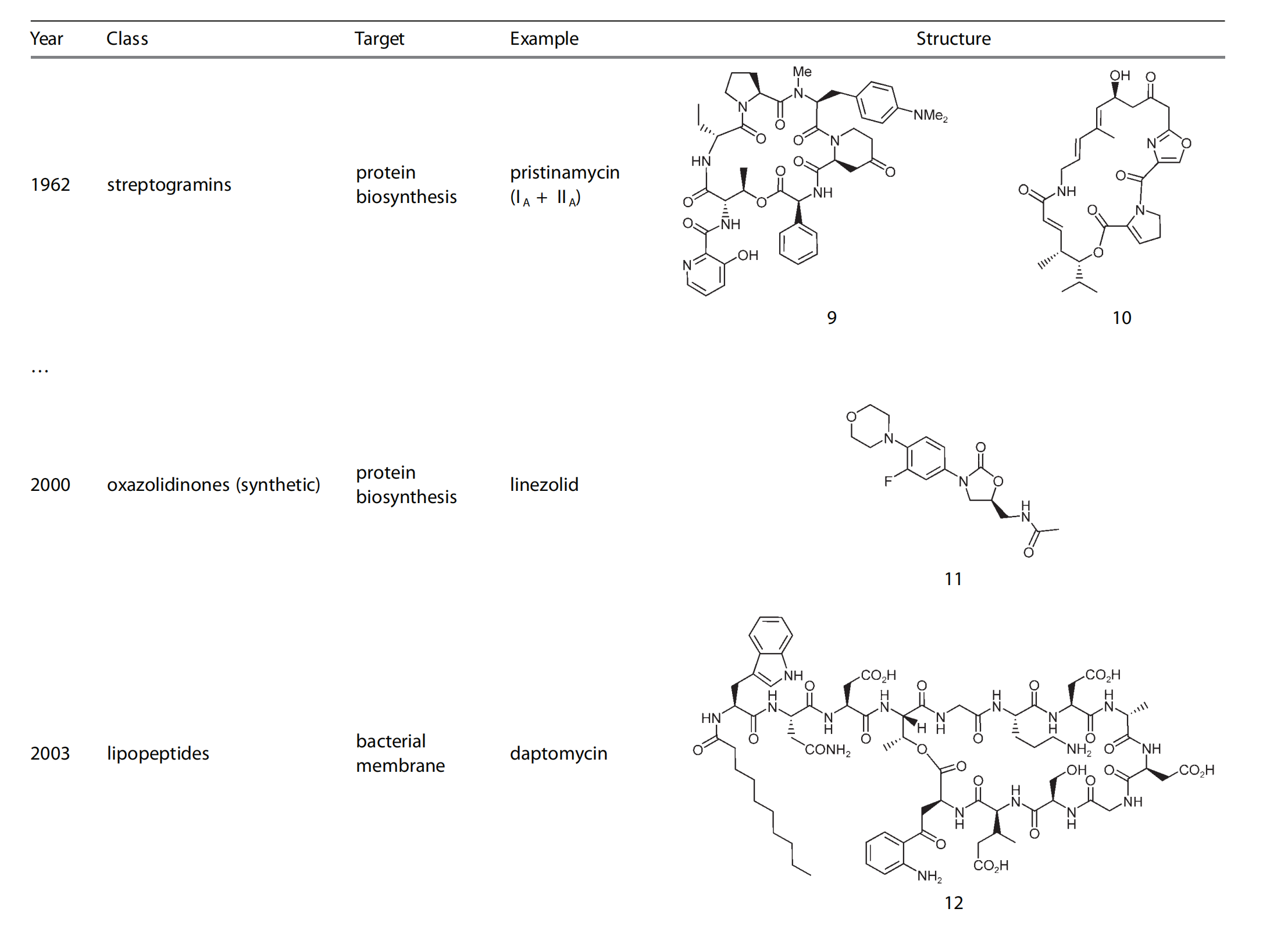
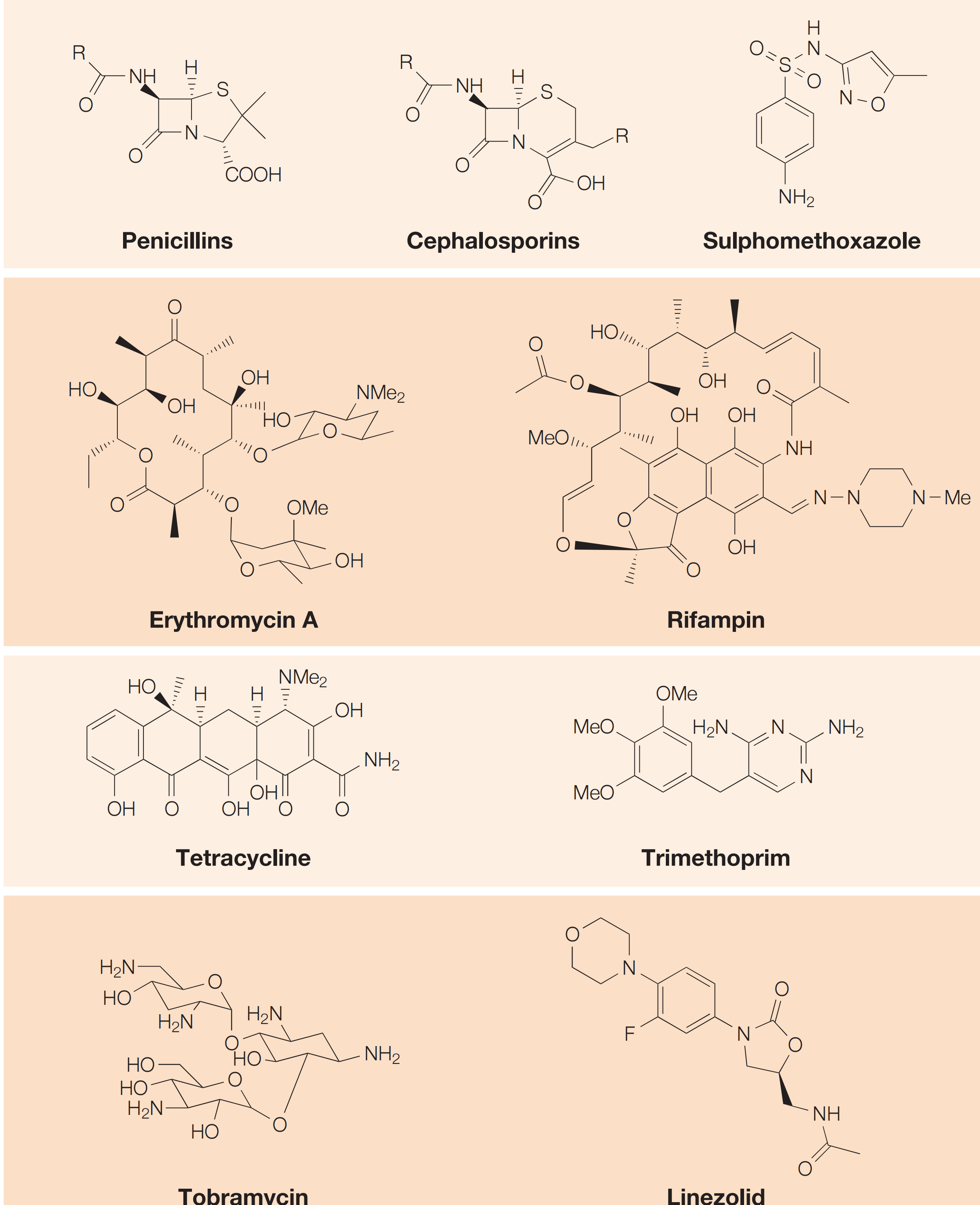
1. History of Antimetabolites
Paul Ehrlich developed staining dyes, and for example discovered mast cells. He tested more than 100 synthetic dyes for biological activity against Trypanosoma equinum, responsible for a disease that horses suffered from.

The first active compound was trepan red
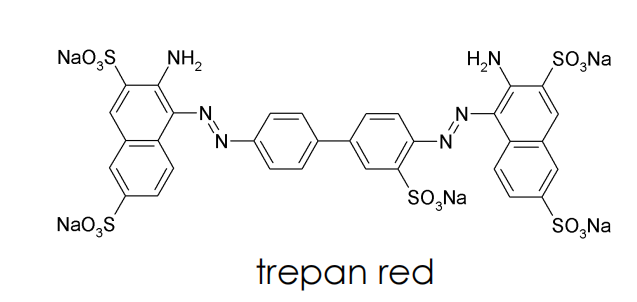
trepan red belongs to the class of azo dyes
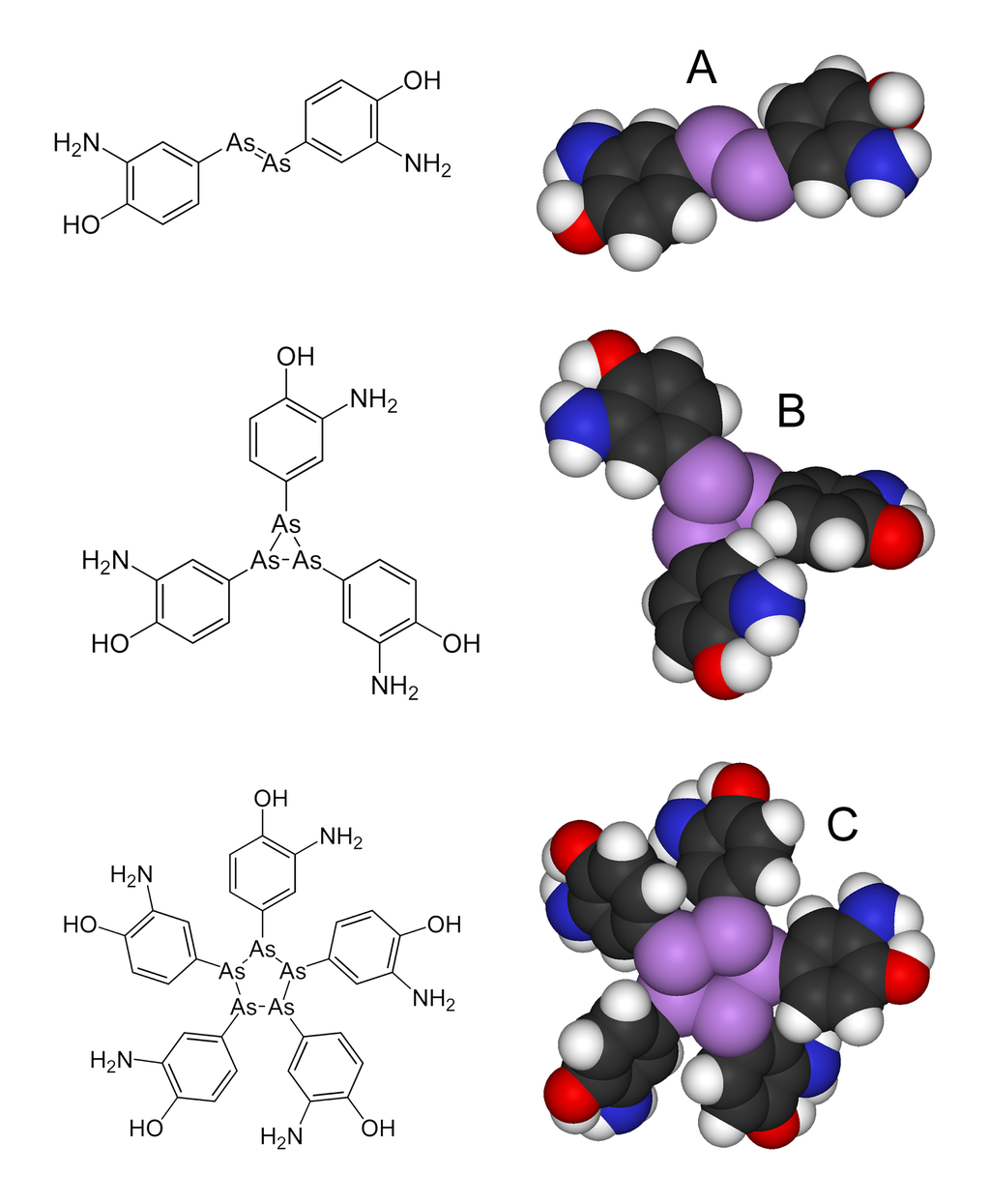
salvarsan is a anti-syphilis compound that is chemically related
Hoechst was a company that produced ago dyes, and the discovery of these antibiotics triggered the transition to pharma industry !
2. Antimetabolites
Sulfonamides
(1) Introduction
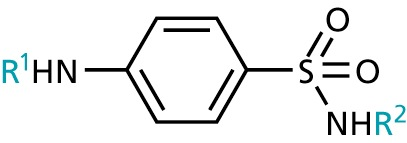
- Sulfonamides
- aromatic ring and sulfonamide required, ring must be para-substituted.
- the sulfonamide nitrogen must be primary or secondary
- R1 must be H (or acyl), R2 is variable
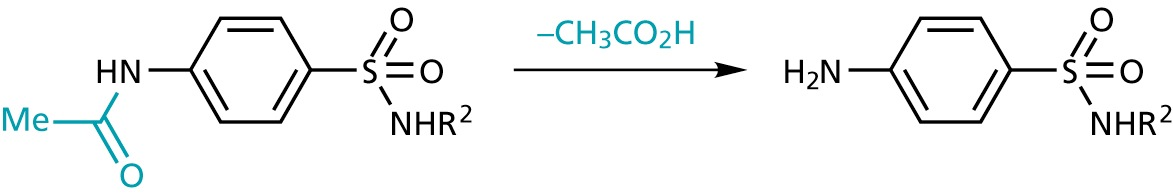
- active form can be formed in vivo (prodrugs)
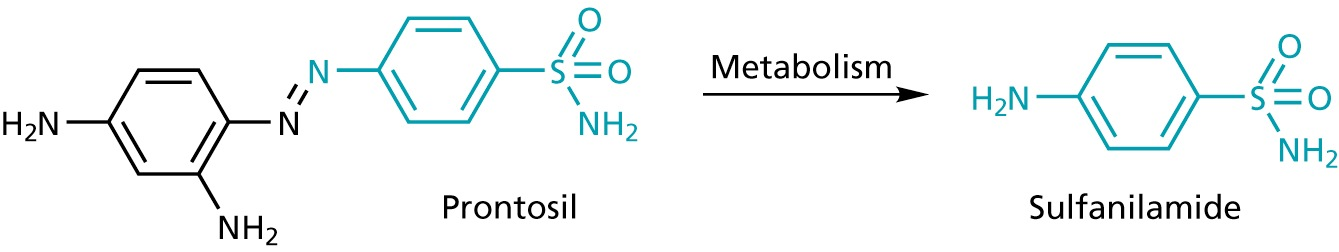
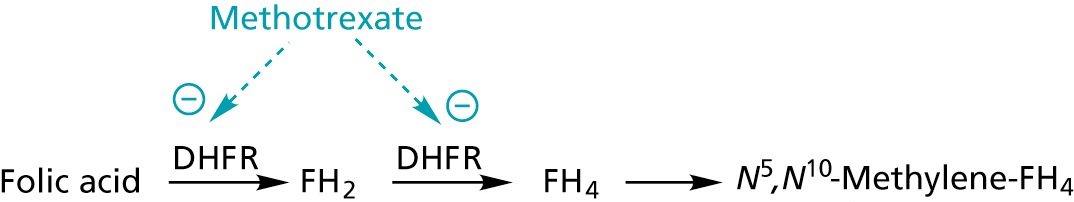
conversion of FH4 to Methlyen-FH4 by the serine hydroxymethyltransferase
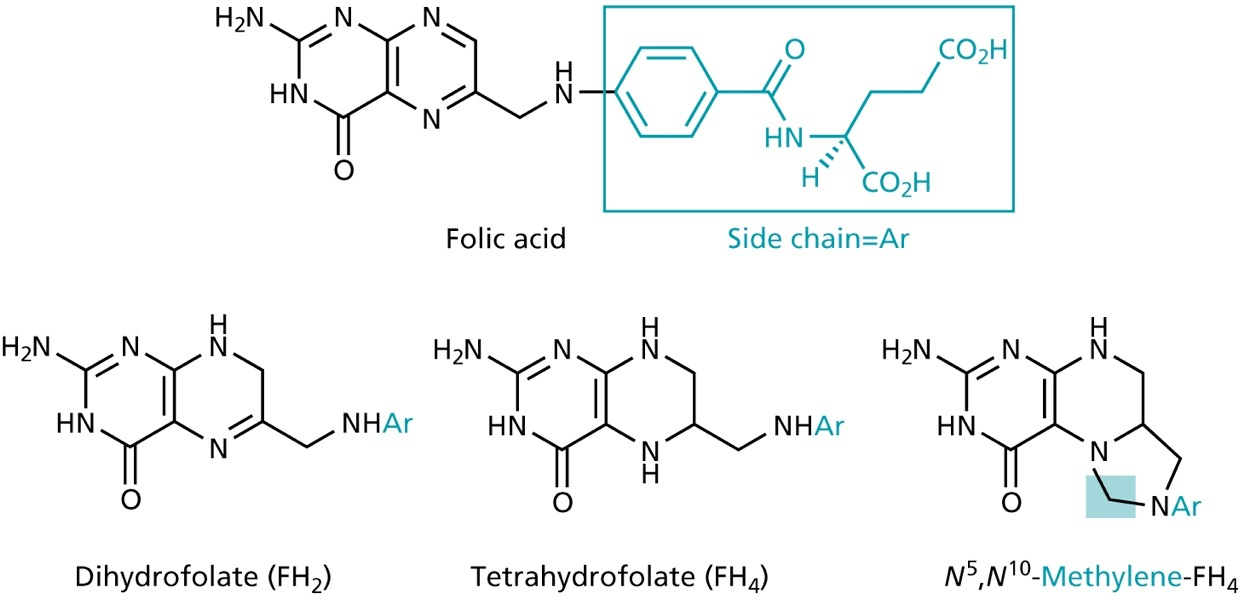
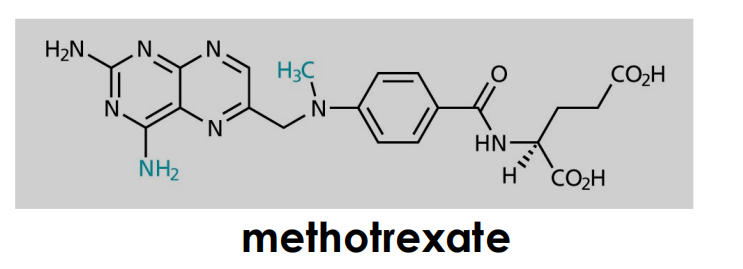
- source of one-C unit for methylations of deoxyuridinemonophosphate (dUMP) to form deoxythymidinemonophosphate (dTMP)
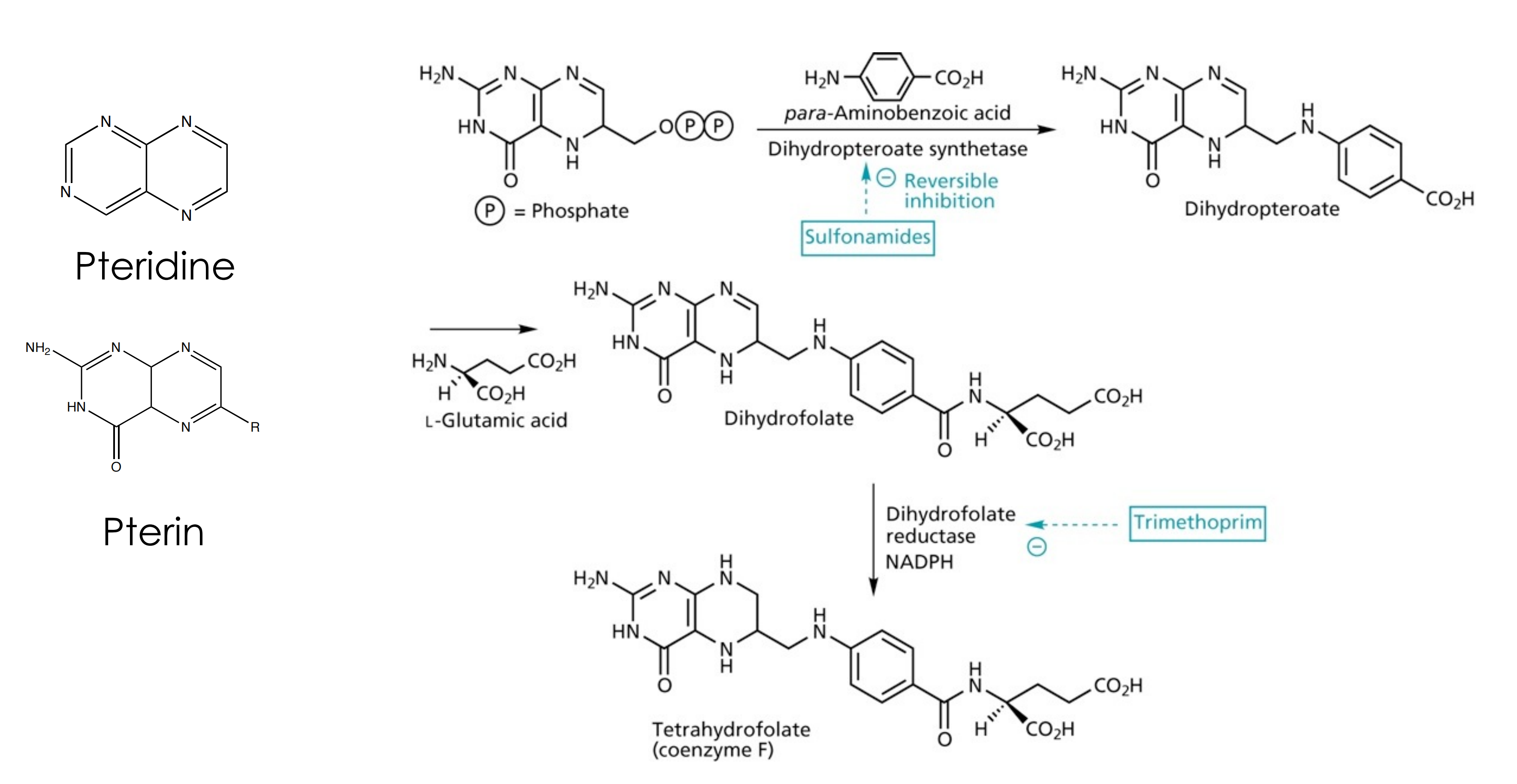
(2) Mode of action of sulfonamides
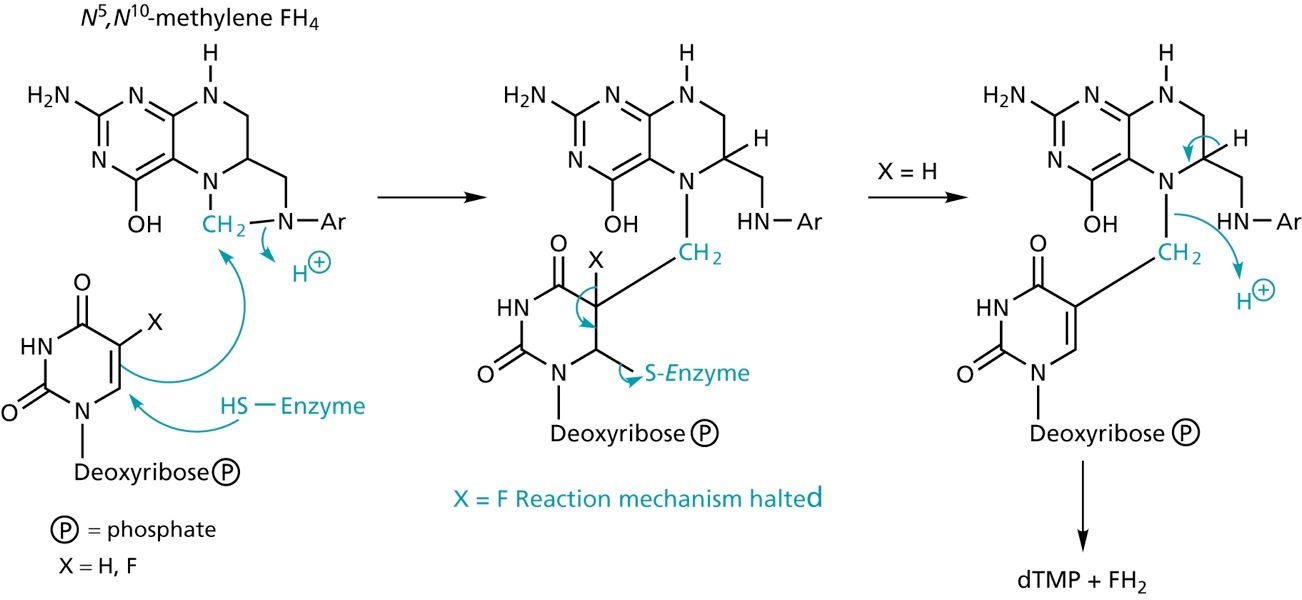
sulfonamides block the biosynthesis of tetrahydrofolate in bacterial cells. Tetrahydrofolate provides C-1 units for the pyrimide biosynthesis required for DNA synthesis.
Sulfonamides mimick p-aminobenzoic acid, a substrate for dihydropteroate synthetase
Thymidylate synthase inhibitors
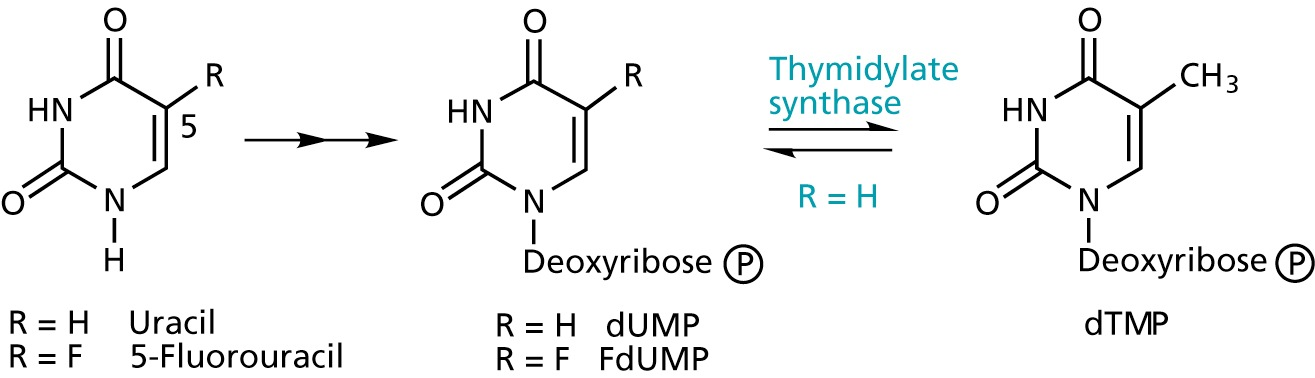
5-Fluorouracil acts as an suicide inhibitor
Other Antimetabolites
(1) Trimethroprim
Trimethroprim: Inhibits dihydrofolate reductase
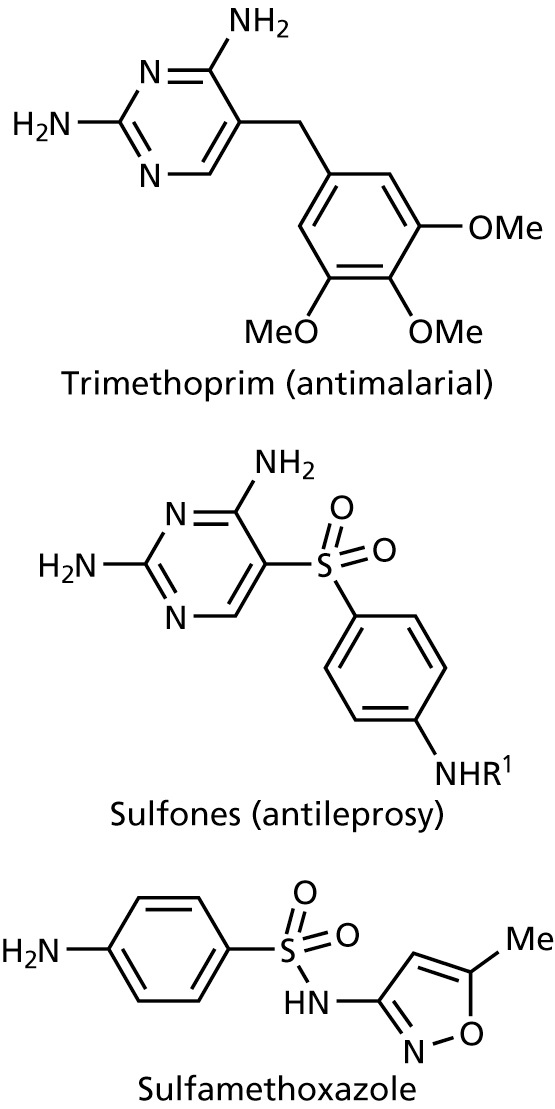
Applications
- treatment of urinary tract infections
- eye lotions
- treatment of infections of mucous membranes
- treatment of gut infections
(2) For lipid synthesis: Penicilins
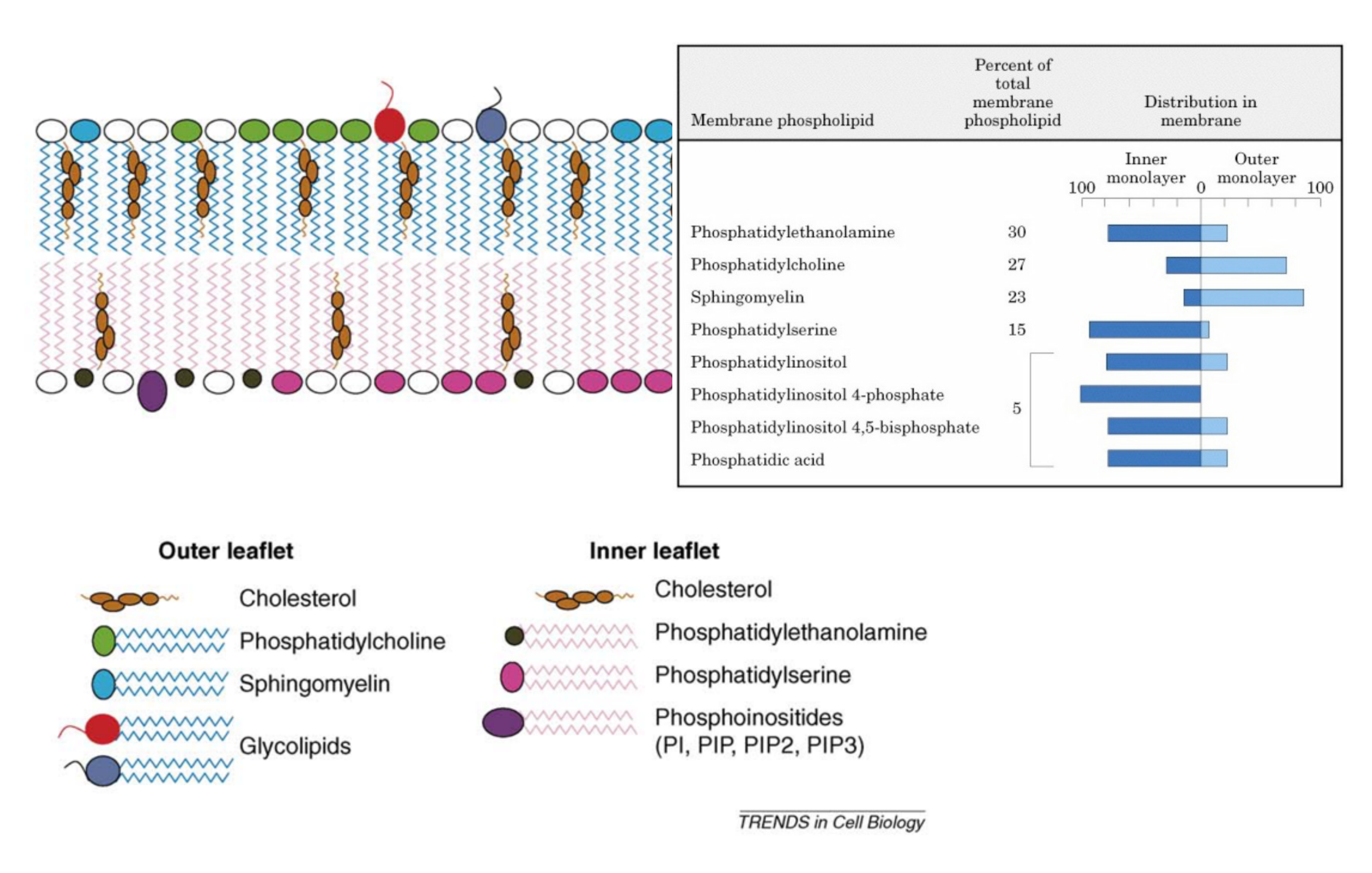
Lipid components of mammalian membranes
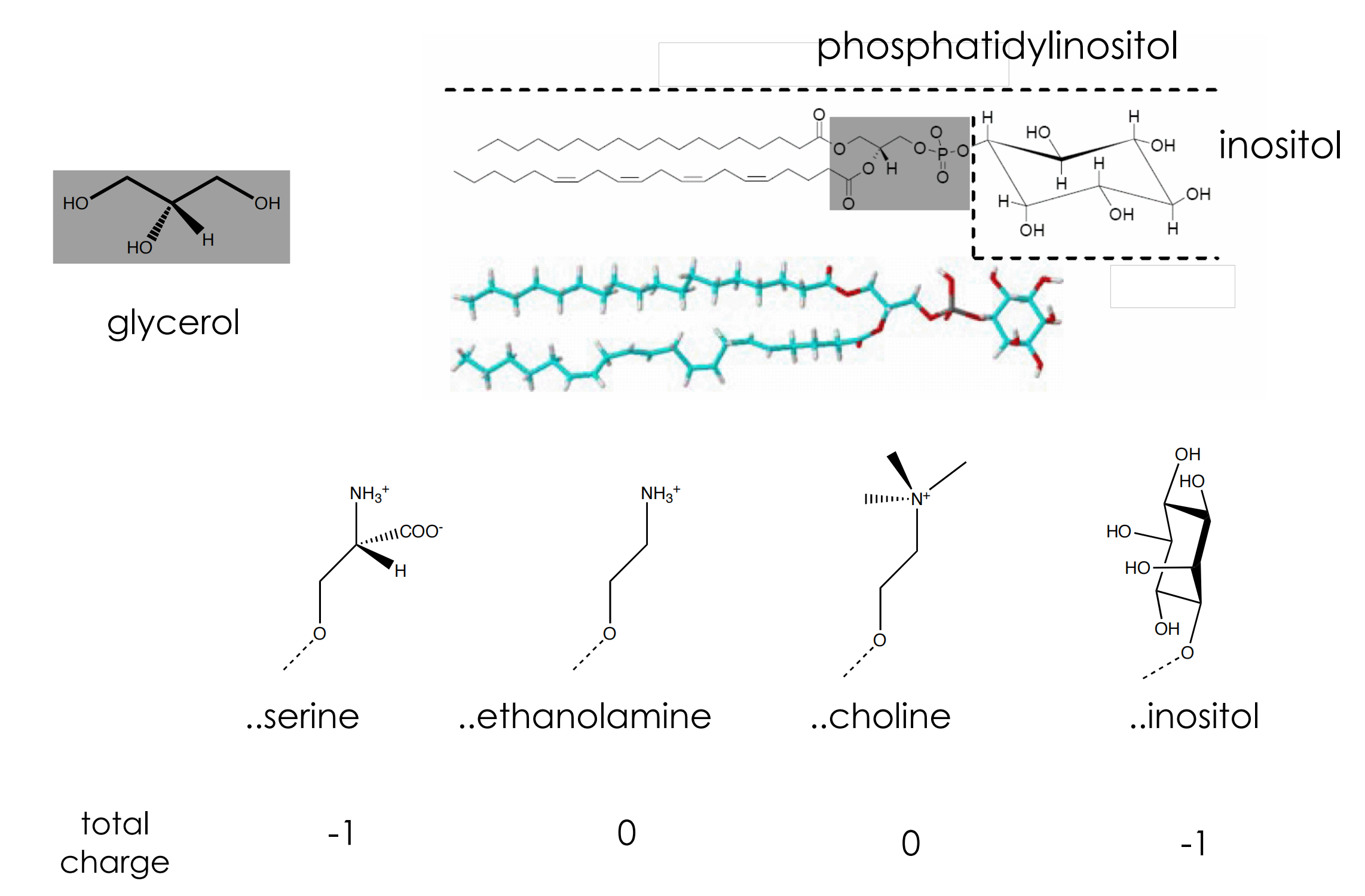
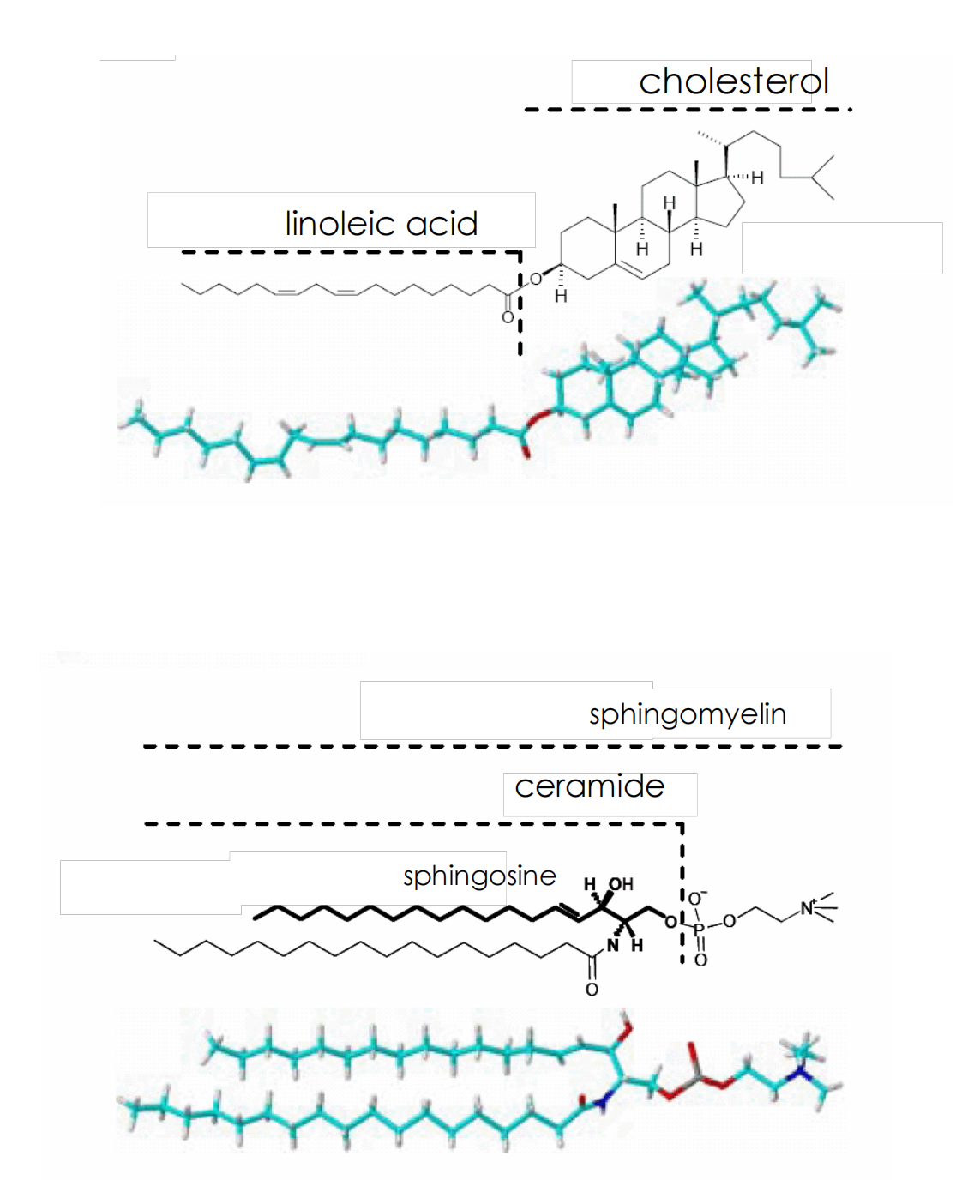
Lipid Asymmetry
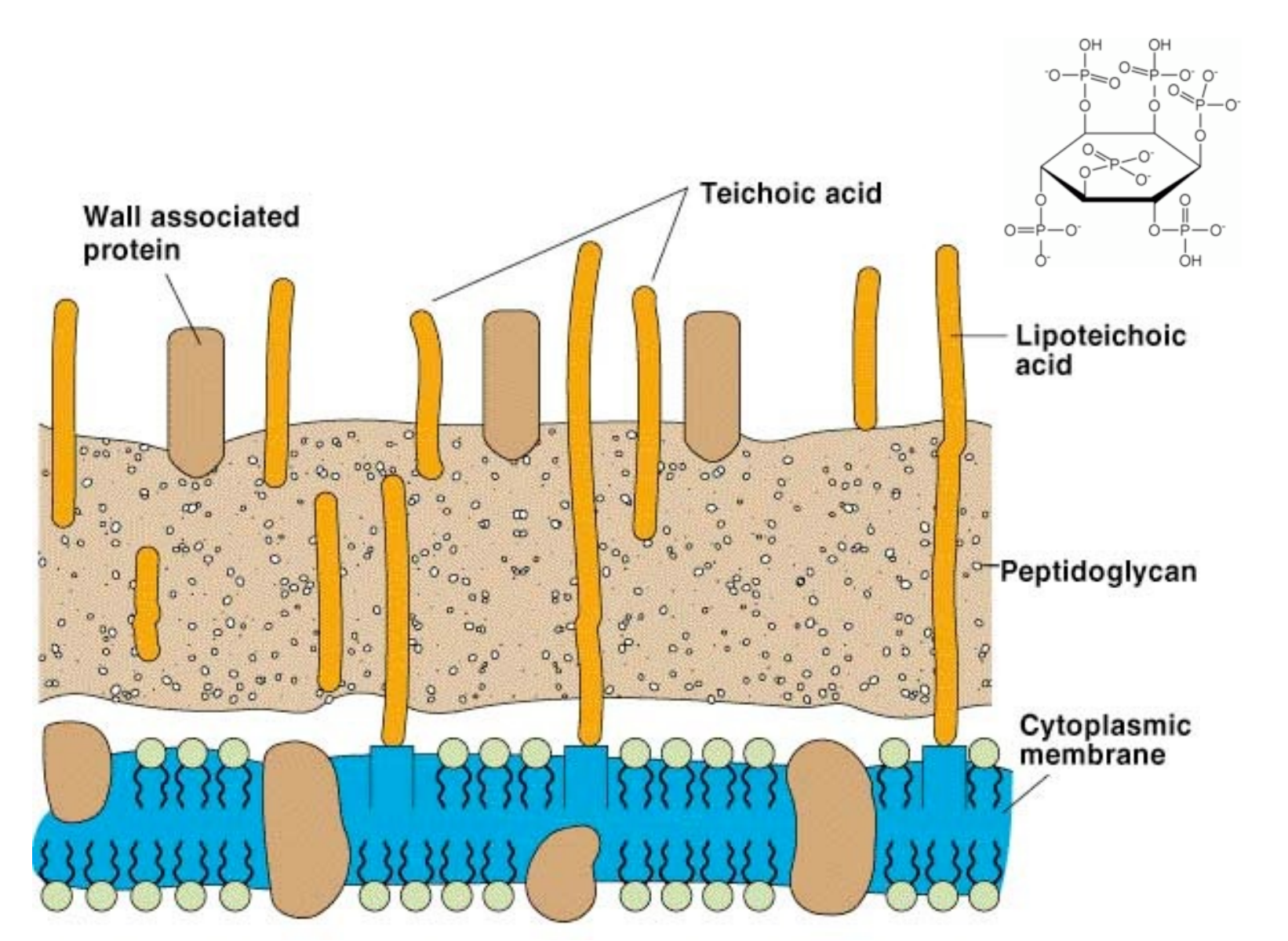
Cell wall of gram-positive bacteria
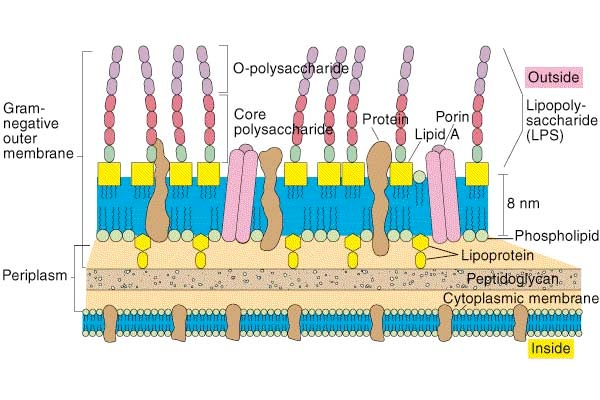
Cell wall of gram-negative bacteria
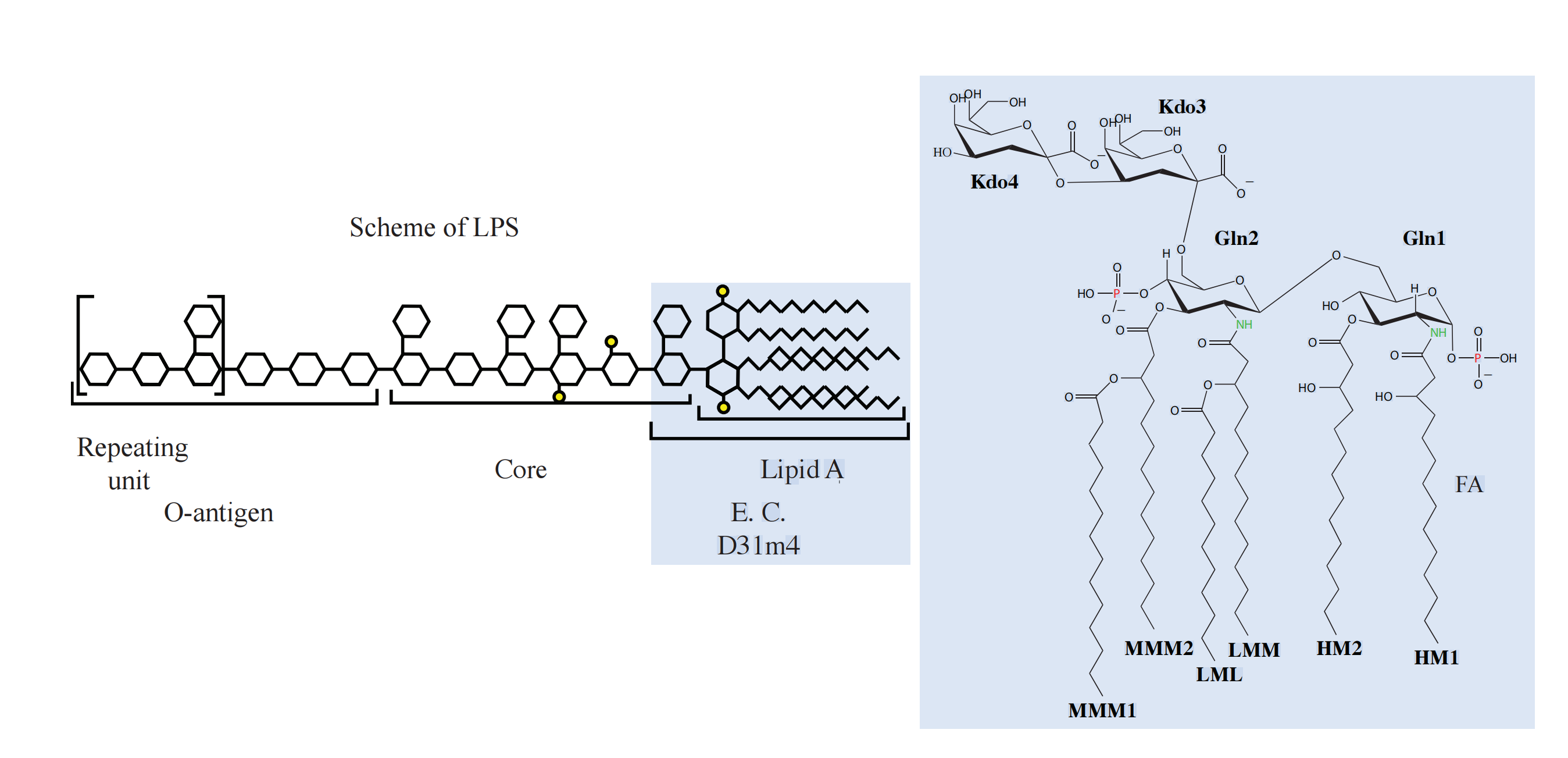
The Structure of Lipopolysaccharide (LPS)
The peptidoglycan layer
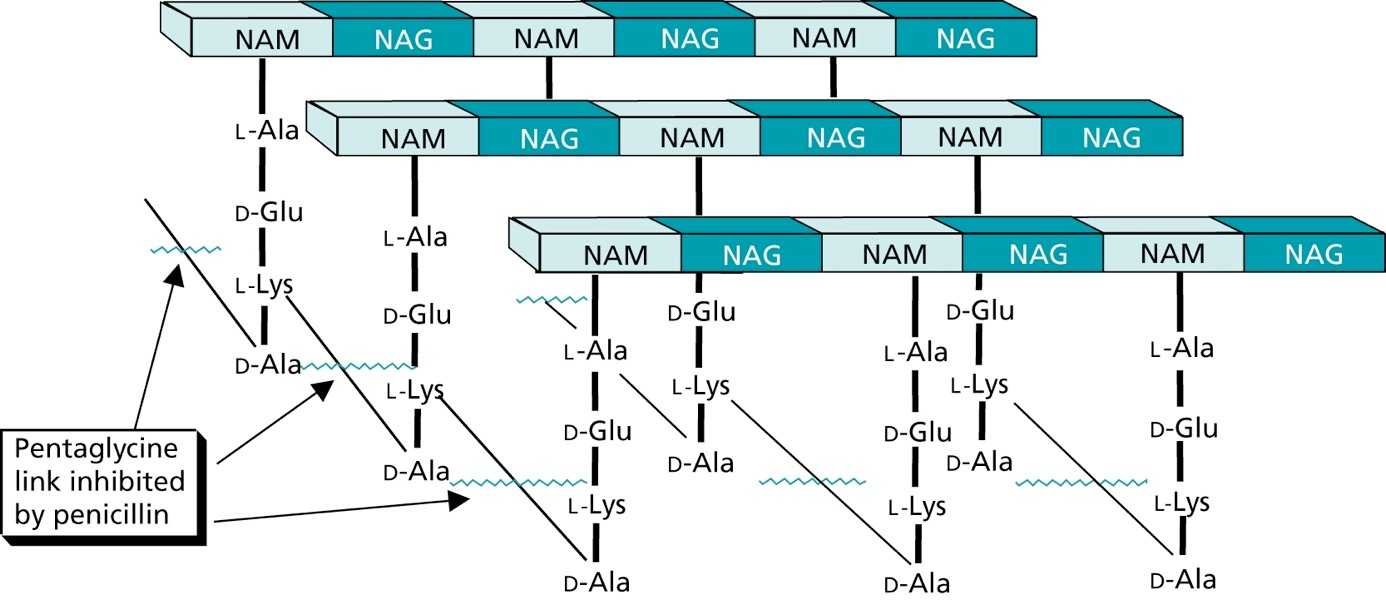
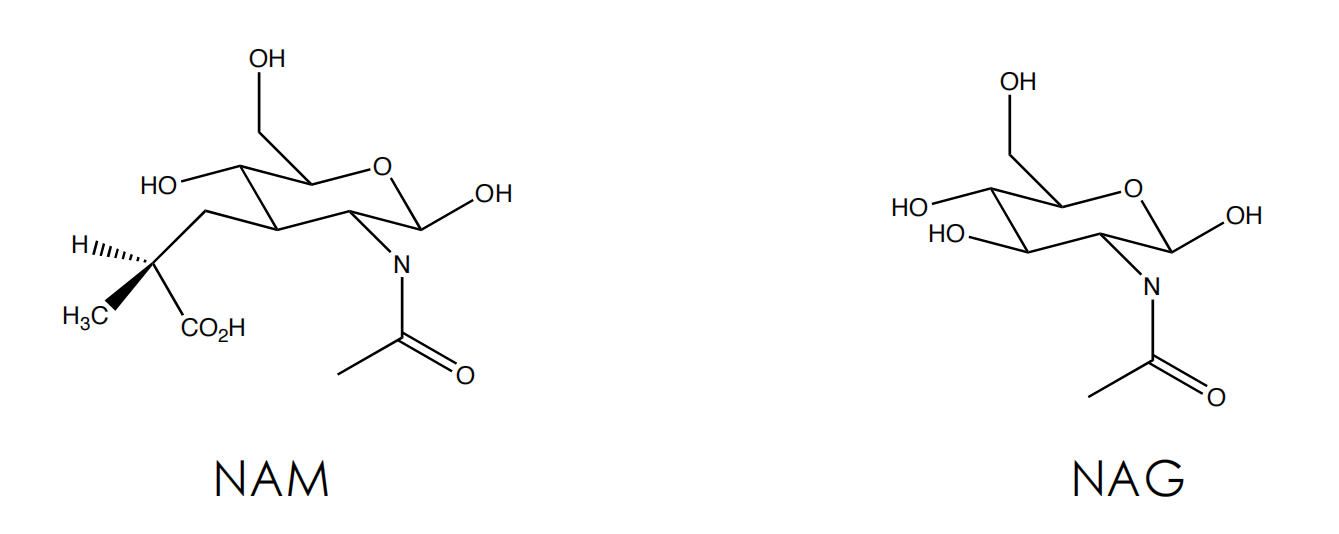
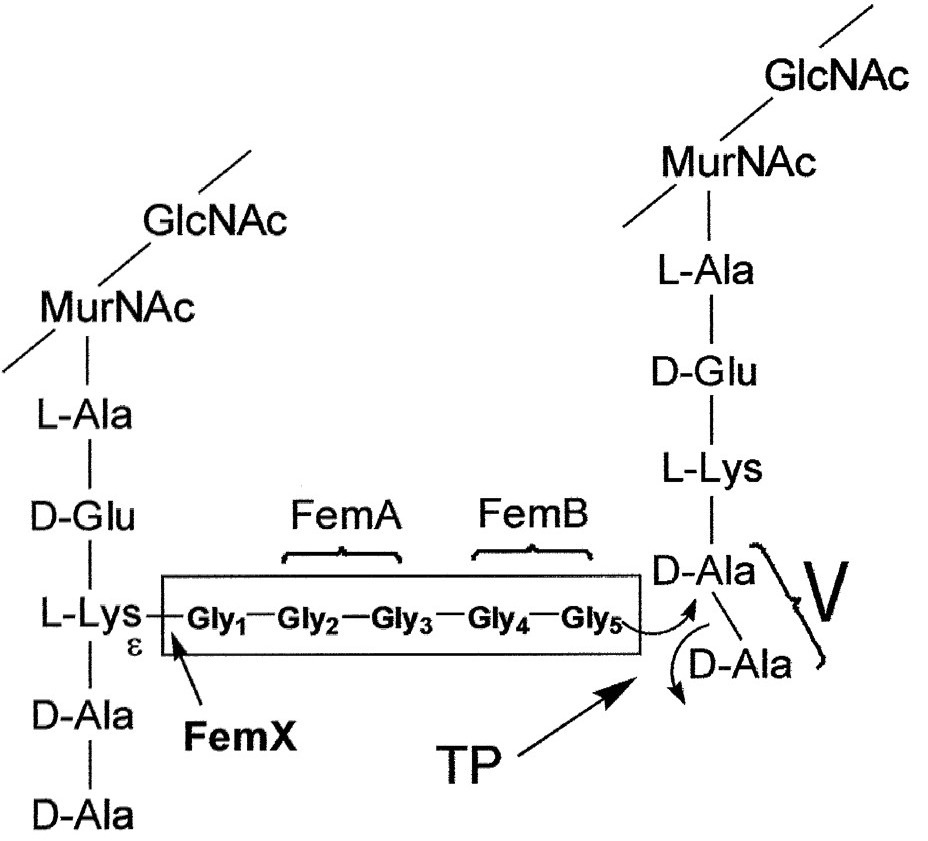
Cell Wall Biosynthesis
Firstly, N-acetylmuramic acid (NAM) is linked to three amino acids (^L^Ala-^D^Glu-^L^Lys)
The tripeptide is then linked to ^D^Ala-^D^Ala
The resulting pentapeptide glycopeptide is attached to a C55 carrier lipid by a translocase enzyme, and carried to the outer surface of the cell membrane
In the following step N-acteylglucosamine (NAG) is added
Afterwards a pentaglycine chain is linked to the peptide part
A transglycosylase enzyme catalyses the attachment of the disaccharide building block to the growing cell wall, and releases the lipid carrier
Chain elongation
Cross-linking
Penecillin
Discovery of Penicillin
- Alexander Flemming discovers in 1928 that a fungus grew on a bacterial plate containing staphylococci. Close to the fungus all bacteria were killed.
- Biotechnological production of penicillins was established during the second world war and helped saving the life of many soldiers. •
- Fleming, Chain und Florey received the Nobel price 1945
Antibacterials that inhibit the cell wall synthesis
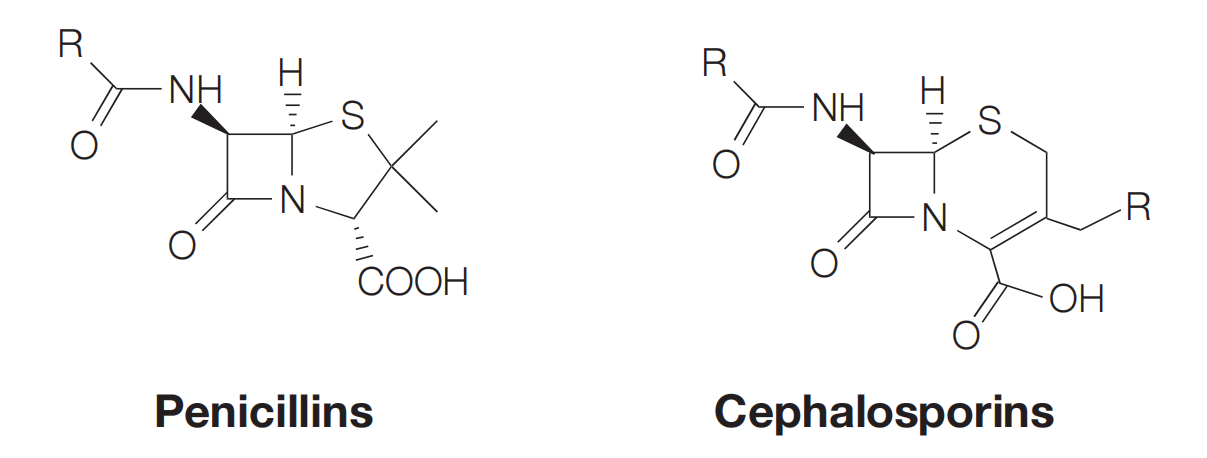
- Antibacterial agents which inhibit bacterial cell wall synthesis
- Discovered by Fleming from a fungal colony (1928)
- Shown to be non toxic and antibacterial
- Isolated and purified by Florey and Chain (1938)
- First successful clinical trial (1941)
- Produced by large scale fermentation (1944)
- Structure established by X-Ray crystallography (1945)
- Full synthesis developed by Sheehan (1957)
- Isolation of 6-APA by Beechams (1958-60) development of semi-synthetic penicillins
- Discovery of clavulanic acid and β-lactamase inhibitors
Mechanism of action bacterial cell wall synthesis
- Penicillin inhibits final crosslinking stage of cell wall synthesis
- It reacts with the transpeptidase enzyme to form an irreversible covalent bond
- Inhibition of transpeptidase leads to a weakened cell wall
- Cells swell due to water entering the cell, then burst (lysis)
- Penicillin possibly acts as an analogue of the L-Ala-γ-D-Ala portion of the pentapeptide chain. However, the carboxylate group that is essential to penicillin activity is not present in this portion
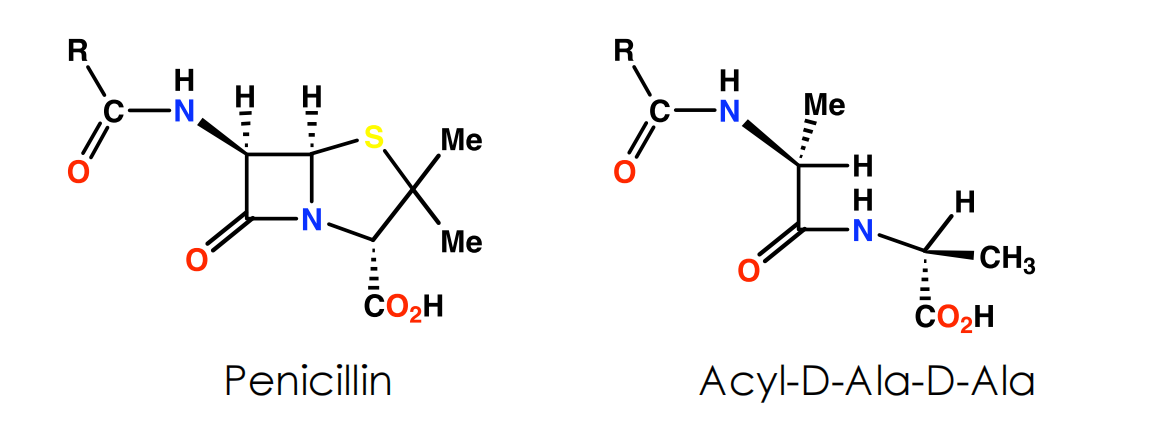

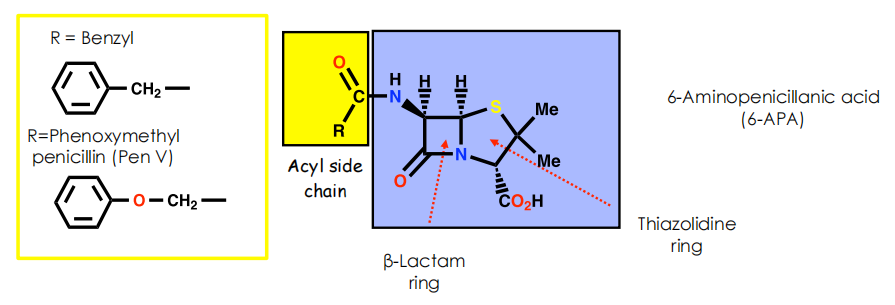
- Side chain varies depending on carboxylic acid present in fermentation medium

Synthesis of Penicillins
Penicillin G can be enzymatically converted into 6-aminopenicillanic acid (6-APA)
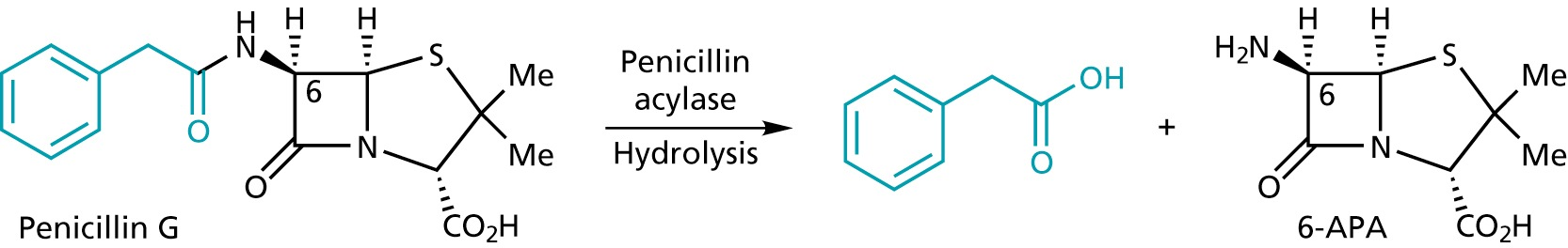
6-APA serves as a convenient starting material for the synthesis of other penicillins
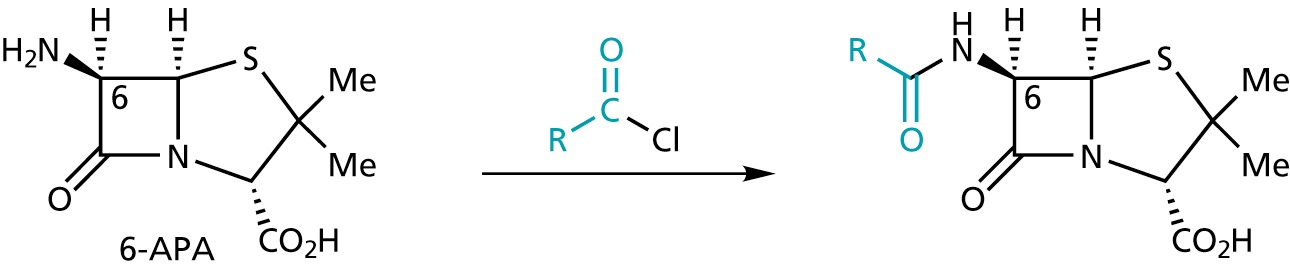
(3) SAR
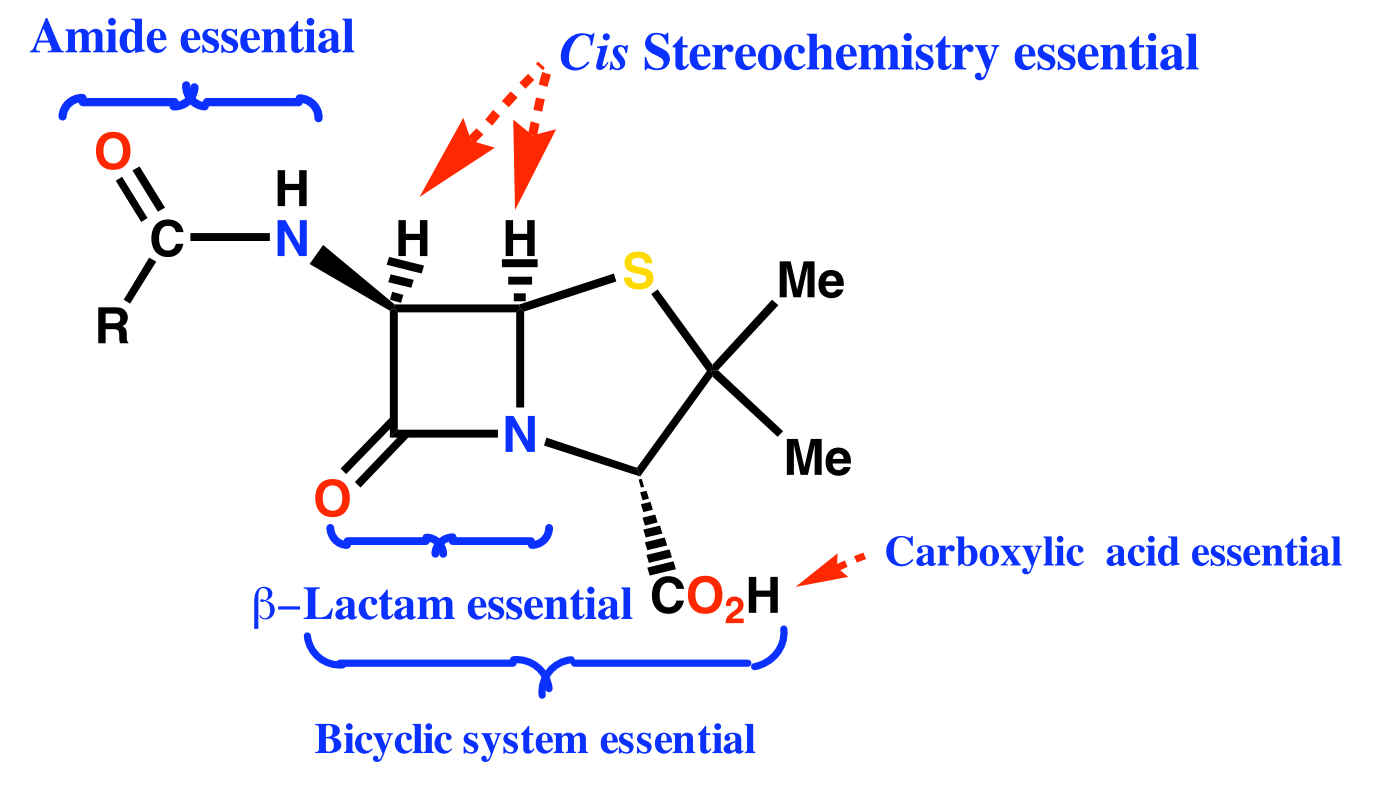
Conclusions
- Amide and carboxylic acid are involved in binding
- Carboxylic acid binds as the carboxylate ion
- Mechanism of action involves the β-lactam ring
- Activity related to β-lactam ring strain (subject to stability factors)
- Bicyclic system increases β-lactam ring strain
- Not much variation in structure is possible
- Variations are limited to the side chain (R)
Resistance to Penicillins
Gram negative bacteria have a lipopolysaccharide (LPS) outer membrane preventing access to the cell wall
- Penicillins can only cross via porins in the outer membrane
- Porins only allow small hydrophilic molecules that can exist as zwitterions to cross
High levels of transpeptidase enzyme may be present
The transpeptidase enzyme may have a low affinity for penicillins (e.g. PBP 2a for S. aureus)
Presence of β-lactamases
Concentration of β-lactamases in periplasmic space
Mutations
Transfer of β-lactamases between strains
Efflux mechanisms pumping penicillin out of periplasmic space
Problems with Penicillin G
It is sensitive to stomach acids
It is sensitive to β-lactamases enzymes which hydrolyse the β-lactam ring
It has a limited range of activity

Sensitivity to β-Lactamases
Enzymes that inactivate penicillins by opening β-lactam rings
Allow bacteria to be resistant to penicillin
Transferable between bacterial strains (i.e. bacteria can acquire resistance)
Important w.r.t. Staphylococcus aureus infections in hospitals
- 80% Staph. infections in hospitals were resistant to penicillin and other antibacterial agents by 1960
Mechanism of action for lactamases is identical to the mechanism of inhibition for the target enzyme
- But product is removed efficiently from the lactamase active site
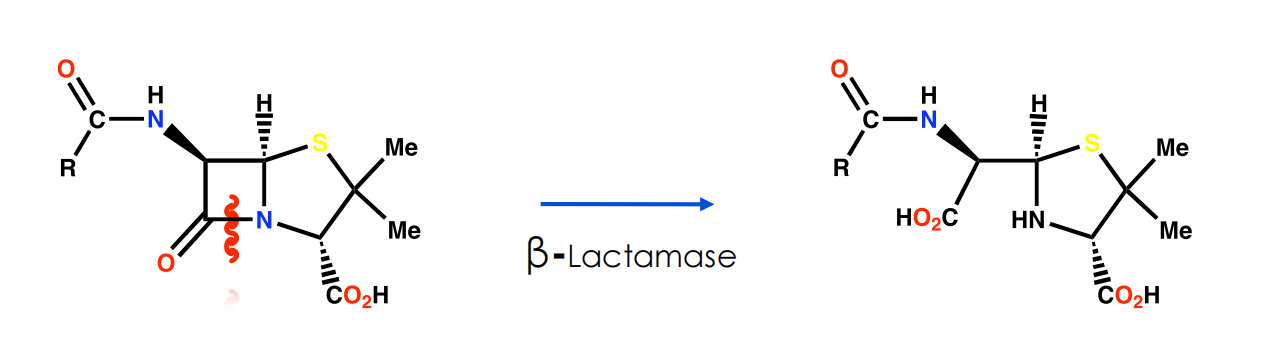
Block access of penicillin to active site of enzyme by introducing bulky groups to the side chain to act as steric shields
Size of shield is crucial to inhibit reaction of penicillins with βlactamases but not with the target enzyme (transpeptidase)
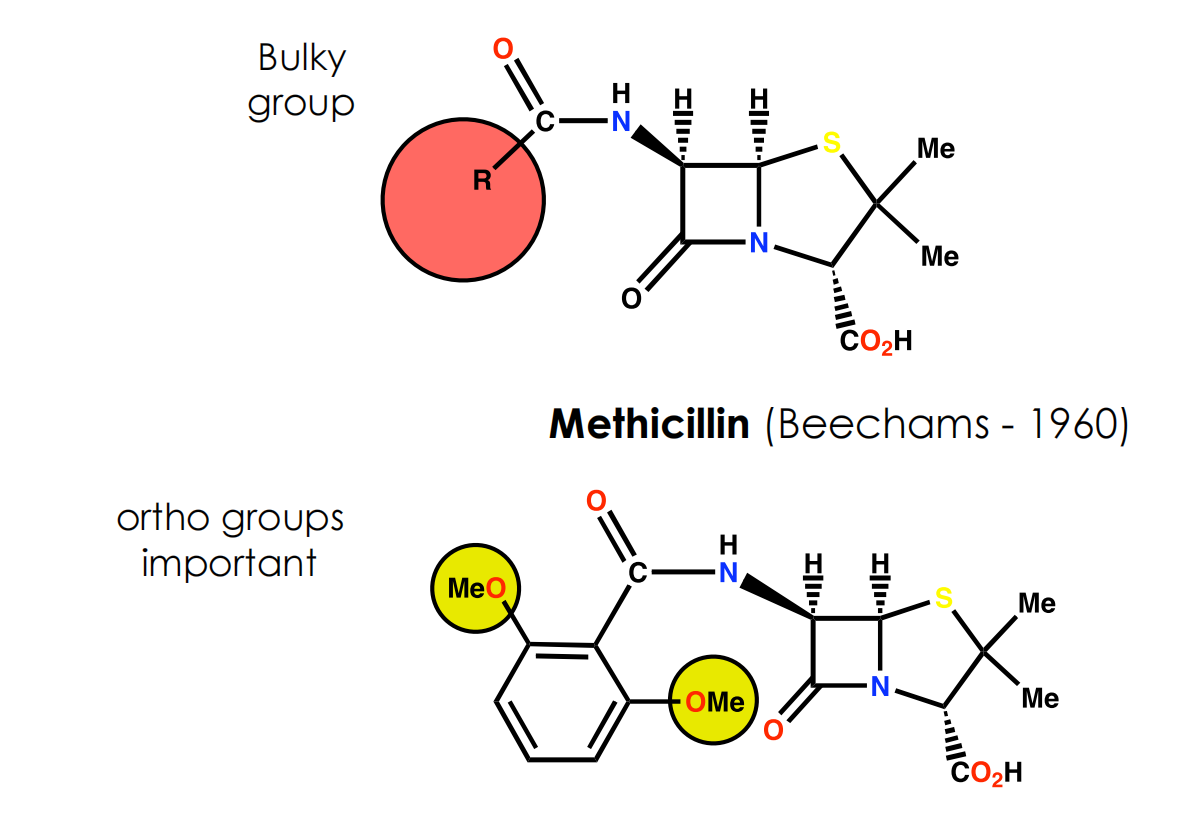
(4) Cephalosporins
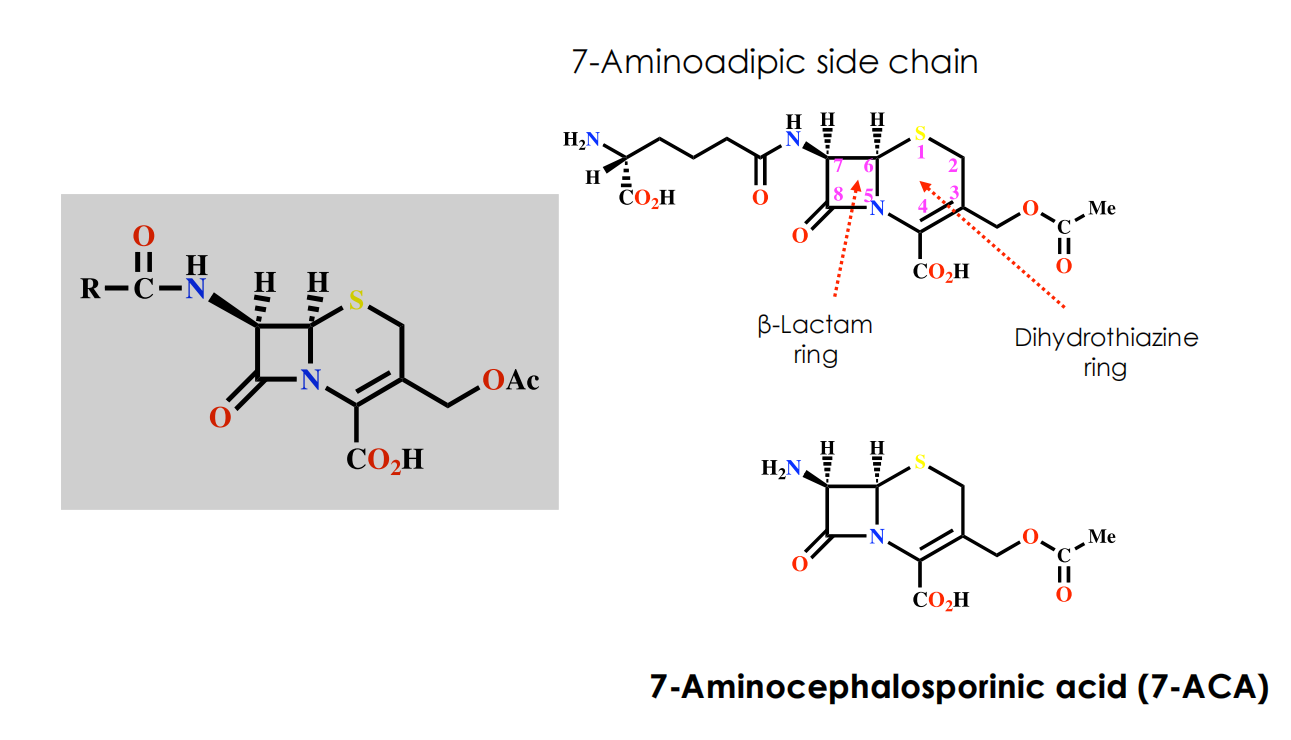
- 7-Aminoadipic side chain
- 7-Aminocephalosporinic acid (7-ACA)
Properties of Cephalosporin C
Disadvantages
- Polar due to the side chain difficult to isolate and purify
- Low potency, not absorbed orally
Advantages
- Non toxic
- Lower risk of allergic reactions compared to penicillins
- More stable to acid conditions
- More stable to β-lactamases
- Ratio of activity vs Gram -ve and Gram +ve bacteria is better
SAR
- The β-lactam ring is crucial to the mechanism
- The carboxylic acid at position 4 is important to binding
- The bicyclic system is important in increasing ring strain
- Stereochemistry is important
- The acetoxy substituent is important to the mechanism
- Possible modifications
- 7-Acylamino side chain
- 3-Acetoxymethyl side chain
- Substitution at C-7
(5) Newer β-Lactam Antibiotics
Thienamycin (Merck 1976)(from Streptomyces cattleya)
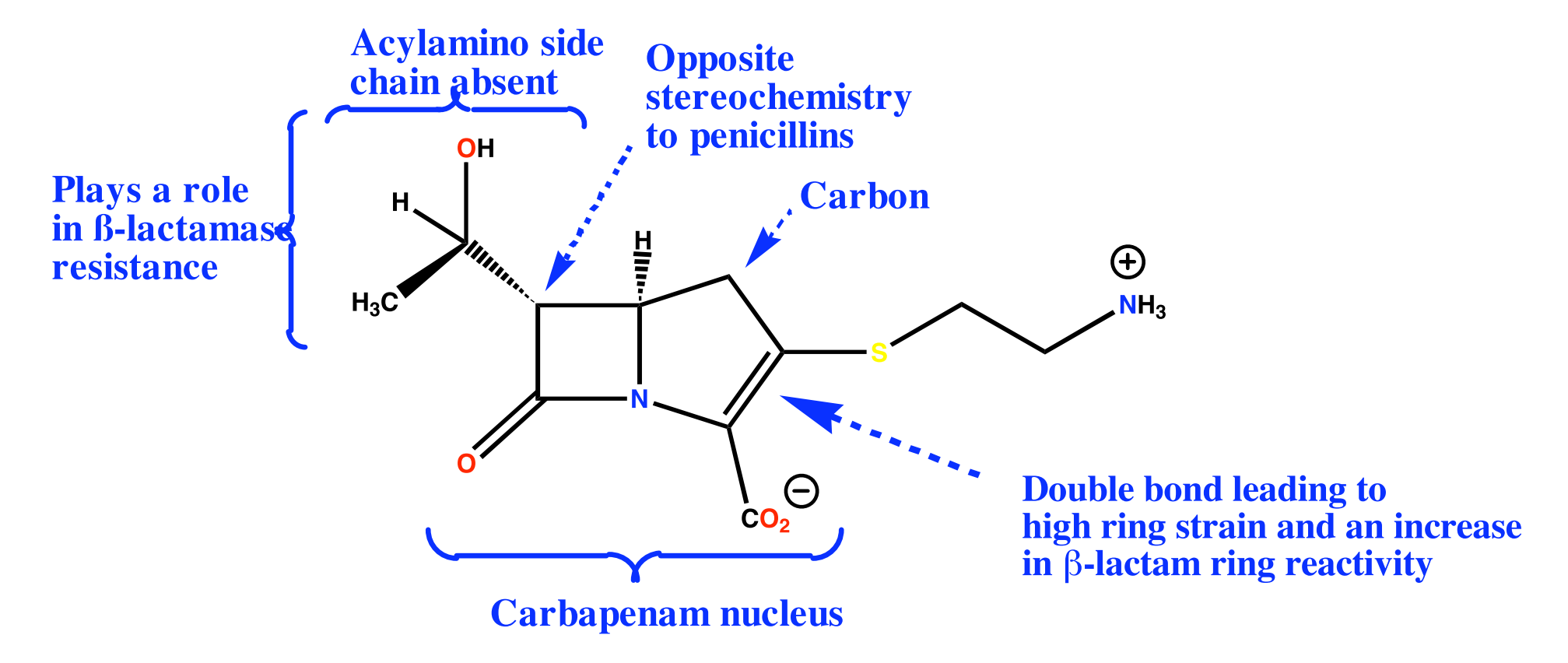
- Potent and wide range of activity vs Gram +ve and Gram -ve bacteria
- Active vs. Pseudomonas aeruginosa
- Low toxicity
- High resistance to β-lactamases
- Poor stability in solution (ten times less stable than Pen G)
(6) β-Lactamase Inhibitors
Clavulanic acid (Beechams 1976)(from Streptomyces clavuligerus)

- Weak, unimportant antibacterial activity
- Powerful irreversible inhibitor of β-lactamases suicide substrate
- Used as a sentry drug for ampicillin
- Augmentin = ampicillin + clavulanic acid
- Allows less ampicillin per dose and an increased activity spectrum
- Timentin = ticarcillin + clavulanic acid
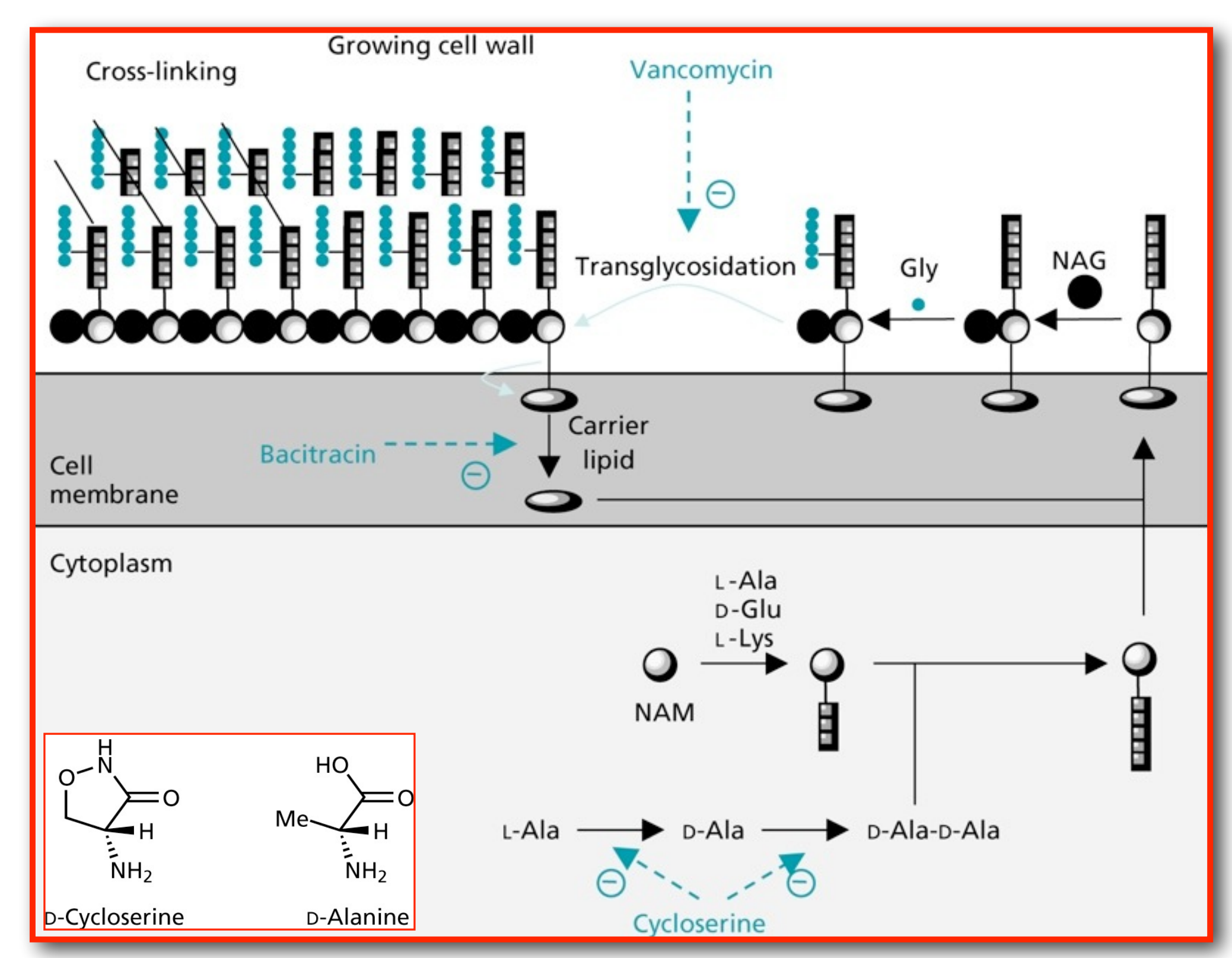
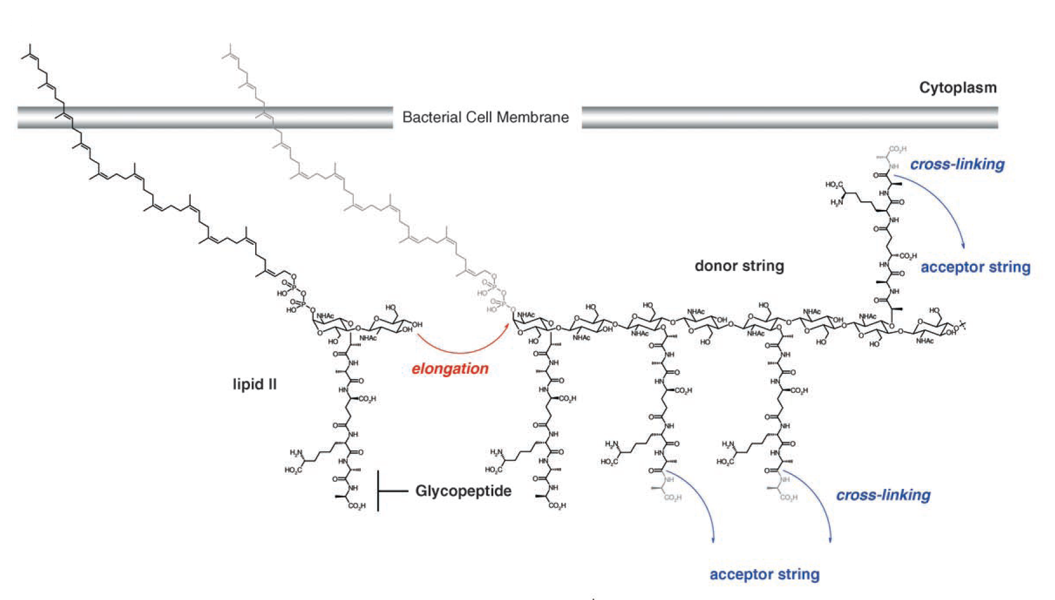
(7) Glycopeptide antibiotics: Vancomycin
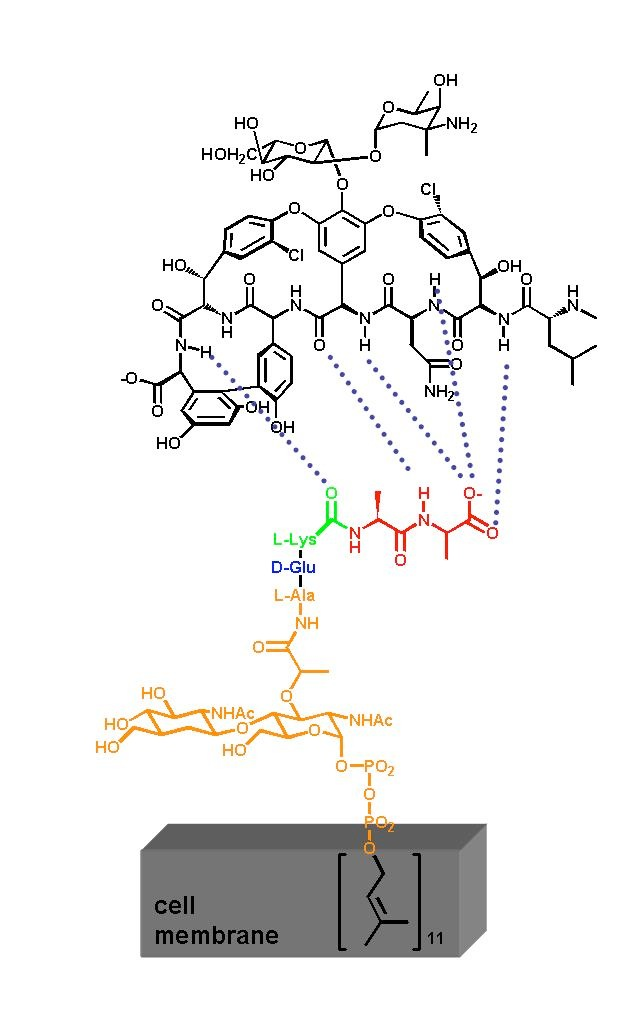
Inhibition of transglycosylation by Vancomycin
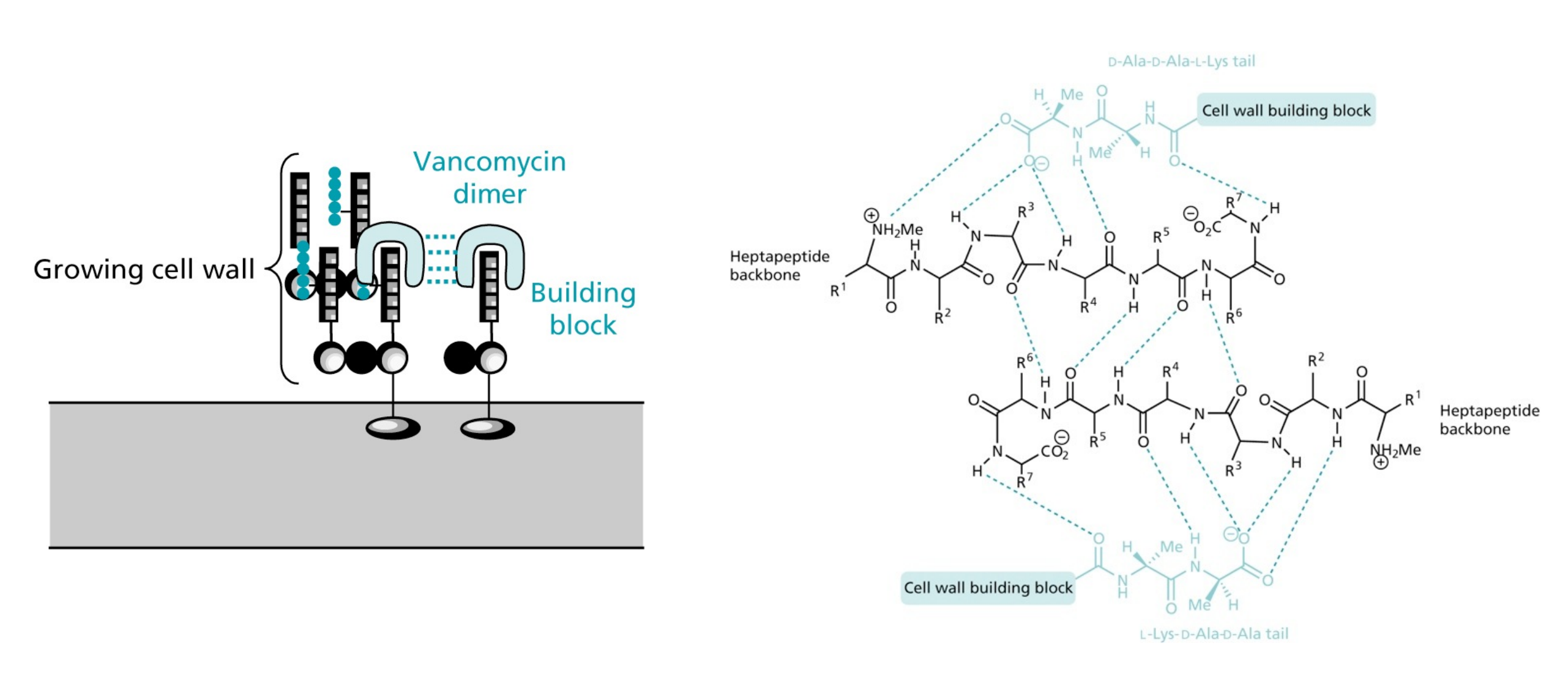
- Vancomycin forms tight hydrogen bonds with the DAla-DAla terminal unit of the pentapeptide, thereby capping the pentapeptides
- Vancomycin can form rather stable head-to-tail dimers
- Due to the large size of Vancomycin it acts as a steric block preventing access from the transglycosylase and transpeptidase enzymes
Structure of the complex between Vacomycin and a tripeptide

3. Agents that act on the plasma membrane
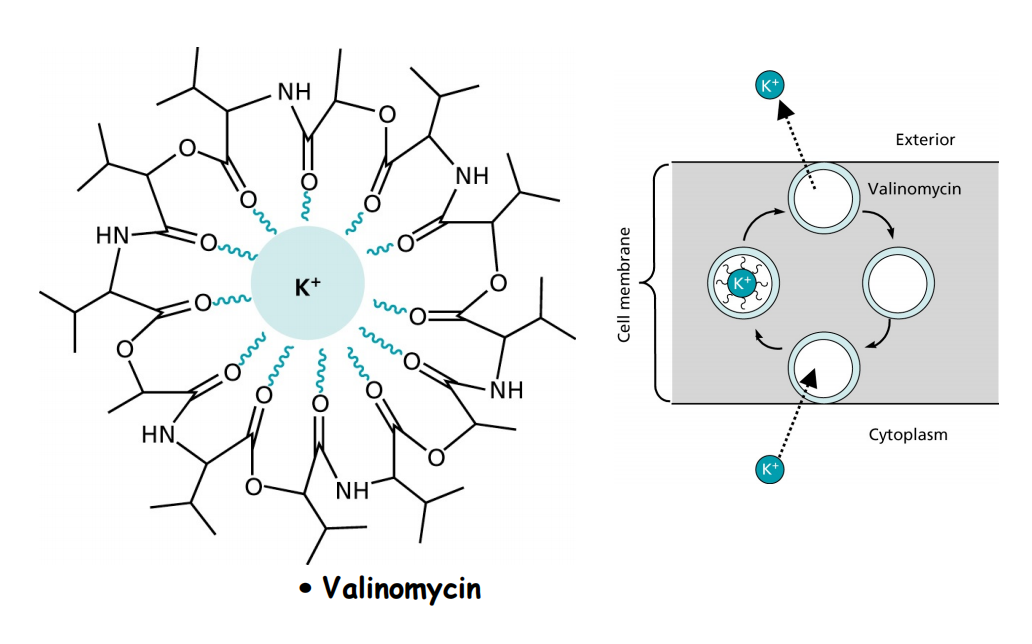
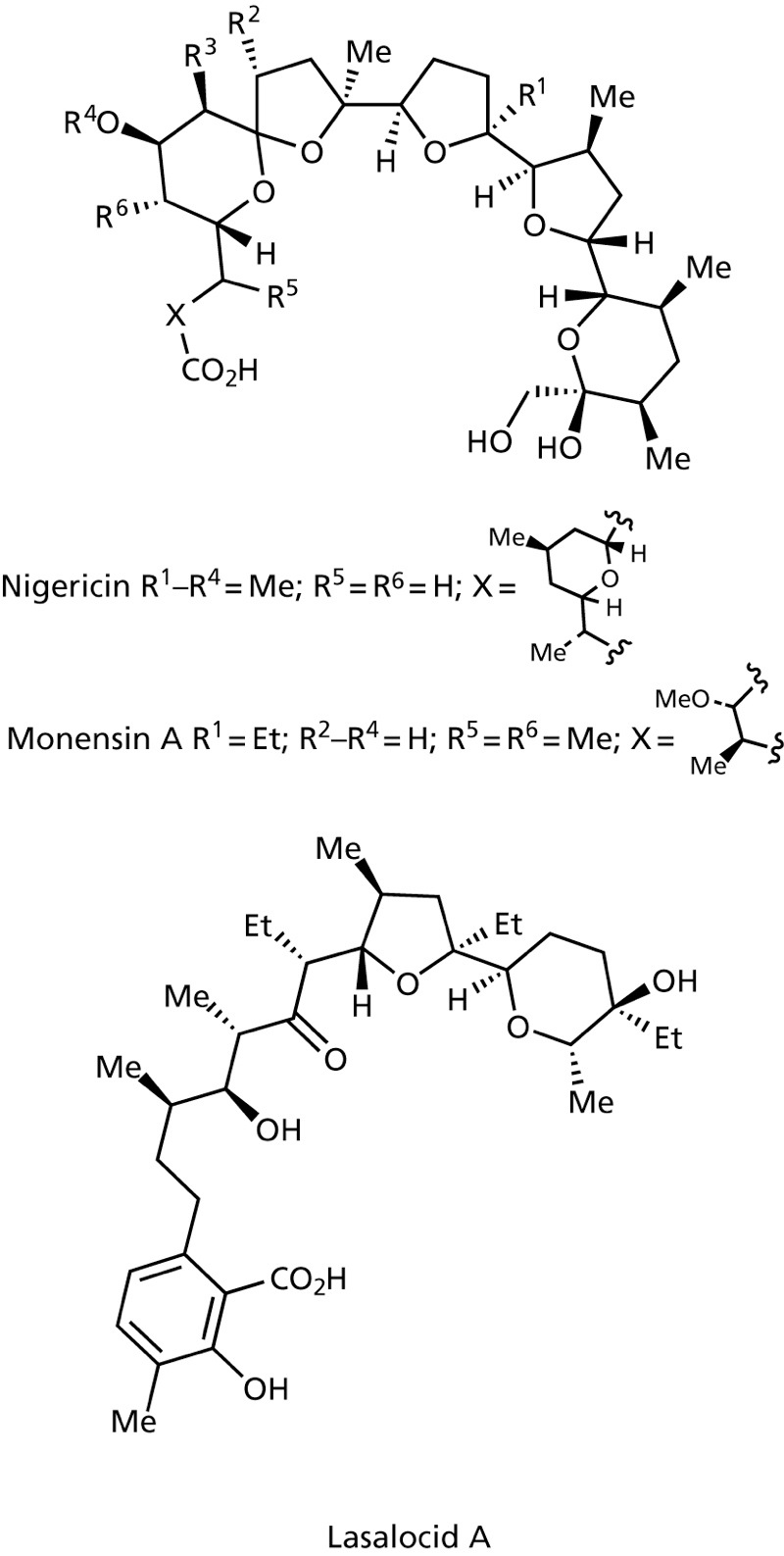
Ionophores such as Valinomycin, Nigericin, Monensin A and Lasalocid A complex ions and transport them in a non-regulated fashion through the membrane disturbing ion concentrations intraand extracellularly.
Gramicidin A forms channels that allow passing of ions, but also of nucleosides. Another such peptide is Polymyxin B, that binds with high selectivity to the bacterial plasma membrane
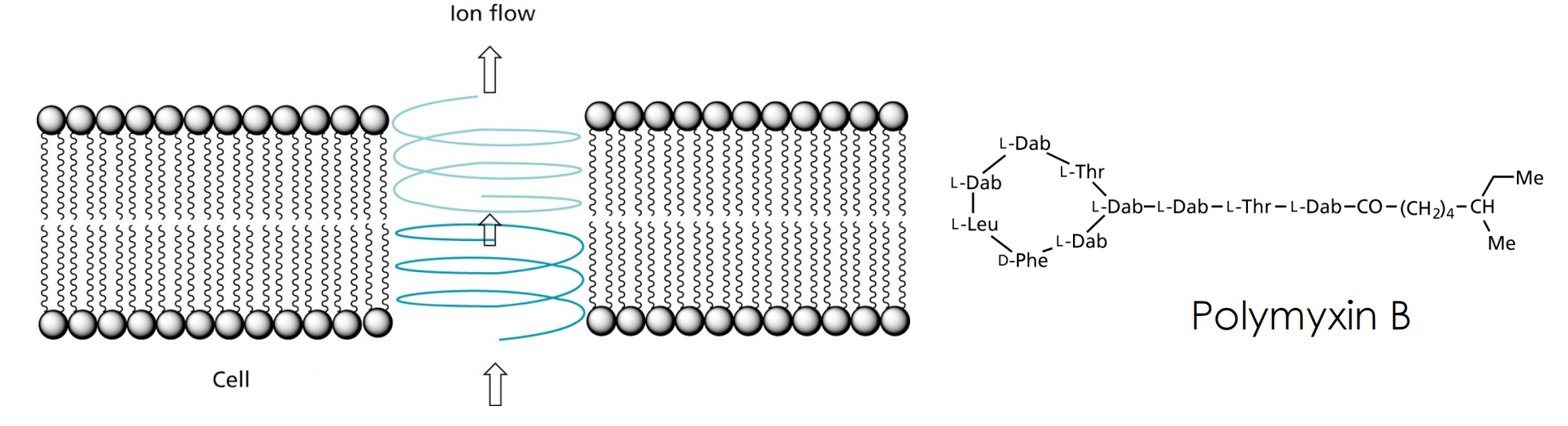
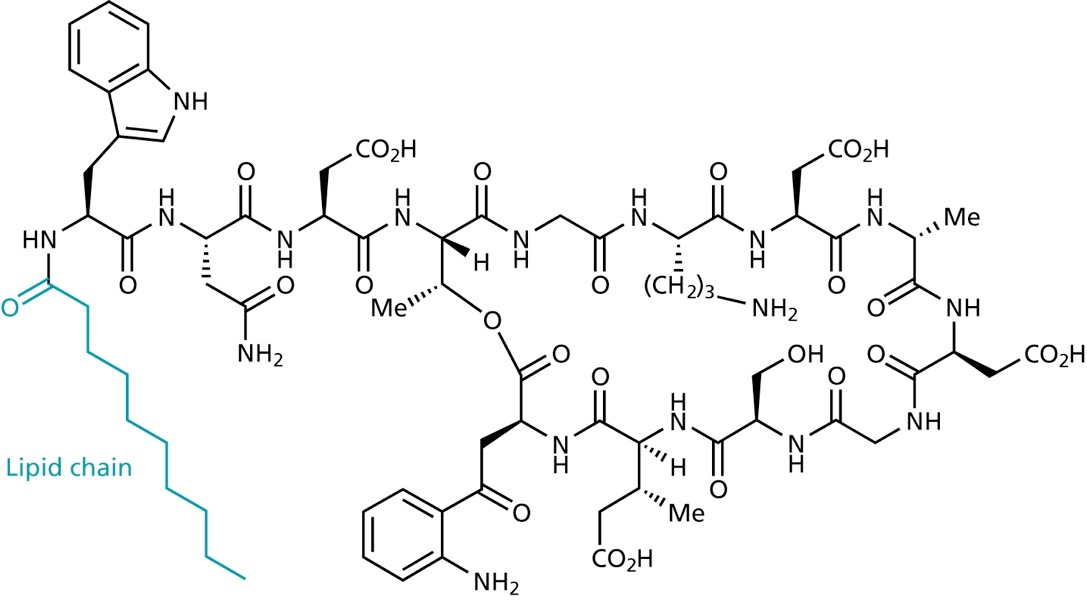
Recently, lipopeptides such as Daptomycin were discovered that disrupt the cell membrane
4. The ribosomes: Drugs that interfere protein synthesis
ribosomes are the location of protein biosynthesis
ribosomes consist of both proteins and RNA
the active site for peptide bond formation consists solely of rRNA
it consists of a large (50S) and a small (30S) subunit
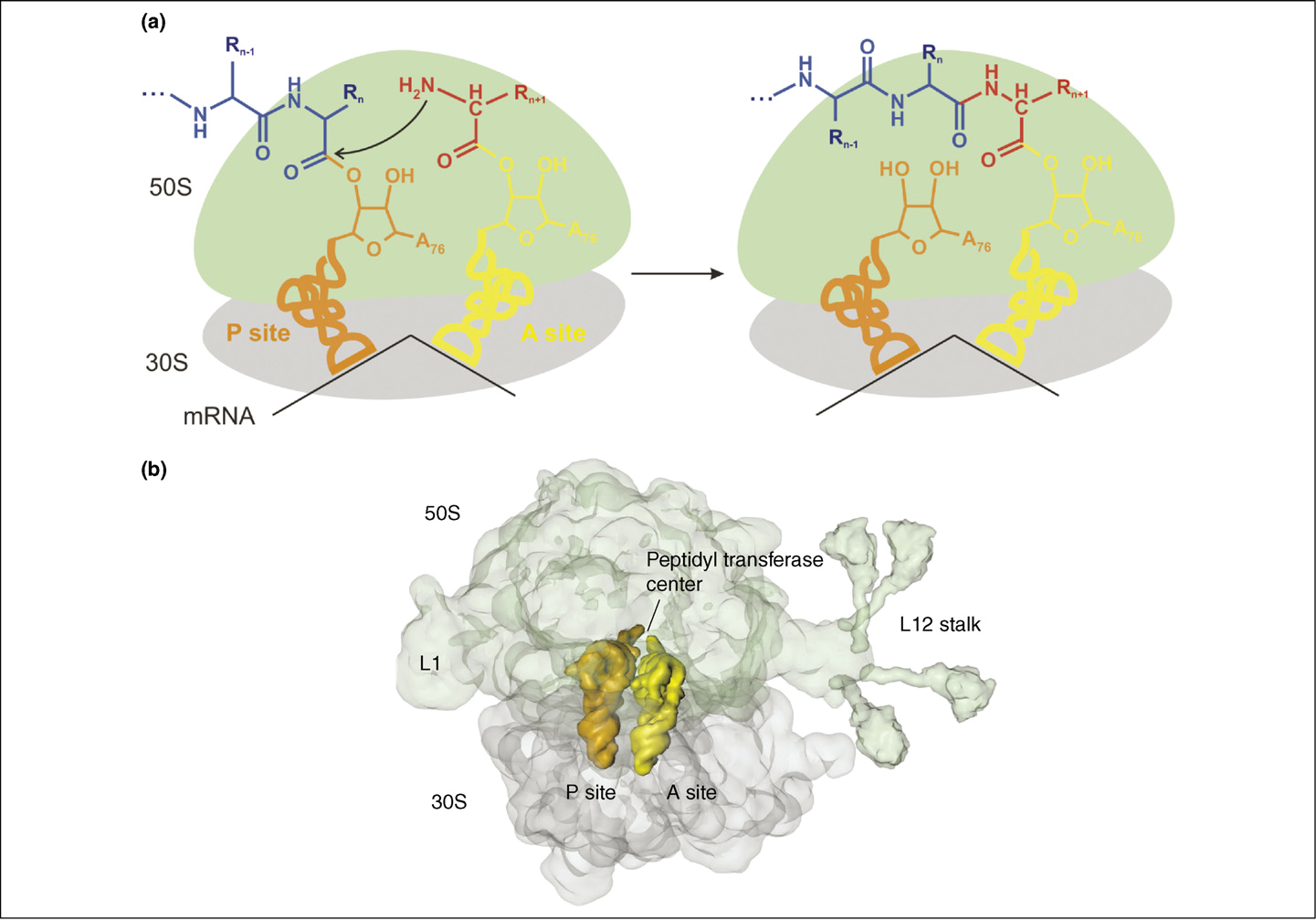
Ribosomes are made of RNA and proteins

- Left: Structure of the 30S subunit from T. thermophilus
- Right: Structure of the 50S subunit from D. radiodurans
Interference with protein synthesis by blocking protein translation

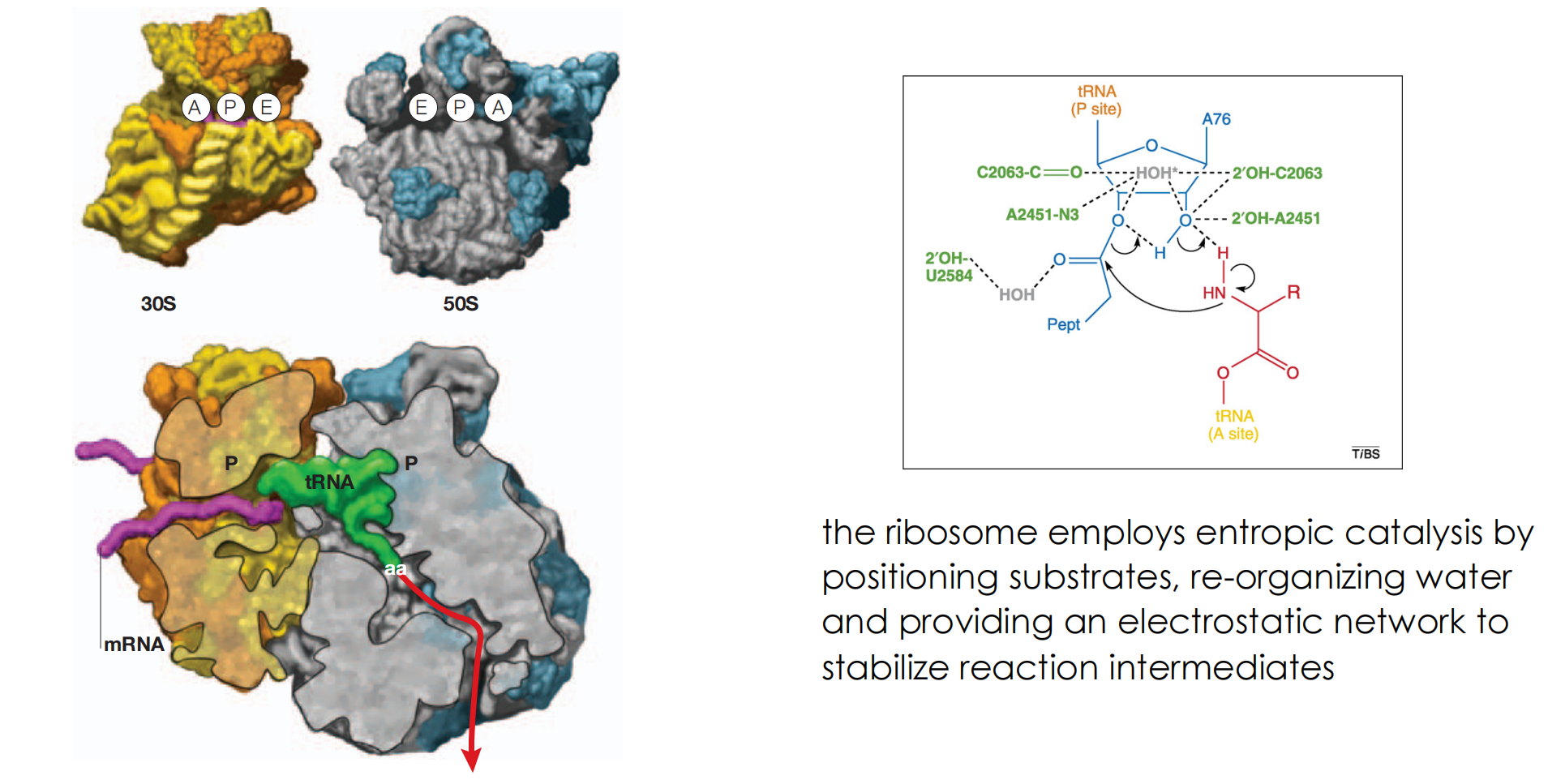
- the ribosome employs entropic catalysis by positioning substrates, re-organizing water and providing an electrostatic network to stabilize reaction intermediates
mRNA passes through two narrow channels on the 30S subunit to be displayed at the interface decoding site where it interacts with the tRNA anticodon
Initially the start codon is translocated into the P site on the 30S subunit to interact with the initiator tRNA charged with Met
the second mRNA codon , in the adjacent A site, accepts the next tRNA. Basepair matching is checked by the decoding site
If it matches, the aminoacyl end of the tRNA is swung to the peptidyl-transferase centre, on the 50S subunit
The ribosome then moves along the mRNA to bring the next codon into the aminoacyl (A) site. In addition, the tRNA holding the nascent peptide strand is translocated into the peptidyl (P) site
At the end the full strand is moved into the E (exit) site, from where it is ejected
Binding sites for antibiotics
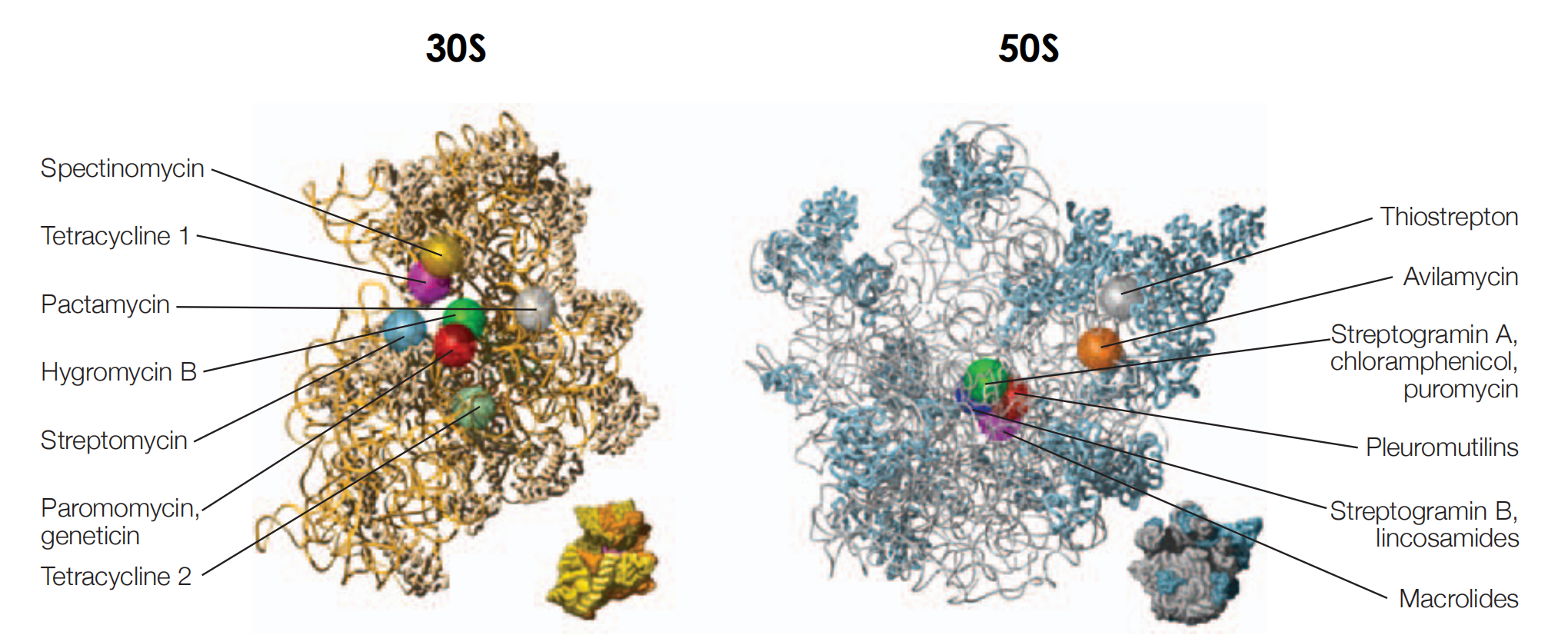
- the chain of the nascent polypeptide passes through a narrow tunnel on the 50S subunit, which is 100 Å in length, and runs from the peptidyltransferase centre to emerge from the back of the ribosome
- the macrolides bind in the narrow tunnel blocking exit of the nascent chain
(1) Binding site on the 30S subunit
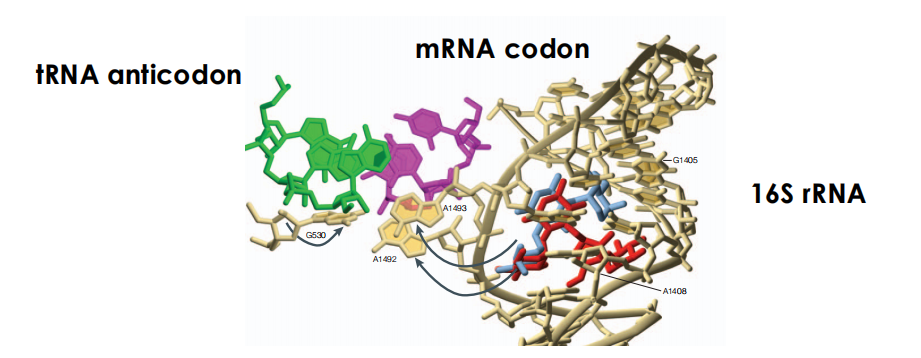
During protein synthesis nucleotides A1492 and A1493 are flipped out of the 16S rRNA helix to interact with the mRNA codon and its cognate tRNA anticodon at the ribosomal A-site
some aminoglycosides antibiotics bind within the helix and induce a similar but not identical flip-out of A1493 and A1492
as a result basepair matching is NOT required leading to the addition of incorrect amino acids to the nascent chain.
Aminoglycosides
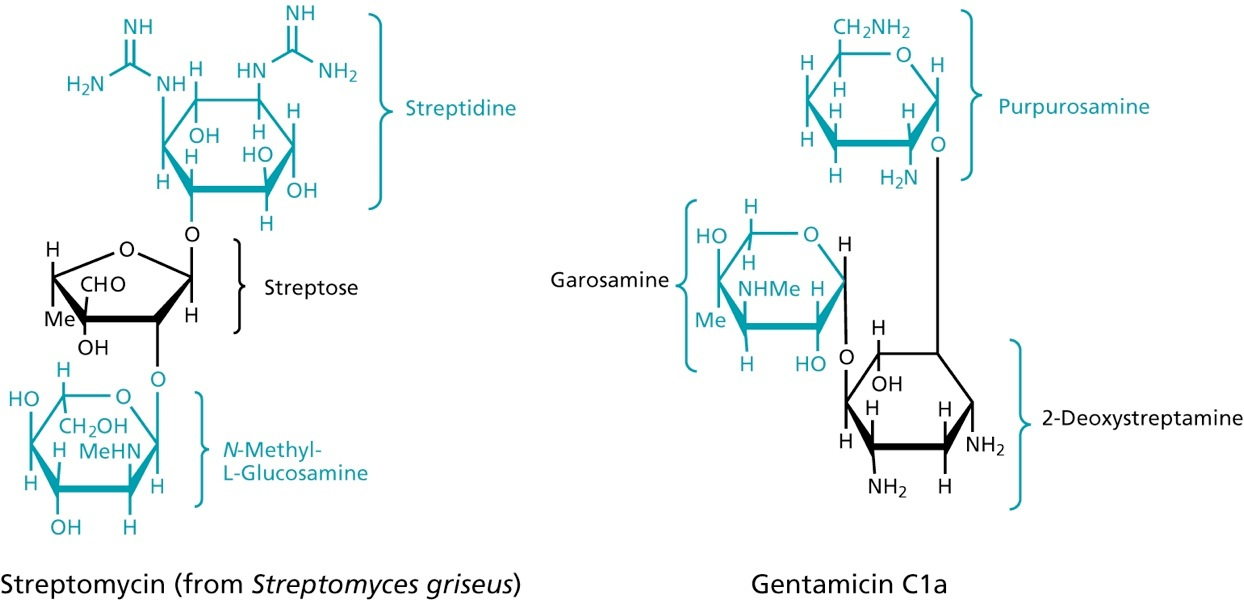
at physiological pH charged -> binding to lipopolysaccharide, phospholipids and permeabilize the membrane
bind to the 30S ribosomal subunit, thereby preventing the movement along the mRNA, so that the triplet code cannot be read
don’t bind to human ribosomes
polar molecules, need to be injected, unable to cross the BBB
Tetracyclines
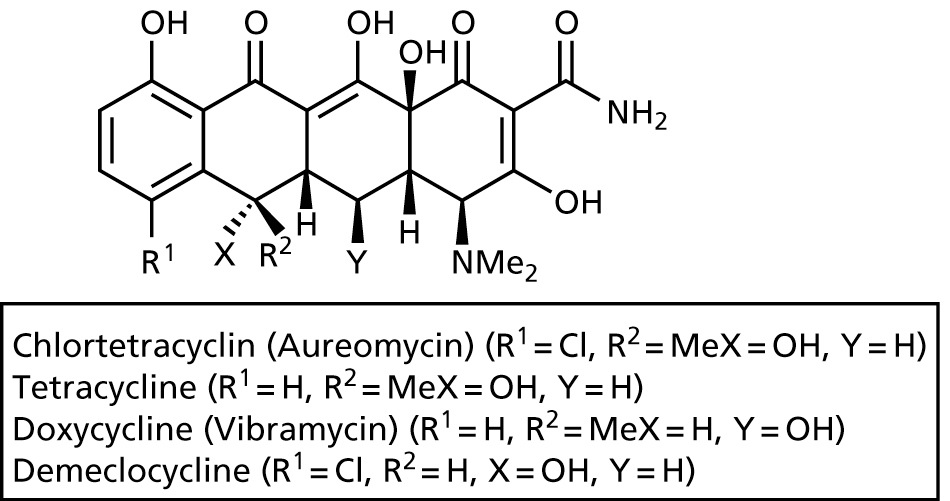
- bacteriostatic, widely prescribed
- broad-band antibiotic against gram-positive and gram-negative bacteria
- bind to 30S subunit of ribosomes, and thereby prevent aminoacyl-tRNA from binding
- passes through membrane via porins
- inhibits also protein synthesis in mammalian cells, but more effective in bacteria
Chloramphenicol
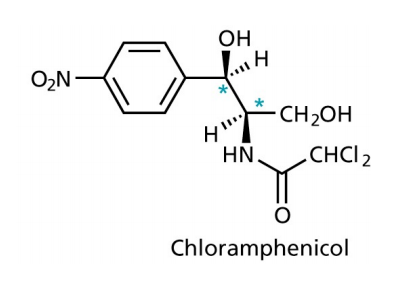
- binds to the 50S subunit of the ribosomes, inhibiting the movement of the ribosomes along the mRNA
- used to cure eye infections and typhoid
- quite toxic
- bacteria that contain the gene for chloramphenicol transferase are resistant
Macrolides
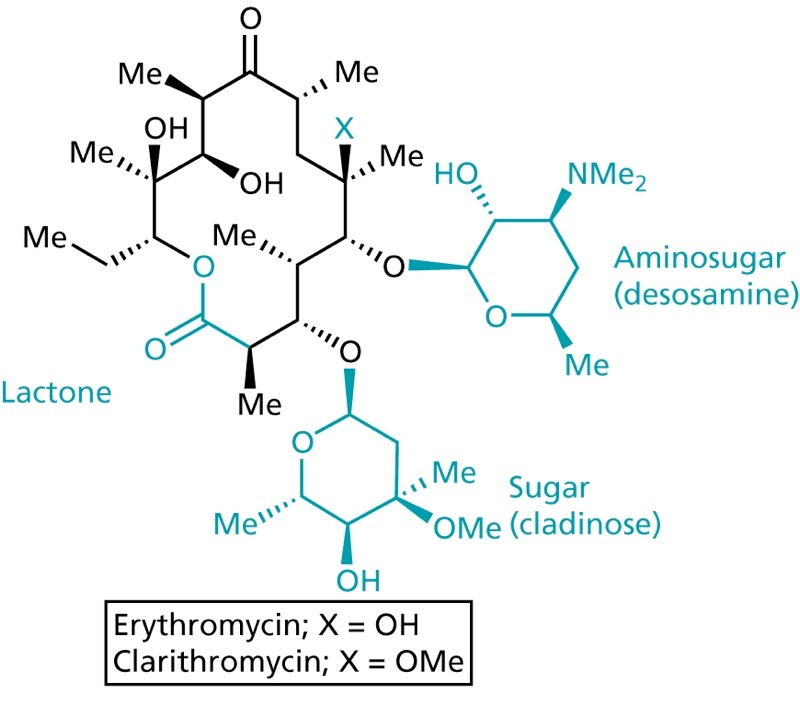
- one of the safest antibiotics
- binds to the 50S subunit of the ribosome, thereby inhibiting translocation
- can be orally taken
- acid sensitive
Oxazolidones
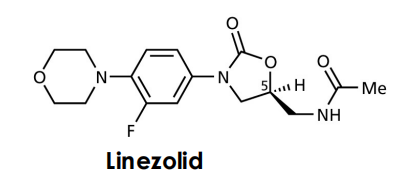
Before protein synthesis the 30S and the 50S subunits must associate to form the 70S ribosome. This step is inhibited
5. Antibiotics inhibiting nucleic acid transcription and replication
Quinolones and fluoroquinolones
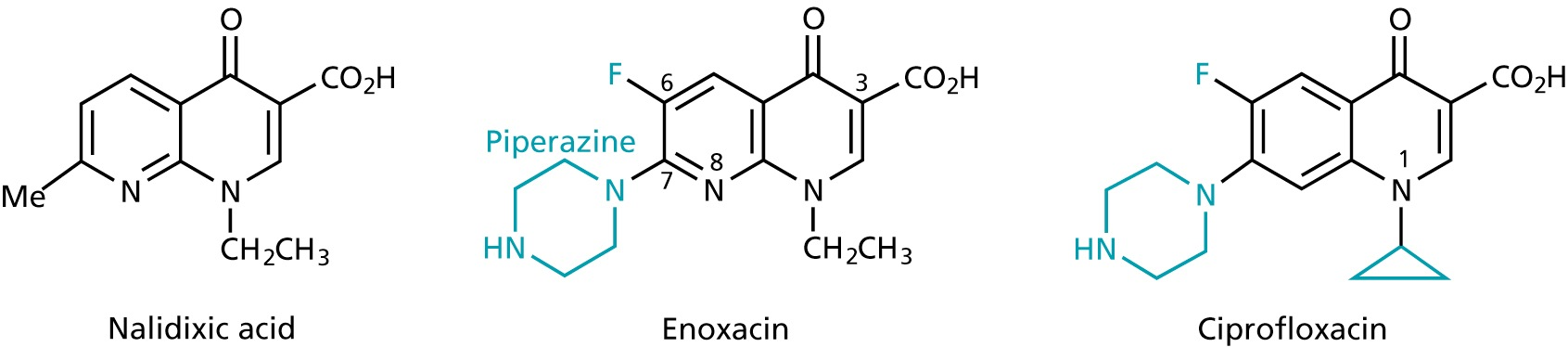
- 1st generation
- broad-band antibiotics
- inhibit the replication and transcription of bacterial DNA by inhibiting the topoisomerases
- used for infections involving the urinary, respiratory and gastrointestinal tracts as well as against infections of skin, bone and joints
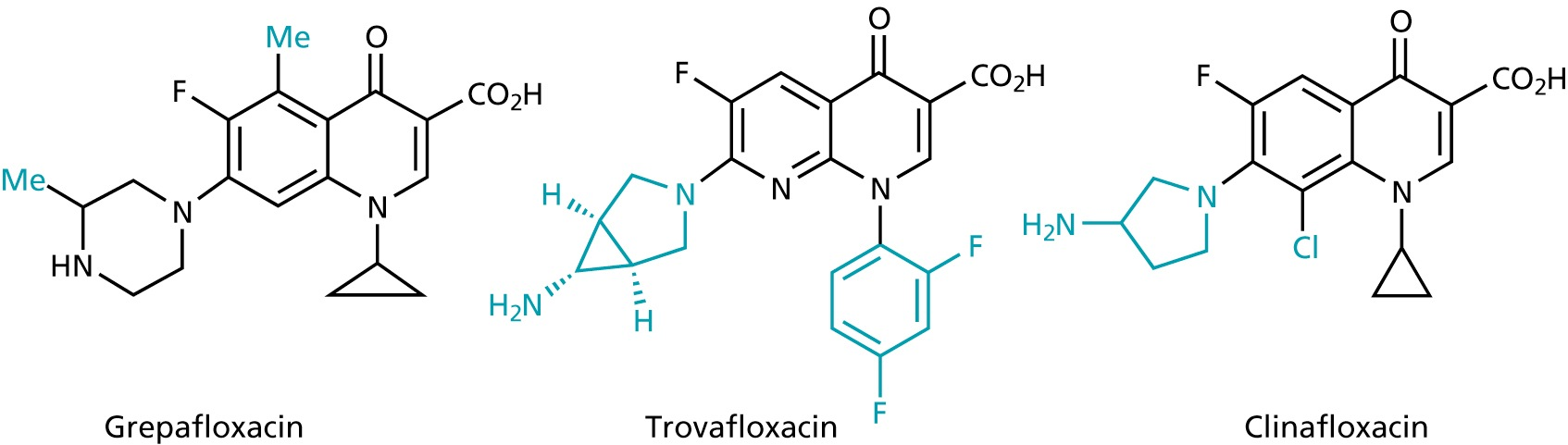
- 2nd generation
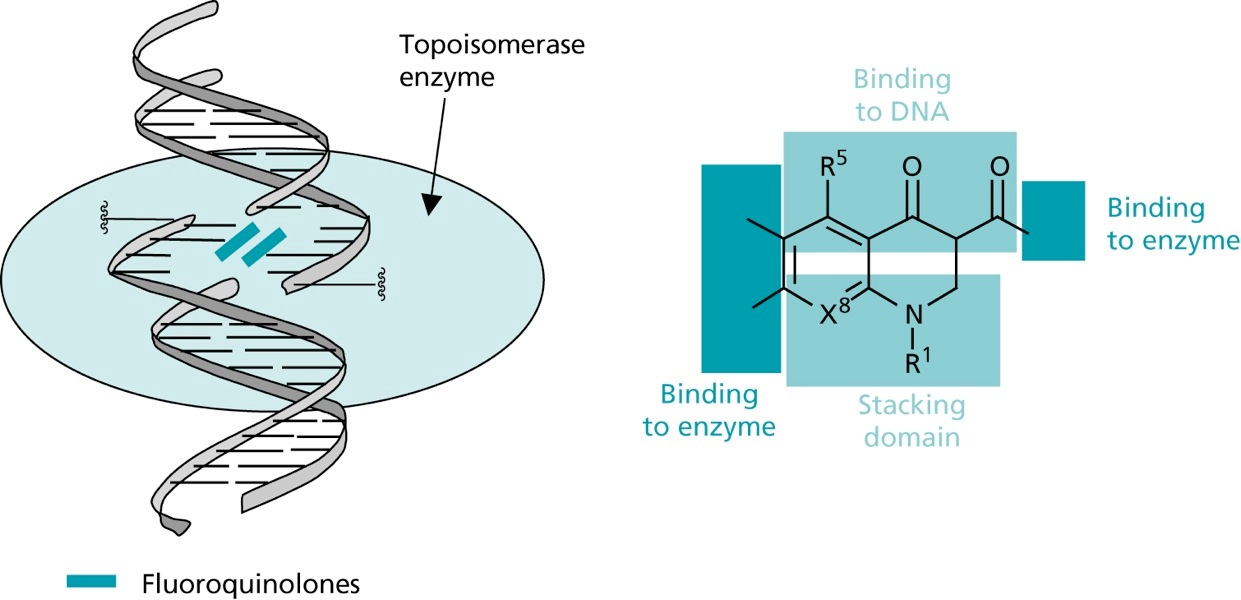
- during replication, supercoiling of the DNA is removed by helicases
- the created tension is released by topoisomerases, that cut break both strands of the DNA and rejoin them
- fluoroquinones inhibit topoisomerase IV in gram +ve bacteria with1000 fold selectivity over the human enzyme
- in gram -ve bacteria, the topoisomerase II (DNA gyrase), that reintroduces supercoiling after replication and transcription is inhibited
Aminoacridines
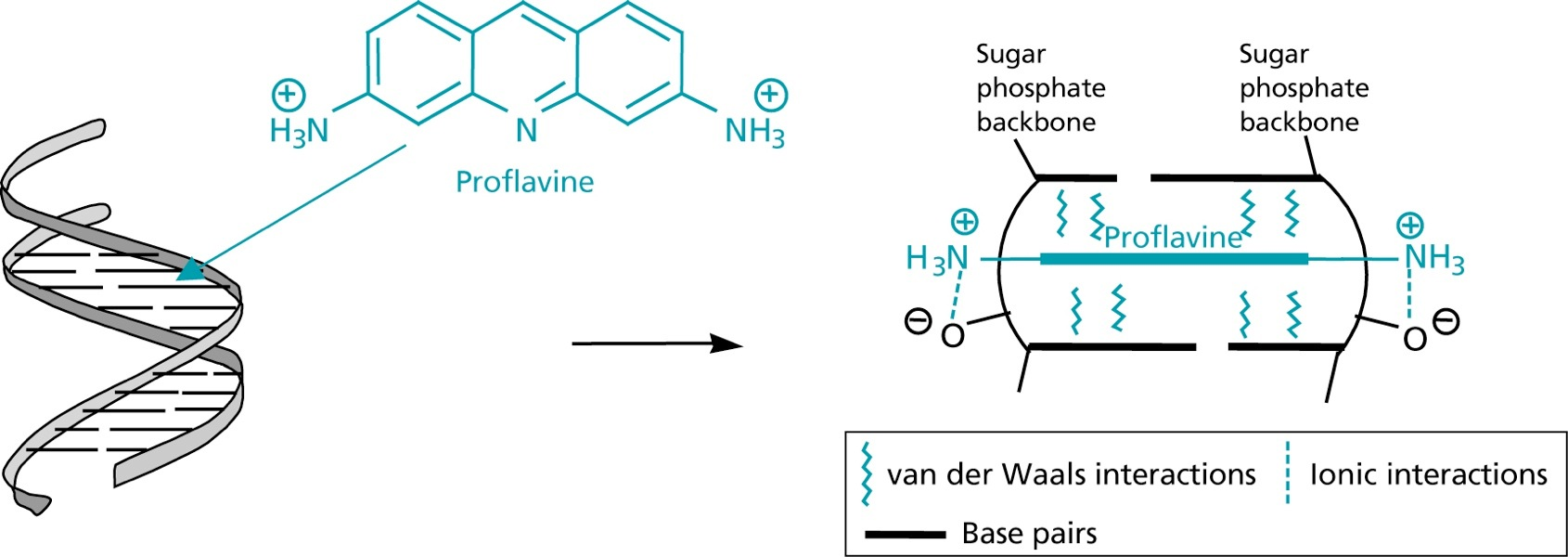
- directly interact with bacterial DNA through intercalation
- thereby prevents replication and transcription (but toxic)
Rifamycins
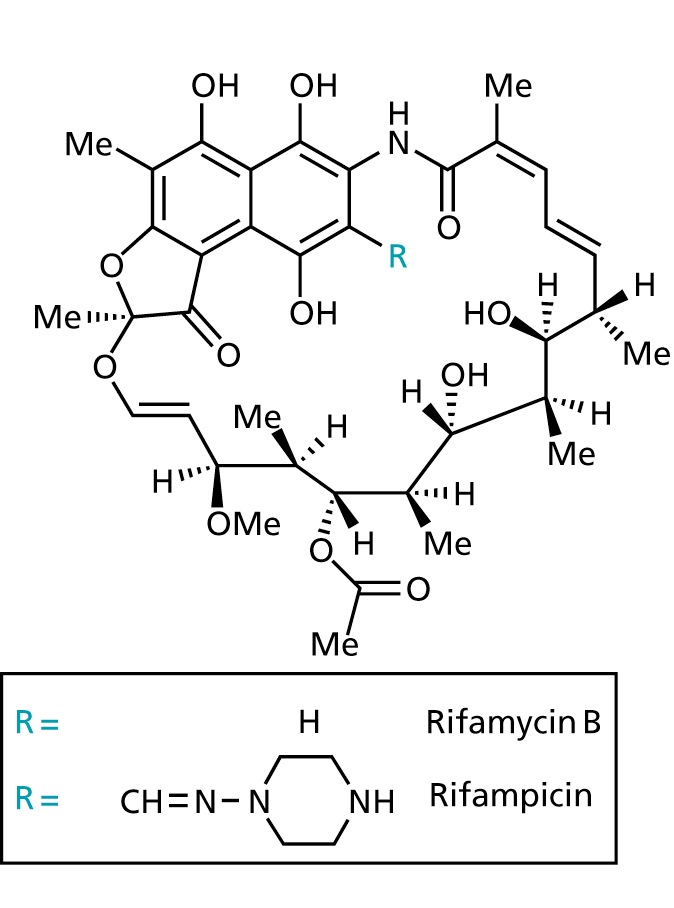
- binds non-covalently to the bacterial DNA-dependent RNA polymerase
- does not attach to eukaryotic RNA polymerase
- bactericidal
- used to treat tuberculosis and staphylococci infections
三、AIDS, HIV
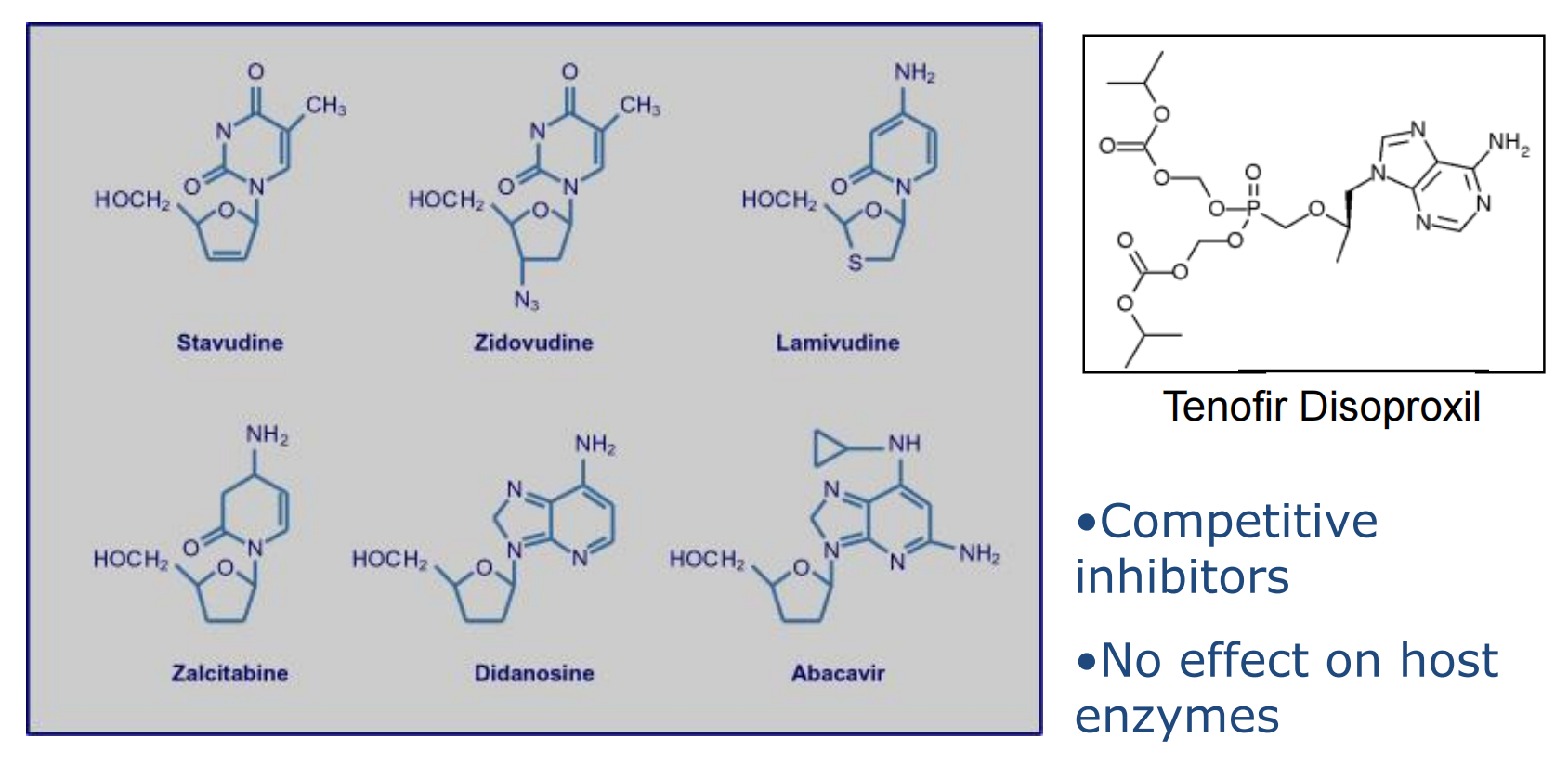
AIDS represents the classic example of immunodeficiency disease caused by extrinsic factors, in this instance the human immunodeficiency virus (HIV)
What is HIV?
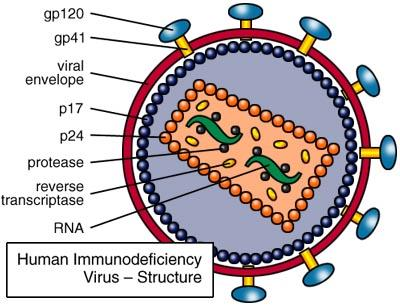
HIV = Human Immunodeficiency Virus
Destroys CD4 cells (T-cells and macrophages)
AIDS = Acquired Immunodeficiency Virus(~10 years after infection)
This virus exhibits a strong tropism for CD4 T helper cells; these become depleted, giving rise to increased frequency of opportunistic infections and malignancies in infected individuals.
The Life Cycle of HIV
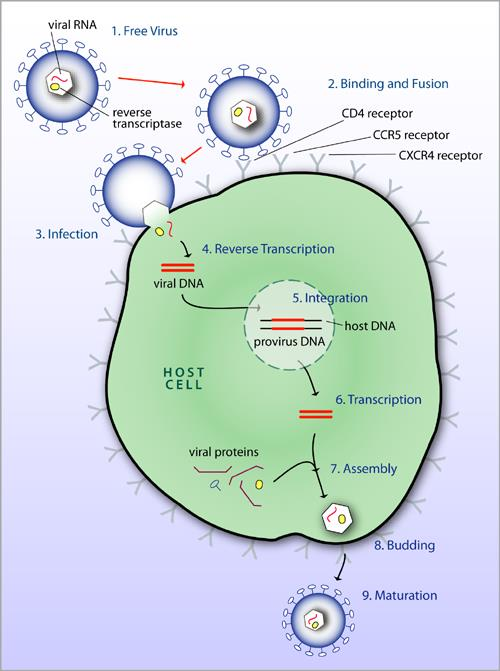
- Free Virus
- Binding and Fusion
- Infection
- Reverse Transcription
- Integration
- Transcription
- Assembly
- Budding
- Maturation
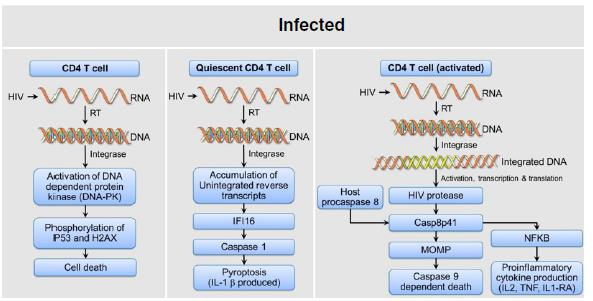
Mechanism of HIV induced CD4+ cell death
Introduction to Drugs
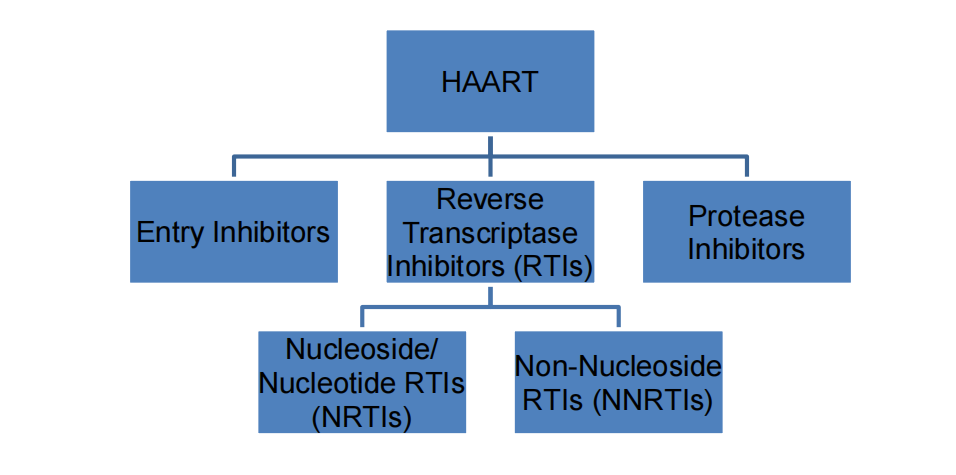
- HAART: highly active anti-retroviral therapy
1. Entry inhibitor: Maraviroc
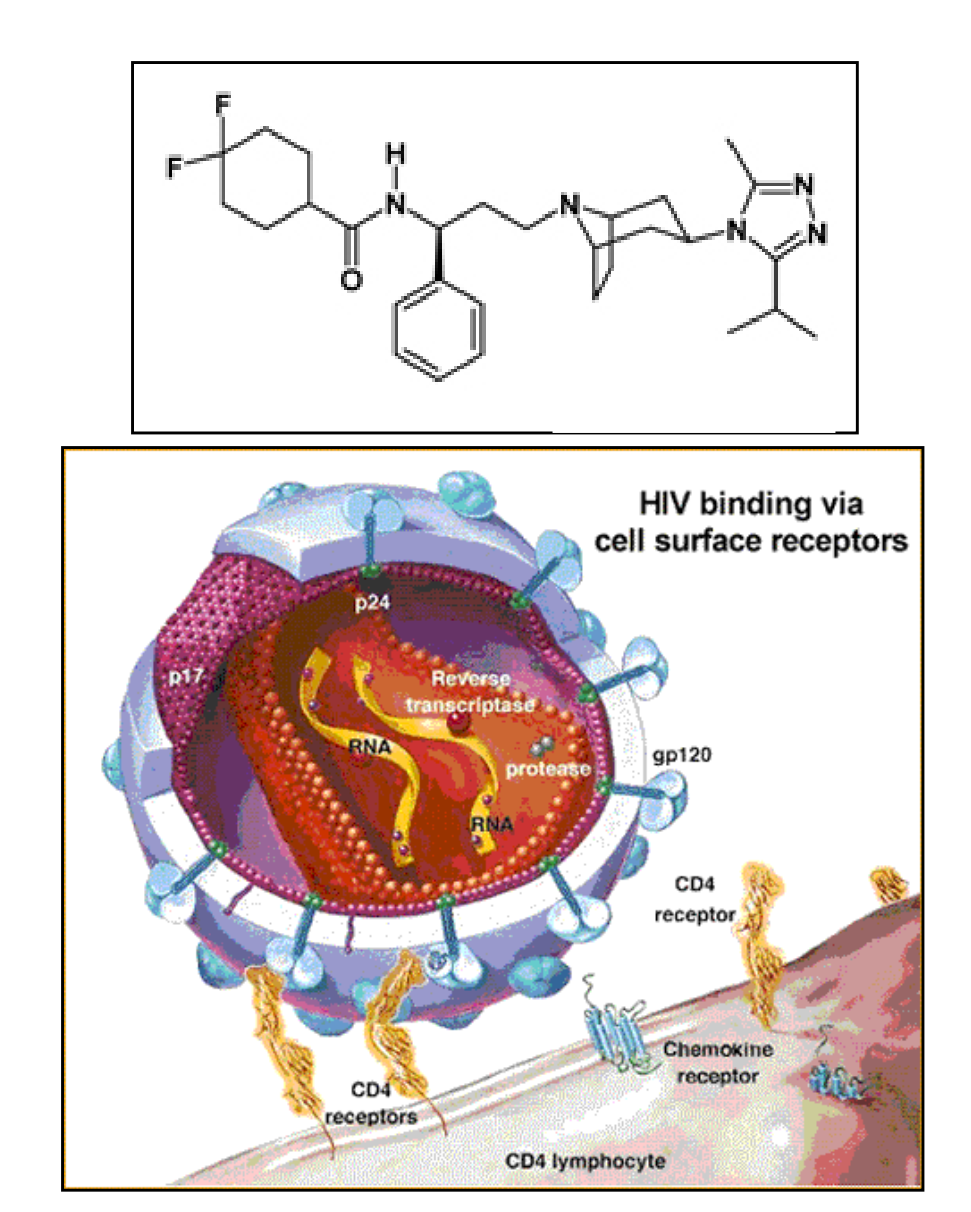
- 1st oral entry inhibitor
- Blocks coreceptor CCR5
- Resistance from one or more of several mutations in the V3loop of gp120 or gp160
- Possible Side Effects:Cough, Fever, Dizziness,Headache, Lowered BP,Nausea, and Bladder Irritation
2. Reverse Transcriptase Inhibitors: NRTIs
Competitive inhibitors
No effect on host
enzymes
NRTI: Zidovudine
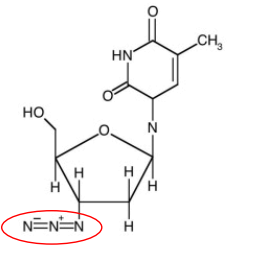
1st approved drug for the treatment of AIDS
Phosphorylated by 3 cellular enzymes to form an active nucleotide triphosphate
Analogue of deoxythymidine where the3’ hydroxyl is replaced by an azide group
The triphosphate is attached to the growing DNA chain, but cannot be extended.
Side effects may include anemia,nausea, headache, changes in body fat,and discoloration of nails.
3. Reverse Transcriptase Inhibitors: NNRTIs
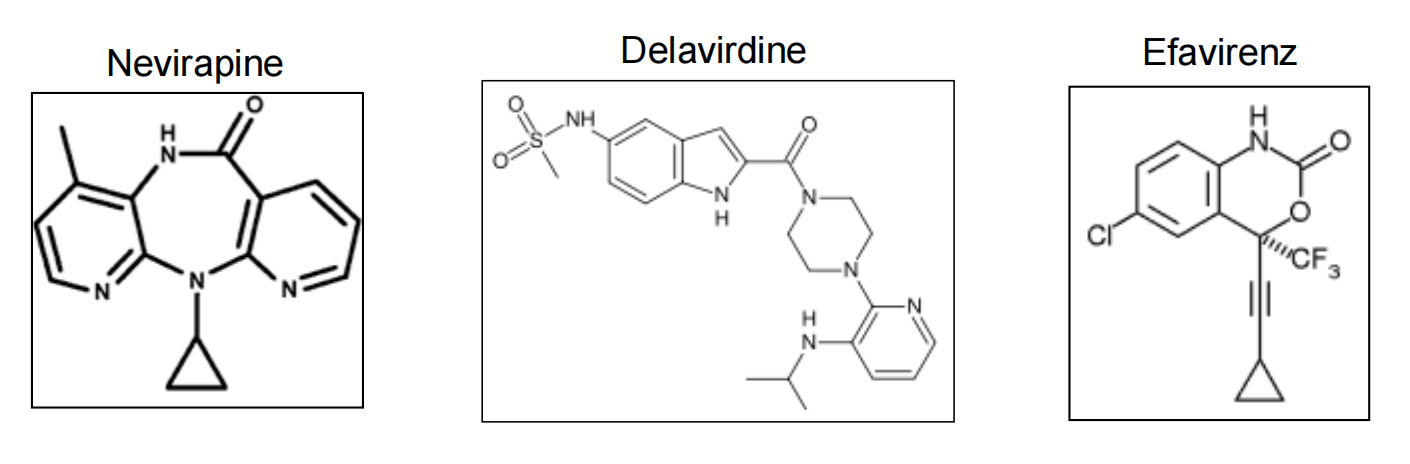
- Noncompetitive inhibitors
- Only active against HIV-1
- Easily vulnerable to resistance
NNRTI: Efavirenz
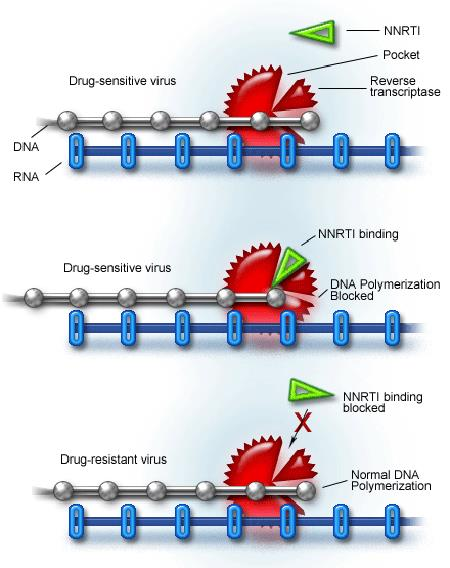
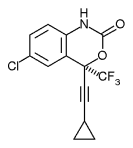
- Made from X-ray crystallography of the RT binding site
- Active against many variants of HIV
- Replacing Lys-103 with asparagine (K103N mutation) causes resistance
- Standard noncompetitive inhibitor
- Possible Side Effects: Insomnia, Depression, Rash, Nausea, and Birth Defects
4. Protease inhibitor: Fosamprenavir
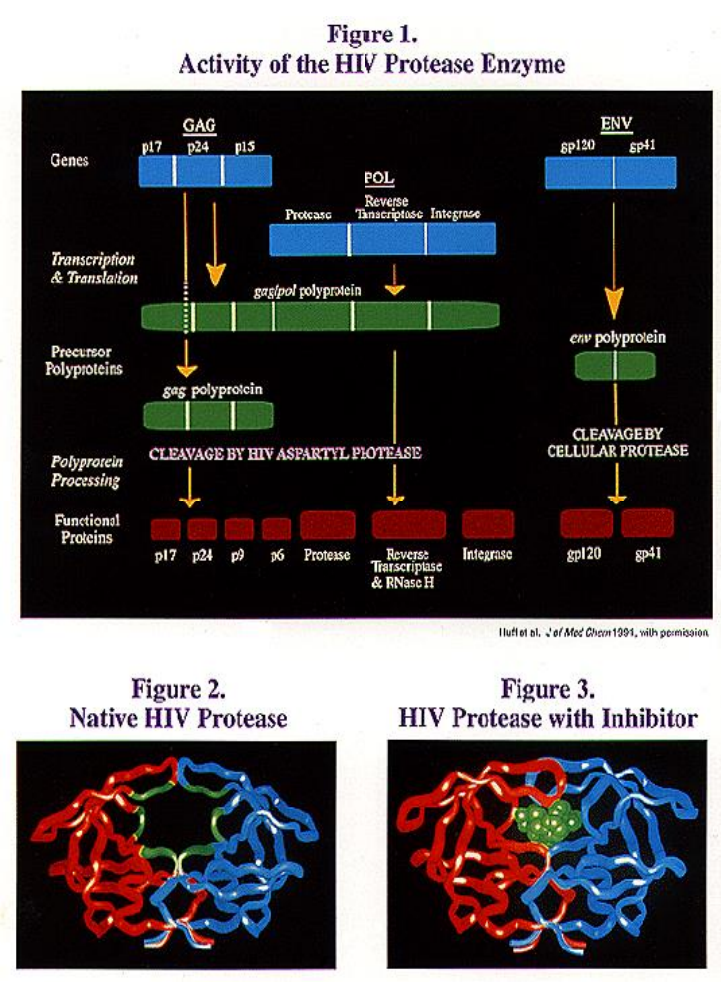

- Increased water solubility and improved oral bioavailability
- Metabolized to form amprenavir, which is the active ingredient
- Because it must be metabolized, it is time released and requires less dosages (4 instead of 16 pills per day)
- Possible Side Effects: Nausea, Vomiting, Diarrhea, Loose Stool, Hyperglycemia, and Fatigue
The Future in Treatment
Integrase inhibitors (raltegravir and elvitegravir) in advanced development
CXCR4 inhibitors currently in development for HIV entry blockage
Drugs with a broader spectrum of activity and less vulnerable to induce resistance
More combination drugs like Atripola (efavirenz, tenofovir, emtricitabine) so that treatment can consist of a single pill taken once daily
Making drugs more affordable and available to more people ($1500/month and $618,000/lifetime)