L03 Citric Acid Cycle and Glyoxylate Cycle
一、Overview of Pyruvate Oxidation and the Citric Acid Cycle
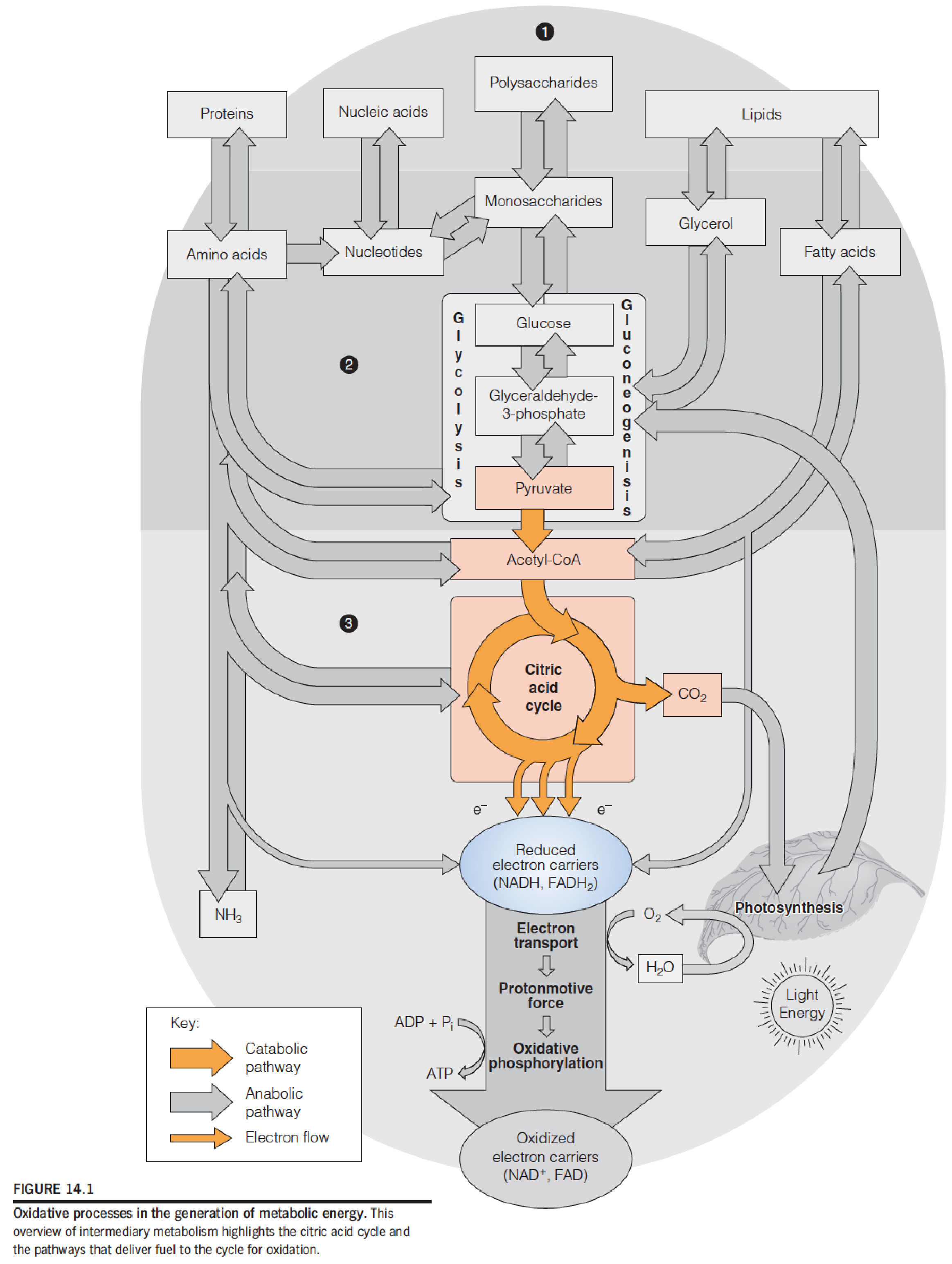
The citric acid cycle is the central oxidative pathway in respiration, the process by which all metabolic fuels—carbohydrate, lipid, and protein—are catabolized in aerobic organisms and tissues.
Most of the energy yield from substrate oxidation in the citric acid cycle comes from subsequent reoxidation of reduced electron carriers.

The three stages of respiration
- Stage 1 - carbon from metabolic fuels is incorporated into acetyl-CoA.
- Stage 2 - the citric acid cycle oxidizes acetyl-CoA to produce CO2, reduced electron carriers, and a small amount of ATP. 经过cycle (Stage 2)之后,消耗了acetyl-CoA里面的两个C,其余的物质都没有改变
- Stage 3 - the reduced electron carriers are reoxidized, providing energy for the synthesis of additional ATP.

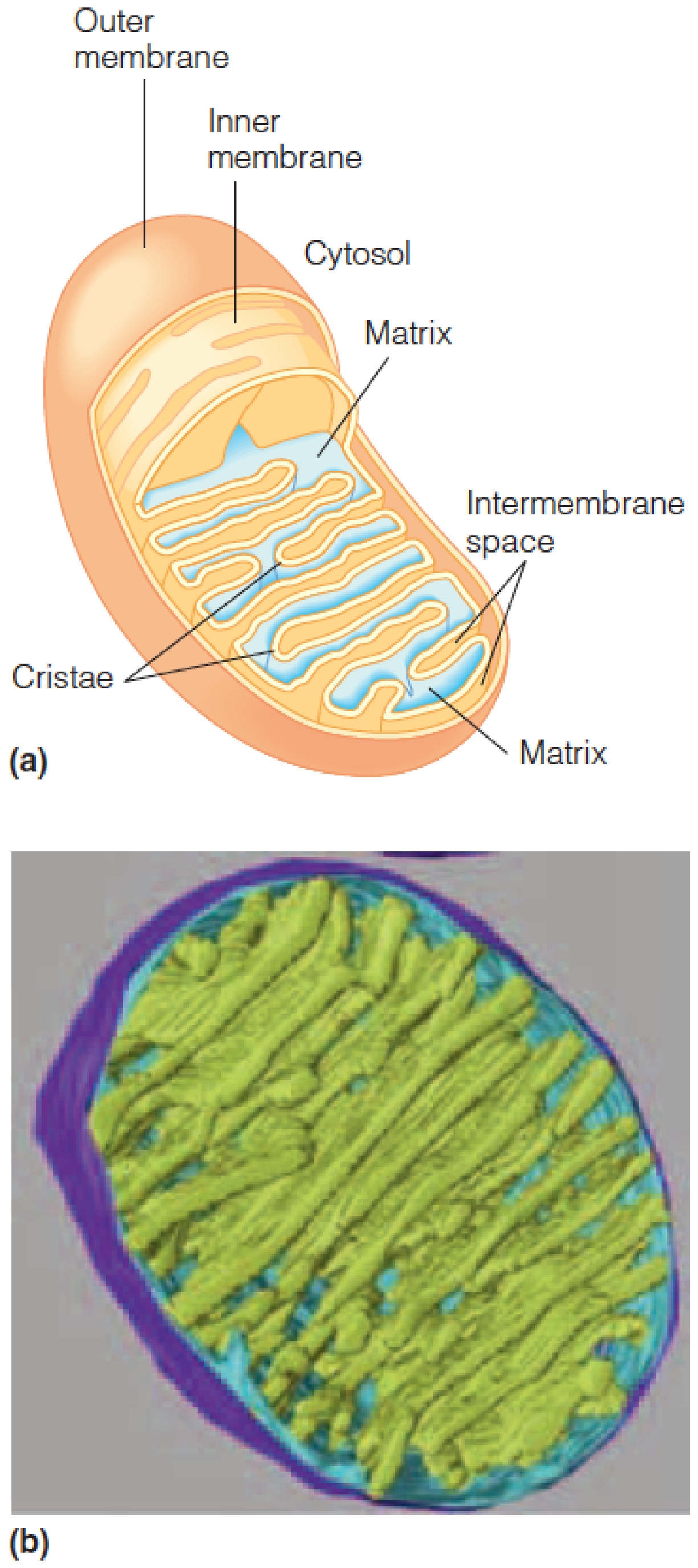
Schematic of a mitochondrion.
Computer model generated from electron tomograms of a mitochondrion.
Dehydrogenases(脱氢酶) catalyze substrate oxidations.
Oxidases(氧化酶) catalyze the subset of oxidations in which O2 is the direct electron acceptor.
Oxygenase(加氧酶): oxygen combines directly with the substrate oxidized.
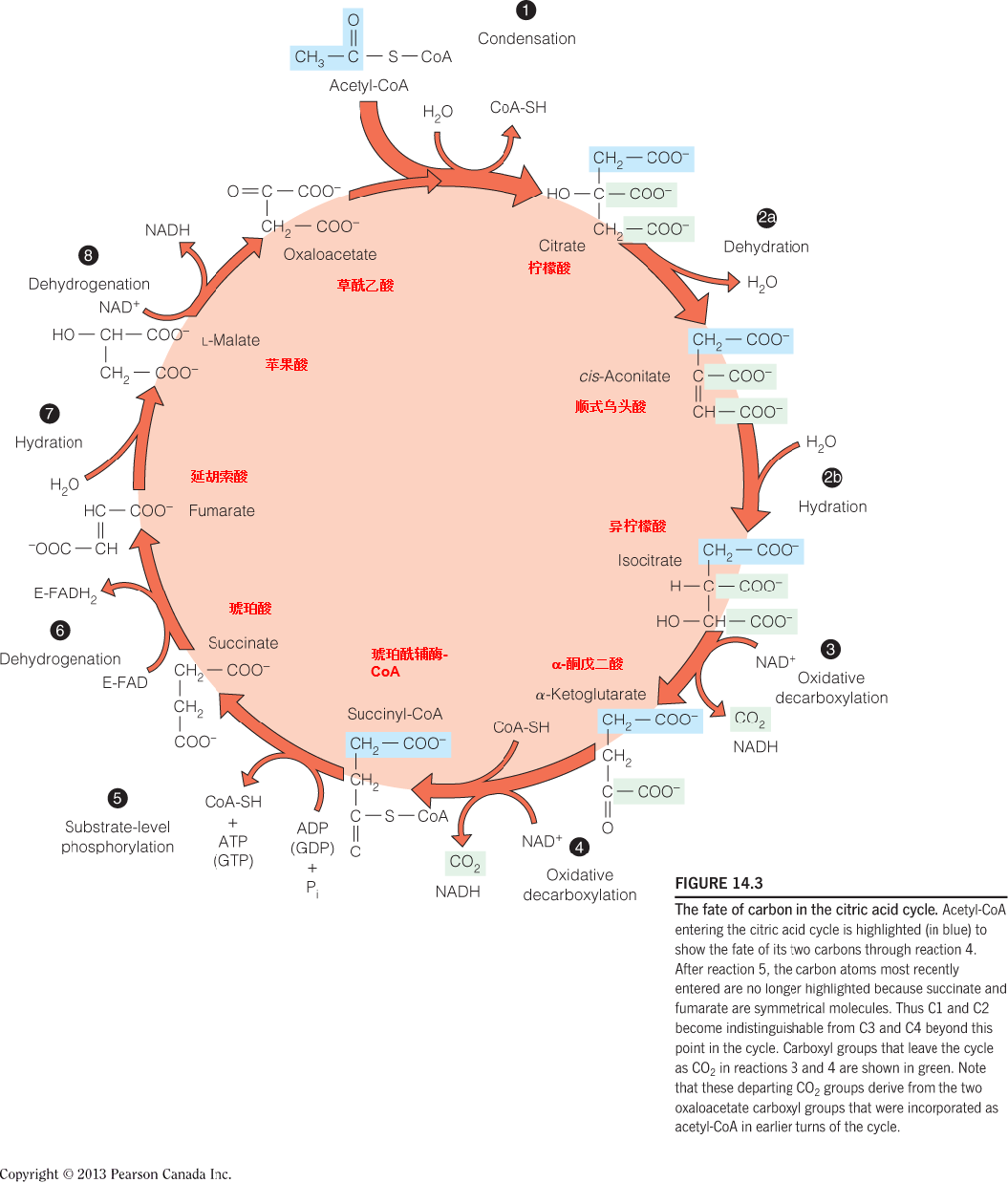
The fate of carbon in the citric acid cycle
Note that these departing CO2 groups derive from the two oxaloacetate carboxyl groups that were incorporated as acetyl-CoA in earlier turns of the cycle.
Other names: Tricarboxylic acid (TCA) cycle; Krebs cycle
Hans Krebs (1937); rejected by Nature
Note that these departing CO2 groups derive from the two oxaloacetate carboxyl groups that were incorporated as acetyl-CoA in earlier turns of the cycle.
二、Pyruvate Oxidation: A Major Entry Route for Carbon into the Citric Acid Cycle
Pyruvate oxidation to acetyl-CoA is catalyzed by the pyruvate dehydrogenase complex (PDH complex, 丙酮酸脱氢酶复合物).
It is an oxidative decarboxylation (氧化脱羧), which is virtually irreversible involving three enzymes and five coenzymes. 由于这个反应不可逆,也不能bypass, 因此偶数碳的脂肪酸不能变成糖
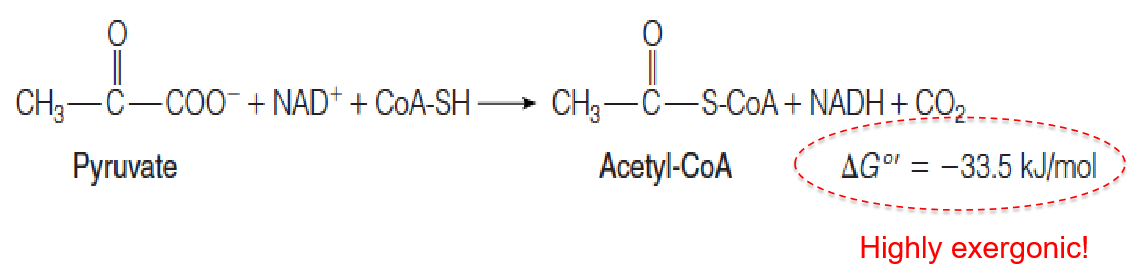
在细胞内条件释放的能量会更多
Structure of the pyruvate dehydrogenase (PDH) complex
- Electron micrograph of the purified pyruvate dehydrogenase complex from E. coli.
- E
2core subcomplex (60 E2monomers). - E
2-E3subcomplex (E2core 12 E3dimers). - Full PDH complex (E
2-E3subcomplex 130 E1~ tetramers). - Cutaway reconstruction of PDH complex.
E1: pyruvate dehydrogenase (24-E.coli)
E2: dihydrolipoamide transacetylase (24)
E3: dihydrolipoamide dehydrogenase(12)
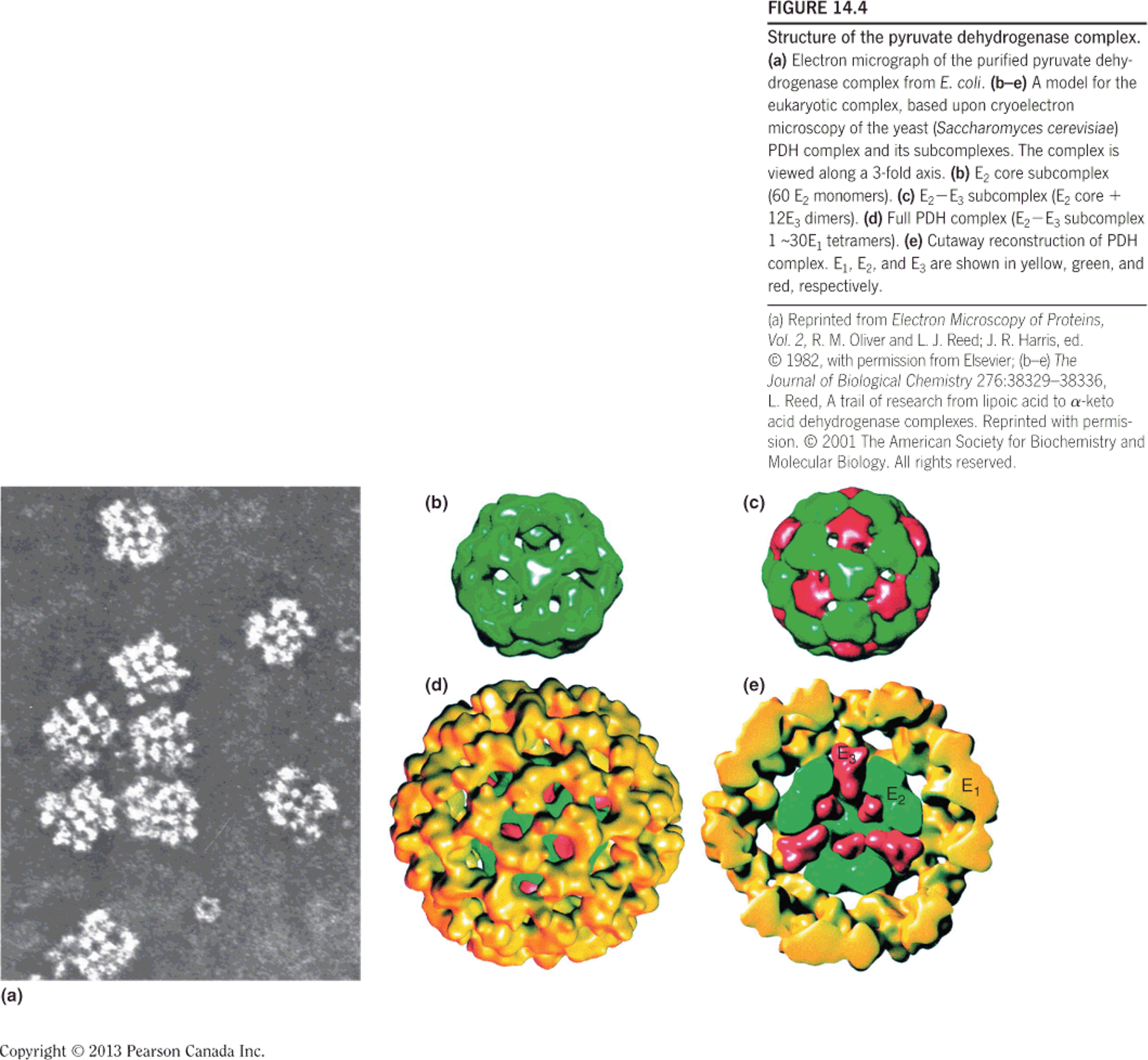
Big complex: 4.6x10^6^ daltons Larger than ribosome
Five-step reactions
Five coenzymes
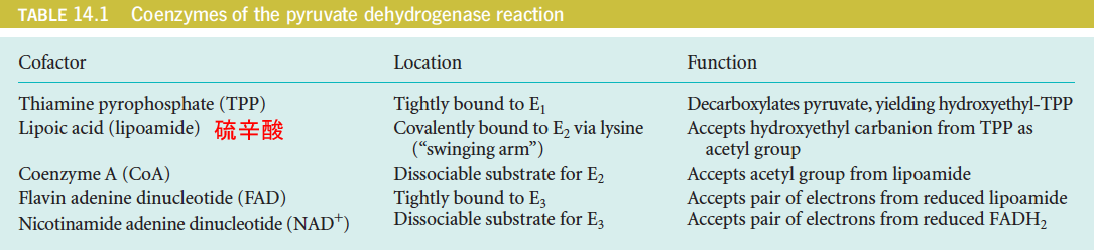
三、Coenzymes Involved in Pyruvate Oxidation and the Citric Acid Cycle
In pyruvate oxidation, the acceptor of the active aldehyde (hydroxyethyl group, 羟乙基) generated by TPP is lipoic acid, which is the internal disulfide of 6,8-dithiooctanoic acid. (6,8-二硫代辛酸)
The coenzyme is joined to via an amide bond linking the carboxyl group of lipoic acid to a lysine -amino group. Thus, the reactive species is an amide, called lipoamide (硫辛酰胺), or lipoyllysine.
Each lipoyllysine side chain is 14Å long, and is located within a flexible lipoyl domain of E2~ (and E3BP), allowing it to function as a “swinging arm (摆臂)” that can interact with the active sites of both the E1 and E3 components of the PDH complex.
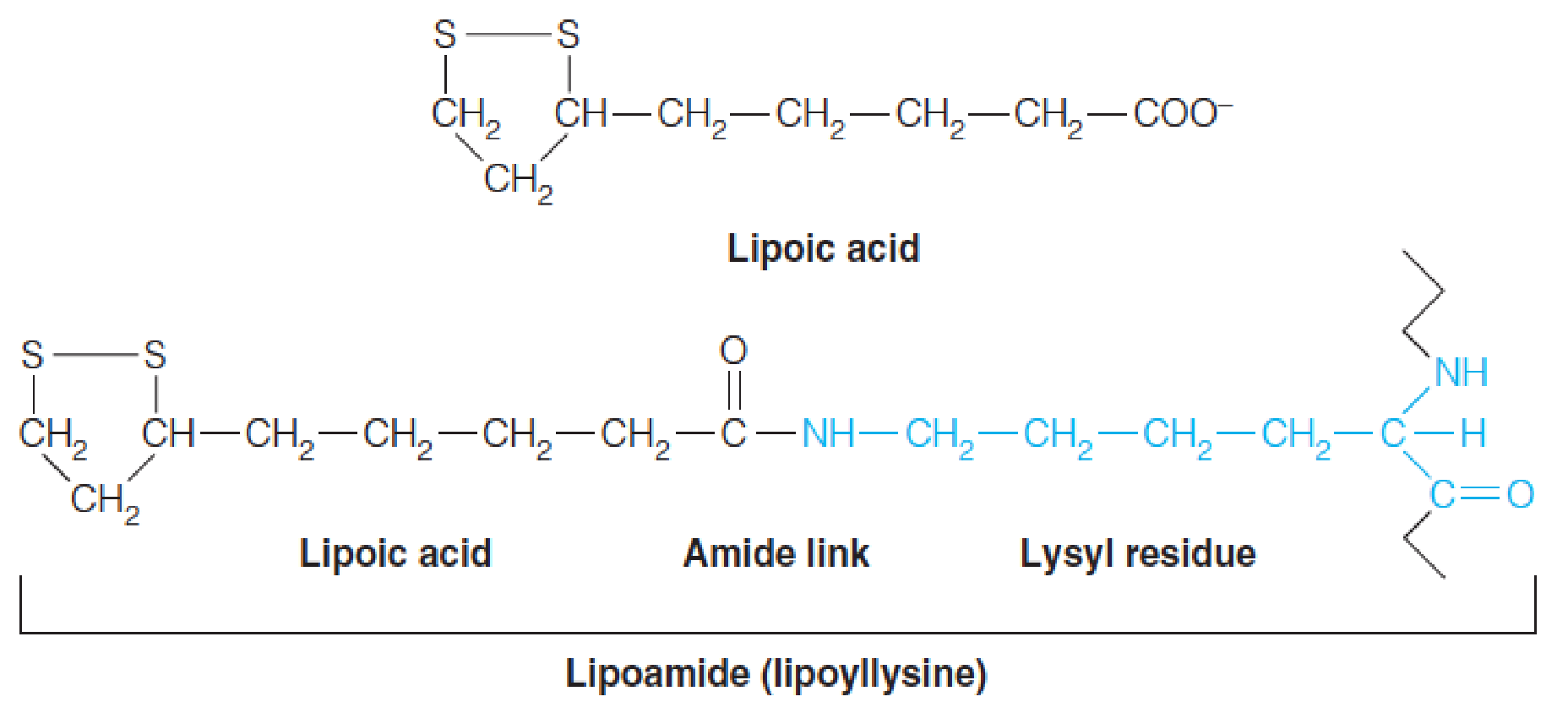
Lipoic acid: 10 tons of pork and beef → 30 mg; “swinging arm” for acetyl group between E1 and E3
Oxidized and reduced forms of lipoamide
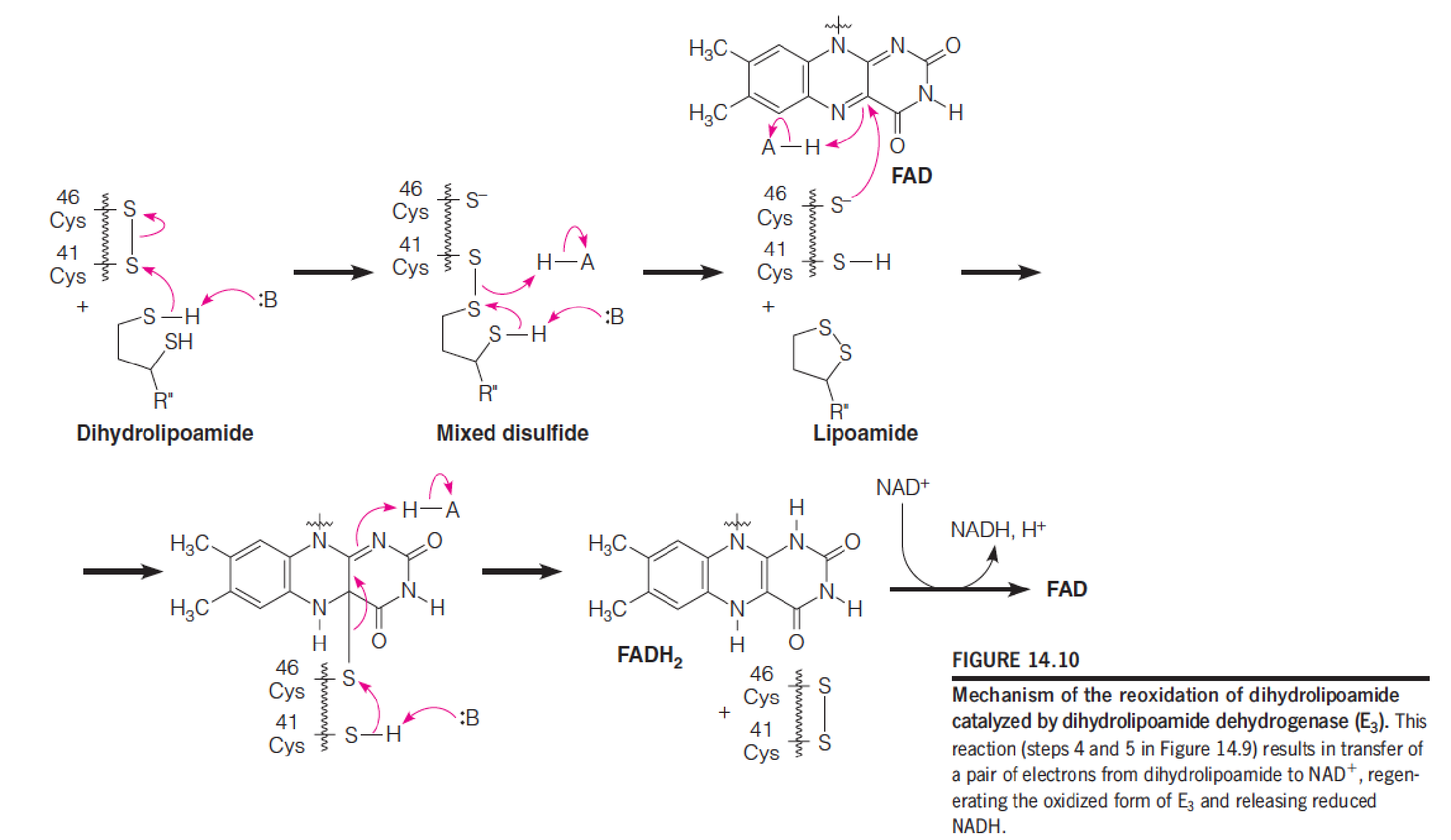
The cyclic disulfide of lipoamide can undergo a reversible two-electron reduction to form the dithiol, dihydrolipoamide (二硫醇,二氢硫辛酸).
In pyruvate dehydrogenase, this reduction is coupled to the transfer of the hydroxyethyl group moiety from TPP, giving an acetyl thioester of the reduced dihydrolipoamide.
Thus, lipoamide is a carrier of both electrons and acyl groups.
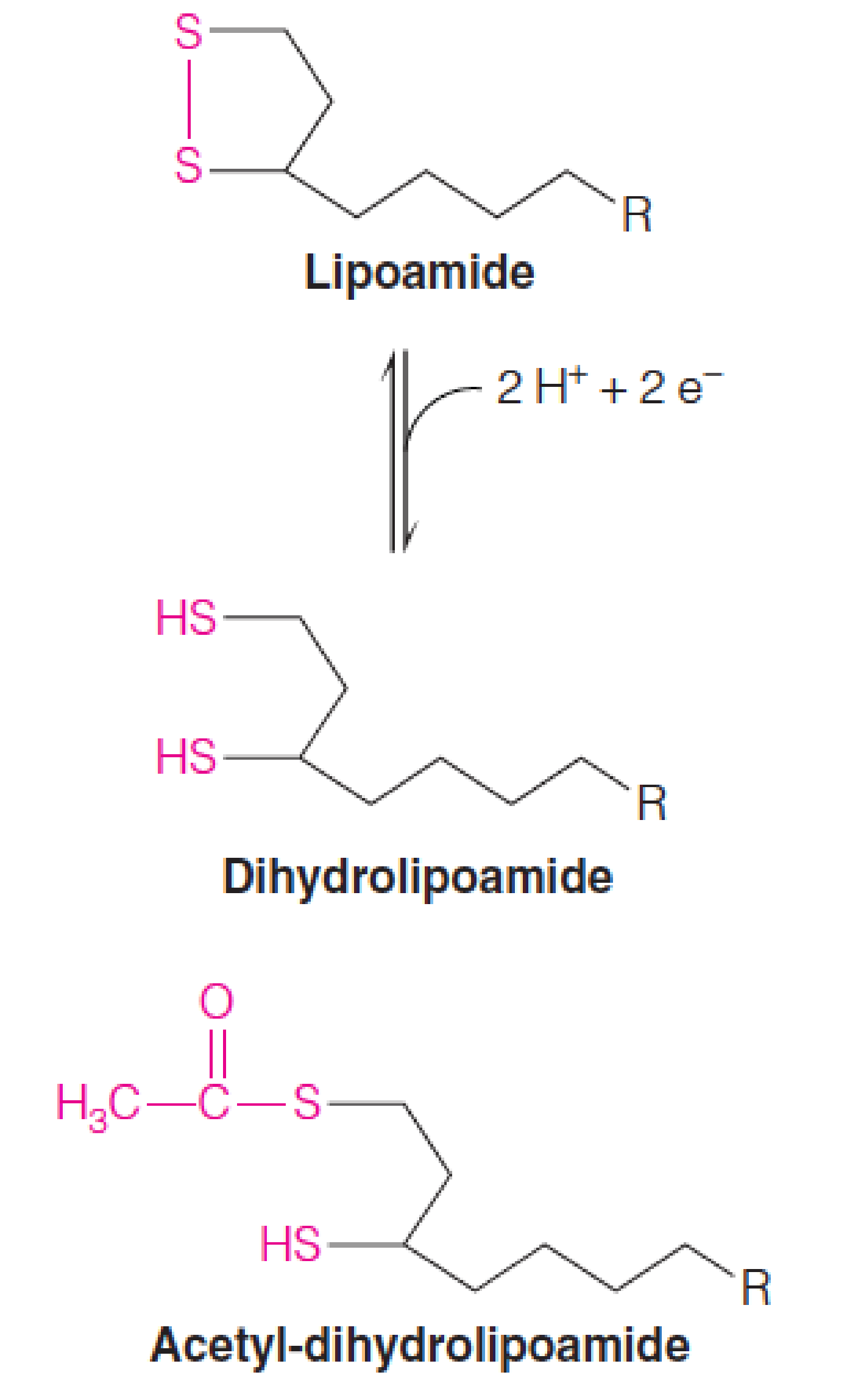
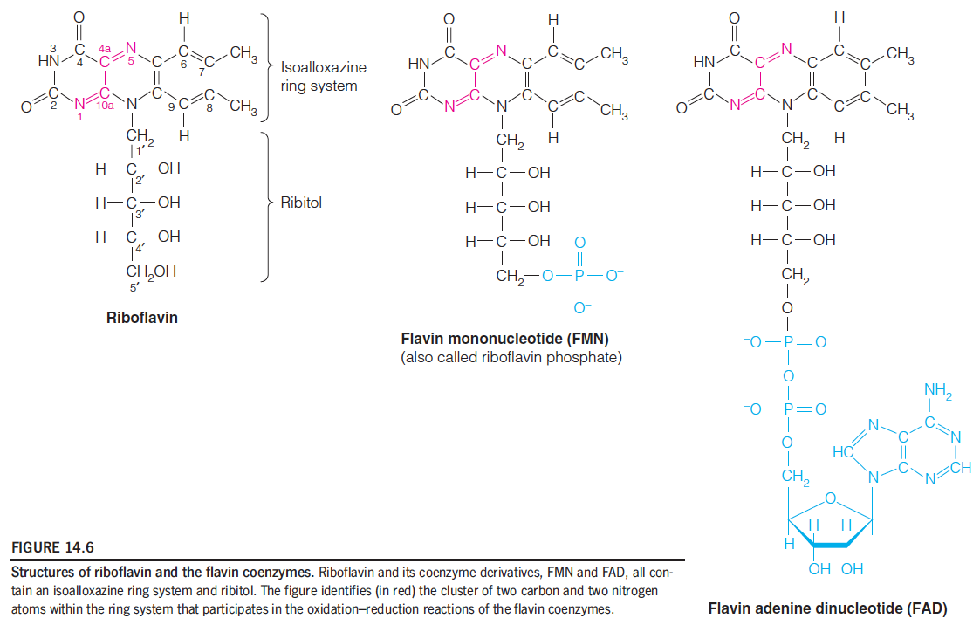
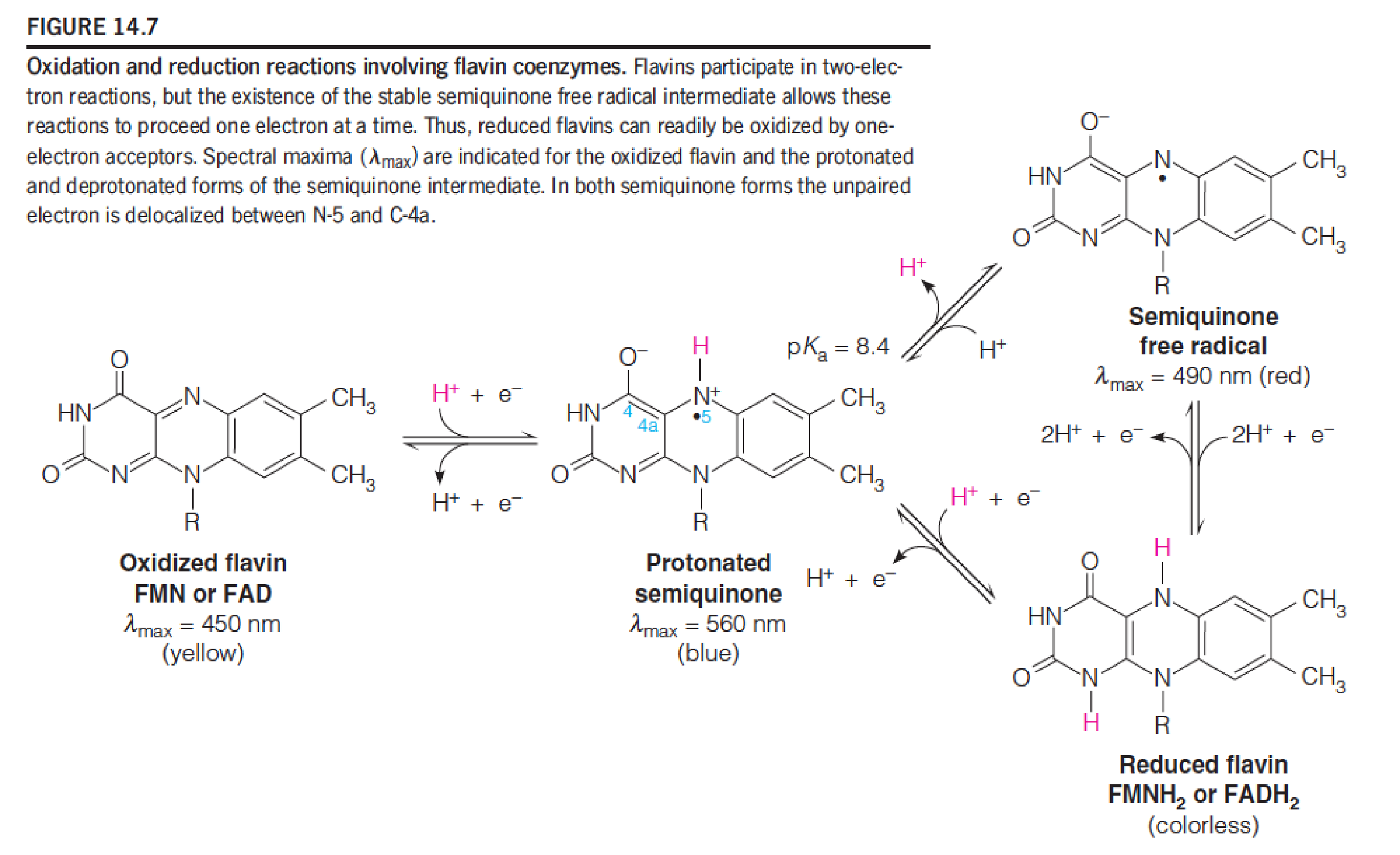
Flavin coenzymes participate in two-electron oxidoreduction reactions that can proceed in 2 one-electron steps.
FAD and FMN(B2, riboflavin): coenzyme; two electrons acceptor, hold electrons, not diffusing them; tightly binds to the protein.
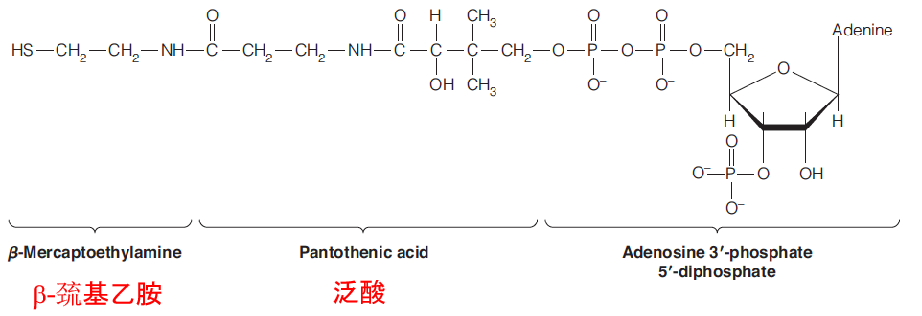
Coenzyme A (A for acyl(脂肪酰基)) participates in activation of acyl groups in general, including the acetyl group derived from pyruvate.
The coenzyme is derived metabolically from ATP, the vitamin pantothenic acid, and β-mercaptoethylamine.
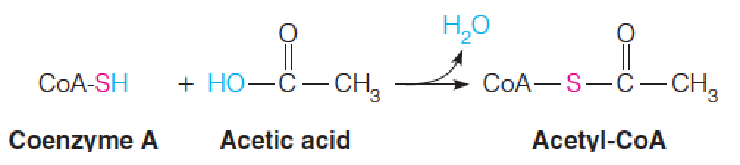
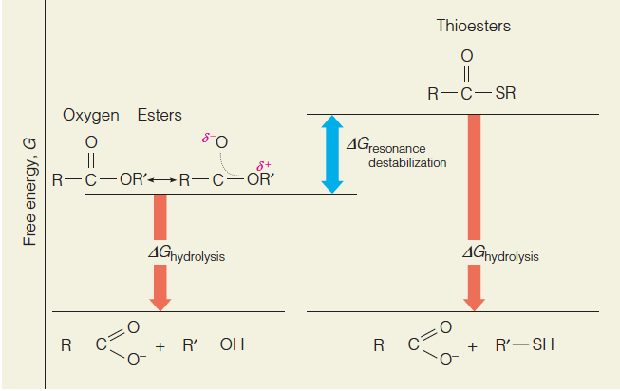
Thioesters (硫酯类) such as acetyl-CoA are energy rich because thioesters are destabilized relative to ordinary oxygen esters.
硫酯键具有较高的能量,高于氧酯键
Comparison of free energies of hydrolysis of thioesters and oxygen esters
Lack of resonance stabilization in thioesters is the basis for the higher $\Delta$G of hydrolysis of thioesters, relative to that of ordinary oxygen esters.
The free energies of the hydrolysis products are similar for the two classes of compounds.
四、Action of the Pyruvate Dehydrogenase Complex
Mechanisms of the pyruvate dehydrogenase complex
Substrate channeling: intermediates of a multiple pathways are “handed off” from one active site to the next without diffusing from the the complex; allows the overall reaction to proceed smoothly and avoids unwanted side reactions.
E1: pyruvate dehydrogenase (丙酮酸脱氢酶)
E2: dihydrolipoamide transacetylase (二氢硫辛酰胺转乙酰酶)
E3: dihydrolipoamide dehydrogenase (二氢硫辛酰胺脱氢酶)
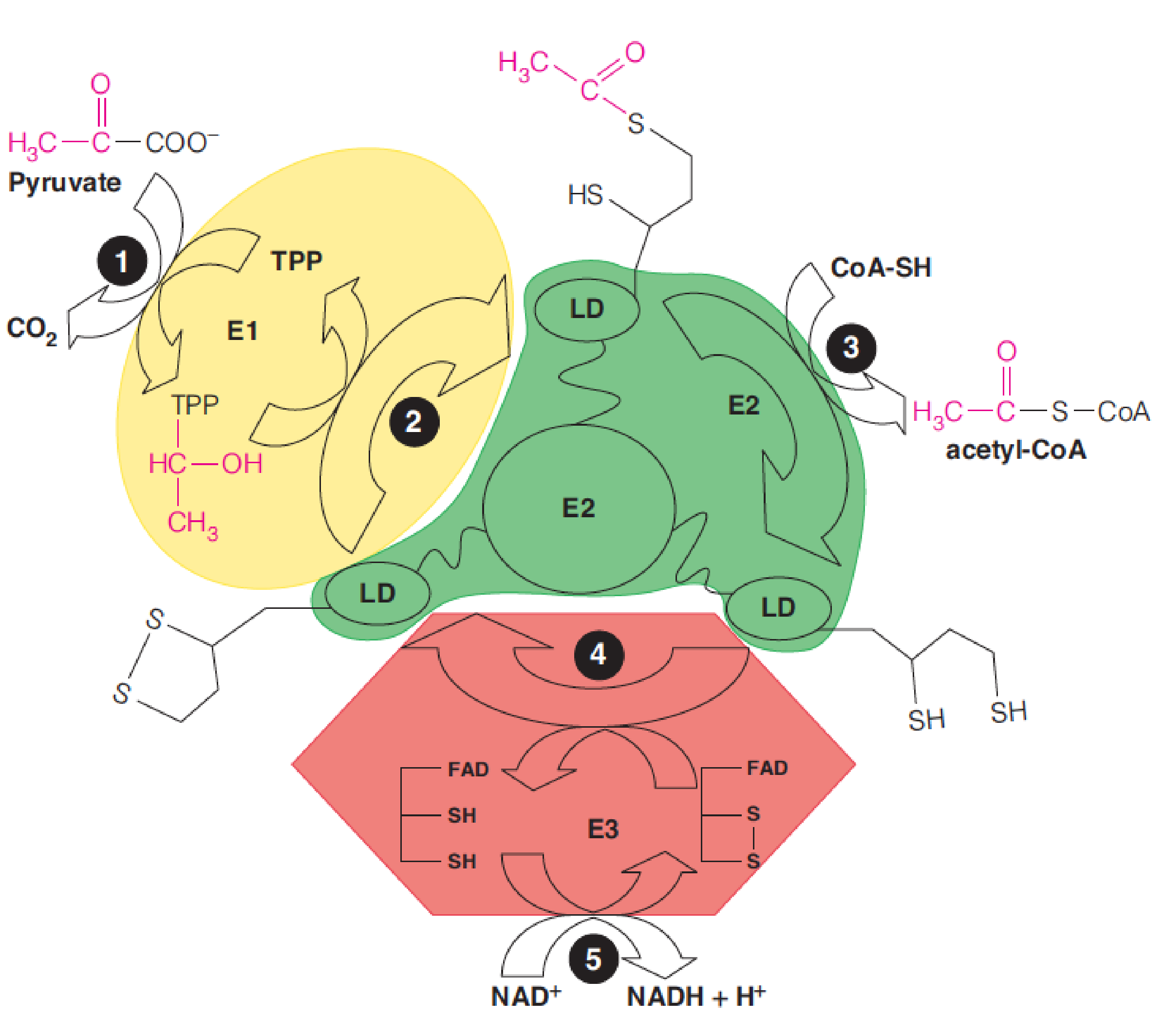
Lipoamide (硫辛酰胺) is tethered to one enzyme (E2) in the PDH complex, but it interacts with all three enzymes via a flexible swinging arm.
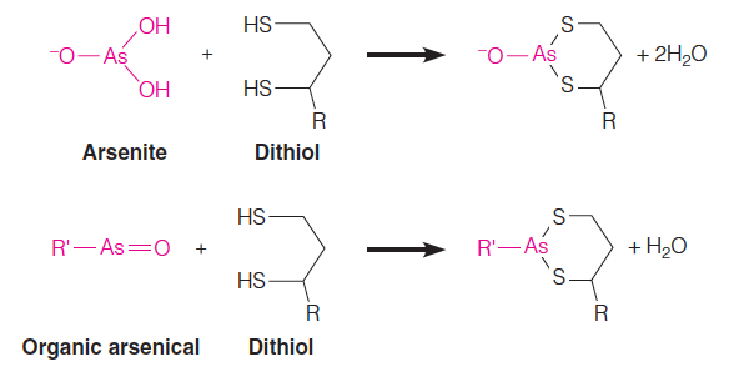
Arsenic poisoning (砷中毒), both intentional and unintentional, has had a long history, dating back to at least the eighth century.
Trivalent As(III) compounds such as arsenite (亚砷酸盐) and organic arsenicals react readily with thiols(硫醇), and they are especially reactive with dithiols(二硫醇类), such as dihydrolipoamide, forming bidentate adducts:
三氧化二砷(砒霜):潘金莲,武大郎;用来治疗leukemia(白血病); syphilis(梅毒)
二硫化砷(雄黄),三硫化砷(雌黄)
Middle ages: impatient heirs ”tonics”
五、The Citric Acid Cycle
Step 1: Introduction of Two Carbon Atoms as Acetyl-CoA
The initial reaction, catalyzed by citrate synthase (柠檬酸合成酶), is akin to an aldol condensation (羟醛缩合).
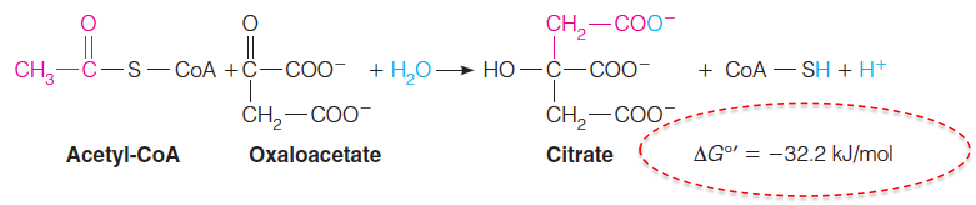
反应放出大量能量,倾向于正向进行,受到Oxaloacetate浓度调节
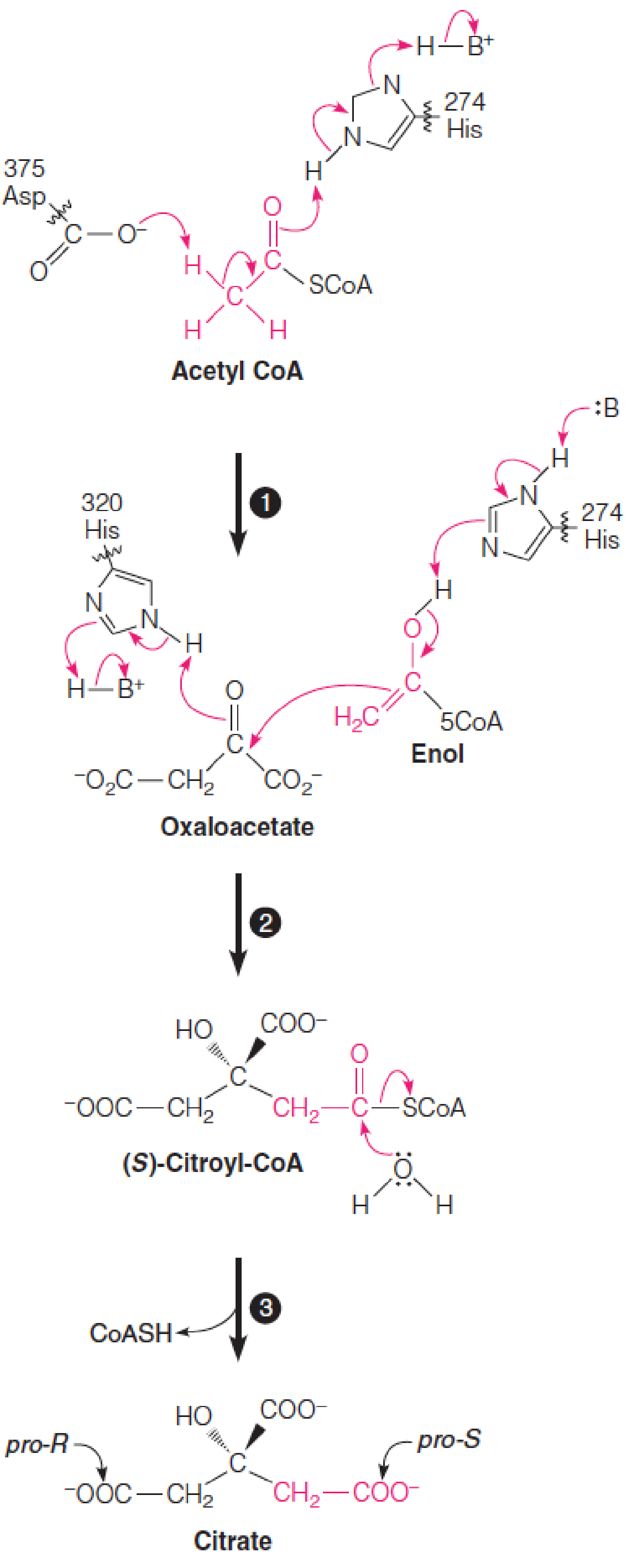
1. Mechanism of the citrate synthase reaction
Mechanism of the citrate synthase reaction:
Step 1: Asp 375 extracts a proton from the methyl group,and His 274 donates a proton to the carbonyl oxygen of acetyl-CoA, creating an enol (烯醇).
Step 2: His 274 deprotonates the acetyl-CoA enol, stabilizing the nucleophilic enolate that attacks the keto carbon of oxaloacetate. His 320 protonates the aldol product (S)-citroyl-CoA.
Step 3:The citroyl-CoA intermediate spontaneously hydrolyzes to citrate by a nucleophilic acyl substitution reaction giving exclusively the S stereoisomer of citroyl-CoA.
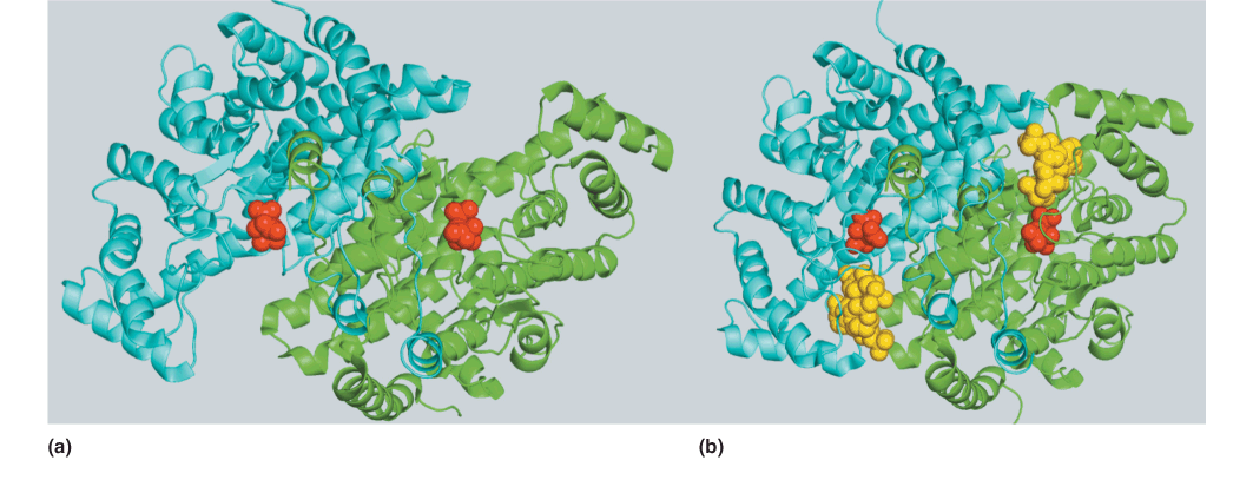
2. Three-dimensional structure of citrate synthase
The two forms of pig heart citrate synthase homodimer shown here were determined by crystallographic methods and support the induced fit model of enzyme catalysis.
- In the absence of CoA-SH the enzyme crystallizes in an “open” form. Citrate binds at the base of large clefts in both catalytic domains of the homodimeric protein.
- Binding of CoA-SH causes the enzyme to adopt a “closed” conformation, with the clefts essentially filled
Step 2: Isomerization of Citrate
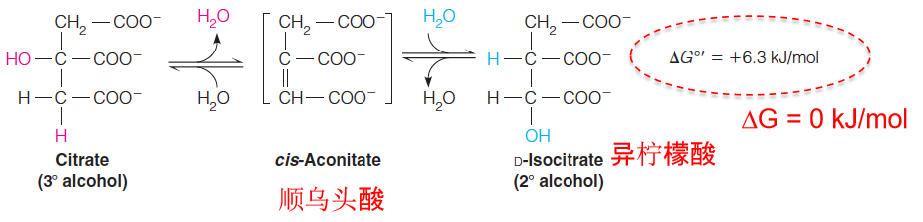
The tertiary alcohol of citrate presents yet another chemical problem: tertiary alcohols cannot be oxidized without breaking a carbon–carbon bond.
To set up the next oxidation in the pathway, citrate is converted to isocitrate, a chiral secondary alcohol, which can be more readily oxidized.
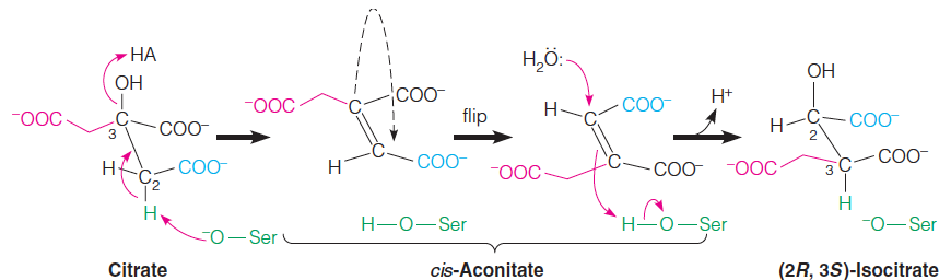
This isomerization reaction, catalyzed by aconitase (乌头酸酶), involves successive dehydration and hydration, through cis-aconitate as a dehydrated intermediate, which remains enzyme-bound.
这个反应是一个可逆反应,在细胞内ΔG = 0
The enzyme contains nonheme iron and acid-labile sulfur in a cluster called a 4Fe–4S iron–sulfur center.
The iron–sulfur cluster coordinates the hydroxyl group and one of the carboxyl groups on the citrate molecule.
The cis-aconitate intermediate must flip 180° during the reaction, presumably by releasing from the enzyme, then rebinding with the iron–sulfur cluster, but in the opposite orientation.
Thus, of the four possible diastereomers (非对映异构体) of isocitrate, only one, the 2R,3S diastereomer, is produced.
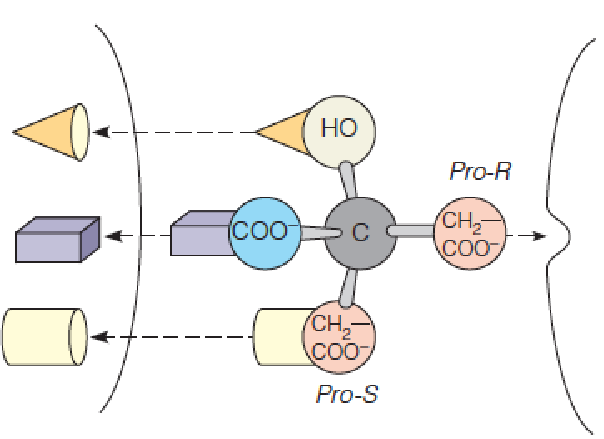
The prochirality (前手性) of citrate when bound to aconitase:
Fluoroacetate (氟乙酸) is an example of a mechanism-based, or suicide, inhibitor of aconitase. (2-Fluorocltrate与柠檬酸结构相似,因此使得aconitase错误识别)
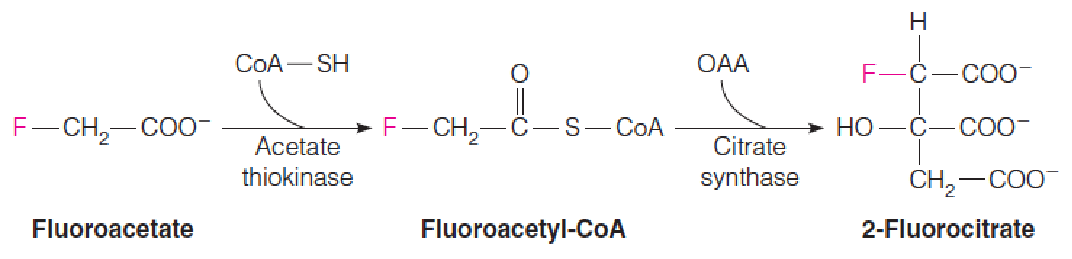
“Suicide substrate”: Not toxic to cell itself, but it resembles a normal metabolic closely enough that it undergoes metabolic transformation to a product that does inhibit a crucial enzyme. The cell “comments suicide” by transforming the analog to a toxic product.
Step 3: Generation of CO2 by an NAD+-Linked Dehydrogenase
The first of two oxidative decarboxylations in the cycle is catalyzed by isocitrate dehydrogenase (异柠檬酸脱氢酶).
Isocitrate is oxidized to a ketone, oxalosuccinate (草酰琥珀酸), an unstable enzyme-bound intermediate that spontaneously β-decarboxylates to give the product, α-ketoglutarate.
The strategy here is to oxidize isocitrate’s secondary alcohol to α keto group β to the carboxyl group to be removed.
The β-keto group acts as an electron sink to stabilize the carbanionic (碳负离子) transition state, facilitating decarboxylation.

Two carbon atoms enter the citric acid cycle as acetyl-CoA, and two are lost as CO2 in the oxidative decarboxylations of steps 3 and 4.
Step 4: Generation of a Second CO2 by an Oxidative Decarboxylation
This is a multistep reaction entirely analogous to the pyruvate dehydrogenase reaction.
An α-keto acid substrate undergoes oxidative decarboxylation, with concomitant formation of an acyl-CoA thioester.
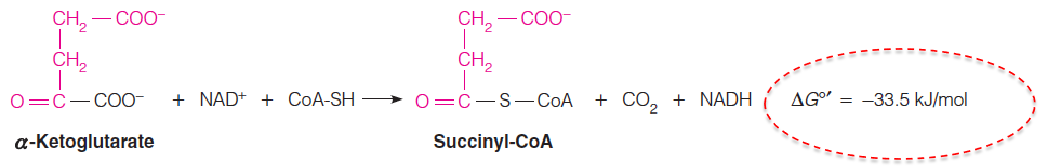
3 enzymes, 5 reactions, 5 coenzymes
1. Decarboxylation of α-ketoglutarate
The first step catalyzed out by the a-ketoglutarate dehydrogenase complex is a decarboxylation catalyzed by a-ketoglutarate decarboxylase (E1 of the complex), producing a four-carbon TPP derivative.
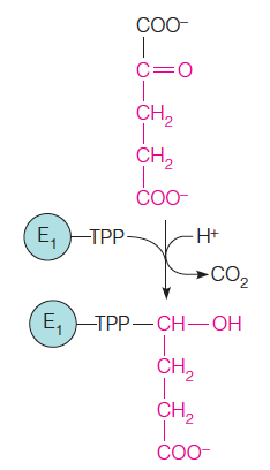
Step 5: A Substrate-Level Phosphorylation
Succinyl-CoA is an energy-rich thioester compound, and its potential energy is used to drive the formation of a nucleoside triphosphate
This reaction, catalyzed by succinyl-CoA synthetase (琥珀酰辅酶A合成酶), is comparable to the two substrate-level phosphorylation reactions that we encountered in glycolysis, except that in animal cells the energy-rich nucleotide product is not always ATP but, in some tissues, GTP.
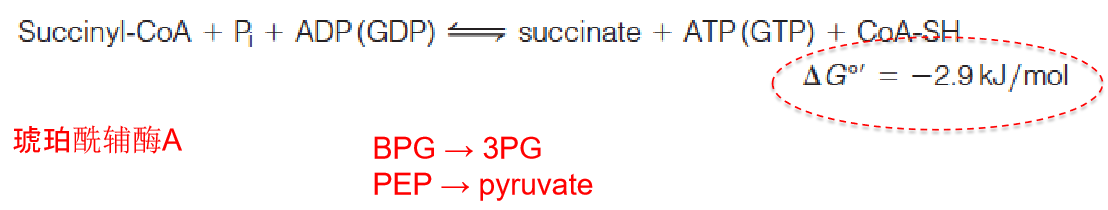
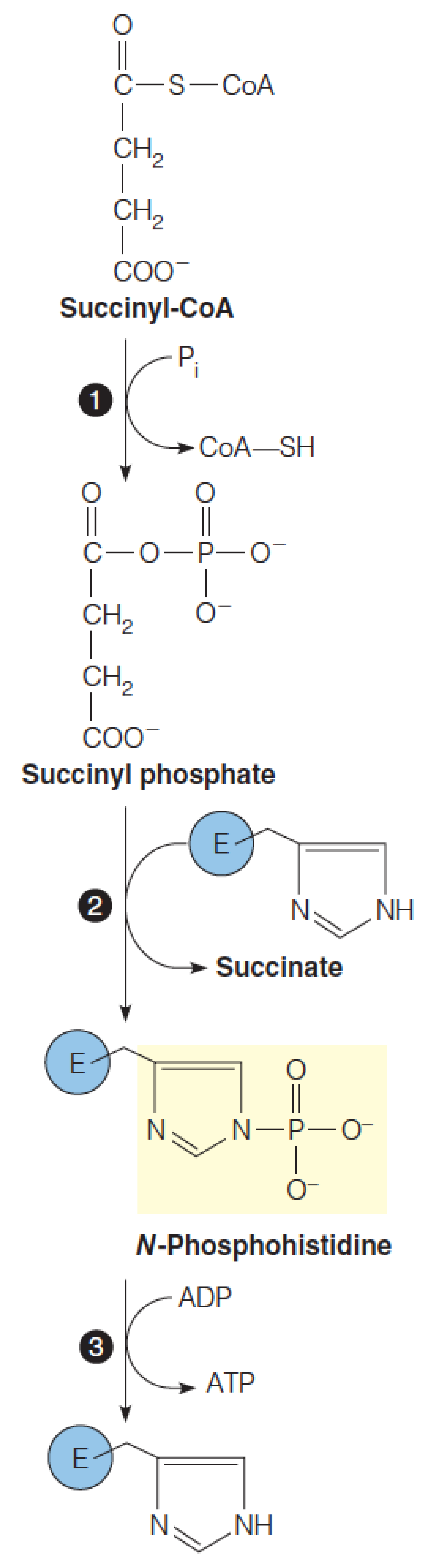
1. Covalent catalysis by the succinyl-CoA synthetase reaction
Three successive nucleophilic substitution reactions conserve the energy of the thioester of succinyl-CoA in the phosphoanhydride bond (磷酸酐键) of ATP (or GTP).
An active site histidine side chain is transiently phosphorylated (N-phosphohistidine) during the reaction.
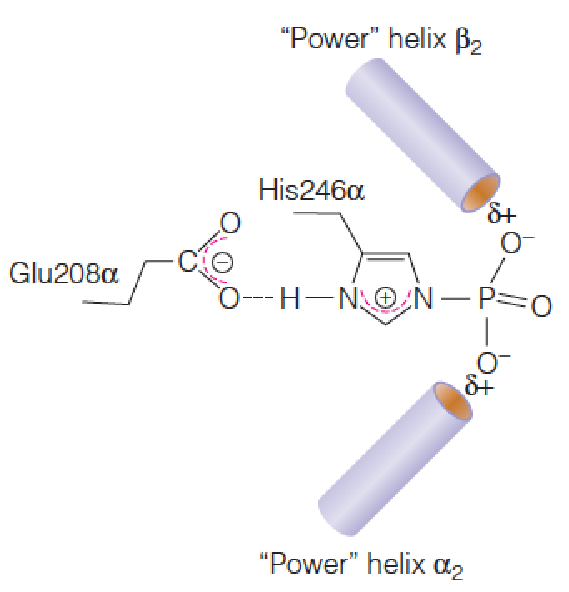
A charge–dipole interaction (电荷偶极相互作用) stabilizes the phosphohistidine intermediate in the succinyl-CoA synthetase reaction.
In this schematic, based on the E. coli enzyme structure, the permanent dipoles of two -helices (the “power” helices) are oriented such that the d+ at their N-termini interact with the negative charges on the phosphate group of the active site N-phosphohistidine, stabilizing this transient reaction intermediate.
Step 6: A Flavin(黄素)-Dependent Dehydrogenation
Completion of the cycle involves conversion of the four-carbon succinate to the four-carbon oxaloacetate.
The first of the three reactions, catalyzed by succinate dehydrogenase (琥珀酸脱氢酶), is the FAD-dependent dehydrogenation of two saturated carbons to a double bond.
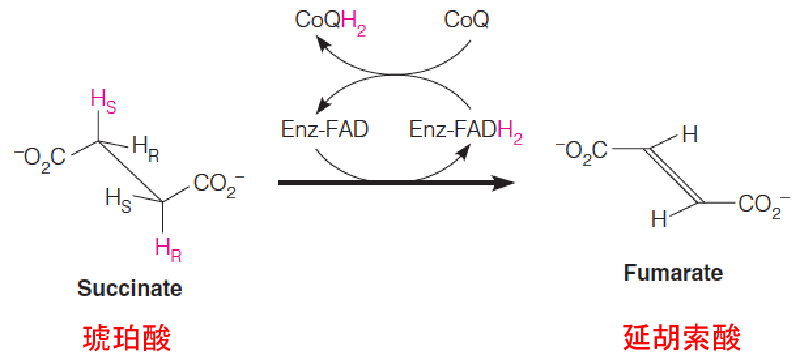
Complex II: Embedded in the membrane, tightly bound to the mitochondrial membrane
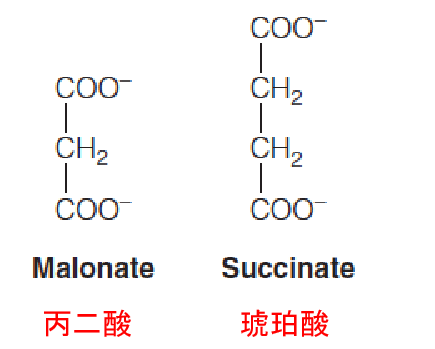
Succinate dehydrogenase is competitively inhibited by malonate (丙二酸), a structural analog of succinate. Malonate inhibition of pyruvate oxidation was one of the clues that led Krebs to propose the cyclic nature of this pathway.
A single C-C bond is more difficult to oxidize than a C-O bond.
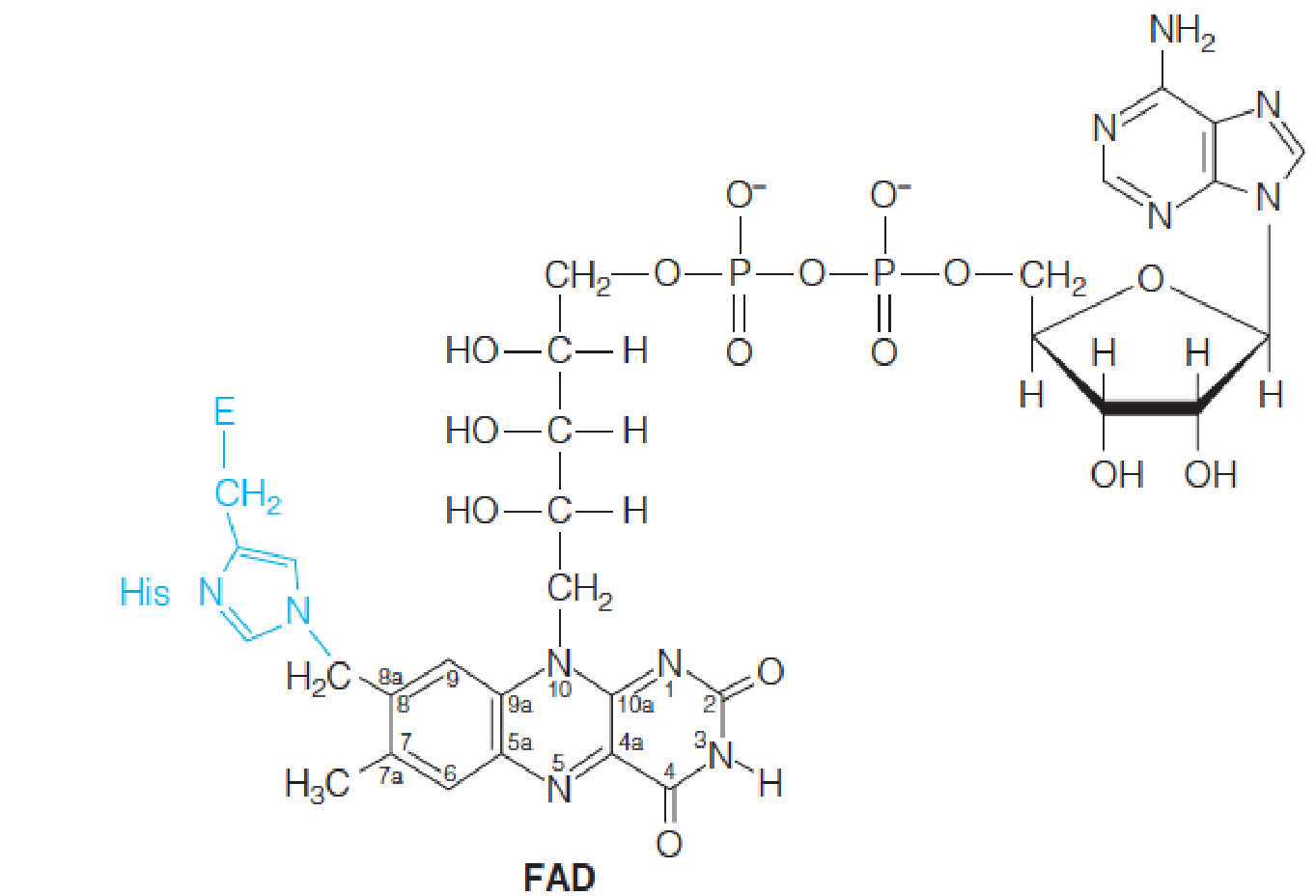
Therefore, the redox coenzyme for succinate dehydrogenase is not NAD+ but the more powerful oxidant FAD.
The flavin is bound covalently to the enzyme protein through a specific histidine residue.
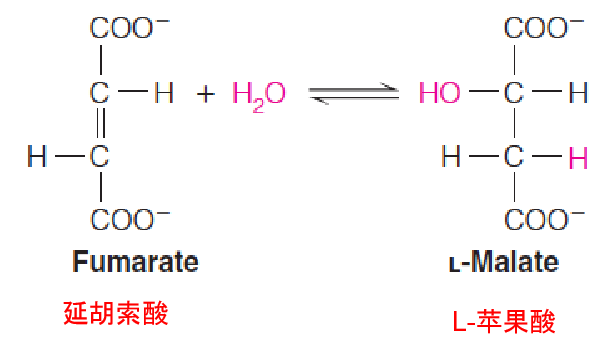
Step 7: Hydration of a Carbon–Carbon Double Bond
The stereospecific(立体特异性) trans hydration of the carbon–carbon double bond is catalyzed by fumarate hydratase, more commonly called fumarase (延胡索酸酶).
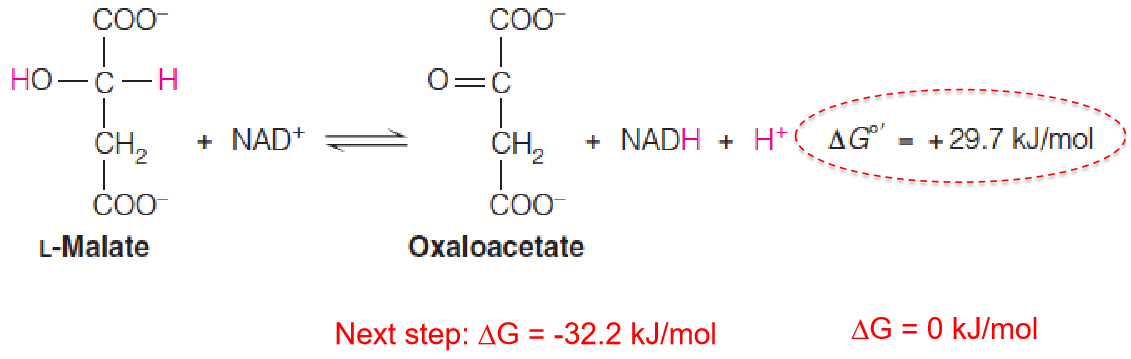
Step 8: A Dehydrogenation that Regenerates
Oxaloacetate
Finally, the cycle is completed with the **NAD+**–dependent dehydrogenation of malate to oxaloacetate, catalyzed by malate dehydrogenase (苹果酸脱氢酶).
六、Stoichiometry and Energetics of the Citric Acid Cycle
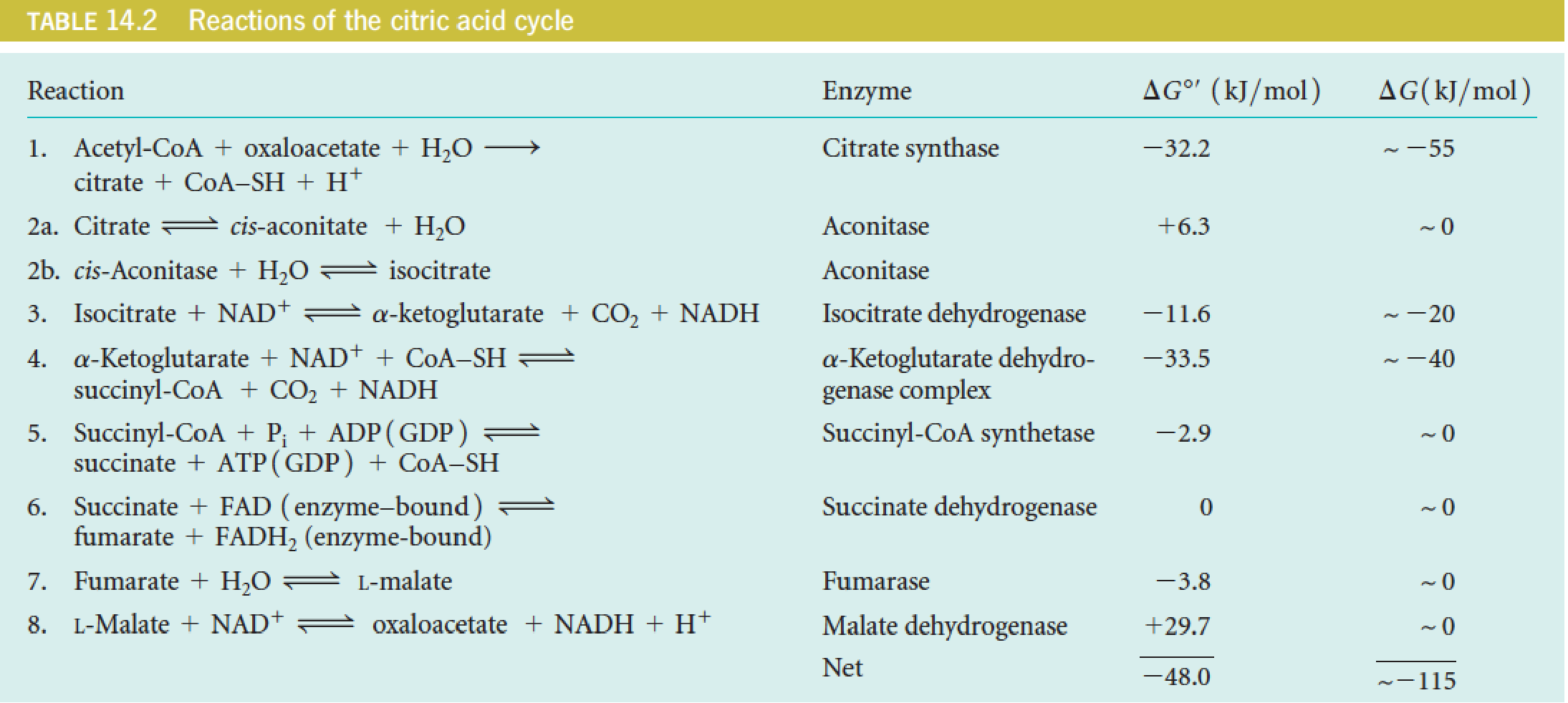
One turn of the citric acid cycle generates one high-energy phosphate (ATP or GTP) through substrate-level phosphorylation, plus three NADH and one FADH2 for subsequent reoxidation in the electron transport chain.
- 1个NADH中的H,最后可以产生2.5ATP;一个FADH
2,只能产生1.5ATP
七、Regulation of Pyruvate Dehydrogenase and the Citric Acid Cycle
Regulation
Major regulatory factors controlling pyruvate dehydrogenase and the citric acid cycle:
Red brackets indicate concentration dependence.
NADH can inhibit through allosteric interactions, but apparent NADH inhibition can also be a reflection of reduced NAD+ availability.
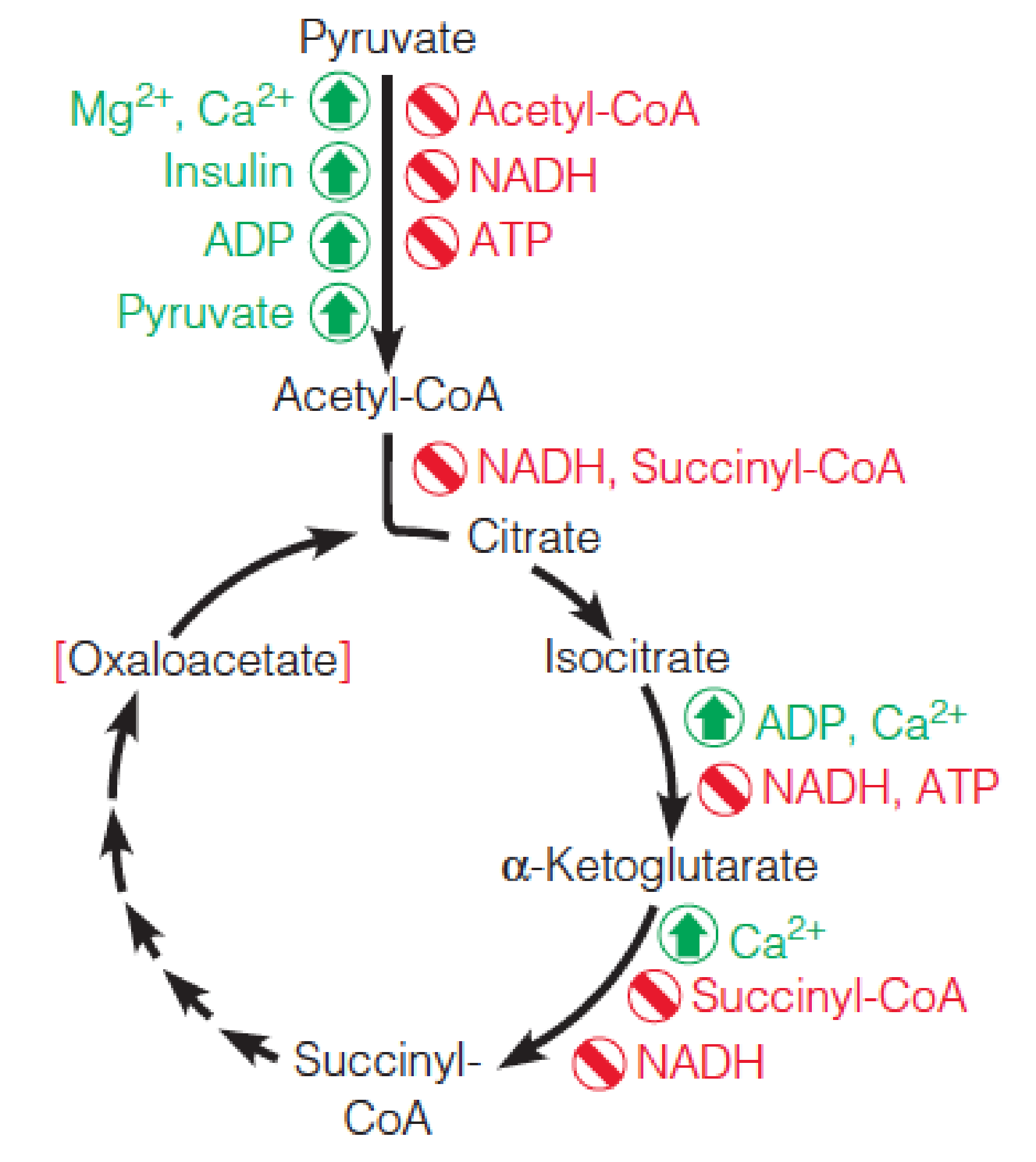
- NADH浓度是重要的调节因子,通过allosteric regulation实现

Pyruvate Dehydrogenase
Regulation of the mammalian pyruvate dehydrogenase complex by feedback inhibition(反馈抑制(allosteric)) and by covalent modification(共价修饰) of E1.
A kinase and a phosphatase inactivate and activate the first component (E1) of the pyruvate dehydrogenase complex by phosphorylating and dephosphorylating, respectively, three specific serine residues.
The active form of the pyruvate dehydrogenase complex is feedback inhibited by acetyl-CoA and NADH.
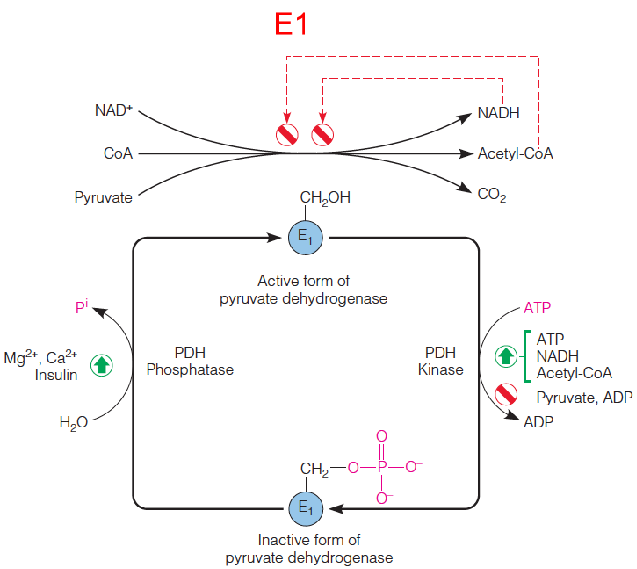
The citric acid cycle is controlled primarily by the relative intramitochondrial concentrations of NAD+ and NADH.
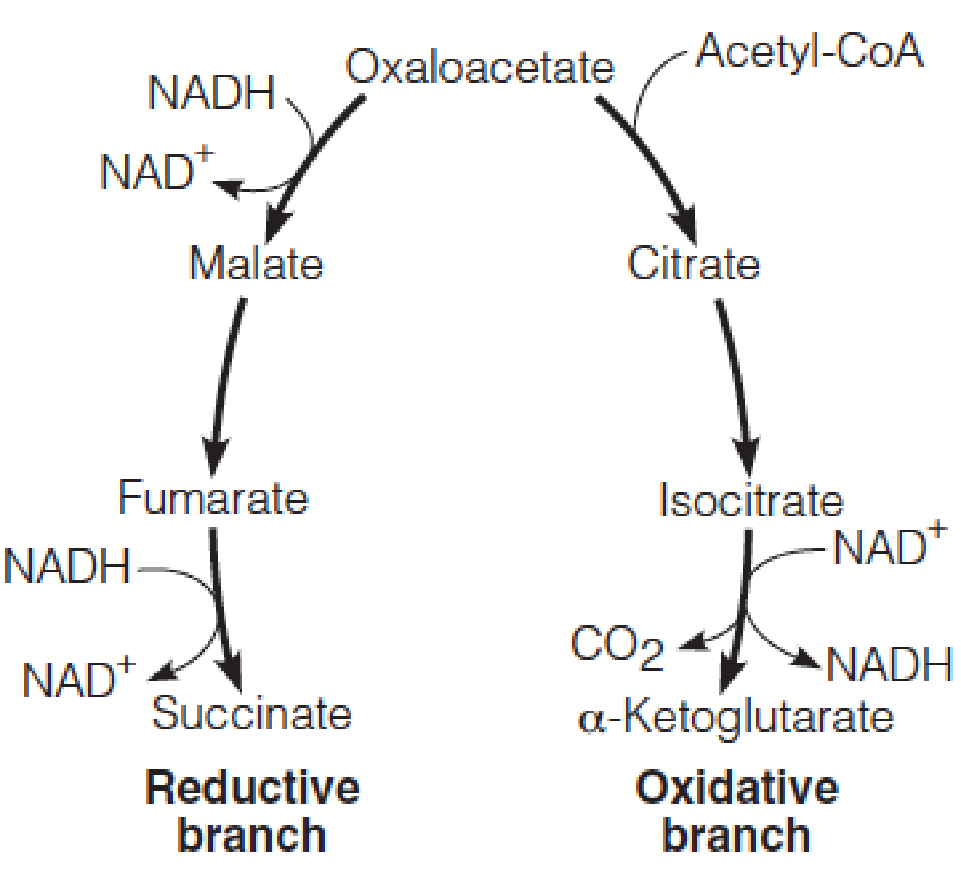
Evolution of CAC:
Metabolic pathways are the products of evolution
Anaerobic chemotrophs (2.5 billions years ago)
八、Anaplerotic Sequences: The Need to Replace Cycle Intermediates
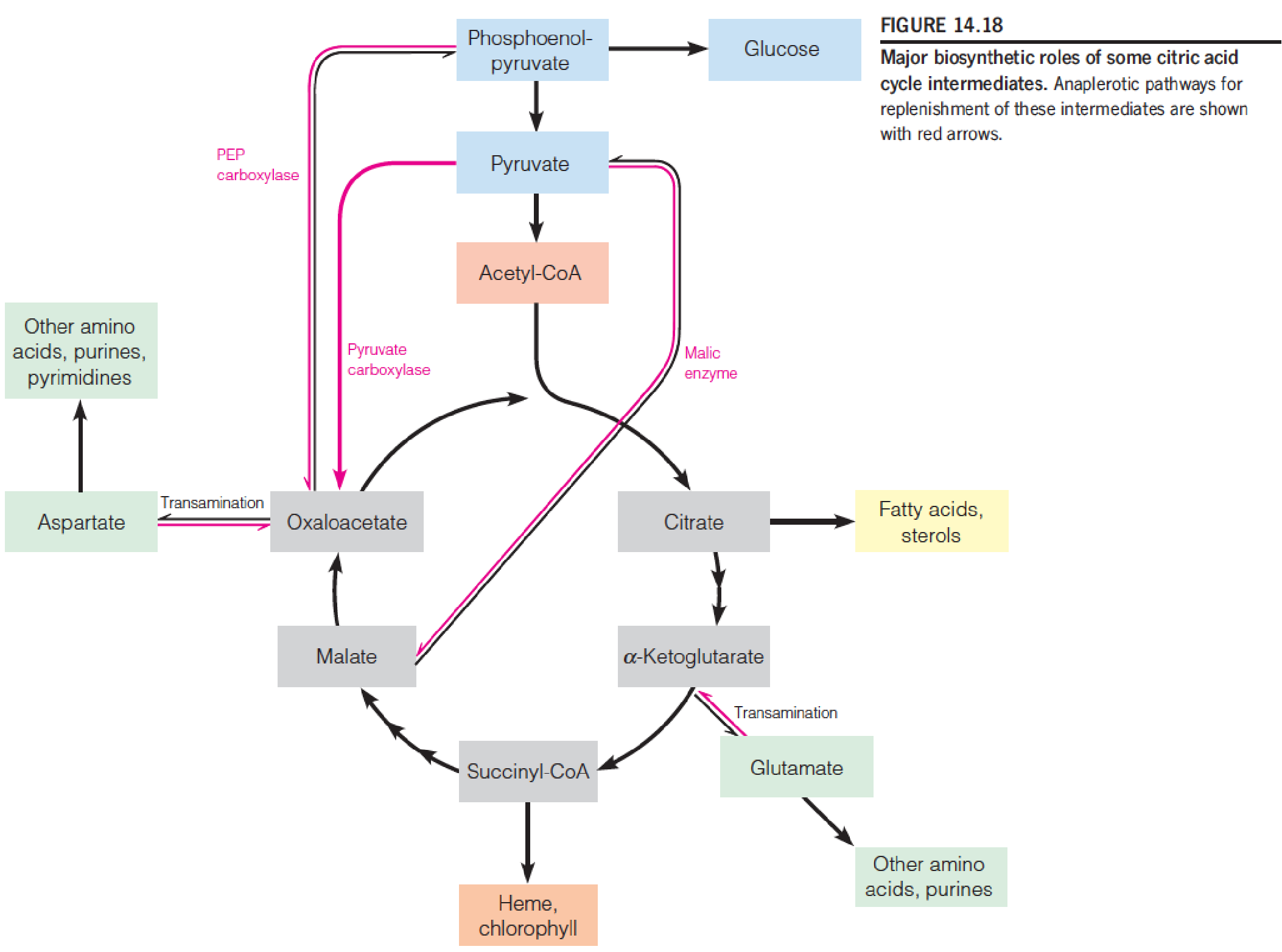
Citric acid cycle intermediates used in biosynthetic pathways must be replenished (补充) to maintain flux through the cycle.
- 因此相关底物的浓度不仅与cycle反应的活性有关,同时与代谢网络内的其他反应有关
Anaplerotic pathways serve this purpose.
1. Pyruvate Carboxylase
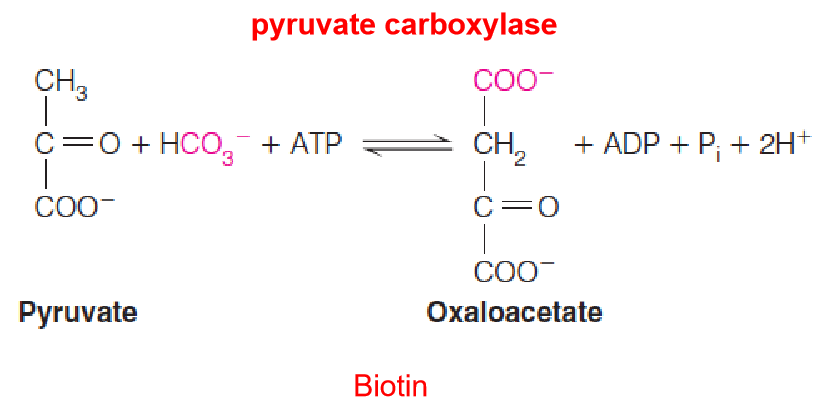
- 这个反应需要cofactor – biotin
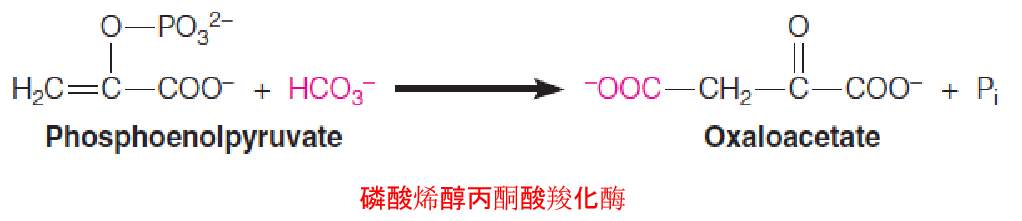
2. phosphoenolpyruvate (PEP)
Because phosphoenolpyruvate is such an energy-rich compound, this reaction, catalyzed by phosphoenolpyruvate carboxylase, requires neither an energy cofactor nor biotin. This reaction is important in the C4 pathway of photosynthetic CO2 fixation
3. malate dehydrogenase
In addition to pyruvate carboxylase and phosphoenolpyruvate carboxylase, a third anaplerotic process is provided by an enzyme commonly known as malic enzyme but more officially as malate dehydrogenase (苹果酸酶) (decarboxylating: NADP+).
The malic enzyme catalyzes the reductive carboxylation of pyruvate to give malate.
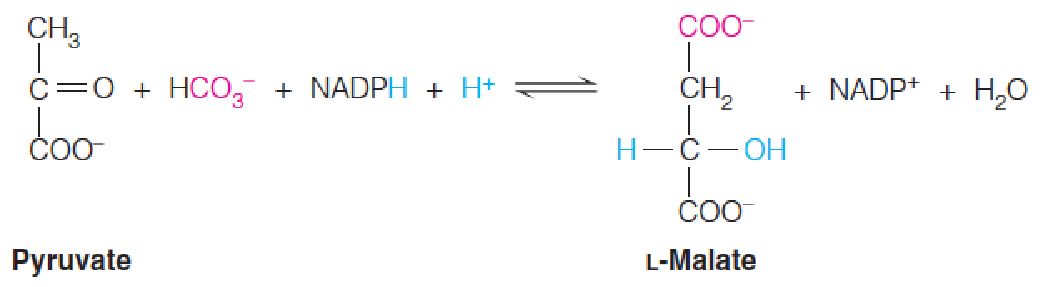
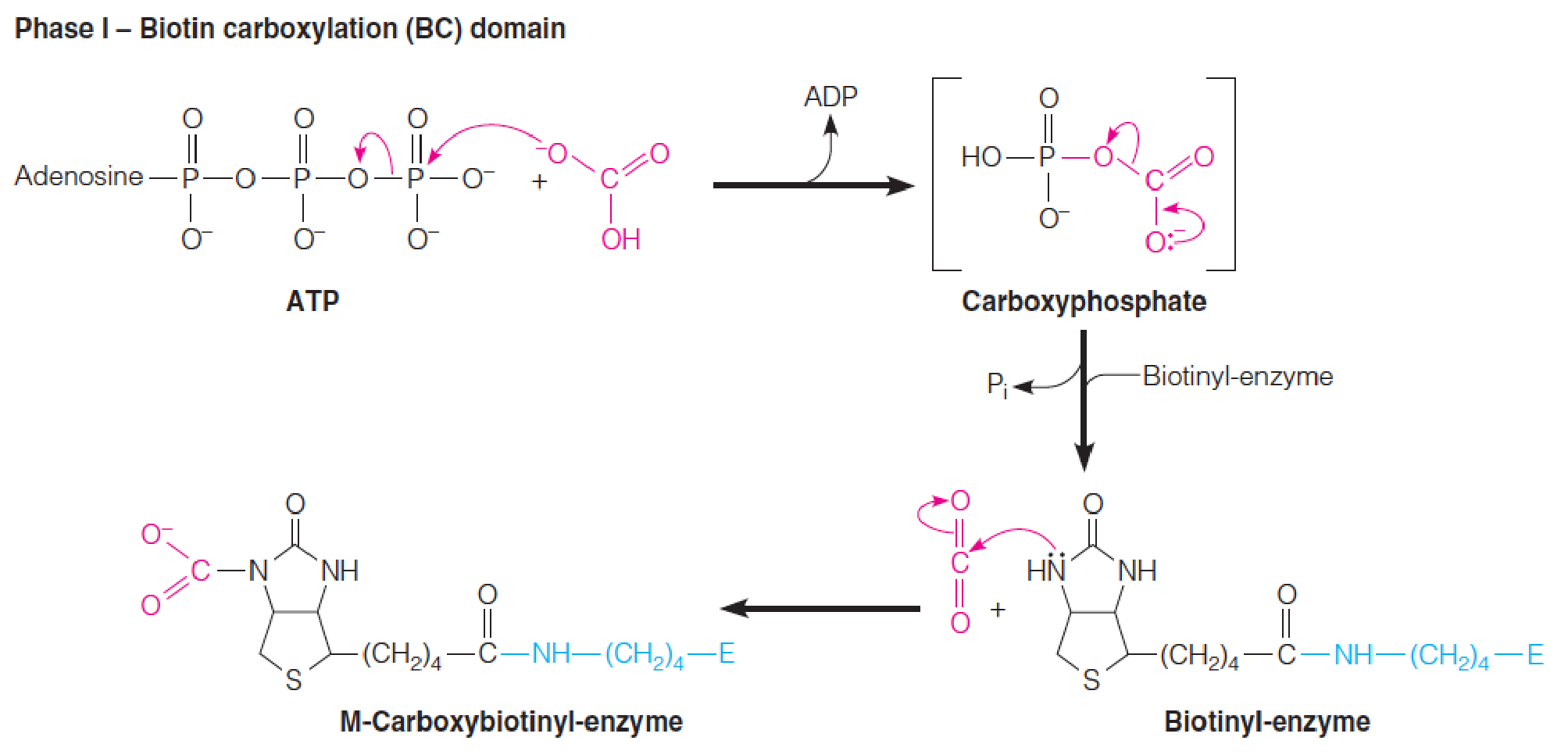
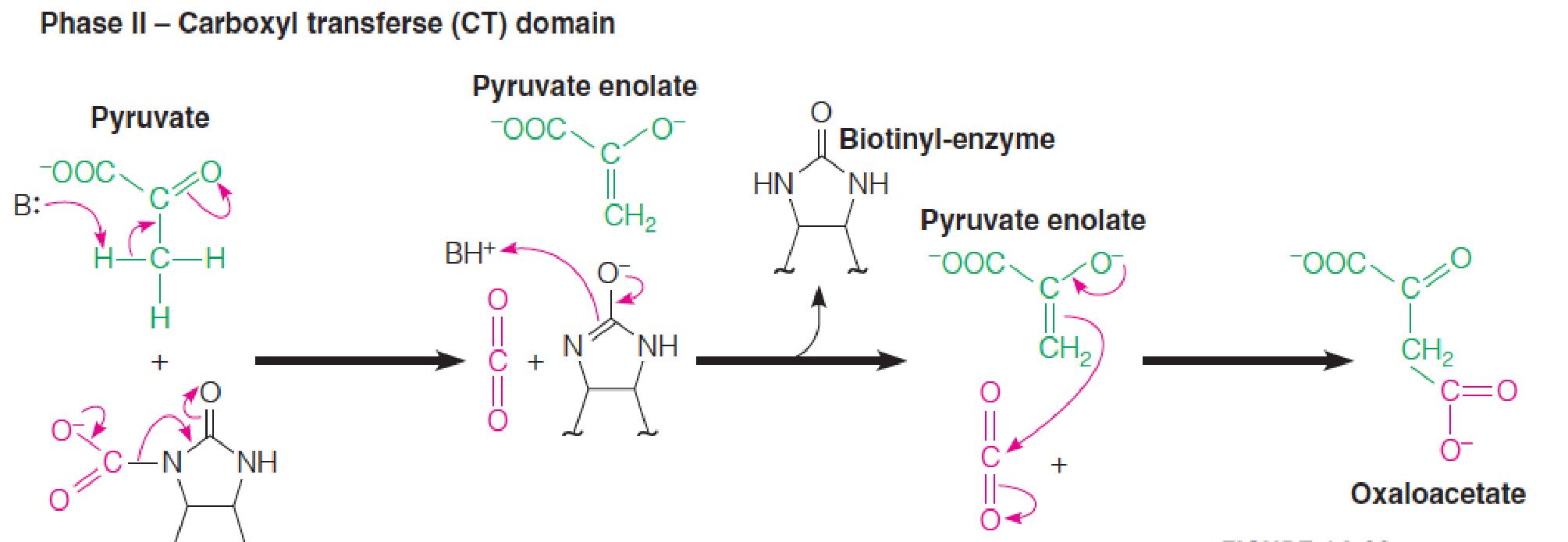
Mechanism of the biotin-dependent pyruvate carboxylase reaction
Phase I is catalyzed by the biotin carboxylation (BC) domain.
Phase II is catalyzed by the carboxyltransferase (CT) domain.
九、Glyoxylate Cycle: An Anabolic Variant of the Citric Acid Cycle
The glyoxylate cycle (乙醛酸循环) allows plants and bacteria to carry out net conversion of fat to carbohydrate, bypassing CO2-generating reactions of the citric acid cycle.
Reactions of the glyoxylate cycle
Two acetyl-CoA molecules enter the cycle, one at the citrate synthase step, and the second at the malate synthase step.
The reactions catalyzed by isocitrate lyase and malate synthase (异柠檬酸裂合酶和苹果酸合成酶) bypass the three citric acid cycle steps between isocitrate and succinate so that the two carbons lost in the citric acid cycle are saved, resulting in the net synthesis of oxaloacetate.
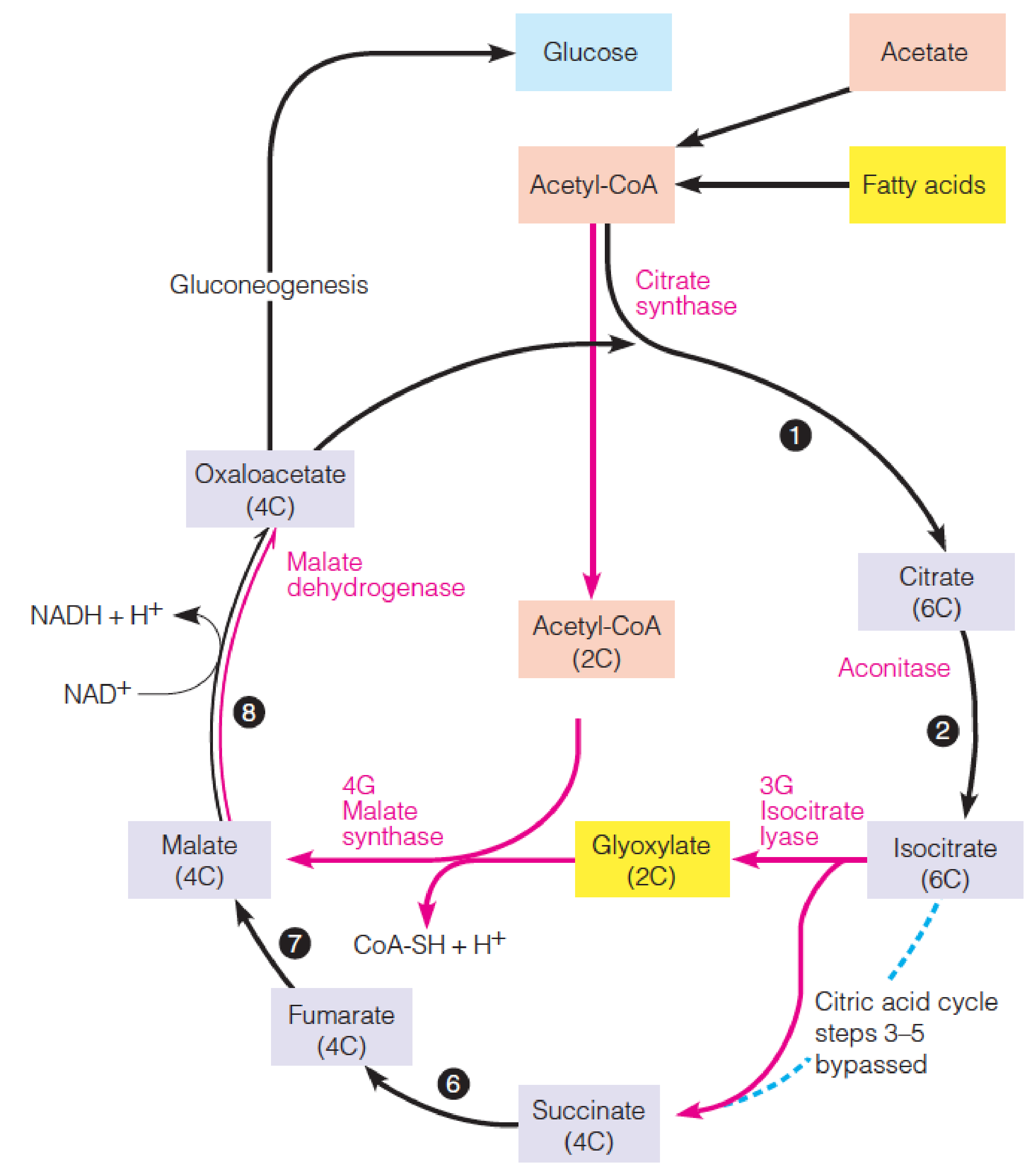
Intracellular relationships involving the glyoxylate cycle in plant cells
Fatty acids released in lipid bodies are oxidized in glyoxysomes to acetyl-CoA, which can also come directly from acetate.
Acetyl-CoA is then converted to succinate in the glyoxylate cycle, and the succinate is transported to mitochondria.
There it is converted in the citric acid cycle to oxaloacetate, which is readily converted to sugars by gluconeogenesis.

Summary
1. Glyoxylate Cycle Summary
- Input 4 carbons (2 Acetyl-CoA)
- Releases 0 CO2 molecules
- Produces one extra oxaloacetate(OA)
- Two oxidations
- 1 NADH, 0 FADH2, 1 (extra) OA per turn of cycle
- Net synthesis of glucose from Acetyl-CoA

2. Citric Acid Cycle Summary
- Input 2 carbons (1 Acetyl-CoA)
- Releases 2 CO2 molecules
- Four oxidations
- 3 NADH, 1 FADH2, 1 GTP per turn of cycle
- No net synthesis of glucose from Acetyl-CoA