L22 Cell Morphology
Cell morphology facilitates cell function
Cells are organized internally!
- Cells use microtubules to position organelles within their cytoplasm.
The cytoskeleton determines cellular organization and polarity
microvilli ((细胞表面的)微绒毛) and cilia (纤毛) are able to maintain a constant location, length, and diameter over the entire lifetime of the cell.
individual actin filaments remain strikingly dynamic and are continuously remodeled and replaced every 48 hours, even within stable cell-surface structures that persist for decades
The large-scale polarity information conveyed by cytoskeletal organization is often maintained over the lifetime of the cell.
- Polarized epithelial cells use organized arrays of microtubules, actin filaments, and intermediate filaments to maintain the critical differences between the apical surface and the basolateral surface.
- They also must maintain strong adhesive contacts with one another to enable this single layer of cells to serve as an effective physical barrier.
一、Overview of cytoskeleton structural elements
Cytoskeleton Elements
3 types of cytoskeleton elements: actin, intermediate filament, and microtubule (Named on basis of physical size)
FUNCTION AND ORIGIN OF THE CYTOSKELETON
The three major cytoskeletal filaments are responsible for different aspects of the cell’s spatial organization and mechanical properties.
- Actin filaments determine the shape of the cell’s surface and are necessary for whole-cell locomotion; they also drive the pinching of one cell into two.
- Microtubules determine the positions of membrane-enclosed organelles, direct intracellular transport, and form the mitotic spindle that segregates chromosomes during cell division.
- Intermediate filaments provide mechanical strength.
The accessory proteins are essential for the controlled assembly of the cytoskeletal filaments in particular locations
Network of filamentous proteins
- filaments formed from a few proteins
- monomer protein forms polymer filaments
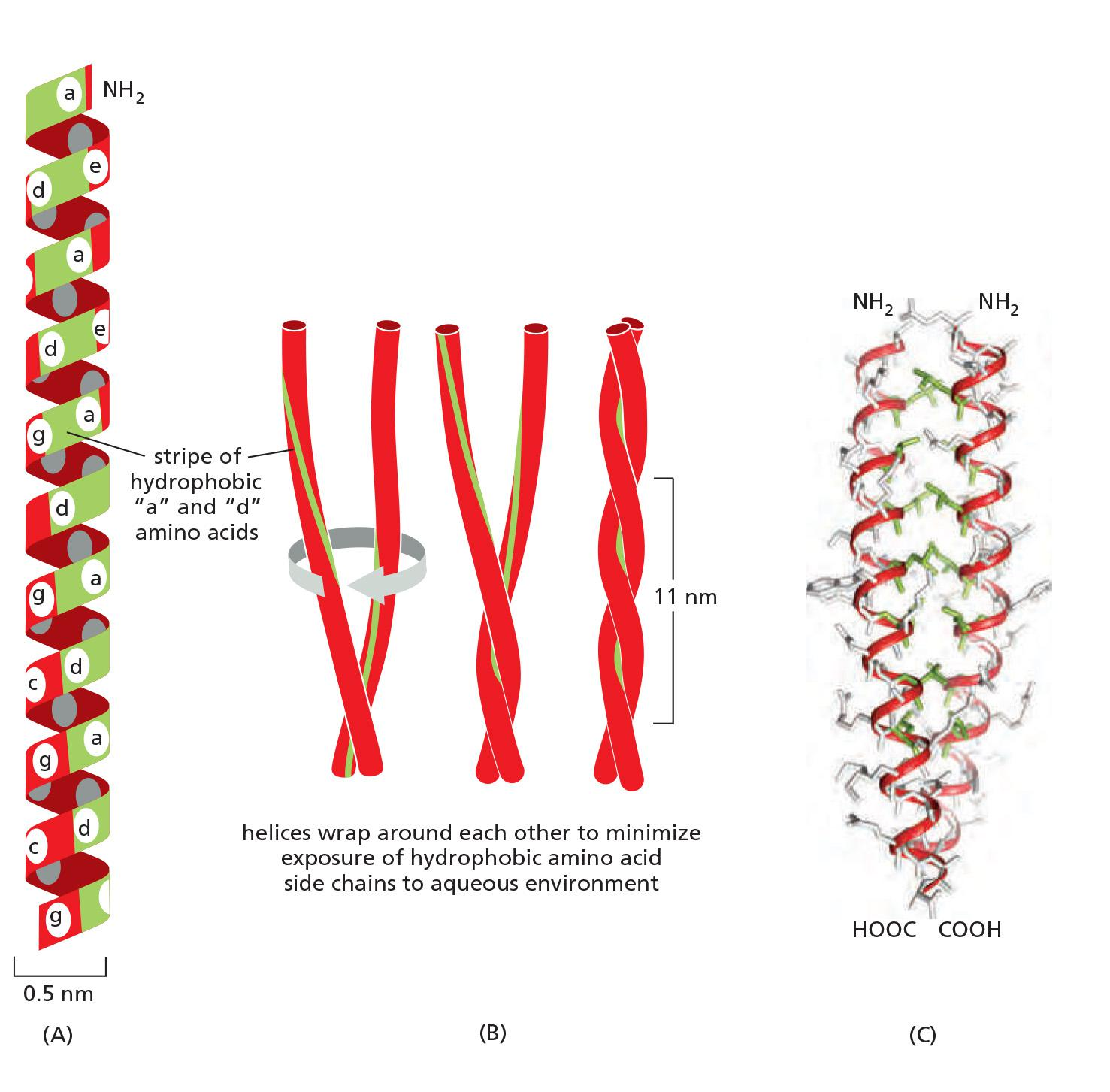
The elements has coiled-coil structures
1. Organization
Located in nucleus and cytoplasmic compartments
- not within organelles
Cytoplasmic
- cortical meshwork under plasma membrane
- three dimensional meshwork through cytoplasm
Nuclear
- cortical meshwork under nuclear envelope
Location based upon cellular function:
Assembly
- some spontaneous
- assembly sites
Dynamic
- variable stability
- high to low stability
- stability can be altered by associated proteins and signals
- drugs can alter stability
The thermal stability of cytoskeletal filament with dynamics ends
Filaments Assemble from Protein Subunits That Impart Specific Physical and Dynamic Properties
Because these subunits are small, they can diffuse rapidly in the cytosol, whereas the assembled filaments cannot. In this way, cells can undergo rapid structural reorganizations, disassembling filaments at one site and reassembling them at another site far away.
All three major types of cytoskeletal filaments form as helical assemblies of subunits (see Figure 3–22) that self-associate, using a combination of end-to-end and side-to-side protein contacts. Differences in the structures of the subunits and the strengths of the attractive forces between them produce important differences in the stability and mechanical properties of each type of filament
It is weak noncovalent interactions that hold together the three types of cytoskeletal polymers.
- The subunits of actin filaments and microtubules are asymmetrical and bind to one another head-to-tail so that they all point in one direction
- In addition, actin and tubulin subunits are both enzymes that catalyze the hydrolysis of a nucleoside triphosphate— ATP and GTP, respectively.
- Intermediate filament subunits also do not catalyze the hydrolysis of nucleotides. Nevertheless, intermediate filaments can be disassembled rapidly when required.
- In mitosis, for example, kinases phosphorylate the subunits, leading to their dissociation.
Cytoskeletal filaments in living cells are not built by simply stringing subunits together in single file
- A thousand tubulin subunits lined up end-to-end, for example, would span the diameter of a small eukaryotic cell
- But a filament formed in this way would lack the strength to avoid breakage by ambient thermal energy, unless each subunit in the filament was bound extremely tightly to its two neighbors
Such tight binding would limit the rate at which the filaments could disassemble, making the cytoskeleton a static and less useful structure
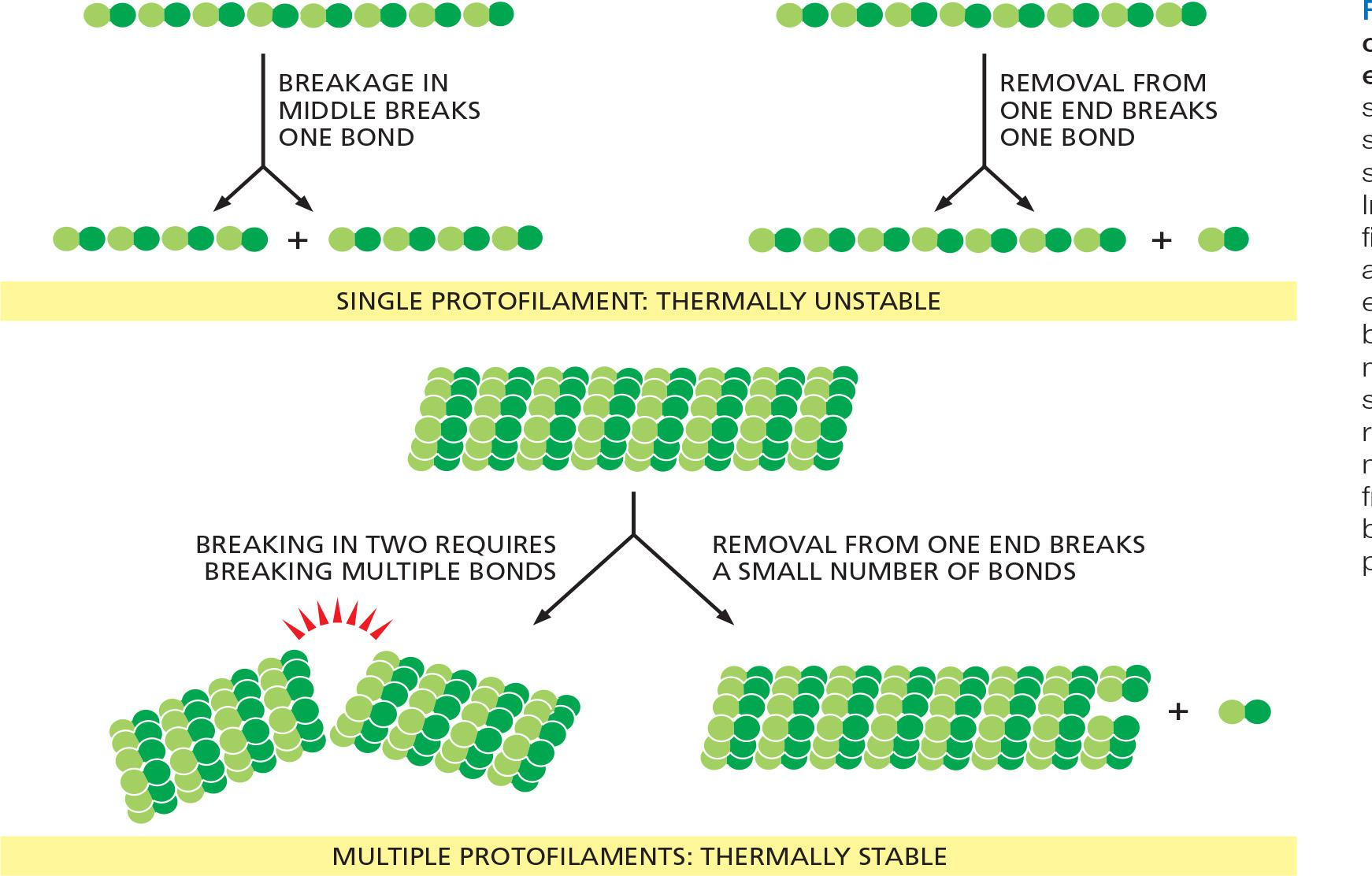
To provide both strength and adaptability, microtubules are built of 13 protofilaments—linear strings of subunits joined end-to-end—that associate with one another laterally to form a hollow cylinder
Cytoskeletal Filaments Adapt to Form Dynamic or Stable Structures
- The addition or loss of a subunit at the end of one protofilament makes or breaks a small number of bonds. In contrast, loss of a subunit from the middle of the filament requires breaking many more bonds, while breaking it in two requires breaking bonds in multiple protofilaments all at the same time
The greater energy required to break multiple noncovalent bonds simultaneously allows microtubules to resist thermal breakage, while allowing rapid subunit addition and loss at the filament ends
Helical actin filaments are much thinner and therefore require much less energy to break. However, multiple actin filaments are often bundled together inside cells, providing mechanical strength, while allowing dynamic behavior of filament ends.
As with other specific protein–protein interactions, many hydrophobic interactions and noncovalent bonds hold the subunits in a cytoskeletal filament together
Actin persistence length: the minimum filament length at which random thermal fluctuations are likely to cause it to bend.
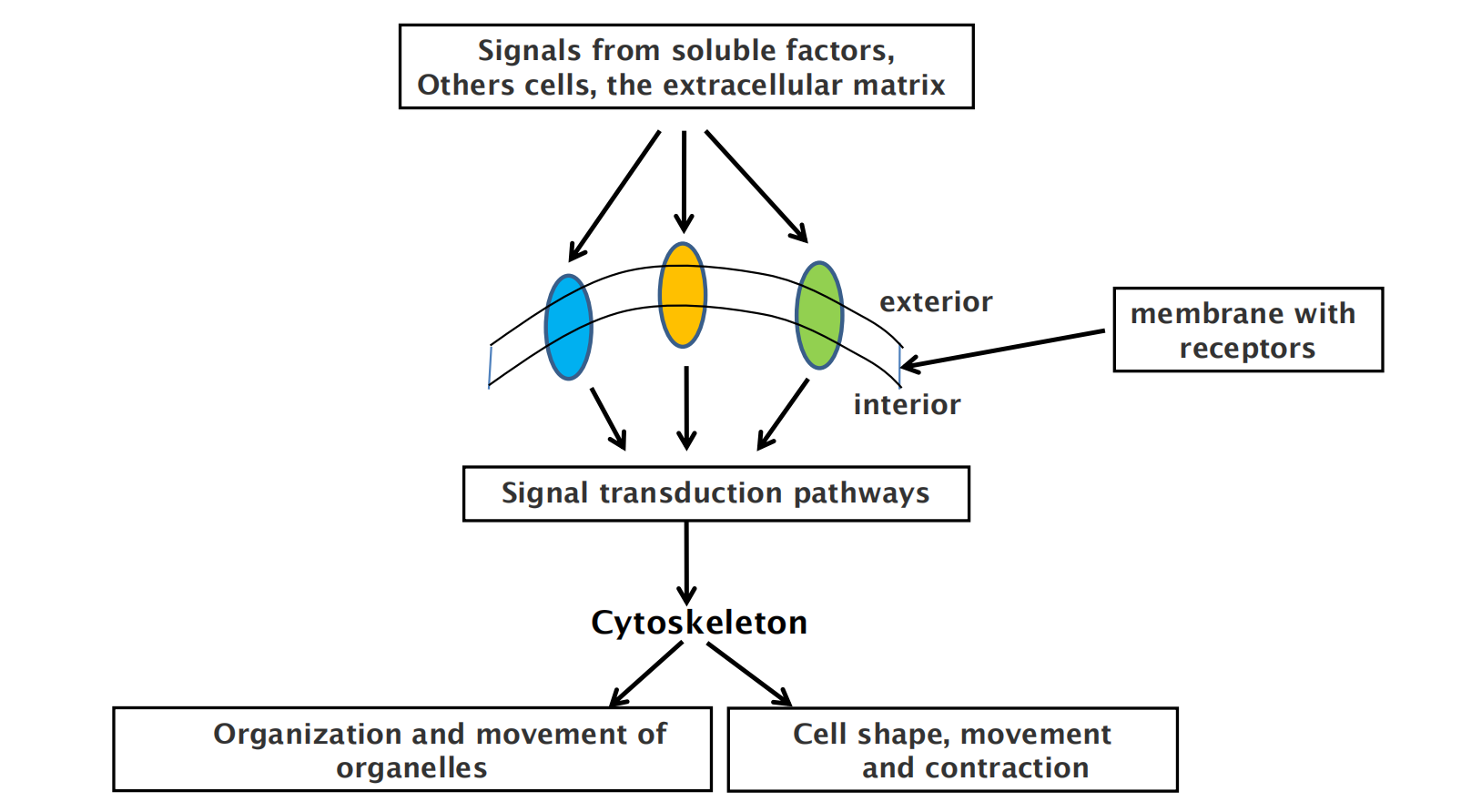
Regulation of cytoskeleton function by cell signaling in time and space
Accessory Proteins and Motors Regulate Cytoskeletal Filaments
Accessory proteins that determine the spatial distribution and the dynamic behavior of the filaments, converting information received through signaling pathways into cytoskeletal action
- These accessory proteins bind to the filaments or their subunits to determine the sites of assembly of new filaments, to regulate the partitioning of polymer proteins between filament and subunit forms, to change the kinetics of filament assembly and disassembly, to harness energy to generate force, and to link filaments to one another or to other cell structures such as organelles and the plasma membrane
Acting together, the accessory proteins enable a eukaryotic cell to maintain a highly organized but flexible internal structure and, in many cases, to move.
Motor proteins. These proteins bind to a polarized cytoskeletal filament and use the energy derived from repeated cycles of ATP hydrolysis to move along it.
2. Functions of cytoskeleton and the relevance for cell viability
| Roles | |
|---|---|
| Actin filament | 1. Determine cell shape and strength to lipid bilayer 2. Whole-Cell locomotion 3. Cell pinching 4. Cell-Surface Projections (lamellipodia, filopodia, stereocilia, microvilli) 5. [In plants] Drives the cytoplasm rapid streaming |
| Microtubule | 1. Determine organelle position 2. Mitotic spindle formation 3. Cilia 4. Tracks for material transportation 5. Cell wall synthesis 6. Protozoans framework |
| Intermediate filaments | 1. Provide mechanical strength 2. [Inner surface of nuclear envelope] DNA protective cage 3. [In cytosol] Strong cables 4. [In hair and fingernails] Tough appendages |
The cytoskeleton fulfills essential tasks:
- Determines cell shape/morphology and provides structure support (integral strength)
- Involved in establishment of cell polarity and cellular organization
- Play roles in cellular motility (e.g. movement during phagocytosis)
- Provides anchor sites for organelles and enzymes to anchor them in specific location in cells
Functions based upon the filaments physical properties
- Muscle contraction
- Chromosome segregation
- Cell division/cytokinesis
- …
signal transduction
- …
二、Actin filament
Actin filaments support and modify cell morphology
Each actin subunit, sometimes called globular- or G-actin, is a 375-amino-acid polypeptide carrying a tightly associated molecule of ATP or ADP
Small variations in actin amino acid sequence can cause significant functional differences:
- In vertebrates, for example, there are three isoforms of actin, termed α, β, and γ, that differ slightly in their amino acid sequences and have distinct functions
- α-Actin is expressed only in muscle cells, while β- and γ-actins are found together in almost all non-muscle cells.
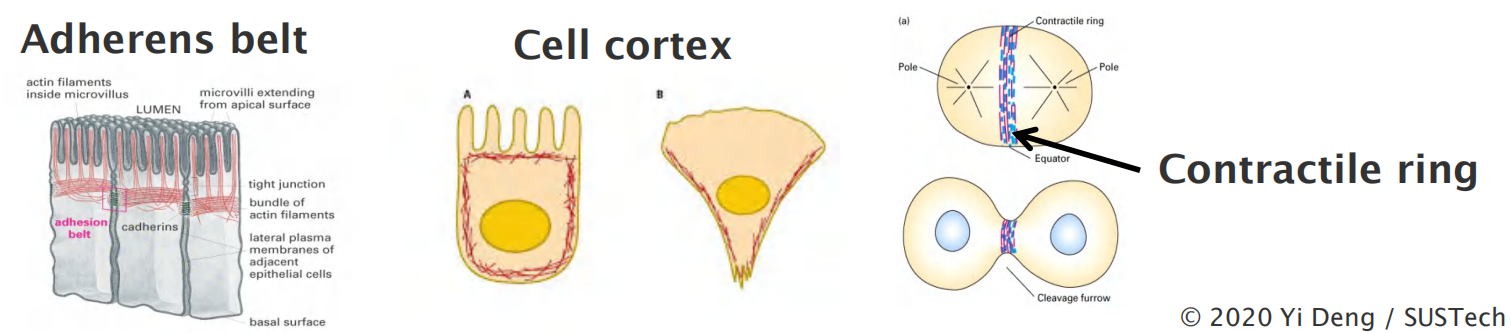
Actin filaments (microfilaments) and structures
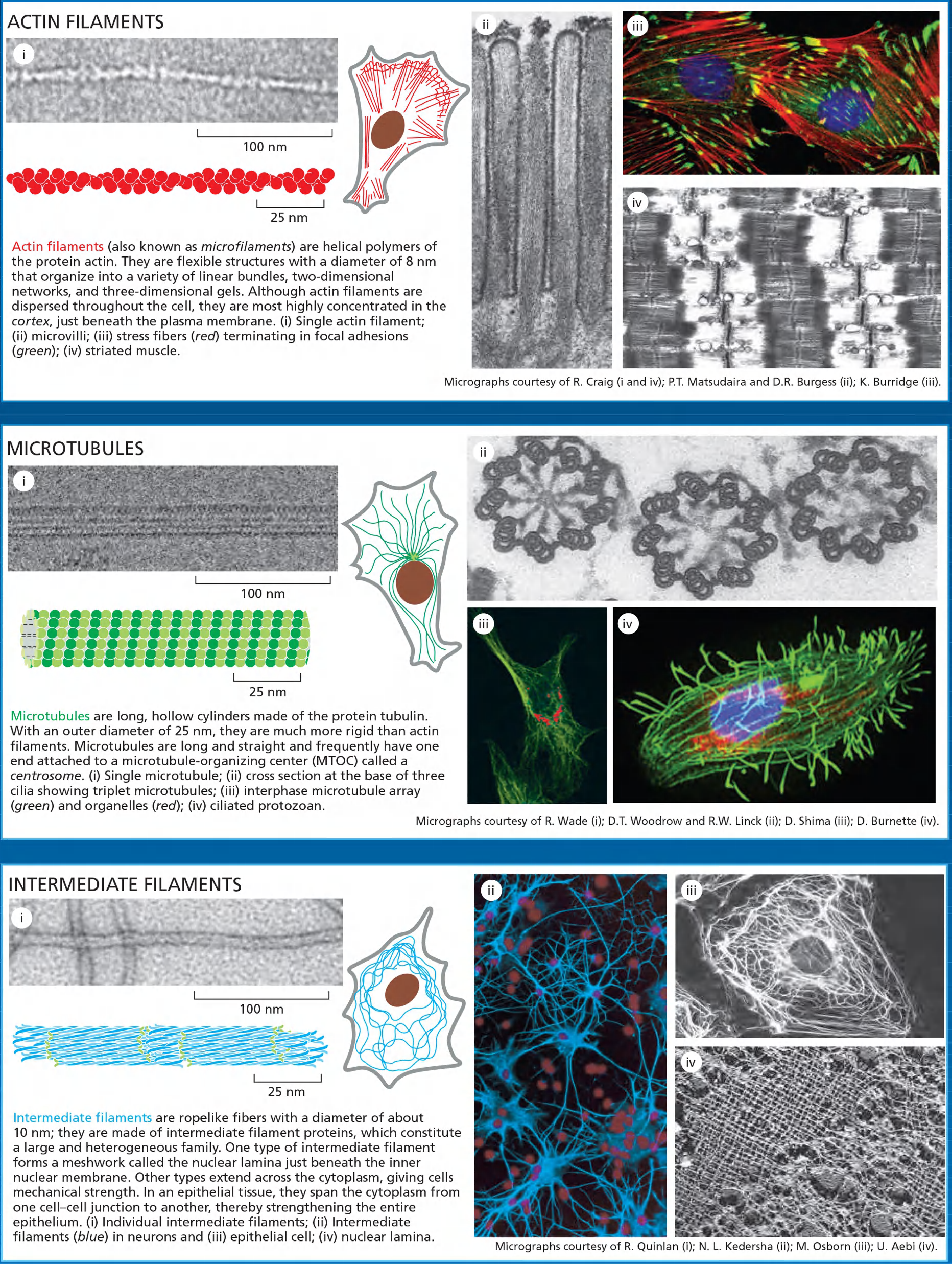
- Microvilli (bundled filaments)
- Cell cortex
- Adherens belt (Adherens junctions)
- Filopodia (bundled actin filaments)
- Lamellipodium/leading edge
- Stress fibers
- Phagocytosis
- Moving endocytic vesicles
- Contractile ring
Actin
Actin Subunits Assemble Head-to-Tail to Create Flexible, Polar Filaments
Actin subunits assemble head-to-tail to form a tight, right-handed helix, forming a structure about 8 nm wide called filamentous or F-actin
Filaments are polar and have structurally different ends: a slower-growing minus end and a faster-growing plus end.
- The minus end is also referred to as the “pointed end (尖头)” and the plus end as the “barbed end,”
The persistence length of an actin filament is only a few tens of micrometers
Highly conserved across species, 80% homology between amoebas and animals
most abundant protein in cells (5% of all cell protein)
- 10-20% of soluble protein
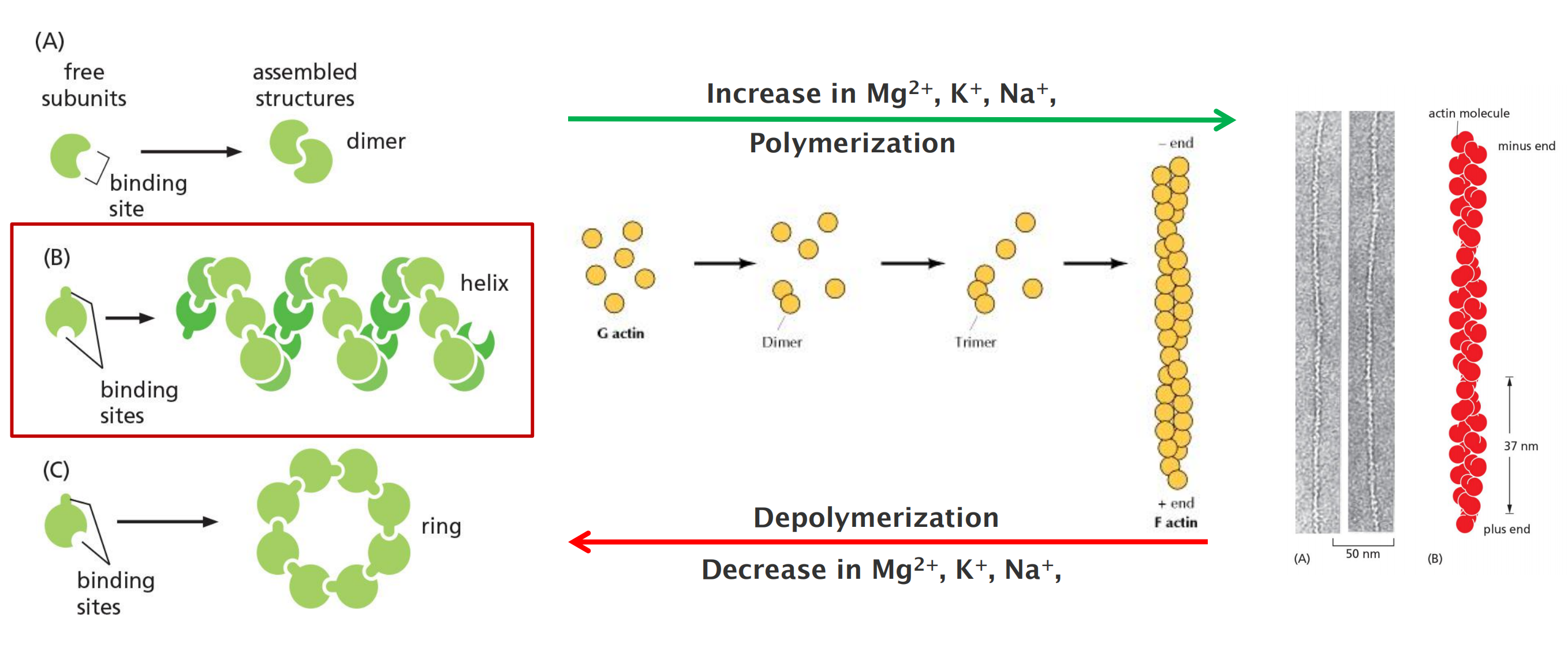
Actin exists in two forms:
- G-actin: globular and monomeric actin, 43 KDa
- F-actin: filamentous, and linear chain of G-actin, twisted chain 7-nm diameter
- Monomer can add to either (+ or - ) end
- Faster at + end
- Actin-ATP hydrolysed (ADP) following addition
- Thinner, more flexible, shorter
- Monomer can add to either (+ or - ) end
Actin binding proteins
- 100 of proteins regulate organization
- many are membrane proteins
Point in same direction
Different organisation in different cellular regions
1. Actin types
Exists in three types (α-actin, β-actin, γ-actin)
- α-actin — contractile structure
- β-actin — leading edge and cell cortex
- γ-actin — stress fibers
2 cytoskeletal isoforms in all non-muscle cells
- Beta (β)
- Gamma (γ)
4 muscle isoforms in different muscle cells
- Alpha (α) skeletal
- Alpha (α) cardiac
- Alpha (α) smooth
- Gamma (γ) smooth
2. Structures of monomeric G-actin and F-actin filaments
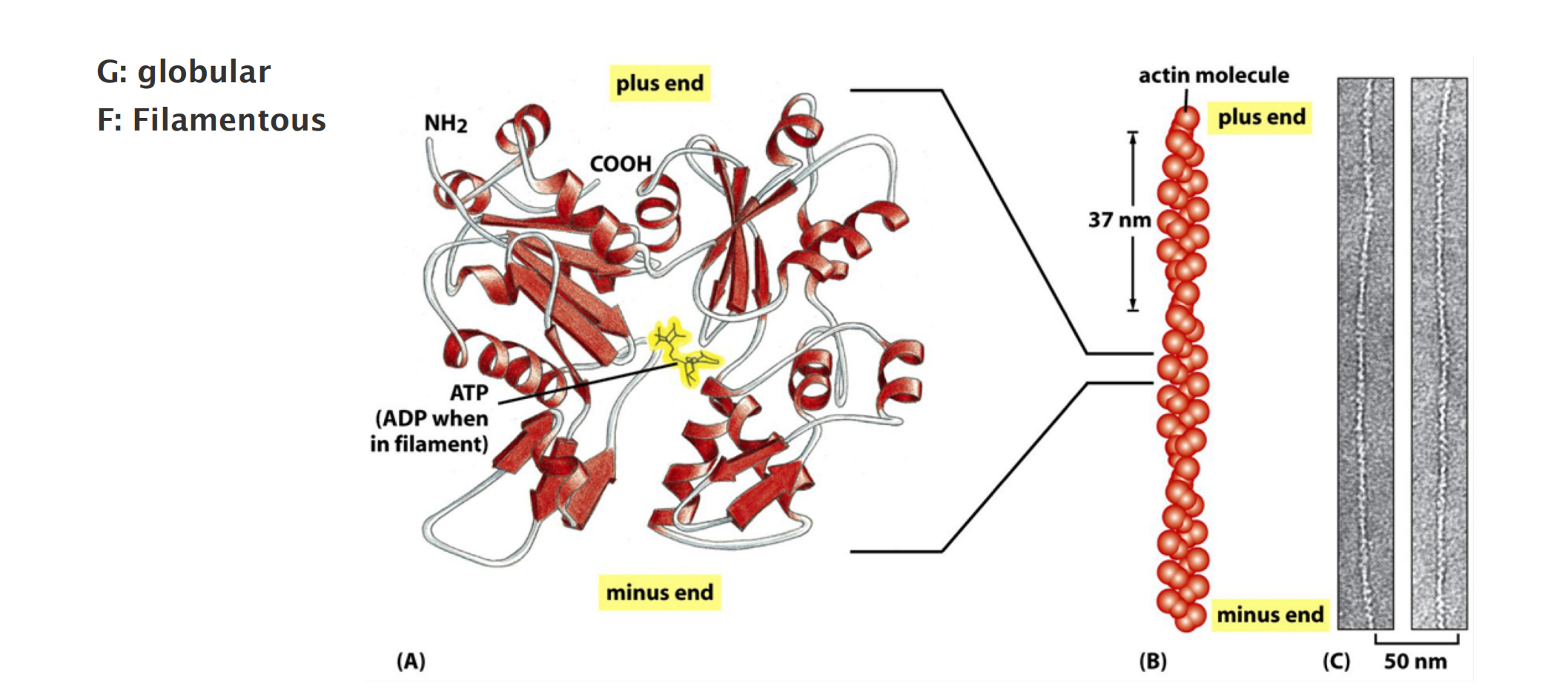
- G-actin, two lobes with a deep cleft in between, binds to ADP/ATP and Mg2+
- Two protofilaments form one filament (held together by lateral contacts)
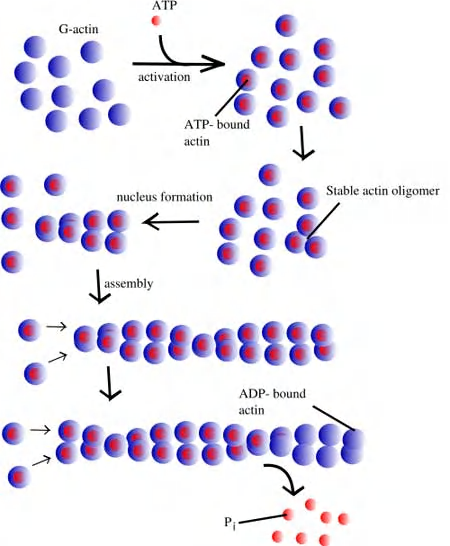
3. Actin polymerization
4. The polarity of an actin filament
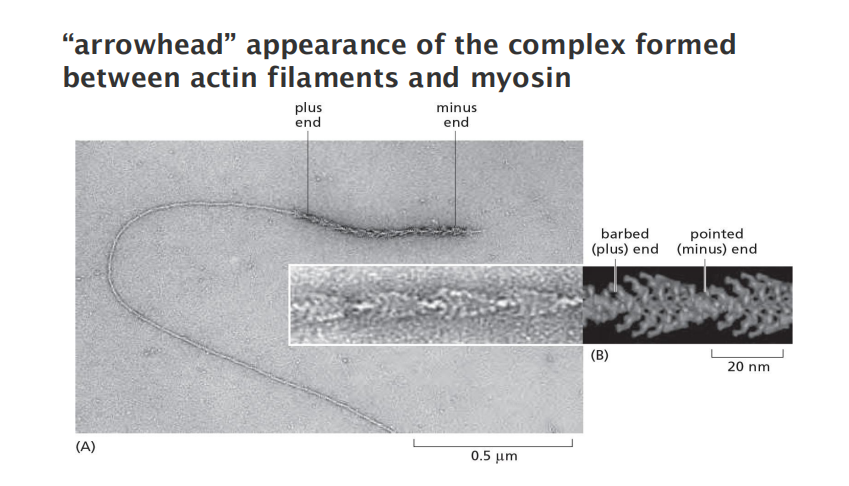
- “arrowhead” appearance of the complex formed between actin filaments and myosin
All actin subunits are oriented the same way
“+” end: end that is favored for addition of actin subunits; ATP-binding cleft of the terminal actin subunits contacts the neighboring subunits
“-” end: end that is favored for subunit dissociation; ATP-binding cleft of the terminal actin subunits is exposed to the solution.
Nucleation
Nucleation Is the Rate-Limiting Step in the Formation of Actin Filaments
Small oligomers of actin subunits can assemble spontaneously, but they are unstable and disassemble readily because each monomer is bound to only one or two other monomers
- For a new actin filament to form, subunits must assemble into an initial aggregate, or nucleus, that is stabilized by multiple subunit–subunit contacts and can then elongate rapidly by addition of more subunits. This process is called filament nucleation.
The instability of smaller actin aggregates creates a kinetic barrier to nucleation
- When polymerization is initiated, this barrier results in a lag phase (滞后阶段) during which no filaments are observed.
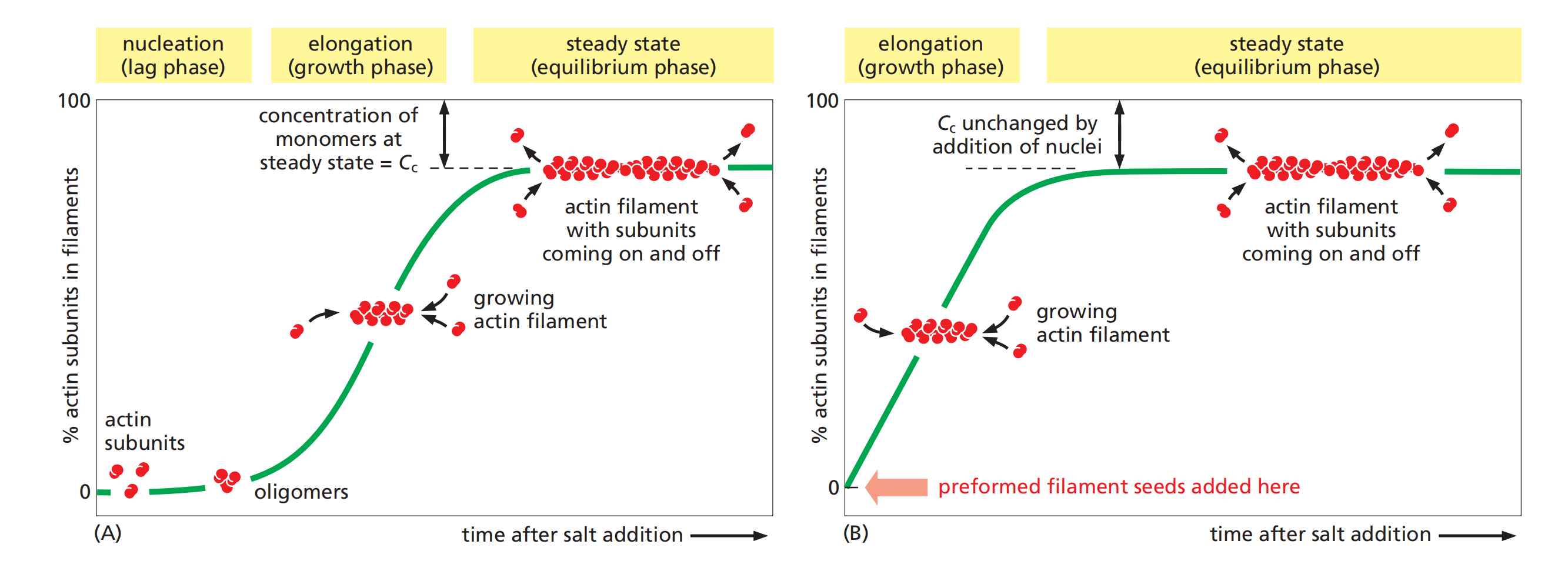
During this lag phase, however, a few of the small, unstable aggregates succeed in making the transition to a more stable form that resembles an actin filament. This leads to a phase of rapid filament elongation during which subunits are added quickly to the ends of the nucleated filaments
Finally, as the concentration of actin monomers declines, the system approaches a steady state at which the rate of addition of new subunits to the filament ends exactly balances the rate of subunit dissociation.
- The concentration of free subunits left in solution at this point is called the critical concentration, C
c.The value of the critical concentration is equal to the rate constant for subunit loss divided by the rate constant for subunit addition—that is, Cc = k
off/kon, which is equal to the dissociation constant, Kd, and the inverse of the equilibrium constant, KThe cell takes great advantage of this nucleation requirement: it uses special proteins to catalyze filament nucleation at specific sites, thereby determining the location at which new actin filaments are assembled.
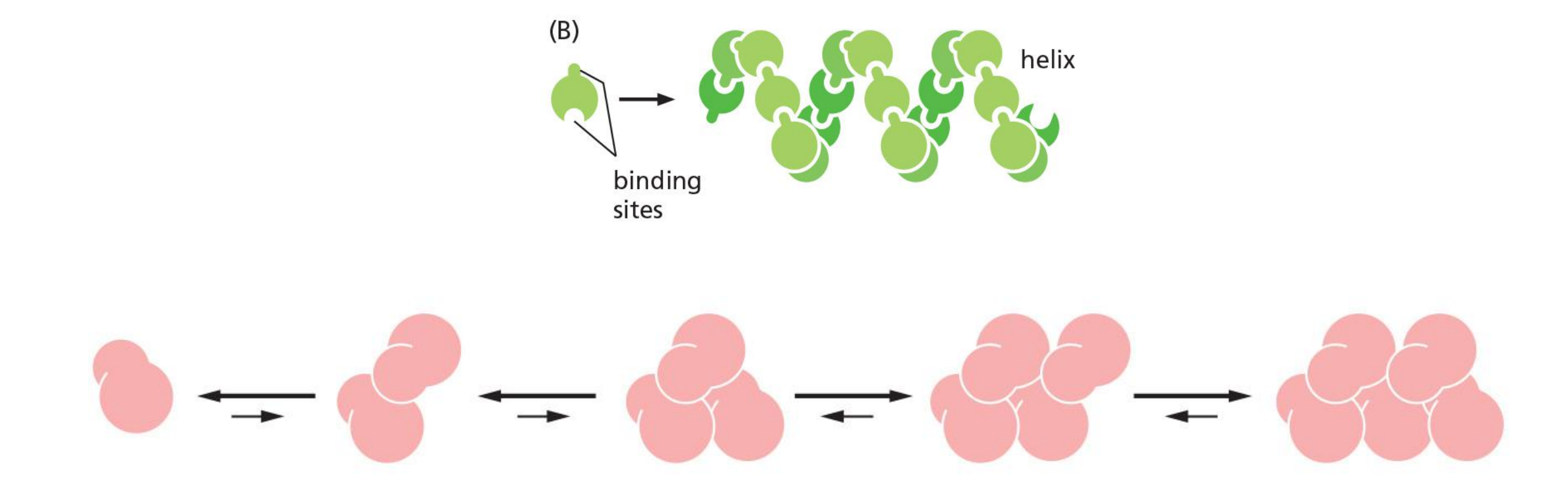
A helical polymer is stabilized by multiple contacts between adjacent subunits.
In the case of actin, two actin molecules bind relatively weakly to each other, but addition of a third actin monomer to form a trimer makes the entire group more stable.
Further monomer addition can take place onto this trimer, which therefore acts as a nucleus for polymerization.
1. Dynamics of actin filaments
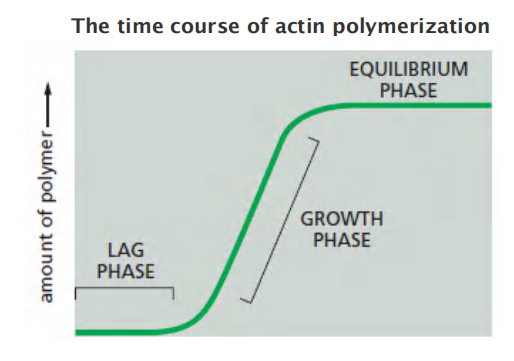
Three stages:
- Nucleation: formation of 3 subunits as seeds for polymerization. This is the rating-limiting step (lag phase).
- Elongation: rapid polymerization from the nucleated seeds (the growth phase)
- Steady-state: addition and removal are balanced, no net increase (equilibrium phase).
2. Molecular mechanism of filament polymerization
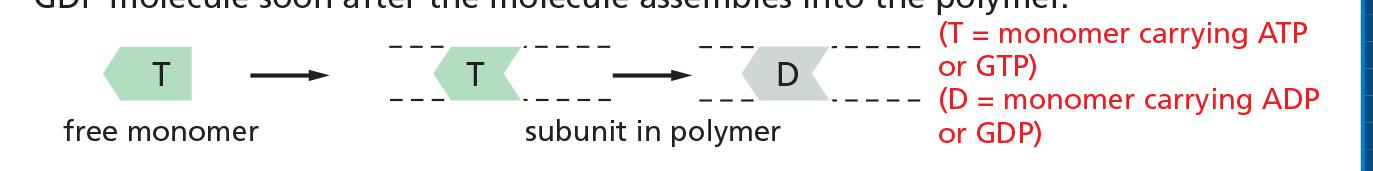
Prerequisites for understanding the dynamics:
- Difference between soluble and filamentous actin:
Actin exists in two forms:
- T-form (ATP-bound)
- D-form (ADP-bound)
In living cells, soluble actin is mainly T-form ([ATP] = 10 [ADP])
- After polymerization, GTP is hydrolyzed
Filament has mainly D-form
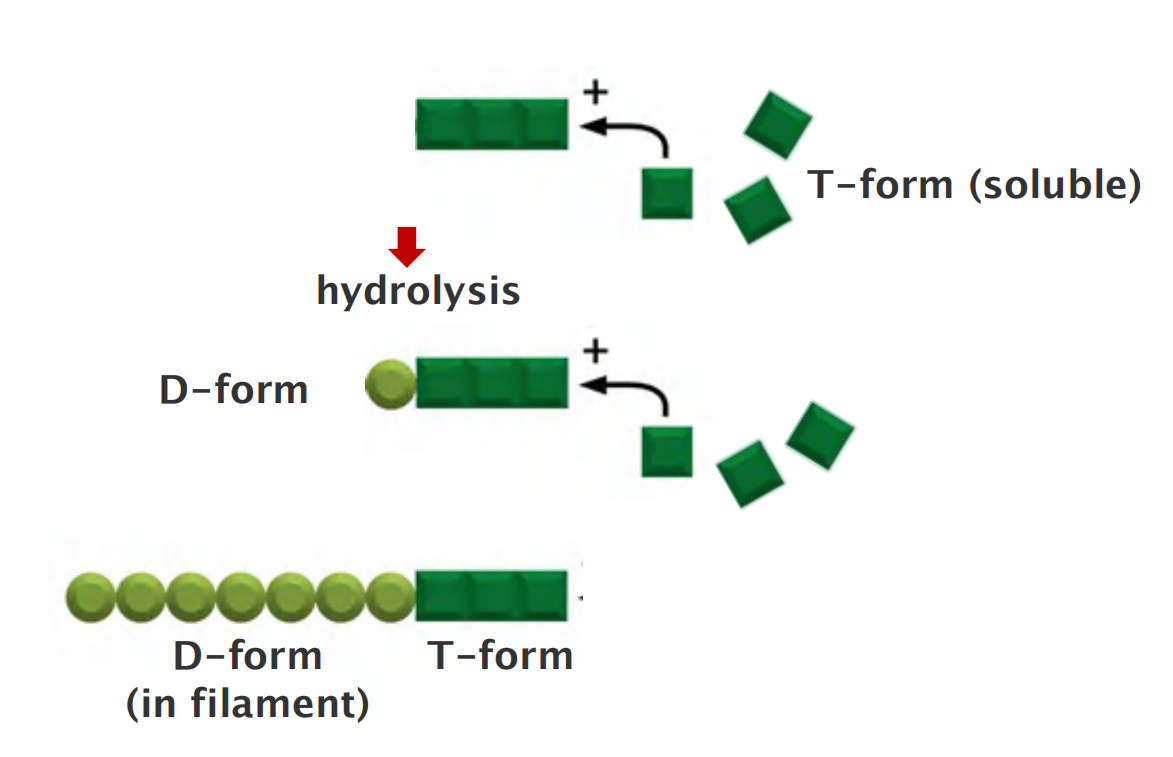
The critical concentration (Cc) for the polymerization of filaments
Critical concentration (Cc): the concentration of subunit at which the rate of subunit addition equals the rate of subunit loss.
$$
K_{on}C = k_{off}\
\text{(C: concentration of G actin) }\
C_c = k_{off}/K_{on} = K_d\
K_d\text{ is the dissociation constant}
$$
Plus and minus end and critical concentration
The conformational change affects the rates at which subunits add to the two ends
For simple polymerization, DG for subunit loss, which determines the equilibrium constant for its association with the end, is identical at both ends. → C > Cc → grow, C < Cc → shrink
With ATP/GTP hydrolysis that accompanies polymerization, this constraint is remove. It is more complex.
Molecular mechanism of filament polymerization
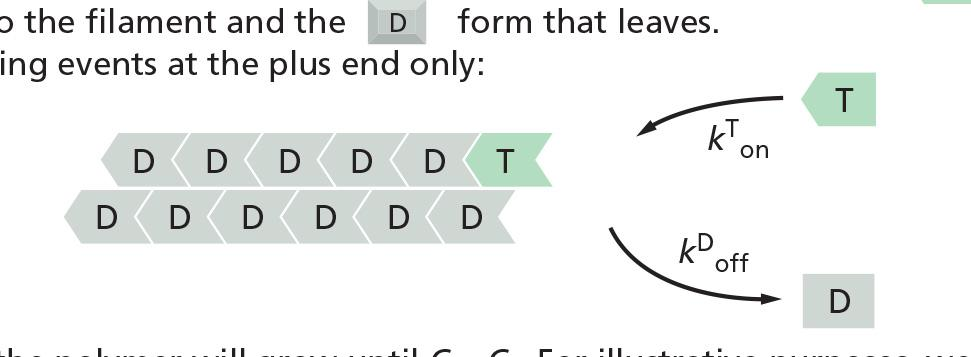
- k^T^
offand k^D^onare too small → ignore
Prerequisites for understanding the dynamics:
- Balance between assembly and dissociation at an end of the filament:
- The conformational change affects the rates at which subunits add to the two ends.
- T-form has high binding affinity but D-form has low binding affinity
- → T-form will get “on” the end of the filament
- → D-form will get “off” the end of the filament
Association (“on”) of the T-form is proportional to the concentration of the soluble actin
Dissociation (“off’) of D-form does not depend on the concentration
If $k^{T}{on} C$ > $k^{D}{off}$ then it will growth, if $k^{T}{on} C$ < $k^{D}{off}$ then it will shrink
- (C: concentration of G actin)
while if $k^{T}{on} C$ = $k^{D}{off}$ then is equilibrium (critical concentration Cc)
- → C = $k^{D}{off}/k^{T}{on}$ → Cc = $k^{D}{off}/k^{T}{on}$
- → minus end Cc(D), plus end Cc(T) → Cc(D) > Cc(T)
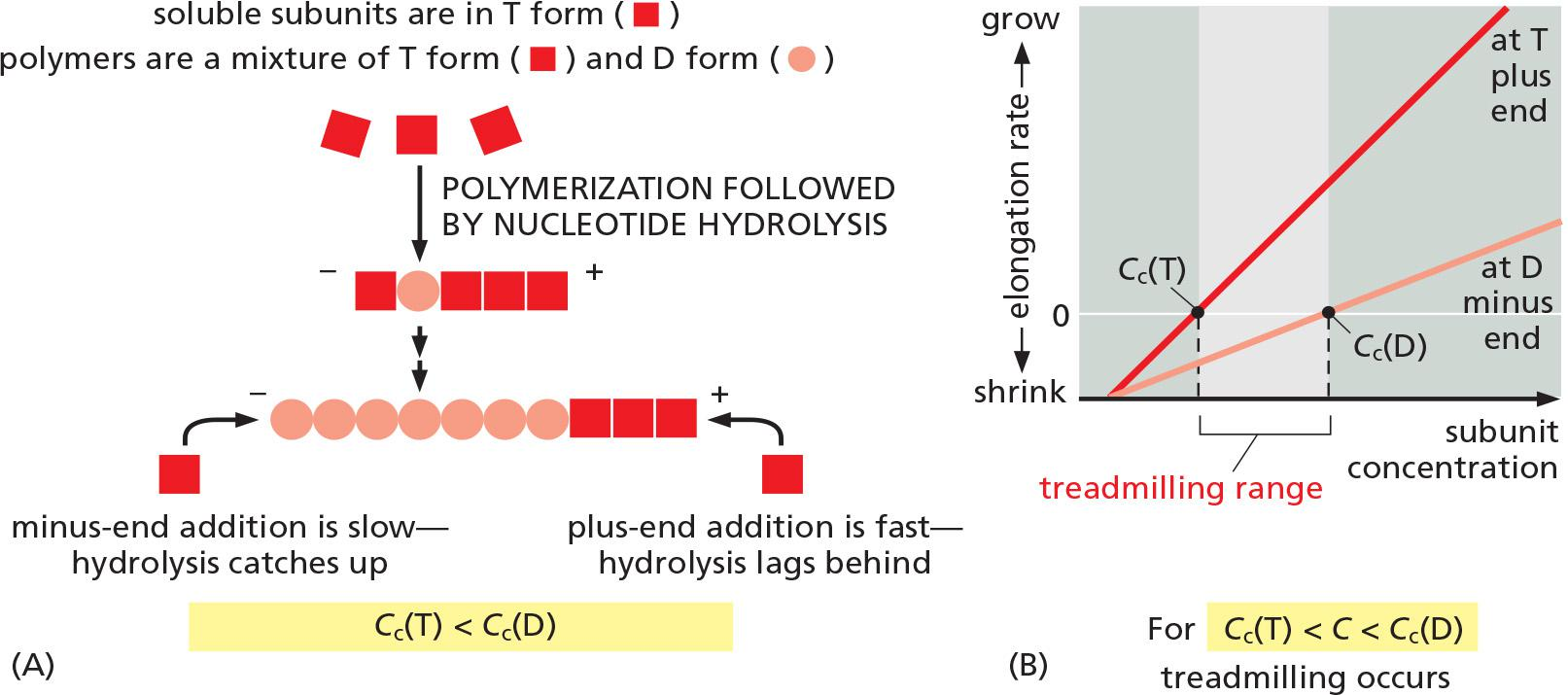
- C = $k^{D}{off}/k^{T}{on}$
- Cc = $k^{D}{off}/k^{T}{on}$
- Minus end Cc is Cc(D)
- Plus end Cc is Cc(T)
- → Cc(D) > Cc(T)
Prerequisites for understanding the dynamics:
- Actin filaments have two different ends and we have to consider both!
- (+) and (-) ends have different critical concentrations (Cc)
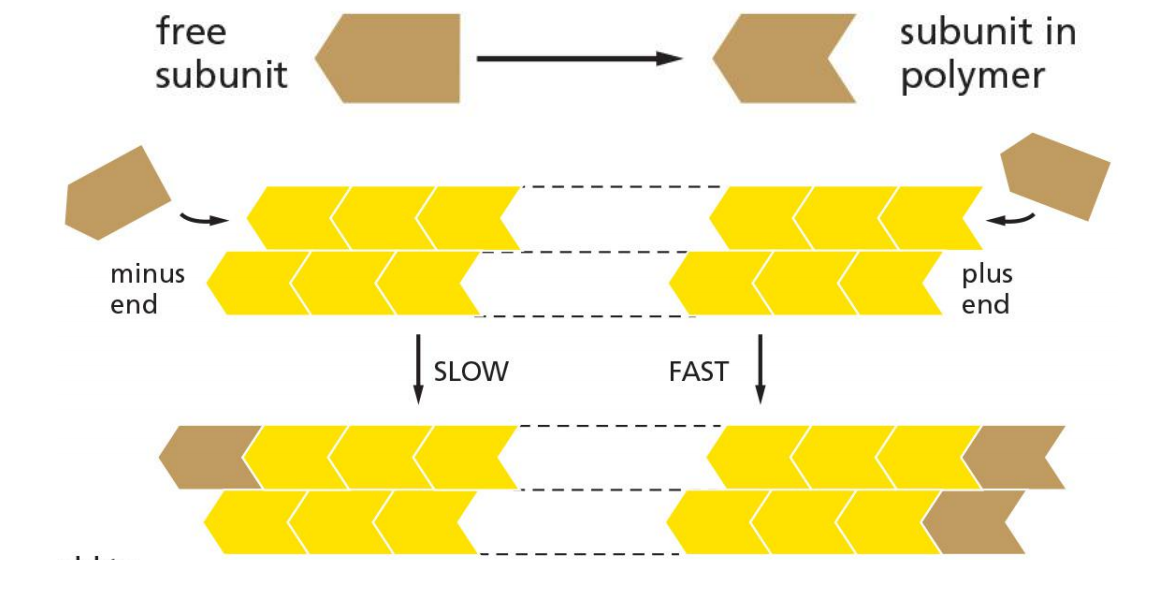
Actin Filaments Have Two Distinct Ends That Grow at Different Rates
The kinetic rate constants for actin subunit association and dissociation— k
onand koff, respectively—are much greater at the plus end than the minus end.
- It is important to note, however, that the two ends of an actin filament have the same net affinity for actin subunits
Addition of a subunit to either end of a filament of n subunits results in a filament of n + 1 subunits. Thus, the free-energy difference, and therefore the equilibrium constant (and the critical concentration), must be the same for addition of subunits at either end of the polymer.
- In this case, the ratio of the rate constants, k
off/kon, must be identical at the two ends, even though the absolute values of these rate constants are very different at each end
Actin treadmilling Cc(T) < C < Cc(D): net flux of molecules
ATP Hydrolysis Within Actin Filaments Leads to Treadmilling at Steady State
- two different types of filament structures can exist, one with the “T form” of the nucleotide bound (ATP), and one with the “D form” bound (ADP).
- Thus, Cc(D) is greater than Cc(T). At certain concentrations of free subunits, D-form polymers will therefore shrink while T-form polymers grow
In living cells, most soluble actin subunits are in the T form, as the free concentration of ATP is about tenfold higher than that of ADP
If the concentration of actin monomers is greater than the critical concentration for both the T-form and D-form polymer, then subunits will add to the polymer at both ends before the nucleotides in the previously added subunits are hydrolyzed
- as a result, the tips of the actin filament will remain in the T form.
At intermediate concentrations of actin subunits, it is possible for the rate of subunit addition to be faster than nucleotide hydrolysis at the plus end, but slower than nucleotide hydrolysis at the minus end. In this case, the plus end of the filament remains in the T conformation, while the minus end adopts the D conformation
The filament then undergoes a net addition of subunits at the plus end, while simultaneously losing subunits from the minus end. This leads to the remarkable property of filament treadmilling
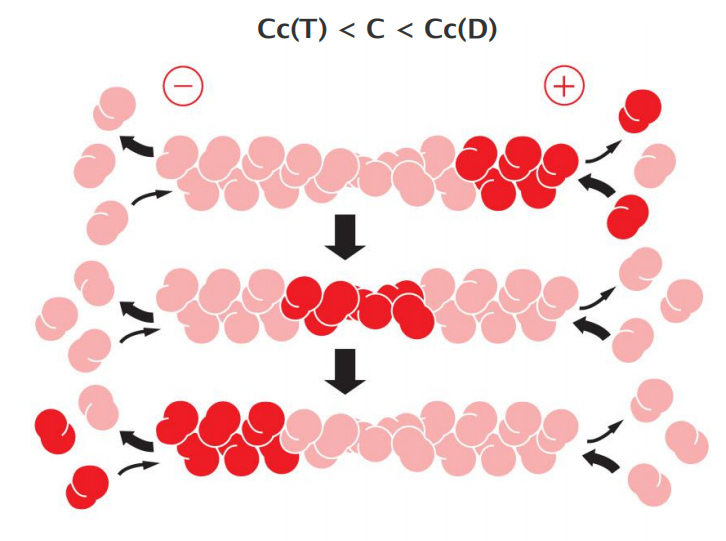
The polymer maintains a constant length, even though there is a net flux of subunits through the polymer, termed treadmilling.
At this steady state, subunits undergo a net assembly at the plus end and a net disassembly at the minus end at an identical rate.
In cells, G-actin levels can be 0.1-0.4 mM, Cc is ~ 0.2 µM
Actin monomer availability controls actin filament assembly
The time course of actin polymerization in a test tube.
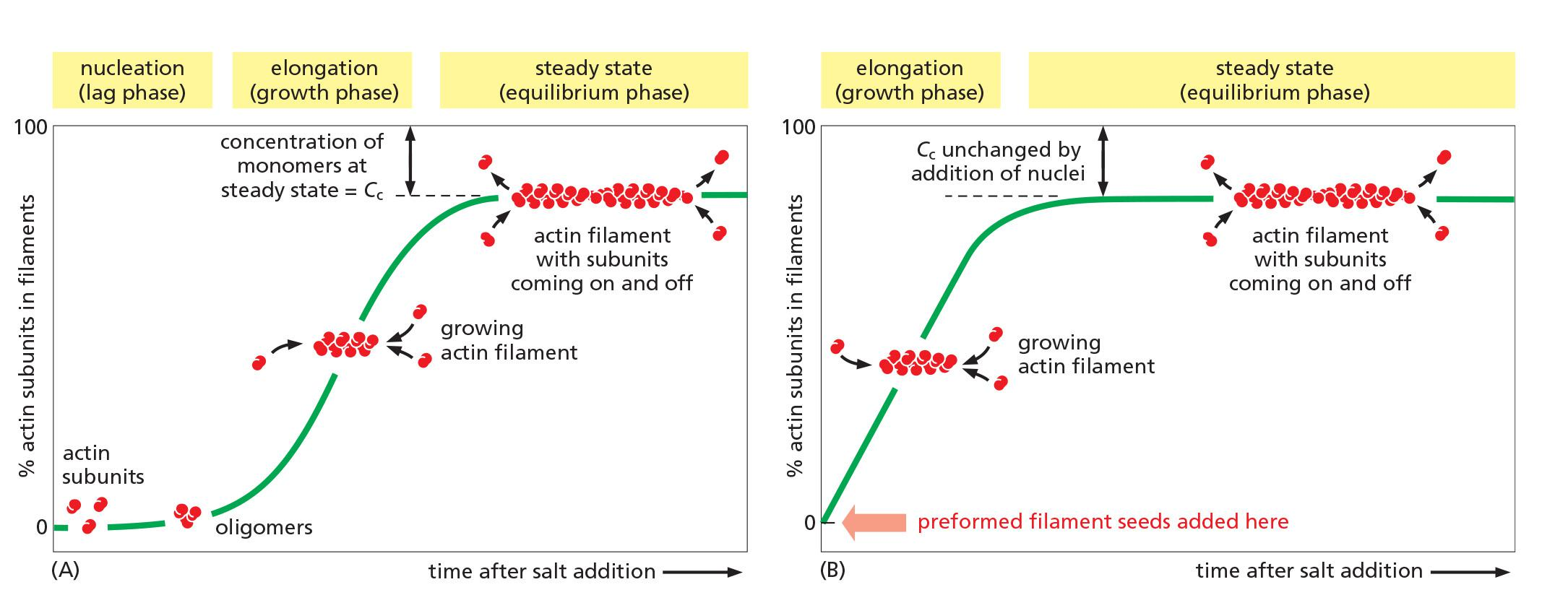
- In the cell, it is more complex in the presence of actin binding proteins.
Mechanisms of actin filament assembly
The “nucleation” is the rating limiting step, what is controlling this critical step?
- Two major classes of actin nucleating proteins accelerate polymerization and generate branched or straight filaments:
- Formin protein family: assembly of long filaments
- Arp (actin-related protein) 2/3 complex: branched filament assembly
Many toxins target actin dynamics
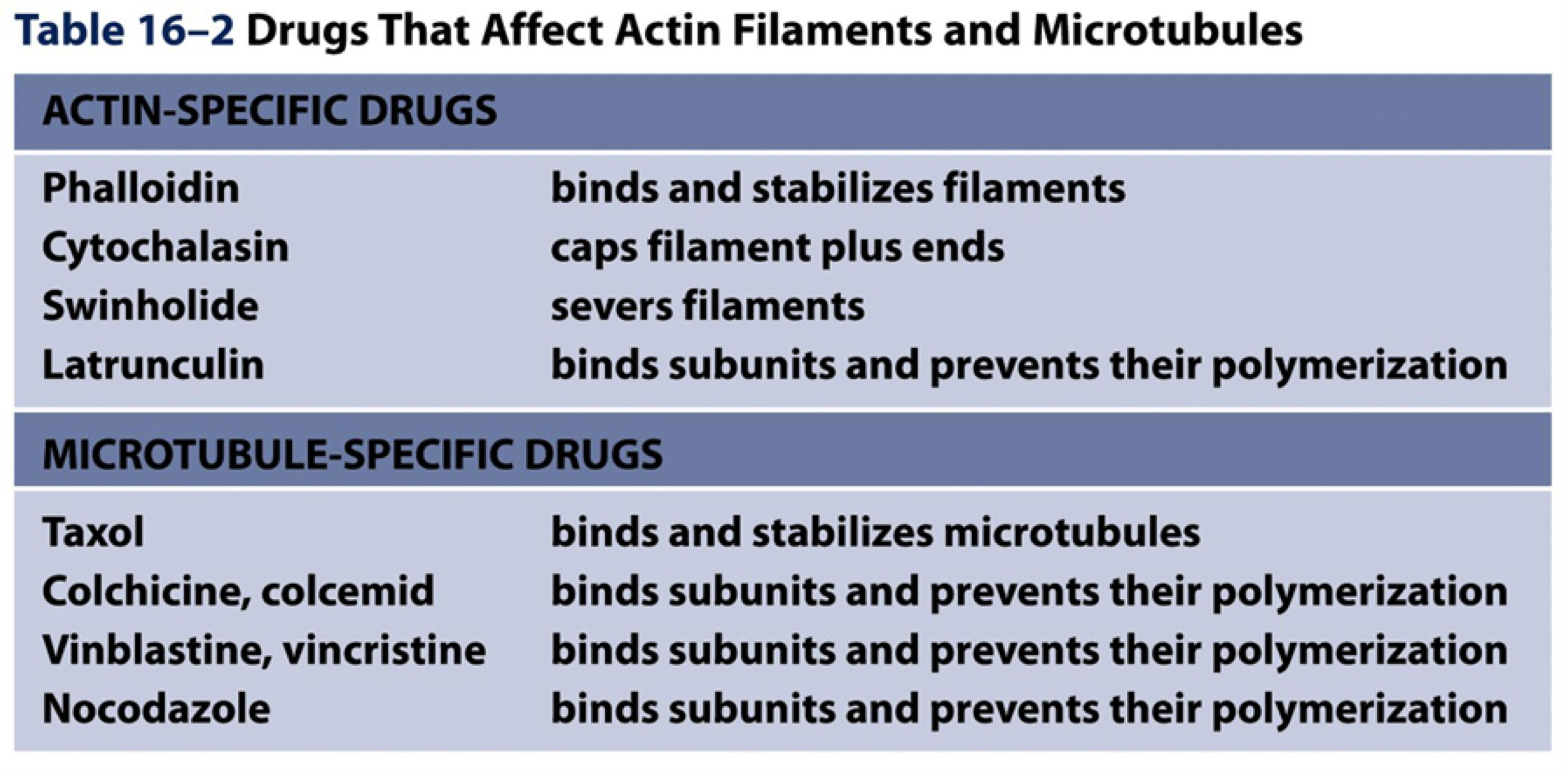
The Functions of Actin Filaments Are Inhibited by Both Polymer stabilizing and Polymer-destabilizing Chemicals
Microfilament depolymerization drugs:
- Cytochalasin D: a fungal alkaloid binds to “+” end of F-actin, blocks addition of subunits.
- Latrunculin: binds to and sequesters G-actin, inhibiting its addition into a filament end.
Microfilament polymerization drugs:
- Jasplakinolide: enhances nucleation by binding and stabilizing actin dimers and lowering the Cc.
- Phalloidin : binds at the interface between subunits in F-actin, locking adjacent subunits together and preventing actin depolymerization.
1. Phalloidin
Actin binding proteins
Actin-Binding Proteins Influence Filament Dynamics and Organization
Actin-binding proteins dramatically alter actin filament dynamics and organization through spatial and temporal control of monomer availability, filament nucleation, elongation, and depolymerization.
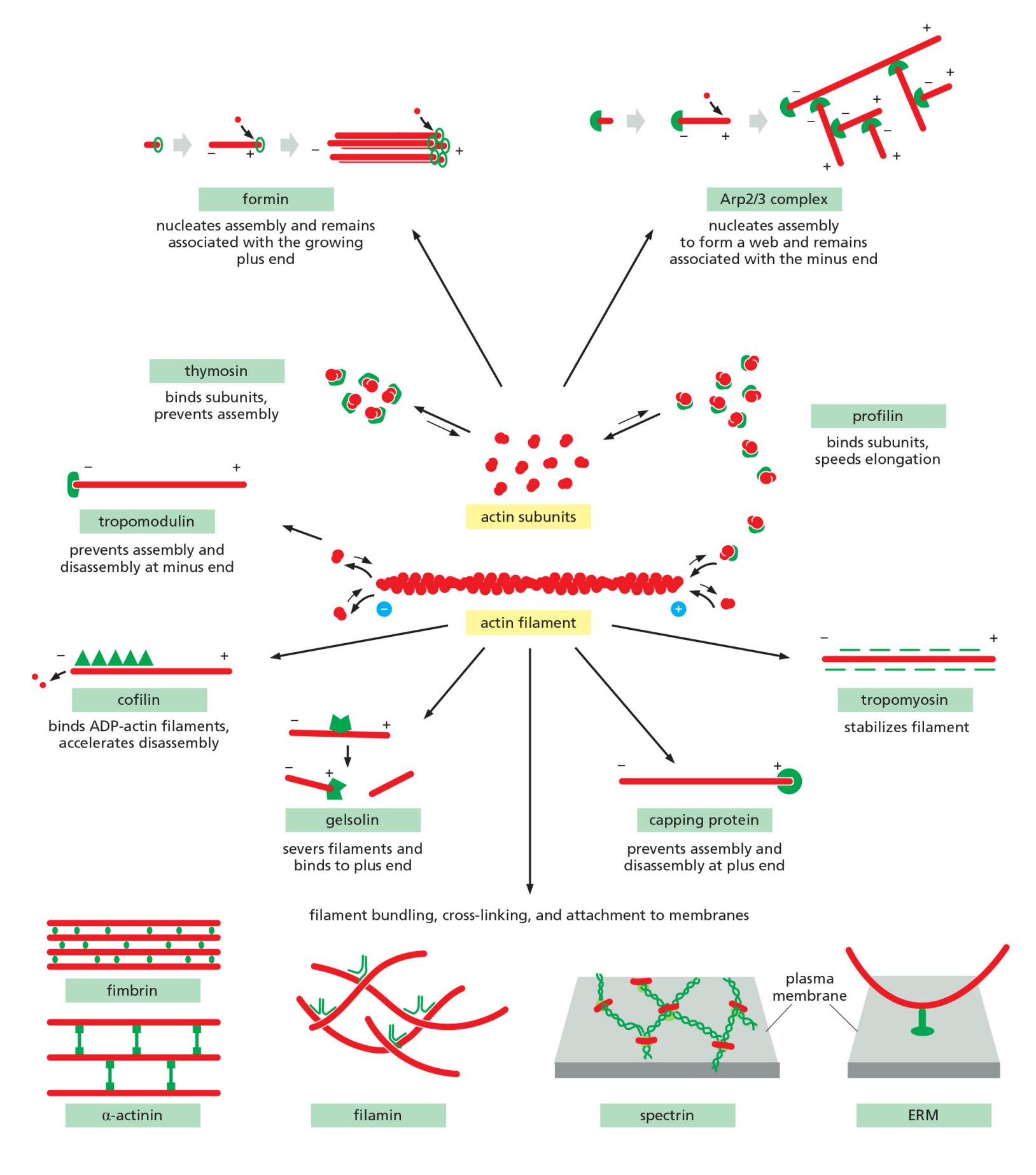
1. Regulation of actin dynamics by actin binding protein
Monomer Availability Controls Actin Filament Assembly
cell contains proteins that bind to the actin monomers and make polymerization much less favorable
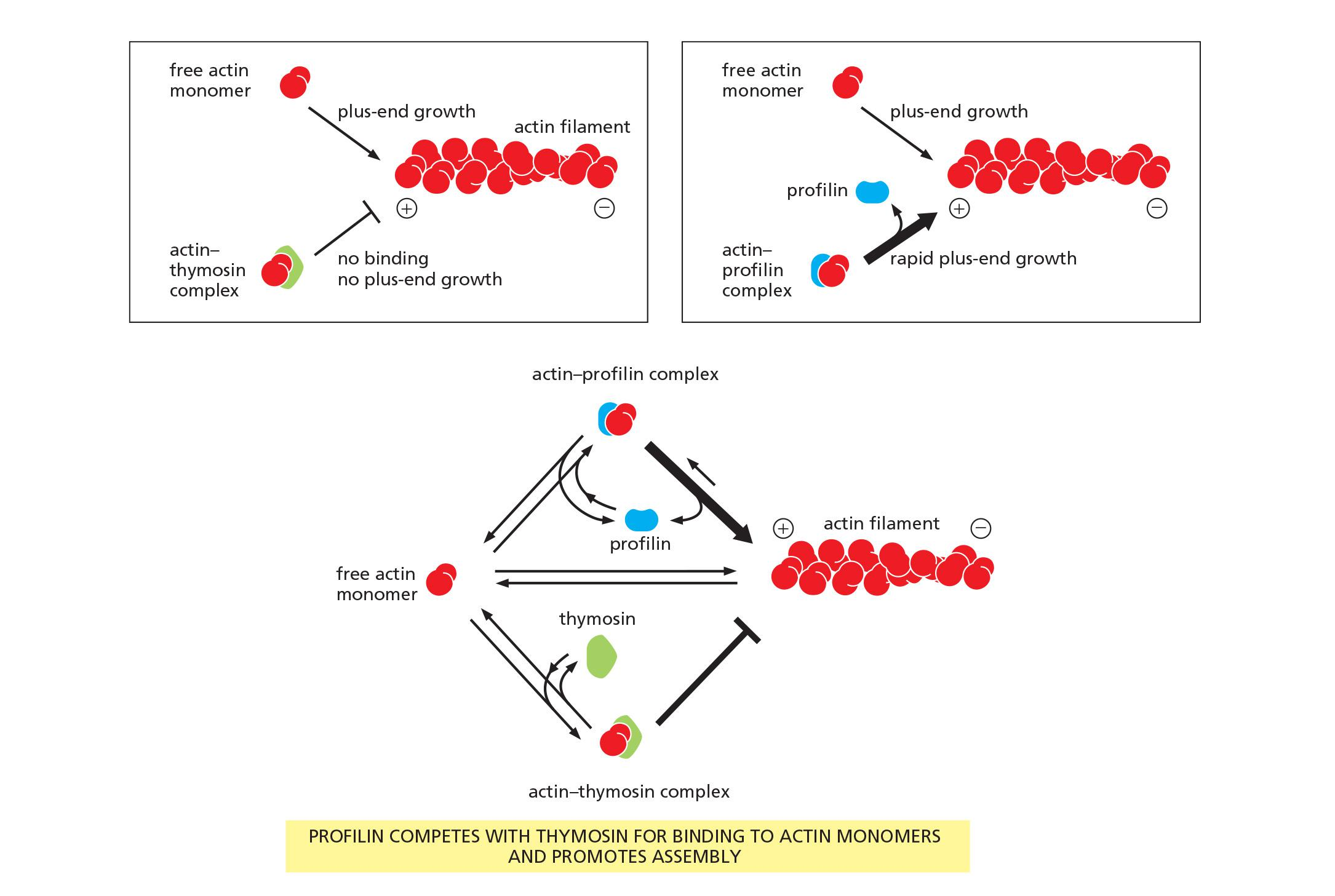
- A small protein called thymosin (胸腺素) is the most abundant of these proteins.
- Actin monomers bound to thymosin are in a locked state, where they cannot associate with either the plus or minus ends of actin filaments and can neither hydrolyze nor exchange their bound nucleotide.
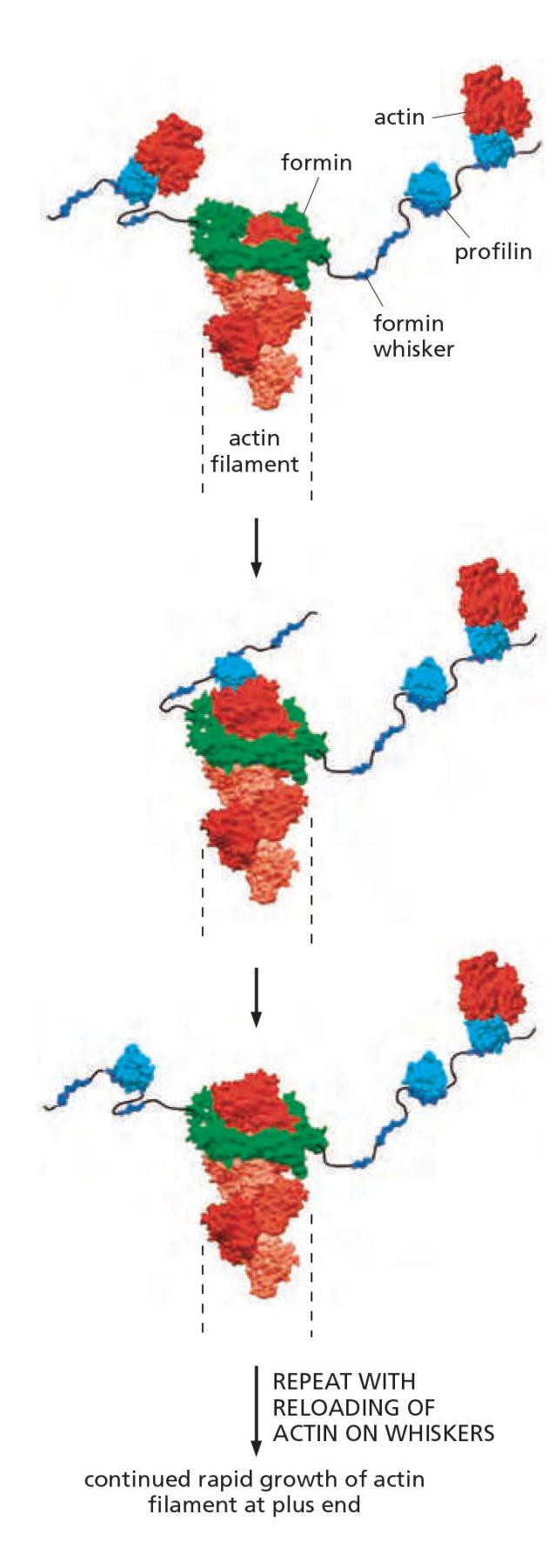
How do cells recruit actin monomers from this buffered storage pool and use them for polymerization? The answer depends on another monomer-binding protein called profilin ([肌动蛋白]抑制蛋白)
- Profilin binds to the face of the actin monomer opposite the ATP-binding cleft, blocking the side of the monomer that would normally associate with the filament minus end, while leaving exposed the site on the monomer that binds to the plus end
- Profilin competes with thymosin for binding to individual actin monomers.
Several mechanisms regulate profilin activity, including profilin phosphorylation and profilin binding to inositol phospholipids. These mechanisms can define the sites where profilin acts
- For example, profilin is required for filament assembly at the plasma membrane, where it is recruited by an interaction with acidic membrane phospholipids
Effects of thymosin and profilin on actin polymerization
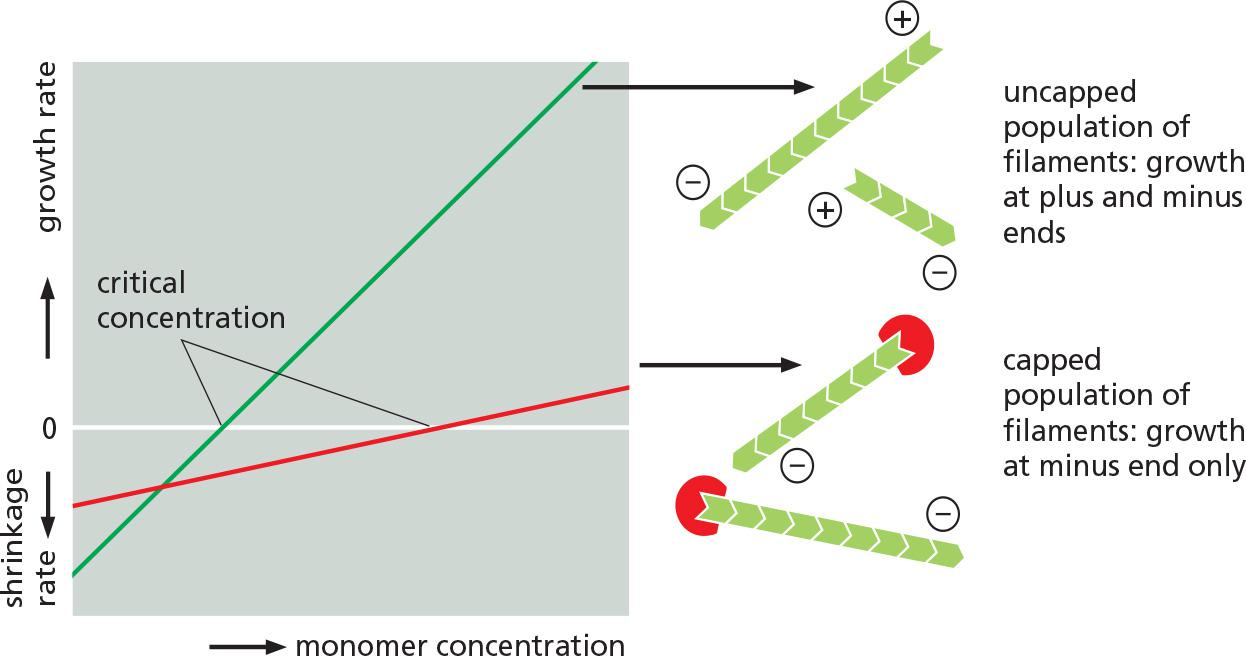
Filament capping and its effects on filament dynamics.
2. Arp2/3 mediates branched filament assembly
Actin-Nucleating Factors Accelerate Polymerization and Generate Branched or Straight Filaments
In addition to the availability of active actin subunits, a second prerequisite for cellular actin polymerization is filament nucleation.
- In most cases, actin nucleation is catalyzed by one of two different types of factors: the Arp 2/3 complex or the formins (形成素 ).
The first of these is a complex of proteins that includes two actin-related proteins, or ARPs, each of which is about 45% identical to actin.
- The Arp 2/3 complex nucleates actin filament growth from the minus end, allowing rapid elongation at the plus end
The complex can attach to the side of another actin filament while remaining bound to the minus end of the filament that it has nucleated, thereby building individual filaments into a treelike web
Comparison of structures: actin versus actin-related protein (Arp) 2 and 3
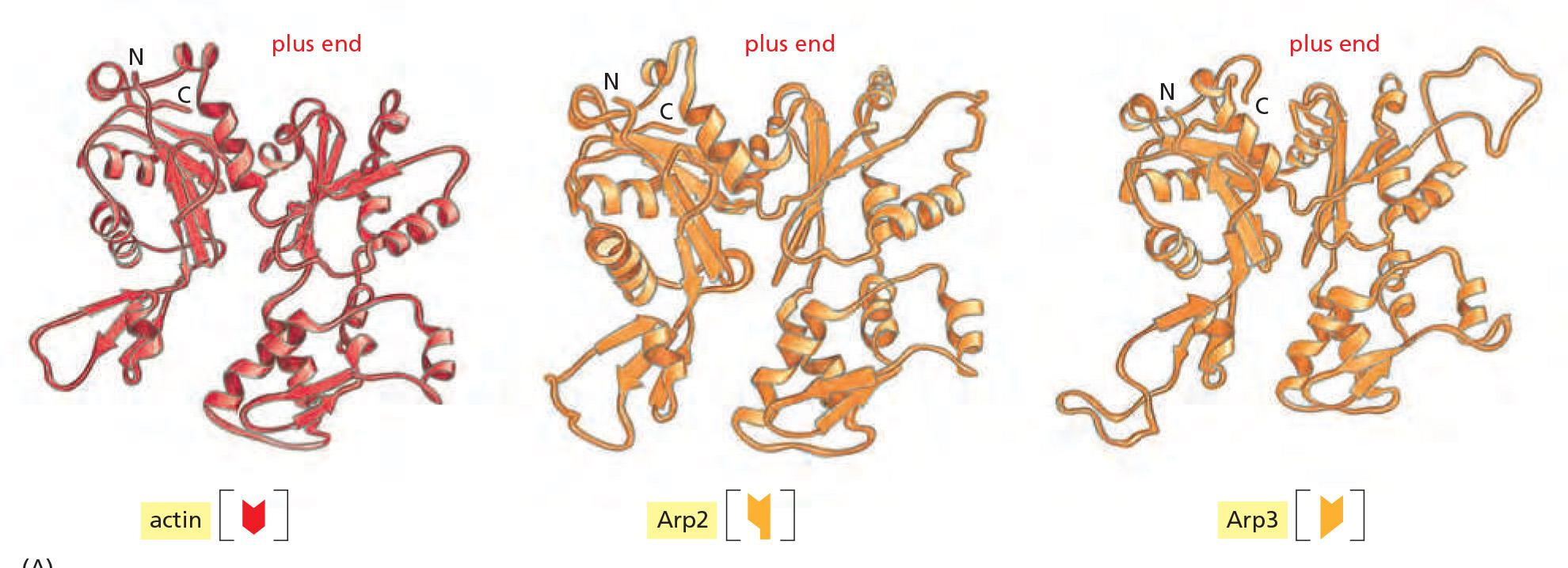
Arp2/3 similar to actin at (+) end but differences at sides and (-) end prevent spontaneous filament formation.
Arp2/3 complex (7 subunits in human)
- actin-related proteins, Arp2 & Arp3, and 5 smaller proteins (ARC41, ARC34, ARC21, ARC20, and ARC16)
Actin nucleation by the Arp2/3 complex
An activating factor is required to bring Arp2 and Arp3 together
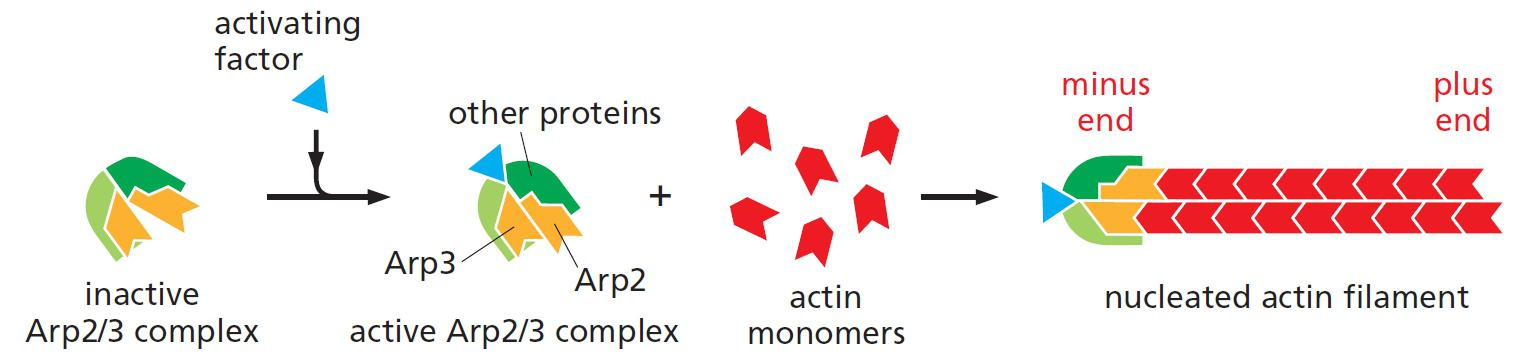
The activated Arp2/3 complex resembles a (+) end of a filament and is used for the actin filament assembly.
Upon activation by a nucleation promoting factor (NPF), Arp2/3 complex binds to the side of an existing actin filament and nucleates assembly of a new filament branch.
Arp2/3 mediates branched filament assembly
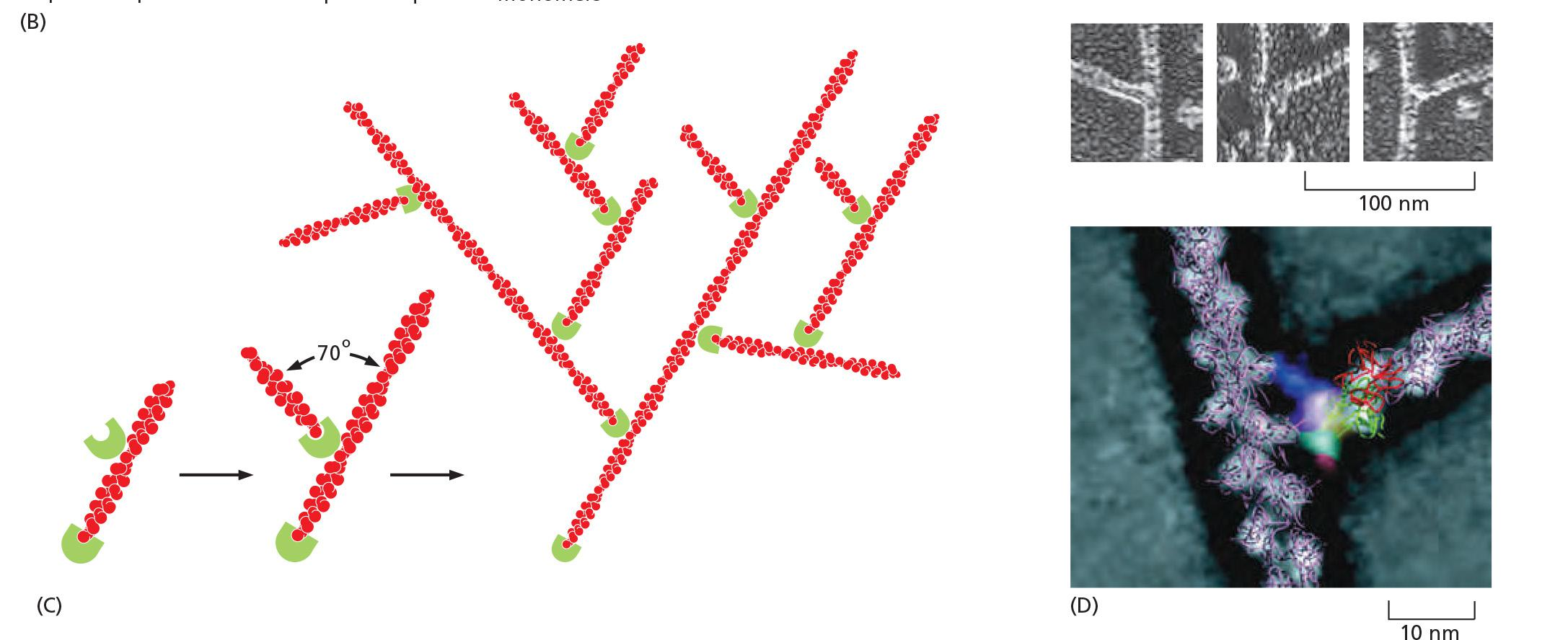
- Branching: actin with Arp2/3 complex fitted according to electron density.
- Mother filament runs from top to bottom
- daughter filament branches to the right
Assembly is most efficient when Arp2/3 is bound to the side of an existing filament
New filament and old filament have an angle of 70 degrees
The resulting branch structure is y-shaped.
3. Formin mediates straight filament assembly
Formins are dimeric proteins that nucleate the growth of straight, unbranched filaments that can be cross-linked by other proteins to form parallel bundles
- Each formin subunit has a binding site for monomeric actin, and the formin dimer appears to nucleate actin filament polymerization by capturing two monomers.
As the newly nucleated filament grows, the formin dimer remains associated with the rapidly growing plus end while still allowing the addition of new subunits at that end
Formin-dependent actin filament growth is strongly enhanced by the association of actin monomers with profilin (Figure 16–18).
Like profilin activation, actin filament nucleation by Arp 2/3 complexes and formins occurs primarily at the plasma membrane, and the highest density of actin filaments in most cells is at the cell periphery
- The layer just beneath the plasma membrane is called the cell cortex, and the actin filaments in this region determine the shape and movement of the cell surface, allowing the cell to change its shape and stiffness rapidly in response to changes in its external environment.
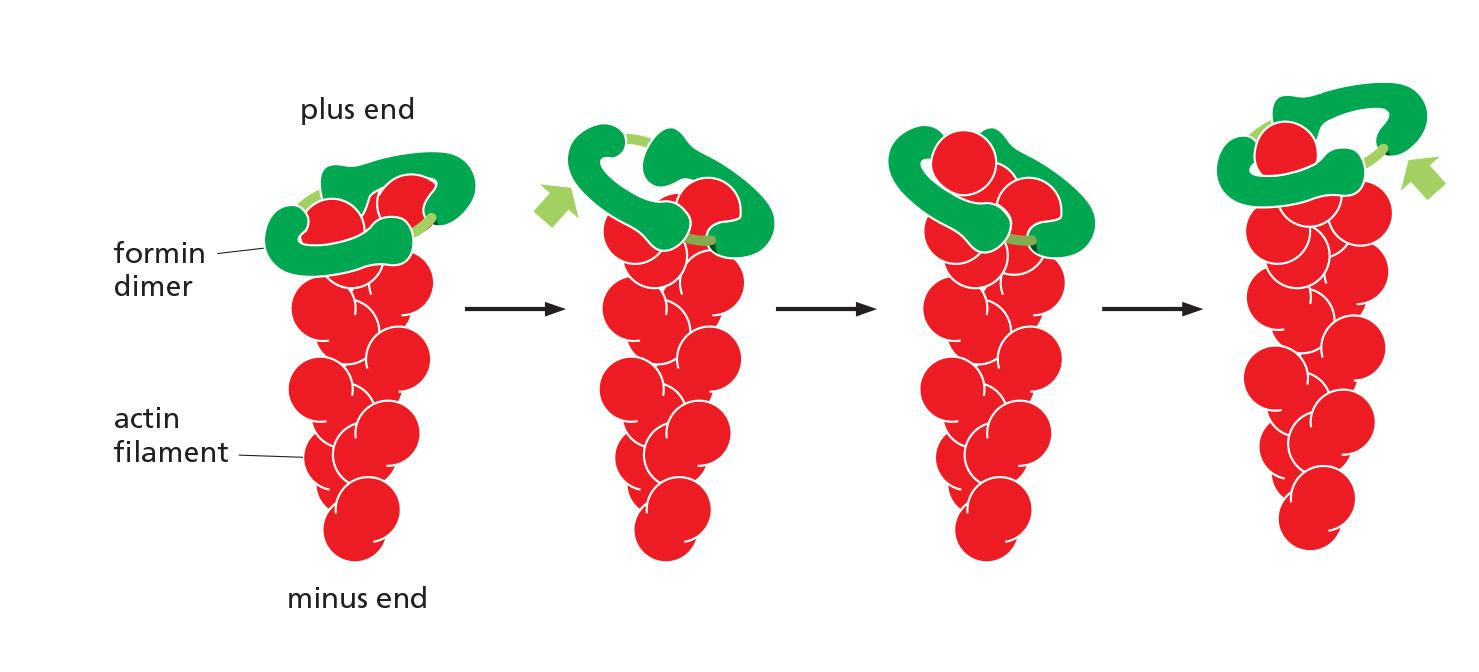
Formins are found at the plus ends of actin filaments.
Formins are dimeric proteins that nucleate the growth of straight, unbranched filaments that can be cross-linked by other proteins to form parallel bundles.
Each formin subunit has a binding site for monomeric actin, and the formin dimer appears to nucleate actin filament polymerization by capturing two monomers.
The newly nucleated filament grows and the formin dimer remains associated with the growing plus end while still allowing the addition of new subunits at that end.
Regulation of formins by Rho-GTPases
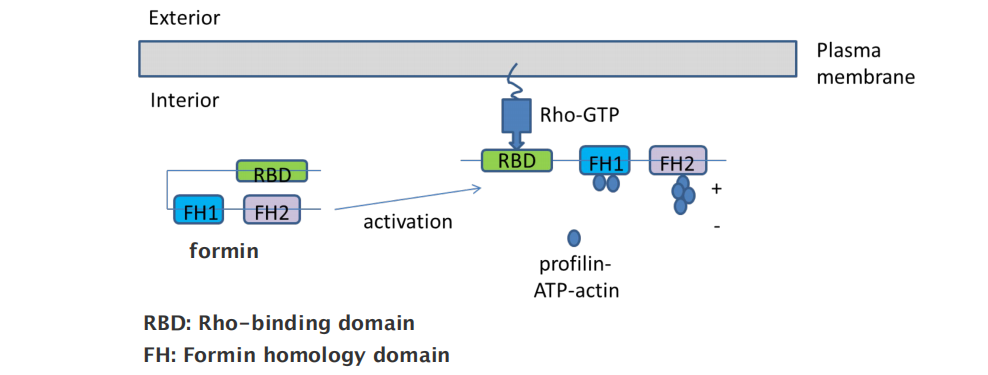
- RBD: Rho-binding domain
- FH: Formin homology domain
4. Actin-Filament-Binding Proteins Alter Filament Dynamics
Actin filament behavior is regulated by two major classes of binding proteins: those that bind along the side of a filament and those that bind to the ends
Side-binding proteins include tropomyosin (原肌球蛋白), an elongated protein that binds simultaneously to six or seven adjacent actin subunits along each of the two grooves of the helical actin filament.
- In addition to stabilizing and stiffening the filament, the binding of tropomyosin can prevent the actin filament from interacting with other proteins
An actin filament that stops growing and is not specifically stabilized in the cell will depolymerize rapidly, particularly at its plus end
The binding of plus-end capping protein (also called CapZ for its location in the muscle Z band) stabilizes an actin filament at its plus end by rendering it inactive, greatly reducing the rates of filament growth and depolymerization
Tropomodulin, best known for its function in the capping of exceptionally long-lived actin filaments in muscle, binds tightly to the minus ends of actin filaments that have been coated and thereby stabilized by tropomyosin.
- It can also transiently cap pure actin filaments and significantly reduce their elongation and depolymerization rates.
For maximum effect, proteins that bind the side of actin filaments coat the filament completely, and must therefore be present in high amounts
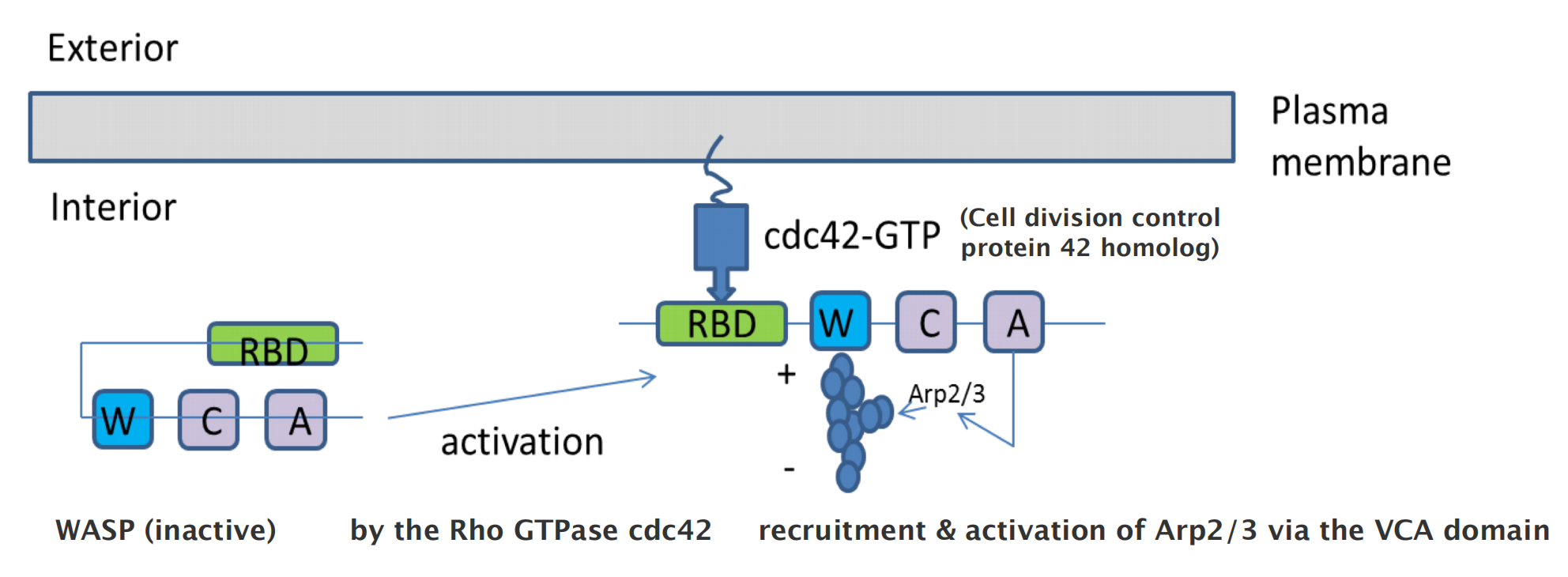
5. Nucleation promoting factor (NPF)
Arp2/3 complex is regulated and recruited by the nucleation promoting factor (NPF): e.g. Wiskott–Aldrich Syndrome protein (WASP)
6. Severing actin filament by cofilin
Severing Proteins Regulate Actin Filament Depolymerization
severing promotes the depolymerization of old filaments, speeding up the depolymerization rate by tenfold or more.
In addition, severing changes the physical and mechanical properties of the cytoplasm: stiff, large bundles and gels become more fluid.
One class of actin-severing proteins is the gelsolin ([肌动蛋白]凝溶胶蛋白) superfamily. These proteins are activated by high levels of cytosolic Ca2+.
- According to one model, gelsolin binds the side of an actin filament until a thermal fluctuation creates a small gap between neighboring subunits, at which point gelsolin inserts itself into the gap to break the filament. After the severing event, gelsolin remains attached to the actin filament and caps the new plus end.
Another important actin-filament destabilizing protein, found in all eukaryotic cells, is cofilin (纤维蛋白)
- Also called actin depolymerizing factor,cofilin binds along the length of the actin filament, forcing the filament to twist a little more tightly
- This mechanical stress weakens the contacts between actin subunits in the filament, making the filament brittle and more easily severed by thermal motions
- Cofilin binds preferentially to ADP-containing actin filaments rather than to ATP-containing filaments.
Actin filaments can be protected from cofilin by tropomyosin binding. Thus, the dynamics of actin in different subcellular locations depends on the balance of stabilizing and destabilizing accessory proteins.
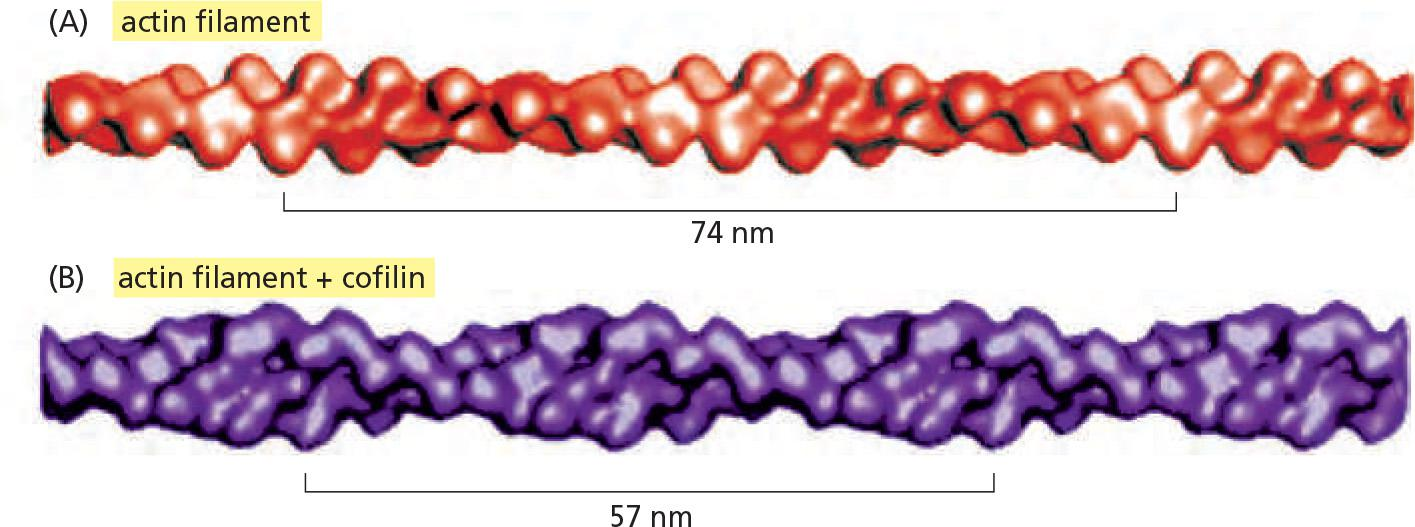
Cofilin tends to dismantle the older but not the newer filaments in the cell.
Cofilin is smaller than actin.
Cofilin binding to actin twists actin filament
###7. Actin cross-linking proteins organize networks
Higher-Order Actin Filament Arrays Influence Cellular Mechanical Properties and Signaling
Actin filaments in animal cells are organized into several types of arrays: dendritic networks, bundles, and weblike (gel-like) networks
- The actin filaments of dendritic networks are nucleated by the Arp 2/3 complex
- while bundles are made of the long, straight filaments produced by formins.
The structural organization of different actin networks depends on specialized accessory proteins.
- As explained earlier, Arp 2/3 organizes filaments into dendritic networks by attaching filament minus ends to the side of other filaments.
Other actin filament structures are assembled and maintained by two classes of proteins:
- bundling proteins, which cross-link actin filaments into a parallel array,
- gel-forming proteins, which hold two actin filaments together at a large angle to each other, thereby creating a looser meshwork.
The spacing and arrangement of these two filament-binding domains determine the type of actin structure that a given cross-linking protein forms.
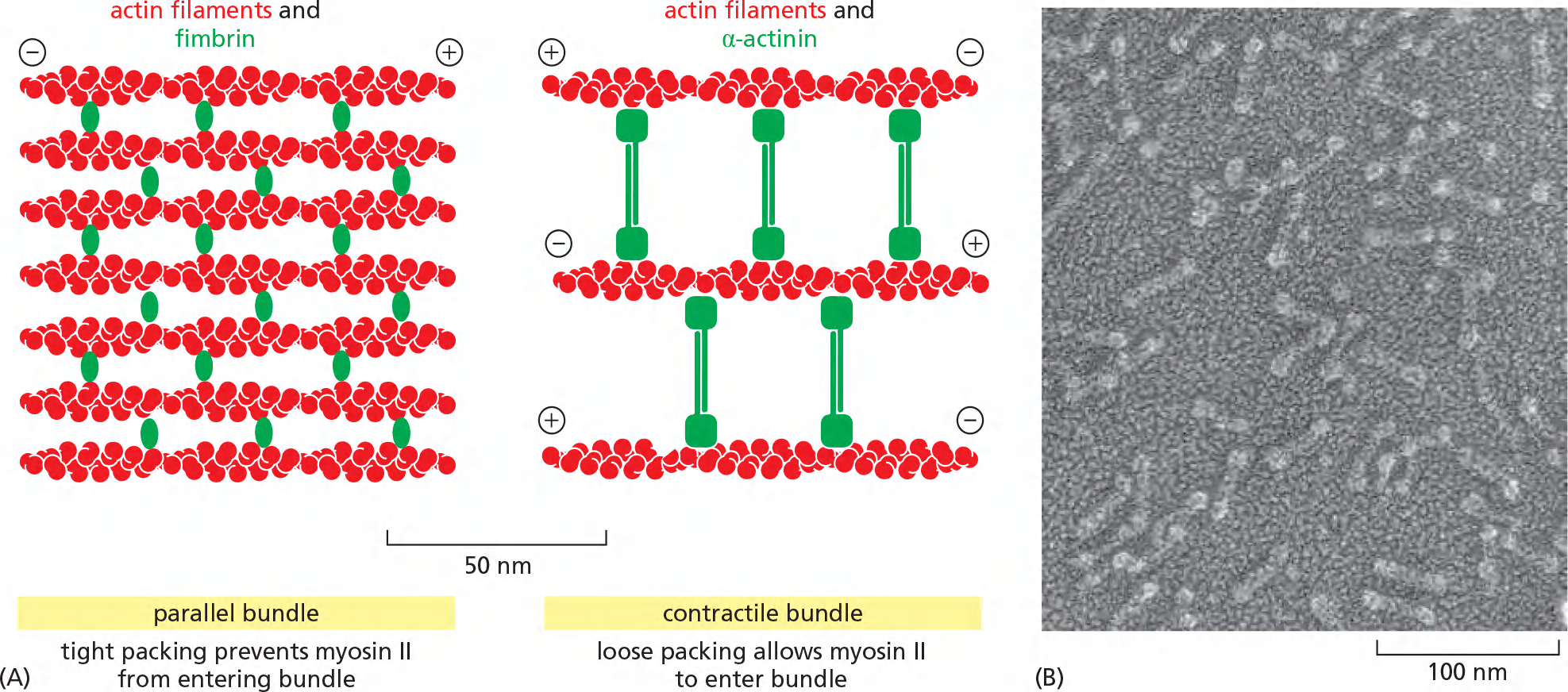
Each type of bundling protein also determines which other molecules can interact with the cross-linked actin filaments. Myosin II is the motor protein that enables stress fibers and other contractile arrays to contract
The very close packing of actin filaments caused by the small monomeric bundling protein fimbrin ([肌动蛋白]丝束蛋白) apparently excludes myosin, and thus the parallel actin filaments held together by fimbrin are not contractile
α-actinin (α-肌动蛋白) cross-links oppositely polarized actin filaments into loose bundles, allowing the binding of myosin and formation of contractile actin bundles
bundling by fimbrin automatically discourages bundling by α-actinin, and vice versa,
Filamin ([肌动蛋白]细丝蛋白) (see Figure 16–22) promotes the formation of a loose and highly viscous gel by clamping together two actin filaments roughly at right angles
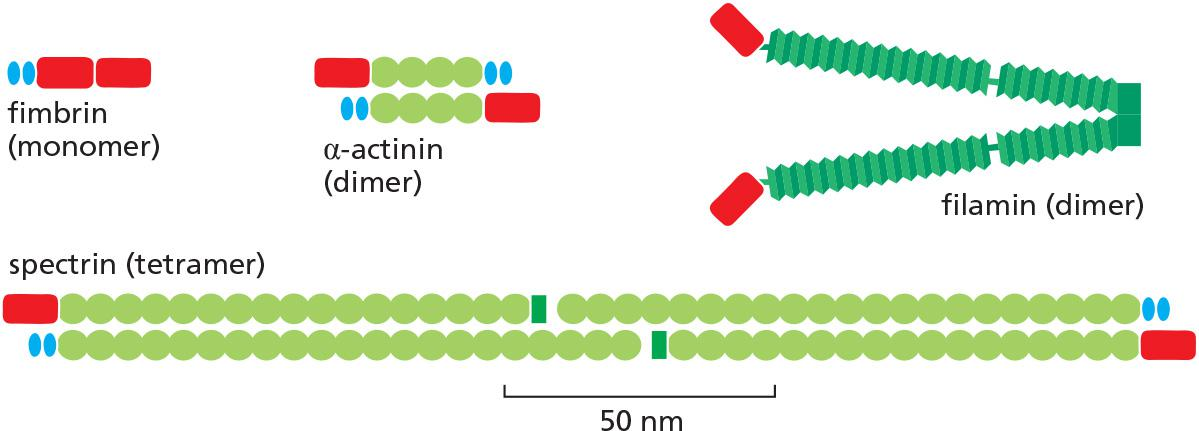
Actin filaments are cross-linked by various proteins.
Two classes of proteins:
- bundling proteins cross-link actin filaments into a parallel array,
- gel-forming proteins hold two actin filaments together at a large angle to each other, thereby creating a looser meshwork.
- → Organization of different actin networks
Filamin cross-links actin filaments into a three-dimensional network and is required for normal neuronal migration
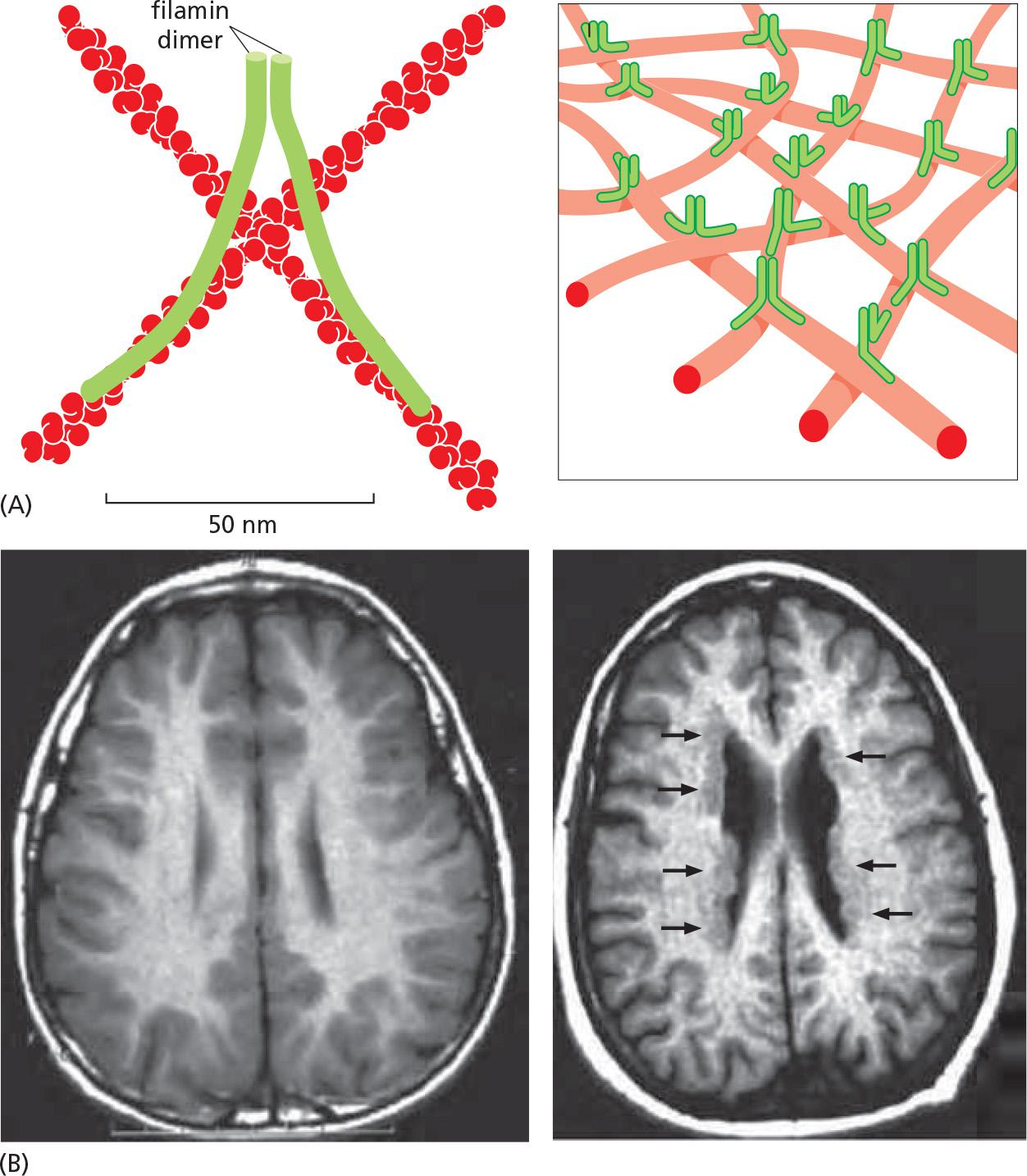
Filamin links actin filaments into a mechanically strong web.
Filamin mutant affect neuron migration
Single cells possess different actin networks
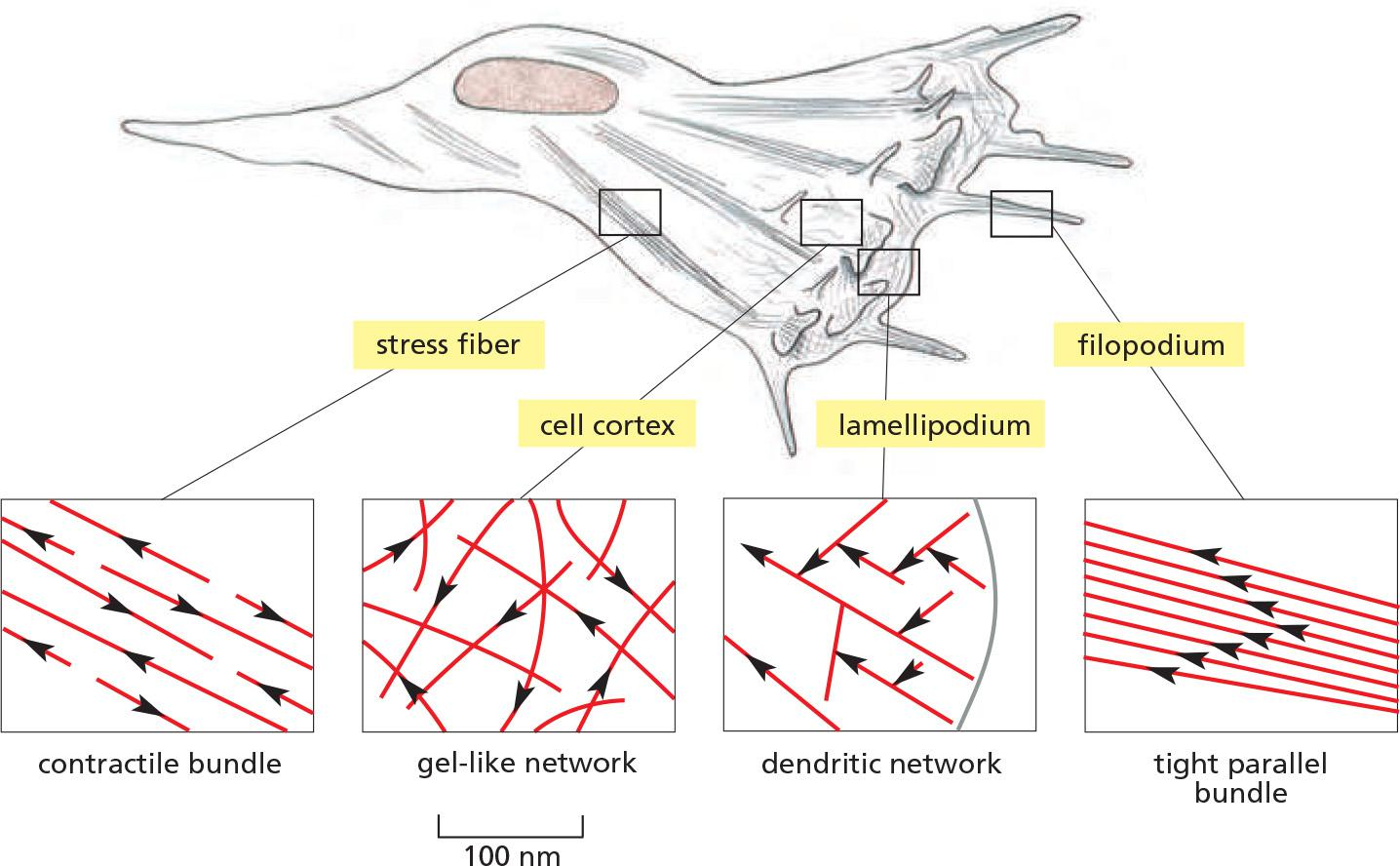
Fibroblasts crawl in tissue-culture with four areas enlarged to show the arrangement of filaments:
- Filopodia are spike-like projections of the plasma membrane to explore the environment.
- Dendritic actin networks enable membrane protrusion at lamellopodia.
- Actin cortex underlies the plasma membrane and consists of gel-like networks.
- Stress fibers are contractile and exert tension
Listeria Monocytogenes Recruiting Actin Inside a Mammalian Cell
Nature cytoskeleton milestone 25: Reconstitution of actin-based motility of Listeria and Shigella using pure proteins
How does Listeria get around in host cells?
Bacteria Can Hijack the Host Actin Cytoskeleton
The Arp 2/3 complex nucleates the assembly of actin filaments that generate a substantial force and push the bacterium through the cytoplasm at rates of up to 1 μm/sec
Arp 2/3 complex, cofilin, and capping protein, illustrating how actin polymerization dynamics generate movement through spatial regulation of filament assembly and disassembly.
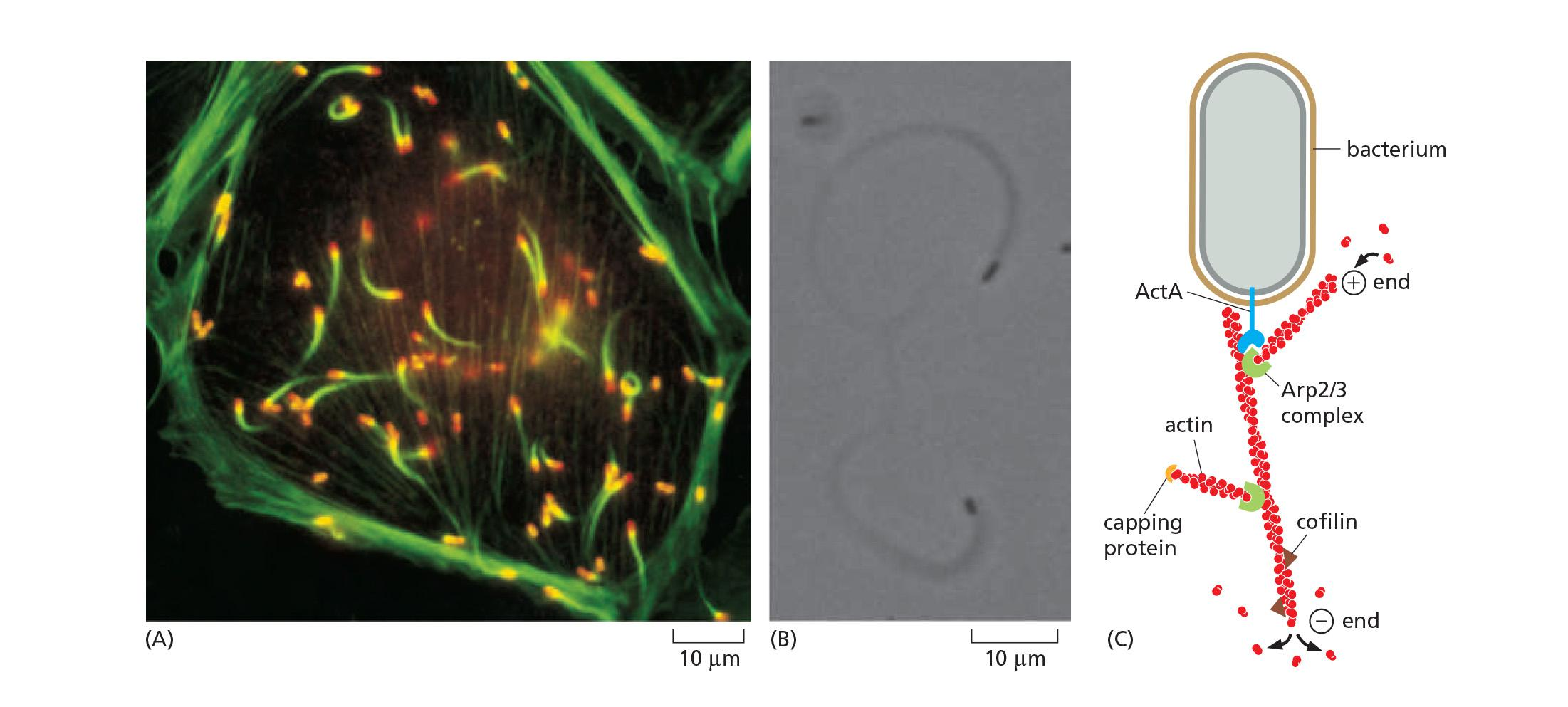
Listeria’s cell surface protein ActA functions as a nucleation promoting factor (NPF), which interacts with VASP to recruit Arp2/3 to enhance ATP-actin assembly.
The recruited Arp2/3 complex nucleates the assembly of actin filaments.
This generates force and pushes the bacterium through the cytoplasm of the cell, at rates of up to 1 mm/sec, leaving behind a long actin “comet tail”.
三、Microtubule structure and organization
Microtubules are polymers of the protein tubulin
The tubulin subunit is itself a heterodimer formed from two closely related globular proteins called α-tubulin and β-tubulin, each comprising 445–450 amino acids, which are tightly bound together by noncovalent bonds
- These two tubulin proteins are found only in this heterodimer, and each α or β monomer has a binding site for one molecule of GTP
- The GTP that is bound to α-tubulin is physically trapped at the dimer interface and is never hydrolyzed or exchanged; it can therefore be considered to be an integral part of the tubulin heterodimer structure.
- The nucleotide on the β-tubulin, in contrast, may be in either the GTP or the GDP form and is exchangeable within the soluble (unpolymerized) tubulin dimer.
Tubulin is found in all eukaryotic cells, and it exists in multiple isoforms. Yeast and human tubulins are 75% identical in amino acid sequence. In mammals, there are at least six forms of α-tubulin and a similar number of β-tubulins, each encoded by a different gene
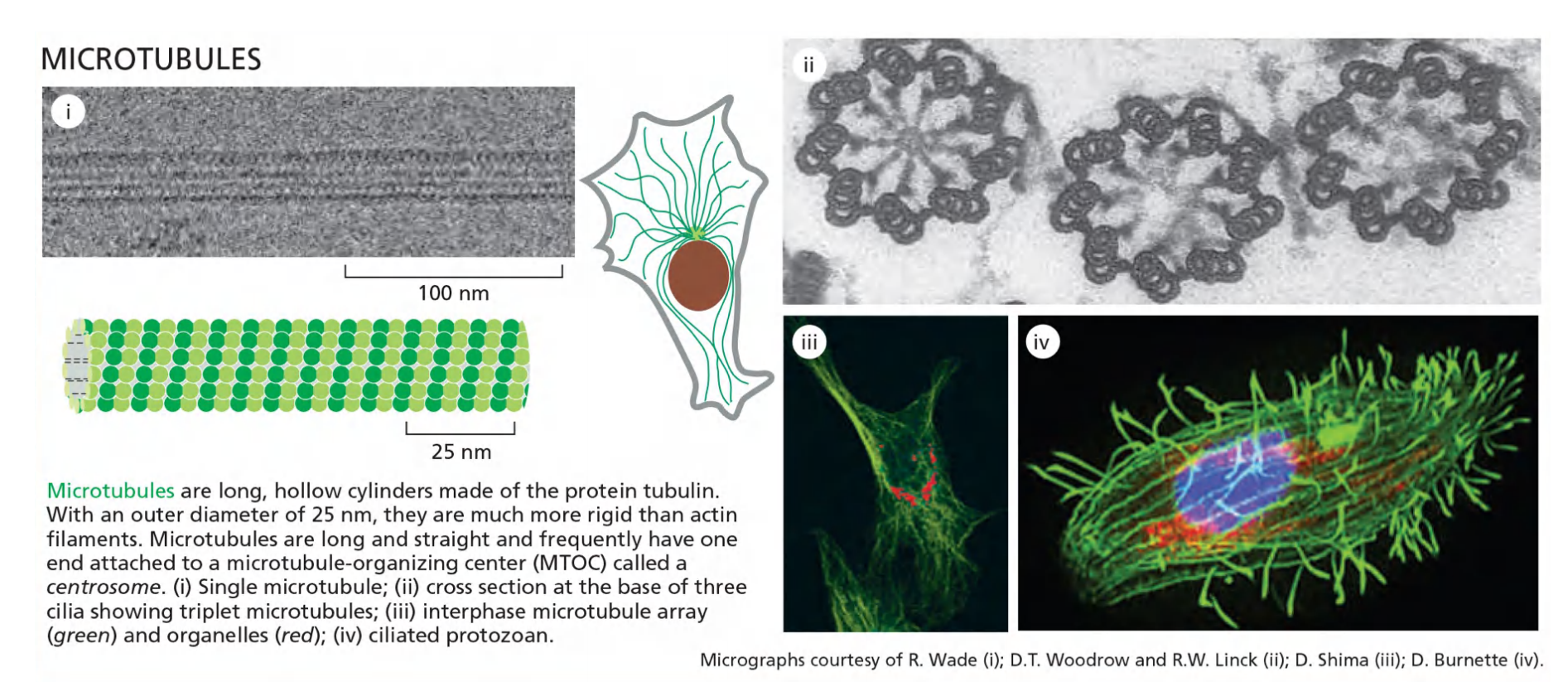
- Grow from centrosome
- Form flagella or cilia
- Form mitotic spindle Organize interior of cell
- Drive intracellular transport
- Structural support in axon
Microtubules: it is not artifacts!
Organization of microtubules
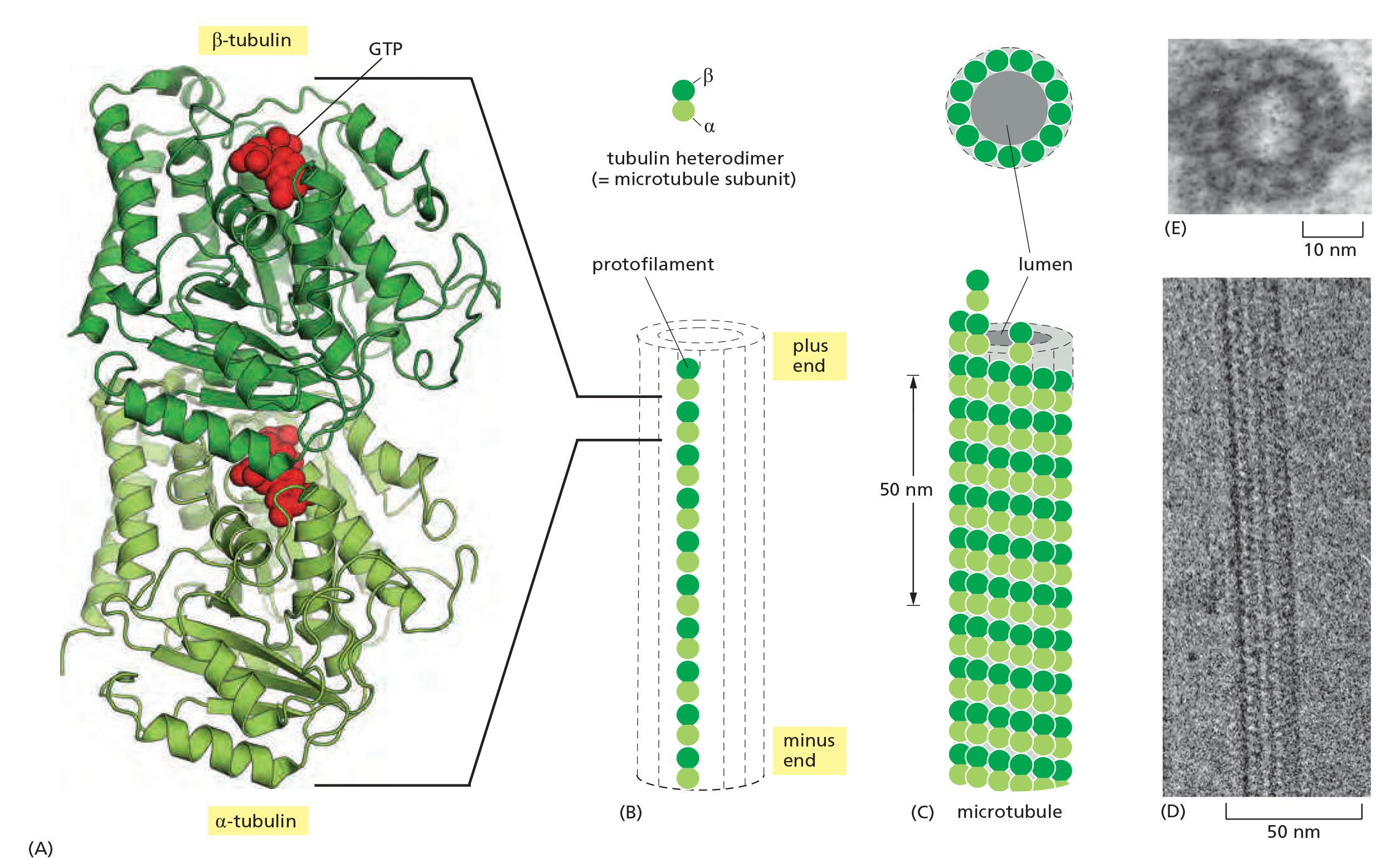
Microtubules Are Hollow Tubes Made of Protofilaments
Along the longitudinal axis of the microtubule, the “top” of one β-tubulin molecule forms an interface with the “bottom” of the α-tubulin molecule in the adjacent heterodimer.
Perpendicular to these interactions, neighboring protofilaments form lateral contacts.
These multiple contacts among subunits make microtubules stiff and difficult to bend. The persistence length of a microtubule is several millimeters, making microtubules the stiffest and straightest structural elements found in most animal cells.
The microtubule lattice itself has a distinct structural polarity, with α-tubulins exposed at the minus end and β-tubulins exposed at the plus end
- As for actin filaments, the regular, parallel orientation of their subunits gives microtubules structural and dynamic polarity (Figure 16–43), with plus ends growing and shrinking more rapidly.
The microtubule is a stiff hollow tube formed from 13 protofilaments aligned in parallel
The microtubule grows faster at one end
Microtubule assembly from microtubule-organizing centers (MTOC)
A Protein Complex Containing γ-Tubulin Nucleates Microtubules
Because formation of a microtubule requires the interaction of many tubulin heterodimers, the concentration of tubulin subunits required for spontaneous nucleation of microtubules is very high
While α- and β-tubulins are the regular building blocks of microtubules, another type of tubulin, called γ-tubulin, is present in much smaller amounts than α- and β-tubulin and is involved in the nucleation of microtubule growth in organisms ranging from yeasts to humans.
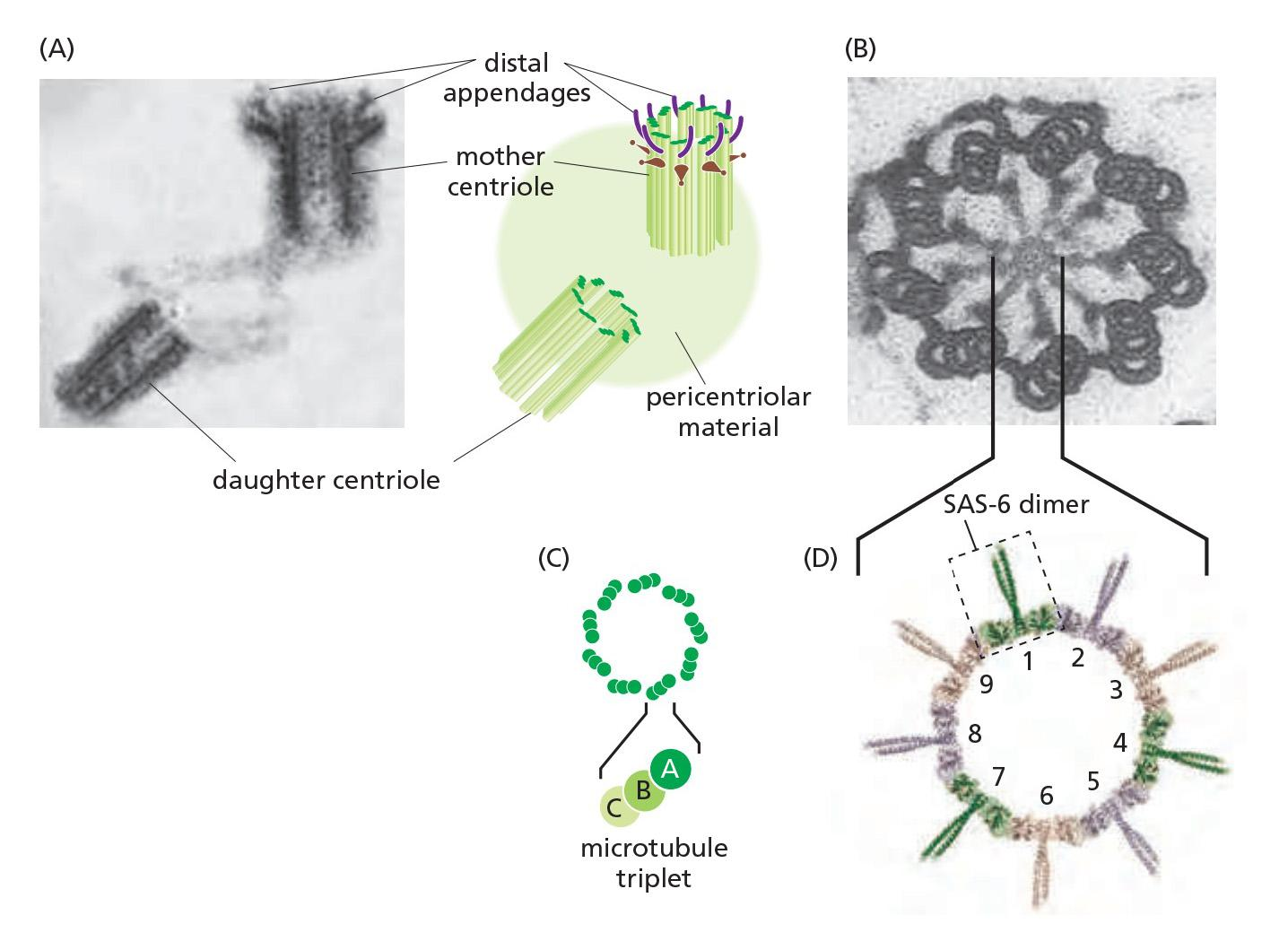
Microtubules are generally nucleated from a specific intracellular location known as a microtubule-organizing center (MTOC) where γ-tubulin is most enriched. Nucleation in many cases depends on the γ-tubulin ring complex (γ-TuRC).
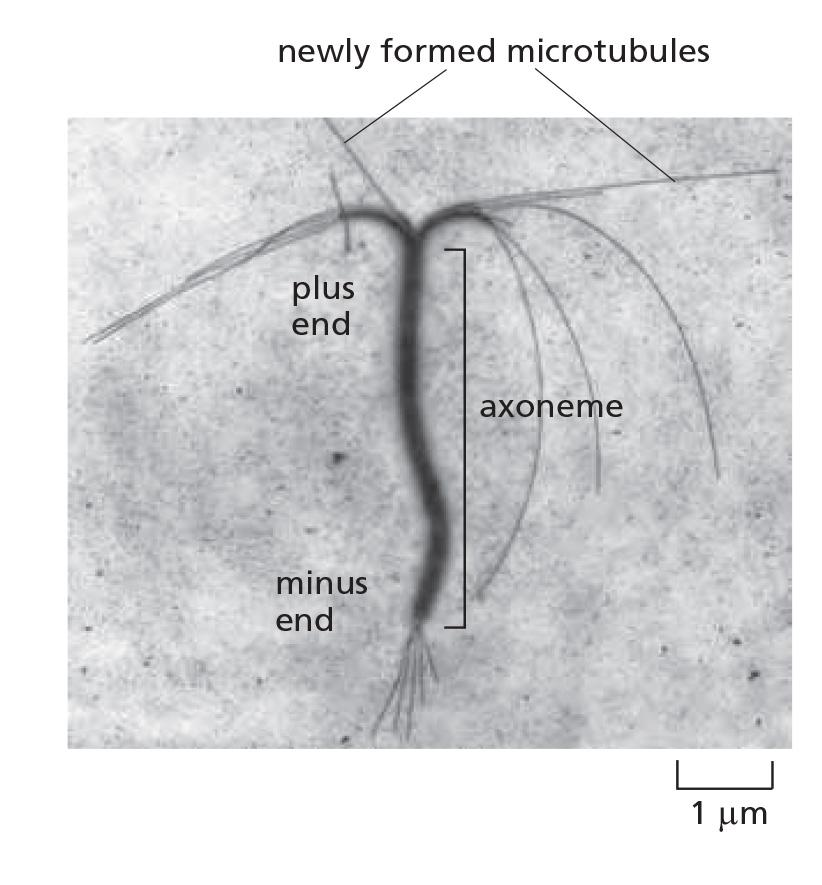
Very rare spontaneous microtubule assembly
All microtubules are nucleated from microtubule-organizing centers (MTOCs): e.g. centrosomes and basal bodies (cilia and flagella)
Plants use additional/other microtubule-nucleating proteins, e.g. SPC98 (different mechanisms to nucleate microtubules) and most plants do not have centrosomes
Like actin, assembly at plus end is much faster than assembly at minus end.
That is why they grow at the plus end!
γ-tubulin ring complex (γ-TuRC) nucleates microtubules
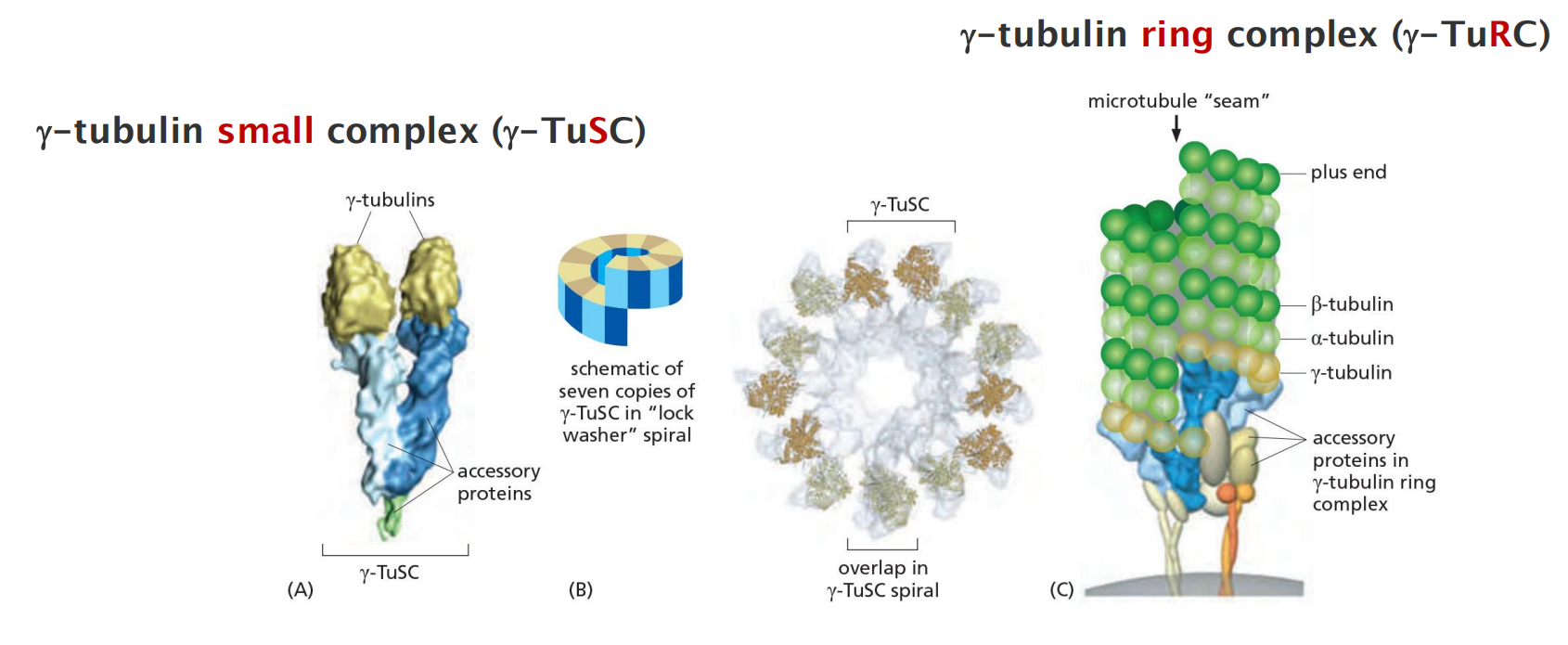
7 γ-TuSCs associate to form a spiral structure in which the last γ-tubulin lies beneath the first, resulting in 13 exposed γ-tubulin subunits in a circular orientation that matches the orientation of the 13 protofilaments in a microtubule
Centrosome
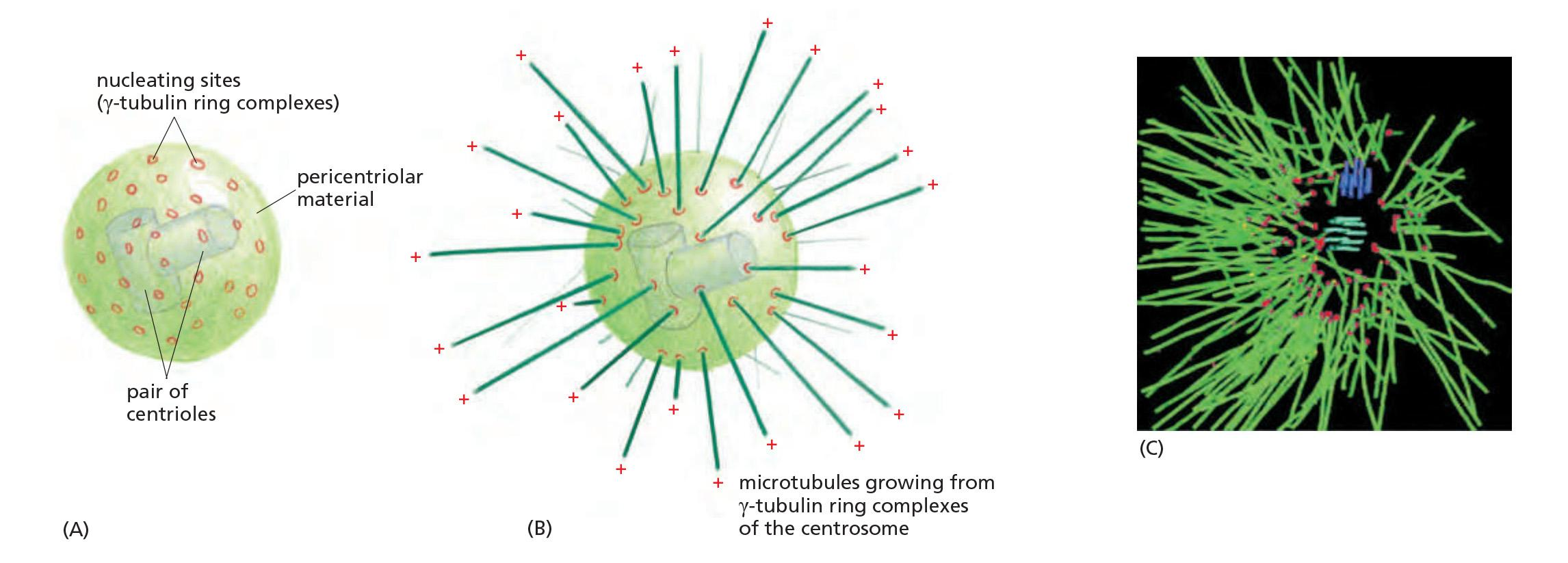
The centrosome: γ-Tubulin ring complexes (γ-TuRC), pericentriolar, are critical to assemble microtubules.
- γ-Tubulin rings: starting places of microtubule growth
- Two perpendicular centrioles (they are similar to basal bodies of flagella)
Centrosome has a fisherman-like behavior: microtubules are constantly growing out of it, then degrading, but some are stabilizing
A centrosome with attached microtubules: The minus end of each microtubule is embedded in the centrosome, having grown from a γ-tubulin ring complex (γ-TuRC), whereas the plus end of each microtubule is free in the cytoplasm
1. Centriole in centrosome
Microtubules Emanate from the Centrosome in Animal Cells
Many animal cells have a single, well-defined MTOC called the centrosome, which is located near the nucleus and from which microtubules are nucleated at their minus ends
A centrosome typically recruits more than fifty copies of γ-TuRC. In addition, γ-TuRC molecules are found in the cytoplasm, and centrosomes are not absolutely required for microtubule nucleation
Embedded in the centrosome are the centrioles, a pair of cylindrical structures arranged at right angles to each other in an L-shaped configuration
- Together with a large number of accessory proteins, the centrioles organize the pericentriolar material, where microtubule nucleation takes place.
Microtubule organization varies widely among different species and cell types.
- In budding yeast, microtubules are nucleated at an MTOC that is embedded in the nuclear envelope as a small, multilayered structure called the spindle pole body, also found in other fungi and diatoms (硅藻).
- Higher-plant cells appear to nucleate microtubules at sites distributed all around the nuclear envelope and at the cell cortex
- Neither fungi nor most plant cells contain centrioles. Despite these differences, all these cells seem to use γ-tubulin to nucleate their microtubules.
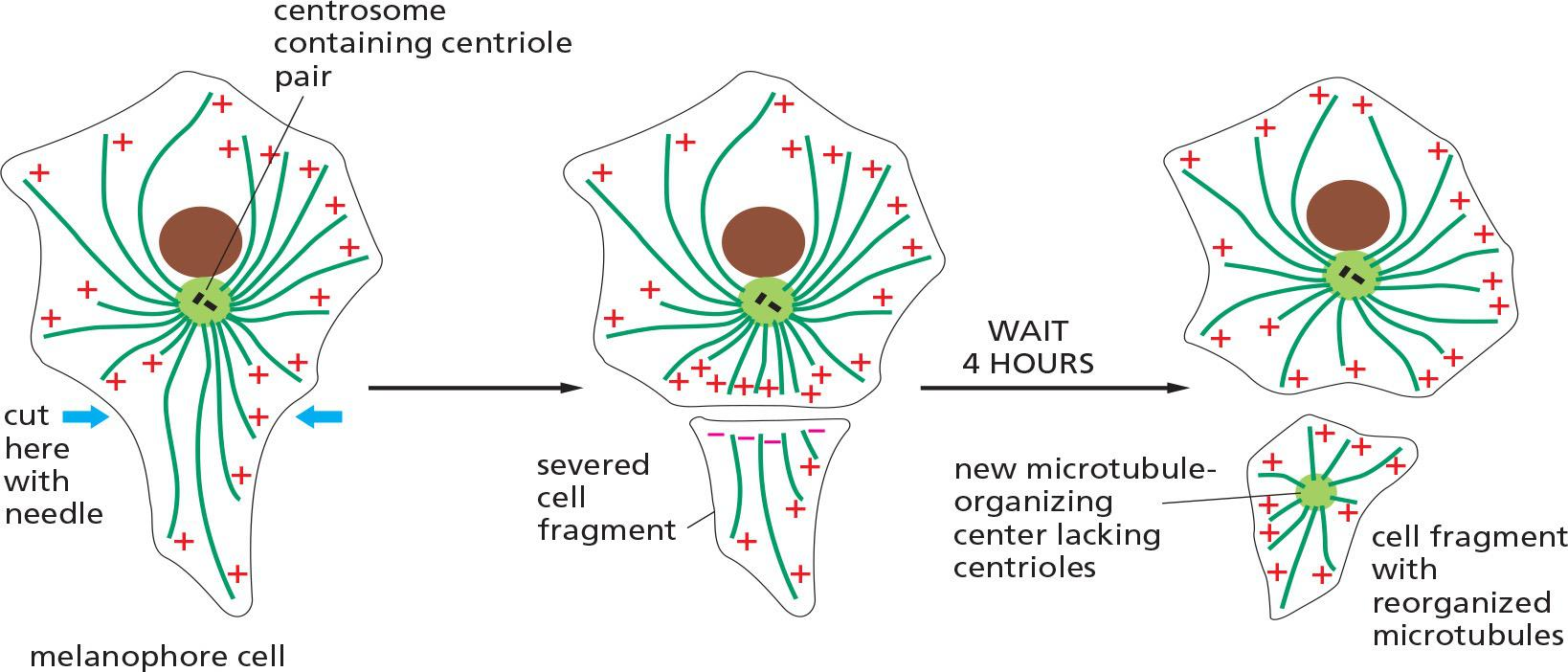
2. A microtubule array can find the center of a cell!
Dynamic microtubules arrange themselves into a star-shaped array with the microtubule minus ends clustered at the center by minus-end-binding proteins
This ability of the microtubule cytoskeleton to find the center of the cell establishes a general coordinate system, which is then used to position many organelles within the cell.
From this asymmetrical location, a microtubule array extends along the long axis of the cell, with plus ends directed toward the basal surface
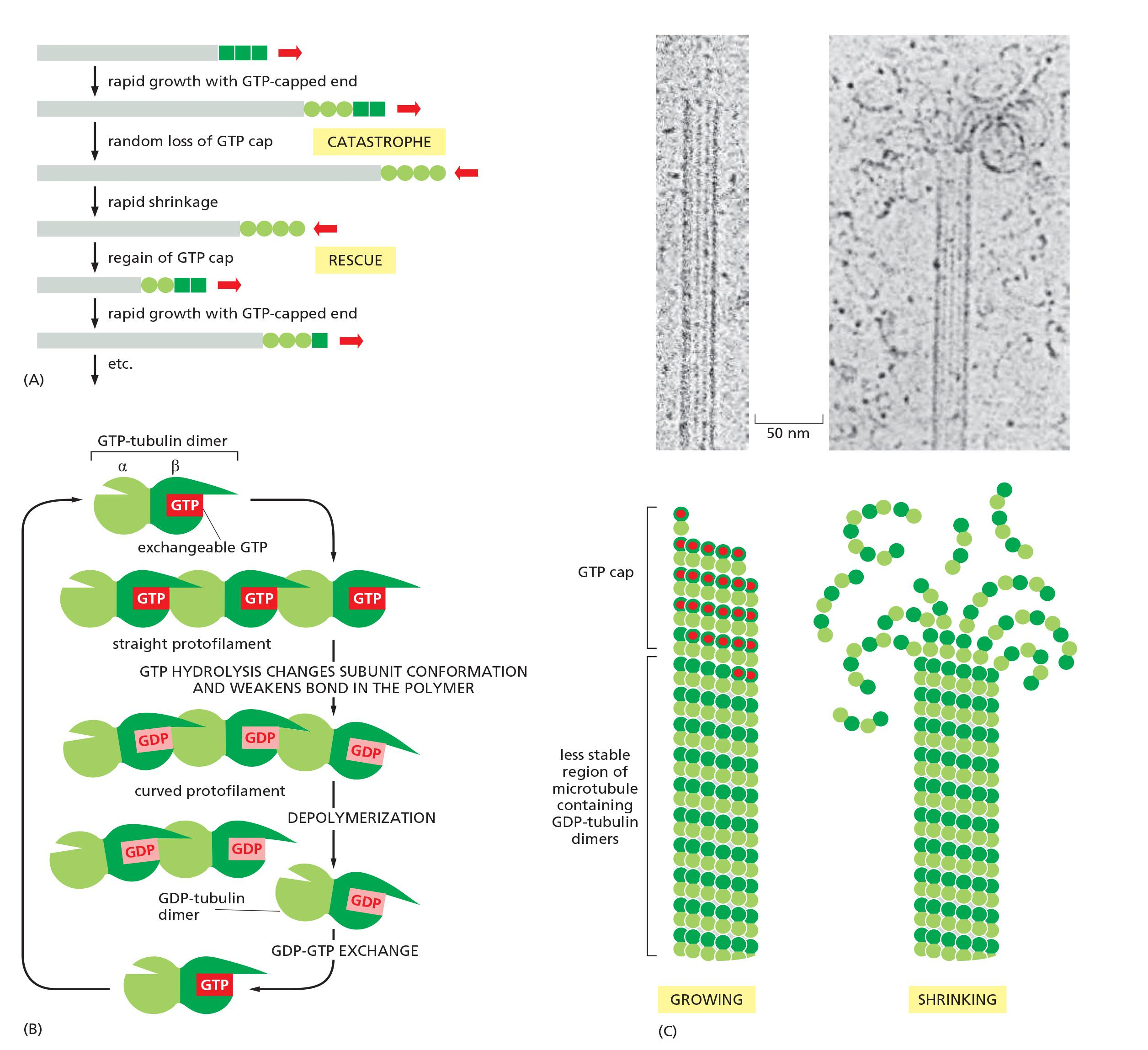
Microtubule dynamic instability
Microtubules Undergo Dynamic Instability
GTP hydrolysis occurs only within the β-tubulin subunit of the tubulin dimer.
As in the case of actin filaments, two different types of microtubule structures can exist, one with the “T form” of the nucleotide bound (GTP) and one with the “D form” bound (GDP).
The energy of nucleotide hydrolysis is stored as elastic strain (弹性应变) in the polymer lattice, making the free-energy change for dissociation of a subunit from the D-form polymer more negative than the free-energy change for dissociation of a subunit from the T-form polymer.
- In consequence the ratio of k
off/konfor GDP-tubulin (its critical concentration [Cc(D)]) is much higher than that of GTP-tubulin. Thus, under physiological conditions, GTP-tubulin tends to polymerize and GDP-tubulin to depolymerize.
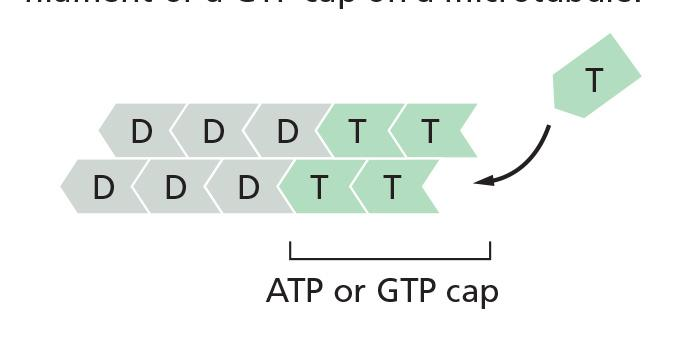
Whether the tubulin subunits at the very end of a microtubule are in the T or the D form depends on the relative rates of GTP hydrolysis and tubulin addition.
If the rate of subunit addition is high—and thus the filament is growing rapidly — then it is likely that a new subunit will be added to the polymer before the nucleotide in the previously added subunit has been hydrolyzed.
- In this case, the tip of the polymer remains in the T form, forming a GTP cap
However, if the rate of subunit addition is low, hydrolysis may occur before the next subunit is added, and the tip of the filament will then be in the D form
- If GTP-tubulin subunits assemble at the end of the microtubule at a rate similar to the rate of GTP hydrolysis, then hydrolysis will sometimes “catch up” with the rate of subunit addition and transform the end to a D form
This transformation is sudden and random, with a certain probability per unit time that depends on the concentration of free GTP-tubulin subunits.
Suppose that the concentration of free tubulin is intermediate between the critical concentration for a T-form end and the critical concentration for a D-form end (that is, above the concentration necessary for T-form assembly, but below that for the D form).
- Now, any end that happens to be in the T form will grow, whereas any end that happens to be in the D form will shrink
- On a single microtubule, an end might grow for a certain length of time in a T form, but then suddenly change to the D form and begin to shrink rapidly, even while the free subunit concentration is held constant
- At some later time, it might then regain a T-form end and begin to grow again
This rapid interconversion between a growing and shrinking state, at a uniform free subunit concentration, is called dynamic instability
The change from growth to shrinkage is called a catastrophe, while the change to growth is called a rescue
An end might grow for a certain time in a T form
A GTP cap favors growth, but if it suddenly changes to the D form, microtubule begins to shrink rapidly, even while the free subunit concentration is held constant.
Microtubules depolymerize about 100 times faster from an end containing GDP-tubulin than from one containing GTP-tubulin.
Later, it might regain a T-form end and begins to grow again.
This rapid interconversion between a growing and shrinking state, is called dynamic instability
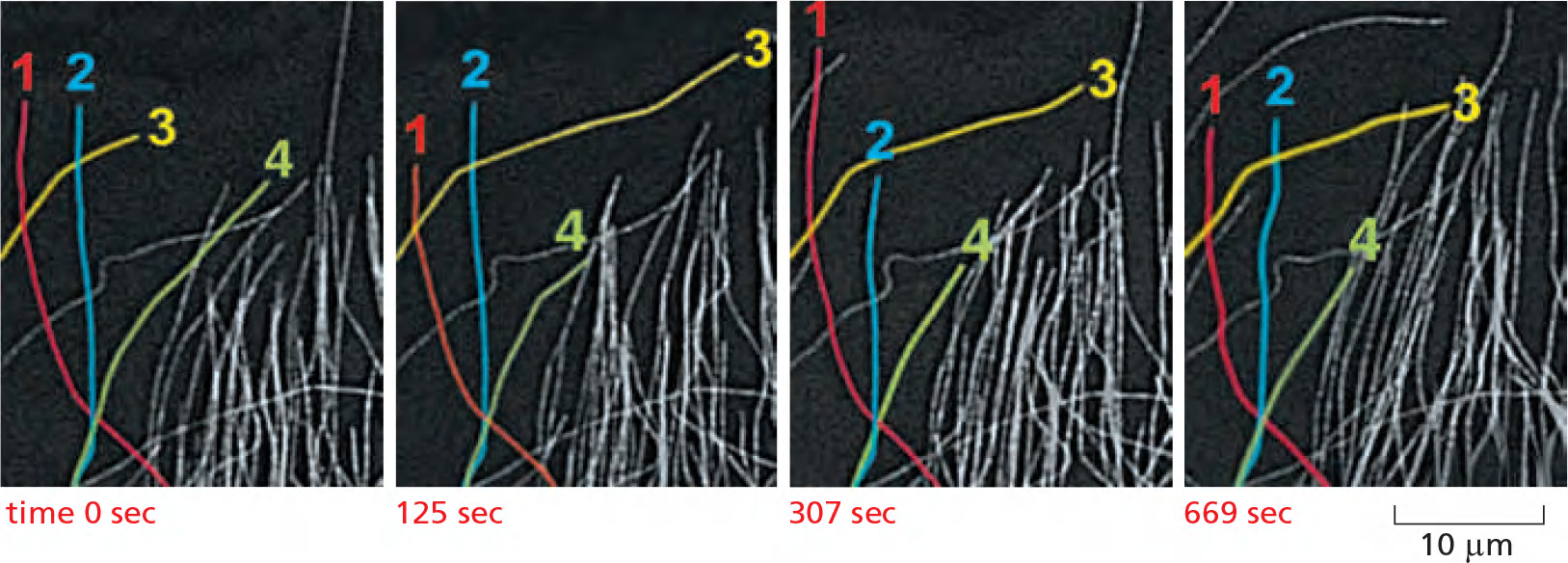
- Rhodamine-labeled tubulin reveals the dynamic instability of microtubules at the edge of the cell: four individual microtubules are highlighted for clarity; each of these shows alternating shrinkage and growth
Growing and shrinking of microtubules
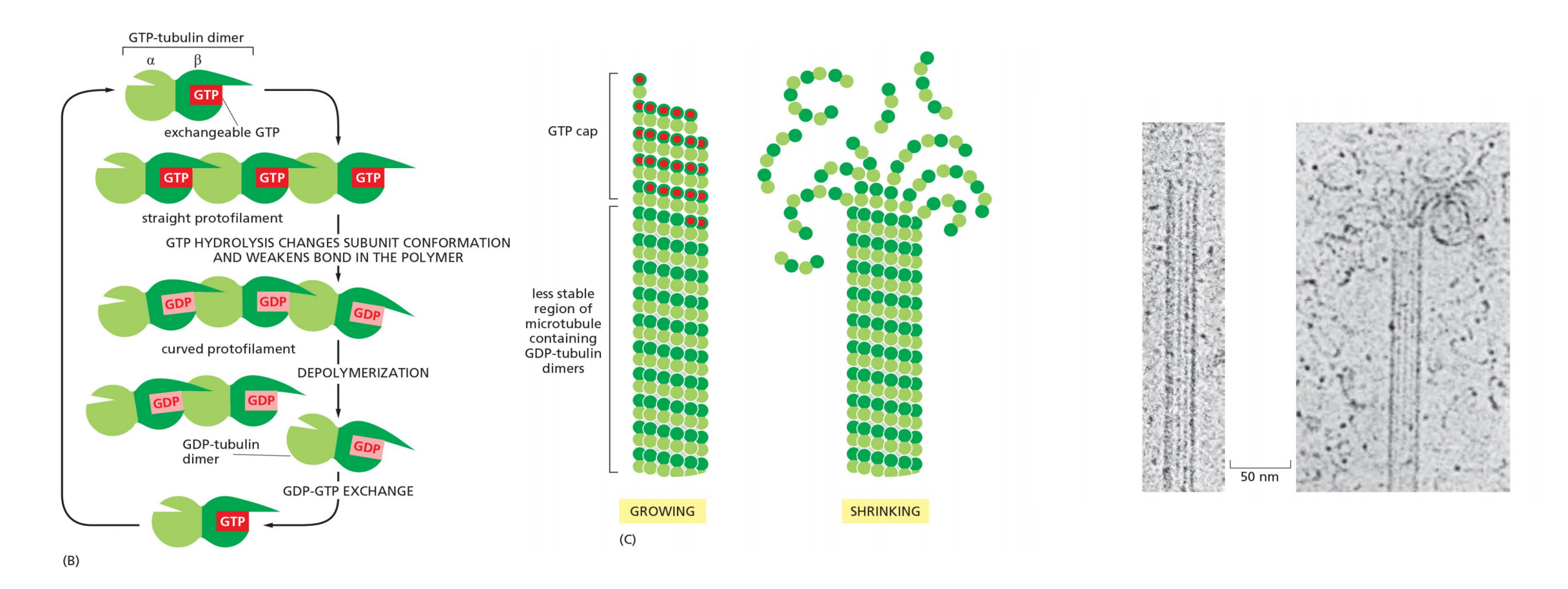
Tubulin dimers bind GTP and form a growing GTP cap of microtubule; if GTP cap is lost, microtubules start to shrink
Capturing the plus end will stabilize microtubule.
Microtubule association proteins (MAPs)
Microtubule-Binding Proteins Modulate Filament Dynamics and Organization
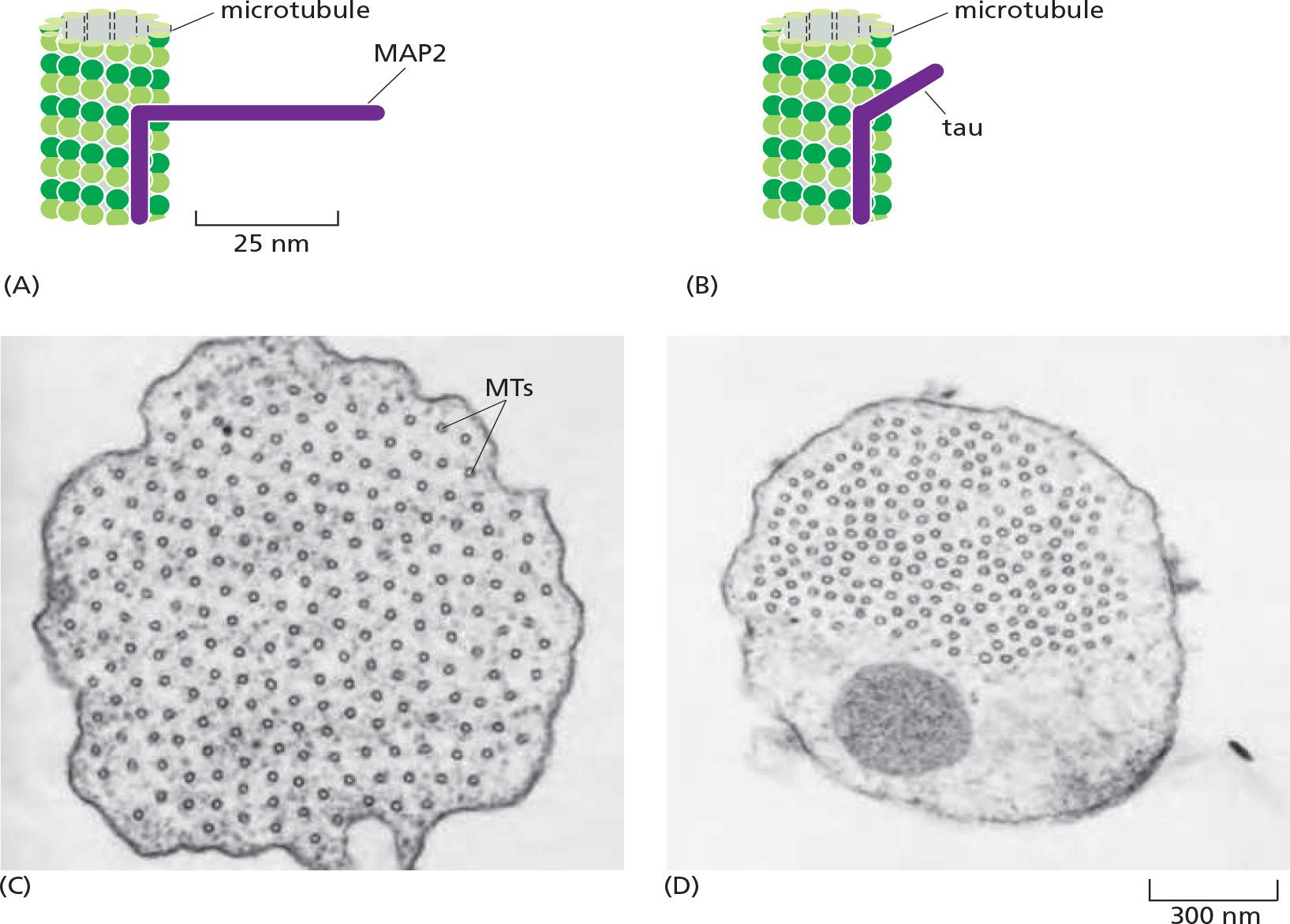
Proteins that bind to microtubules are collectively called microtubule-associated proteins, or MAPs
Cells overexpressing MAP2, which has a long projecting domain, form bundles of stable microtubules that are kept widely spaced, while cells overexpressing tau, a MAP with a much shorter projecting domain, form bundles of more closely packed microtubules
MAPs are the targets of several protein kinases, and phosphorylation of a MAP can control both its activity and localization inside cells.
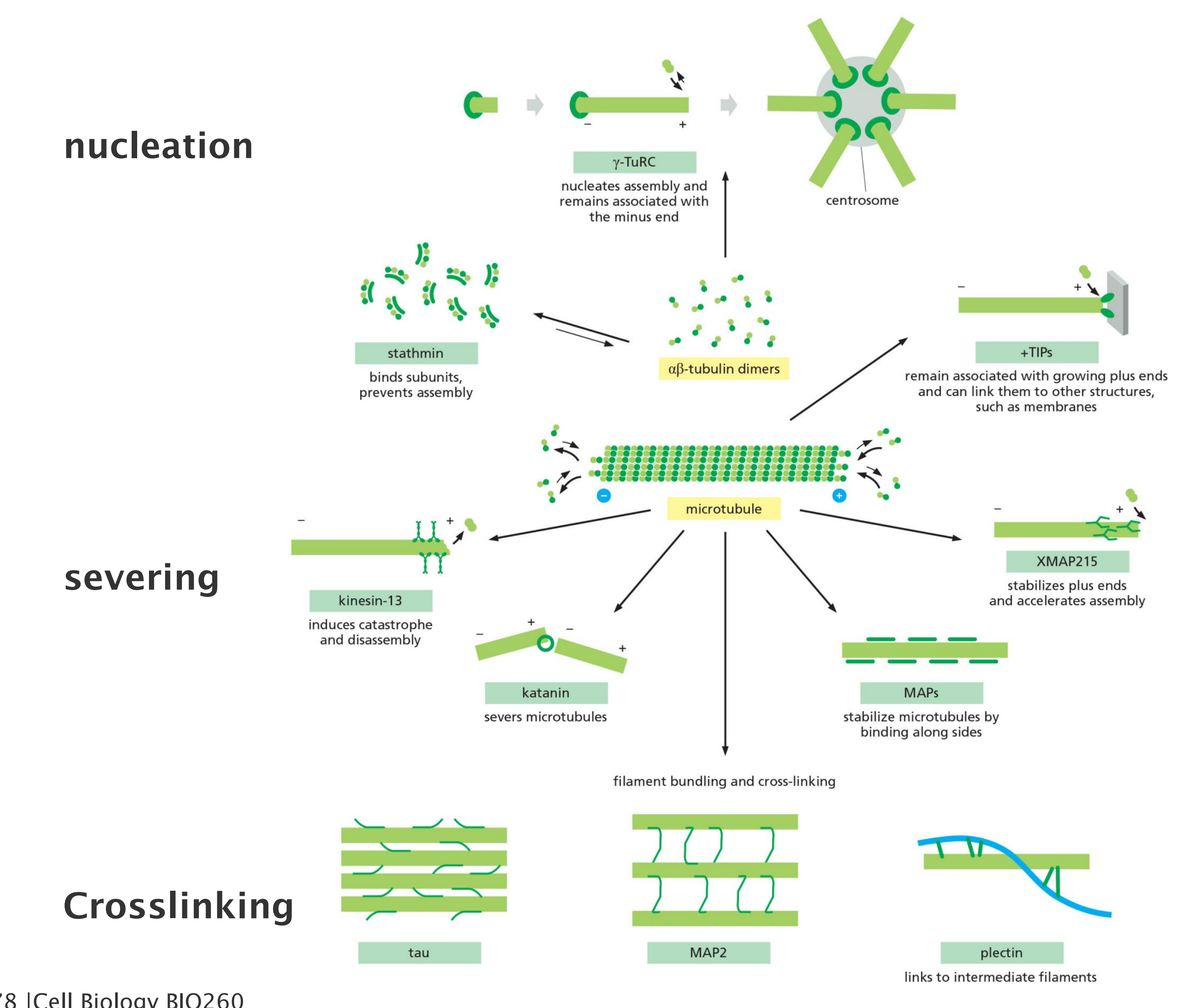
Plus end binding proteins:
- XMAP215
- Plus-end tracking proteins (+TIPs), e.g. EB protein
- kinesin-13
- Stathmin/OP18
Microtubules are stabilized by side-binding proteins:
- Tau
- MAP2
- MAP4
- their activity can be regulated by phosphorylation
1. Microtubule plus end-binding proteins
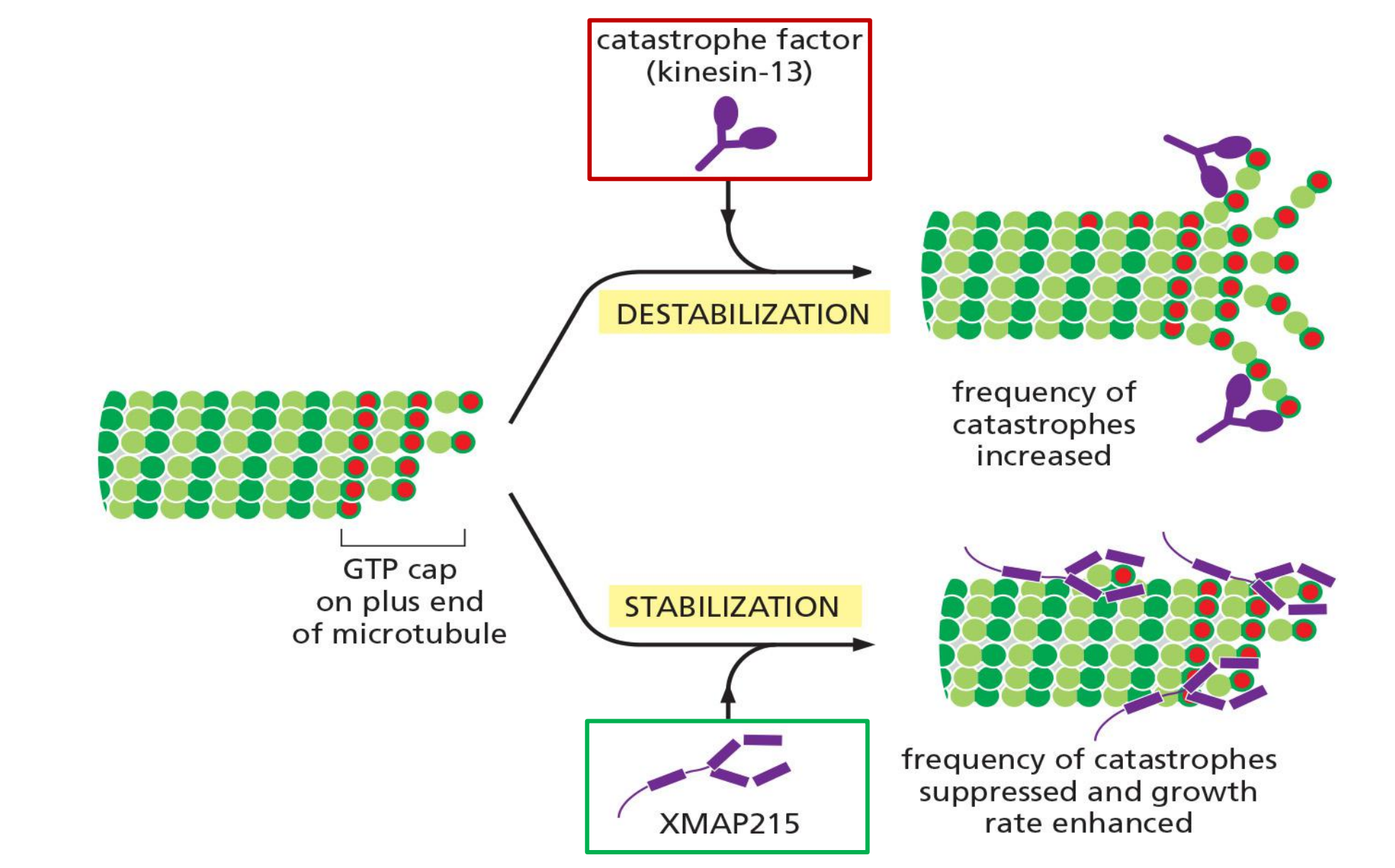
Members of a family of kinesin-related proteins known as catastrophe factors (or kinesin-13) bind to microtubule ends and appear to pry protofilaments apart, lowering the normal activation-energy barrier that prevents a microtubule from springing apart into the curved protofilaments that are characteristic of the shrinking state
- Another protein, called Nezha or Patronin, protects microtubule minus ends from the effects of catastrophe factors.
While very few microtubule minus-end-binding proteins have been characterized, a large subset of MAPs has been identified that are enriched at microtubule plus ends
- A particularly ubiquitous example is XMAP215, which has close homologs in organisms that range from yeast to humans.
- XMAP215 binds free tubulin subunits and delivers them to the plus end, thereby promoting microtubule polymerization and simultaneously counteracting catastrophe factor activity
2. +TIP proteins
In many cells, the minus ends of microtubules are stabilized by association with a capping protein or the centrosome , or else they serve as microtubule depolymerization sites.
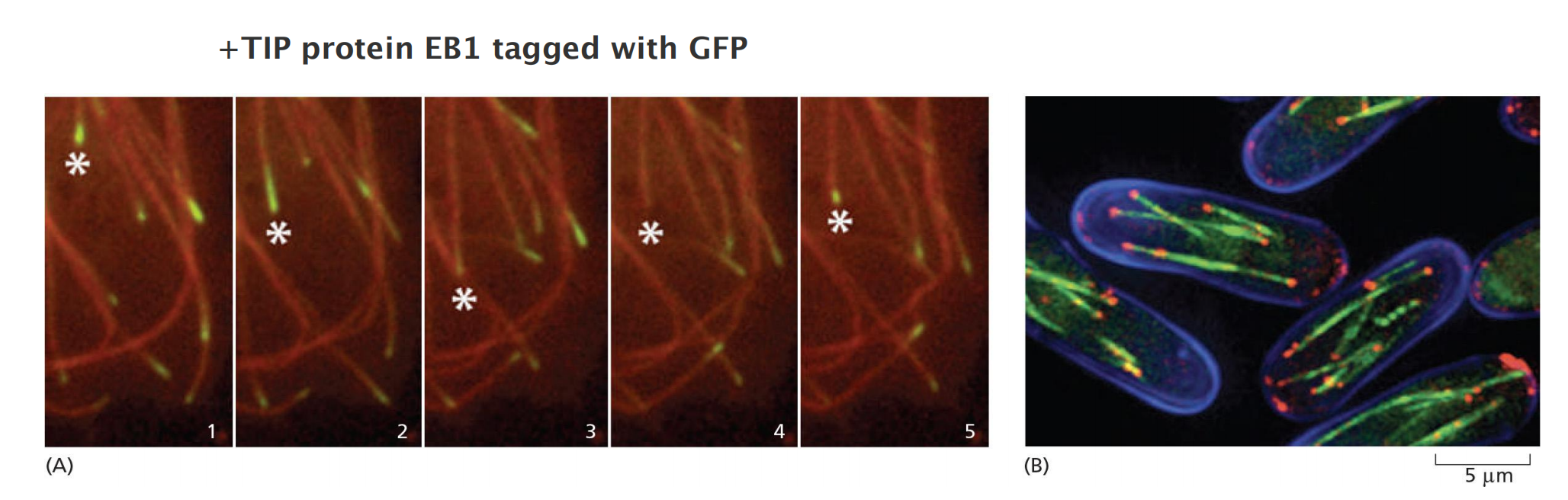
The plus ends, in contrast, efficiently explore and probe the entire cell space. Microtubule-associated proteins called plus-end tracking proteins (+TIPs<) accumulate at these active ends and appear to rocket around the cell as passengers at the ends of rapidly growing microtubules, dissociating from the ends when the microtubules begin to shrink
- The kinesin-related catastrophe factors and XMAP215 mentioned above behave as +TIPs and act to modulate the growth and shrinkage of the microtubule end to which they are attached
Other +TIPs control microtubule positioning by helping to capture and stabilize the growing microtubule end at specific cellular targets, such as the cell cortex or the kinetochore of a mitotic chromosome.
- EB1 and its relatives, small dimeric proteins that are highly conserved in animals, plants, and fungi, are key players in this process.
By attaching to the plus end, these factors allow the cell to harness the energy of microtubule polymerization to generate pushing forces that can be used for positioning the spindle, chromosomes, or organelles.
3. Sequestration of tubulin by stathmin
Tubulin-Sequestering and Microtubule-Severing Proteins Destabilize Microtubules
As it does with actin monomers, the cell sequesters unpolymerized tubulin subunits to maintain a pool of active subunits at a level near the critical concentration
- One molecule of the small protein stathmin (also called Op18) binds to two tubulin heterodimers and prevents their addition to the ends of microtubules
- Stathmin thus decreases the effective concentration of tubulin subunits that are available for polymerization (an action analogous to that of the drug colchicine (秋水仙碱,秋水仙素)), and enhances the likelihood that a growing microtubule will switch to the shrinking state
- Stathmin has been implicated in the regulation of both cell proliferation and cell death.
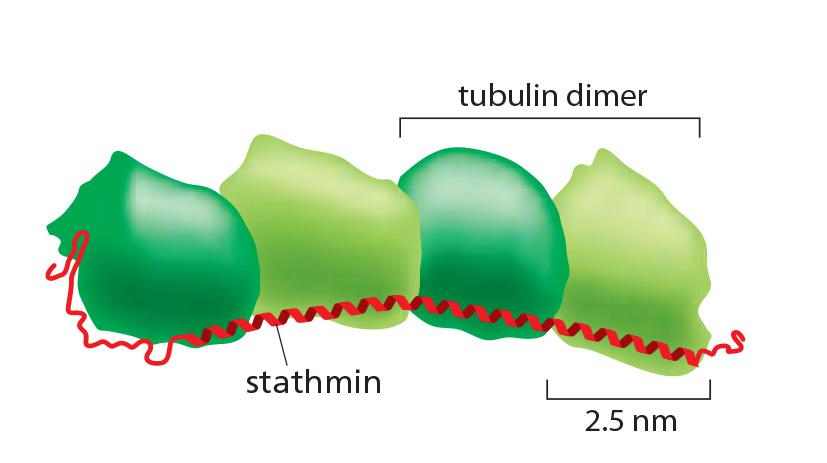
One stathmin/OP18 binds to two tubulin heterodimers and prevents their addition to the ends of microtubules
→ decreases the effective concentration of tubulin subunits that are available for polymerization → enhances the chance to shrink.
Phosphorylation of stathmin inhibits its binding to tubulin,
4. Severing of microtubule by katanin
Severing is another mechanism employed by the cell to destabilize microtubules. To sever a microtubule, thirteen longitudinal bonds must be broken, one for each protofilament.
- The protein katanin, named after the Japanese word for “sword,” accomplishes this demanding task
- Katanin is made up of two subunits: a smaller subunit that hydrolyzes ATP and performs the actual severing, and a larger one that directs katanin to the centrosome
- Katanin releases microtubules from their attachment to a microtubule-organizing center and is thought to contribute to the rapid microtubule depolymerization observed at the poles of spindles during mitosis.
To sever a microtubule, thirteen longitudinal bonds must be broken, one for each protofilament., releasing microtubules from their attachment to a microtubule-organizing center
The protein katanin (in Japanese which means “sword,”) can serve microtubule.
Katanin is made up of two subunits: a smaller subunit that hydrolyzes ATP and performs the actual severing, and a larger one that directs katanin to the centrosome.
May play a role in the rapid microtubule depolymerization at the poles of spindles during mitosis.
5. Organizing microtubule bundles by microtubule-associated proteins (MAP)
Microtubule-specific drugs
四、Intermediate filament
All eukaryotic cells contain actin and tubulin. But the third major type of cytoskeletal protein, the intermediate filament, forms a cytoplasmic filament only in some **metazoans (后生动物)**—including vertebrates, nematodes, and mollusks
- Intermediate filaments are particularly prominent in the cytoplasm of cells that are subject to mechanical stress and are generally not found in animals that have rigid exoskeletons, such as arthropods (节肢动物) and echinoderms (棘皮动物).
Intermediate filaments integrate cells into a mechanical network
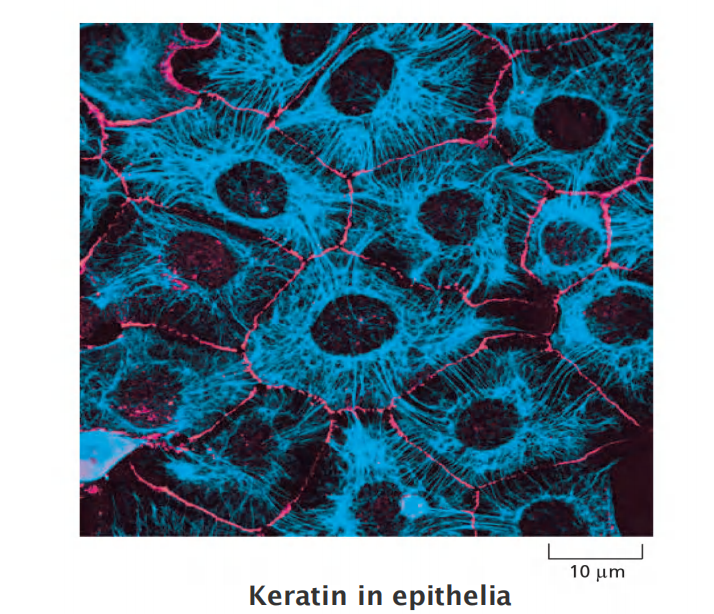
Intermediate filaments integrate cells into a mechanical network: e.g. in the epidermis of the skin
Intermediate filaments provide tensile strength
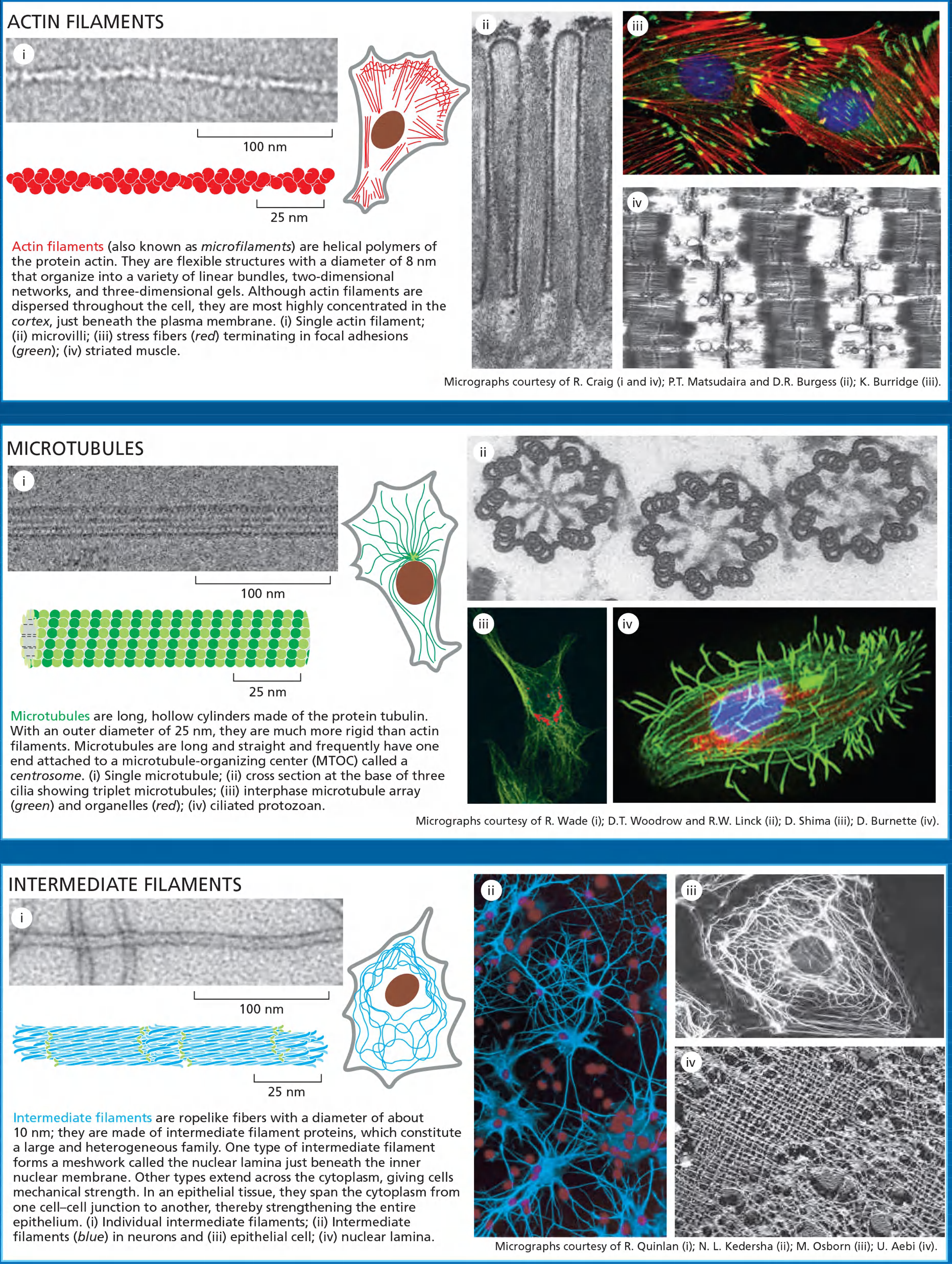
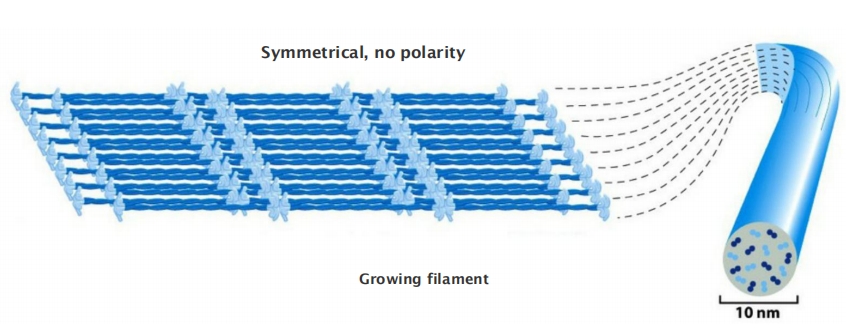
- No polarity
- Tensile and stable
- Difficult to solubilize
- Very heterogeneous substances
- Defects in genes for intermediate filaments are associated with ~50 clinical disorders
- Not in all eukaryotic cells: fungi and plants do not have IFs
Major types of intermediate filament proteins
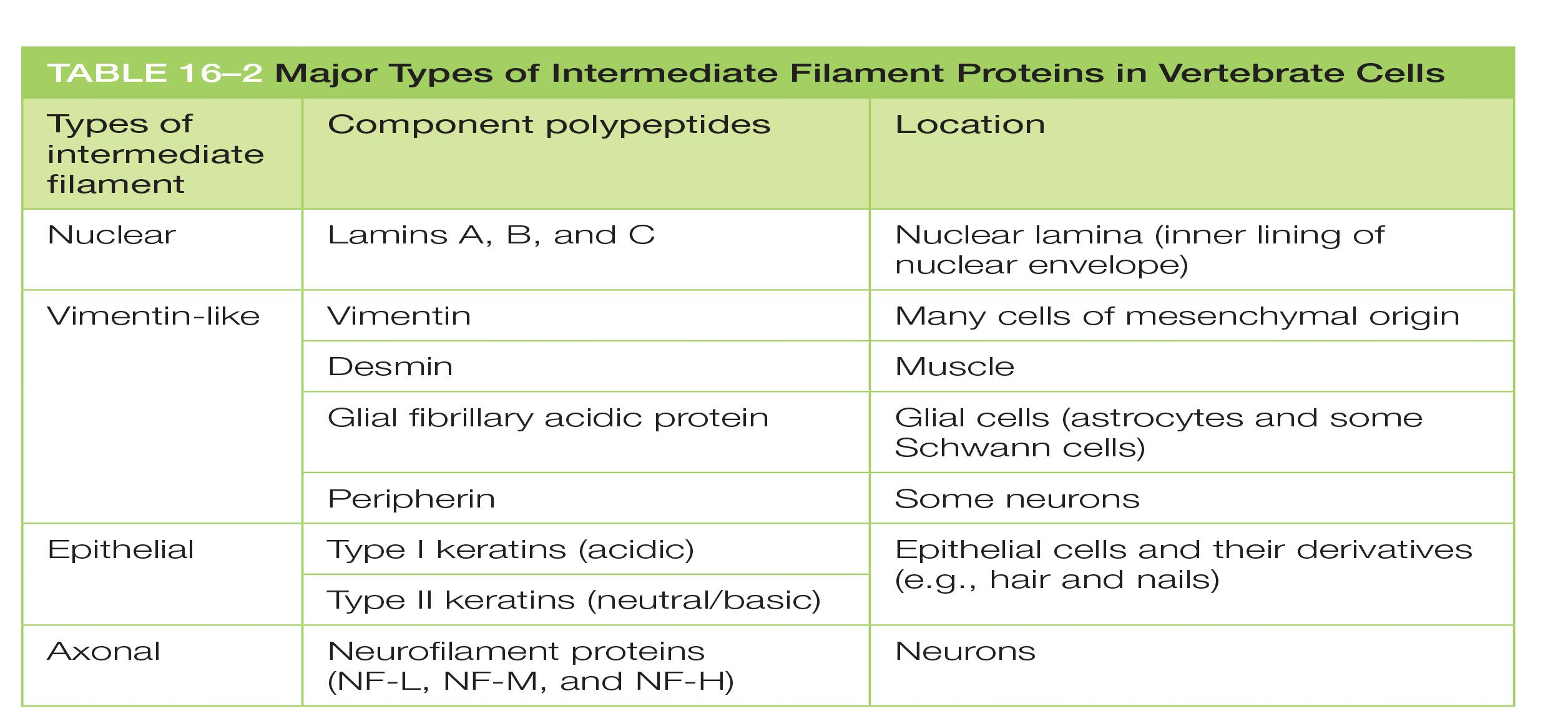
| Types of intermediate filament | Component polypeptides | Location |
|---|---|---|
| Nuclear | Lamins A, B, and C | Nuclear lamina (inner lining of nuclear envelope) |
| Vimentin-like | Vimentin | Many cells of mesenchymal origin |
| Desmin | Muscle | |
| Glial fibrillary acidic protein | Glial cells (astrocytes and some Schwann cells) | |
| Peripherin | Some neurons | |
| Epithelial | Type I keratins (acidic) | Epithelial cells and their derivatives (e.g., hair and nails) |
| Type II keratins (neutral/basic | ||
| Axonal | Neurofilament proteins (NF-L, NF-M, and NF-H) | Neurons |
1. Intermediate filament are a family of proteins with tissue-specific expression
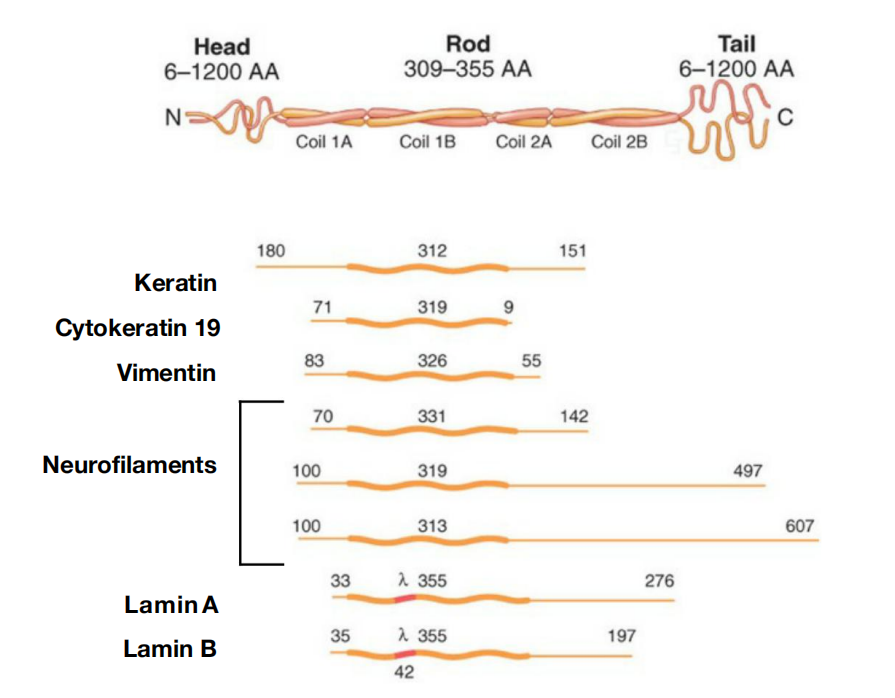
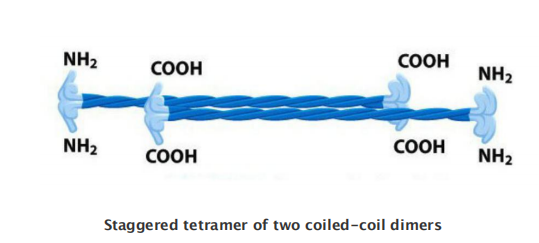
2. Intermediate filaments assemble through coiled coil interactions
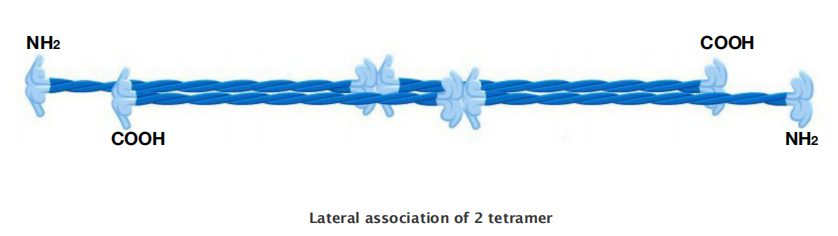
Intermediate Filament Structure Depends on the Lateral Bundling and Twisting of Coiled-Coils
- Although their aminoand carboxy-terminal domains differ, all intermediate filament family members are elongated proteins with a conserved central α-helical domain containing 40 or so heptad repeat motifs that form an extended coiled-coil structure with another monomer
Unlike actin or tubulin subunits, intermediate filament subunits do not contain a binding site for a nucleotide.
The assembled intermediate filament therefore lacks the overall structural polarity that is critical for actin filaments and microtubules.
This large number of polypeptides all lined up together, with the strong lateral hydrophobic interactions typical of coiled-coil proteins, gives intermediate filaments a ropelike character.
- They can be easily bent, with a persistence length of less than one micrometer (compared to several millimeters for microtubules and about ten micrometers for actin), but they are extremely difficult to break and can be stretched to over three times their length.
In pure protein solutions, intermediate filaments are extremely stable due to tight association of subunits, but some types of intermediate filaments, including vimentin (波形蛋白), form highly dynamic structures in cells such as fibroblasts
Protein phosphorylation probably regulates their disassembly, in much the same way that phosphorylation regulates the disassembly of nuclear lamins in mitosis

3. Defects in keratin results in skin blistering
Intermediate Filaments Impart Mechanical Stability to Animal Cells
Keratins (角蛋白) are the most diverse intermediate filament family
- There are about 20 found in different types of human epithelial cells and about 10 more that are specific to hair and nails
- Every keratin filament is made up of an equal mixture of type I (acidic) and type II (neutral/basic) keratin proteins; these form a heterodimer filament subunit
Cross-linked keratin networks held together by disulfide bonds can survive even the death of their cells, forming tough coverings for animals
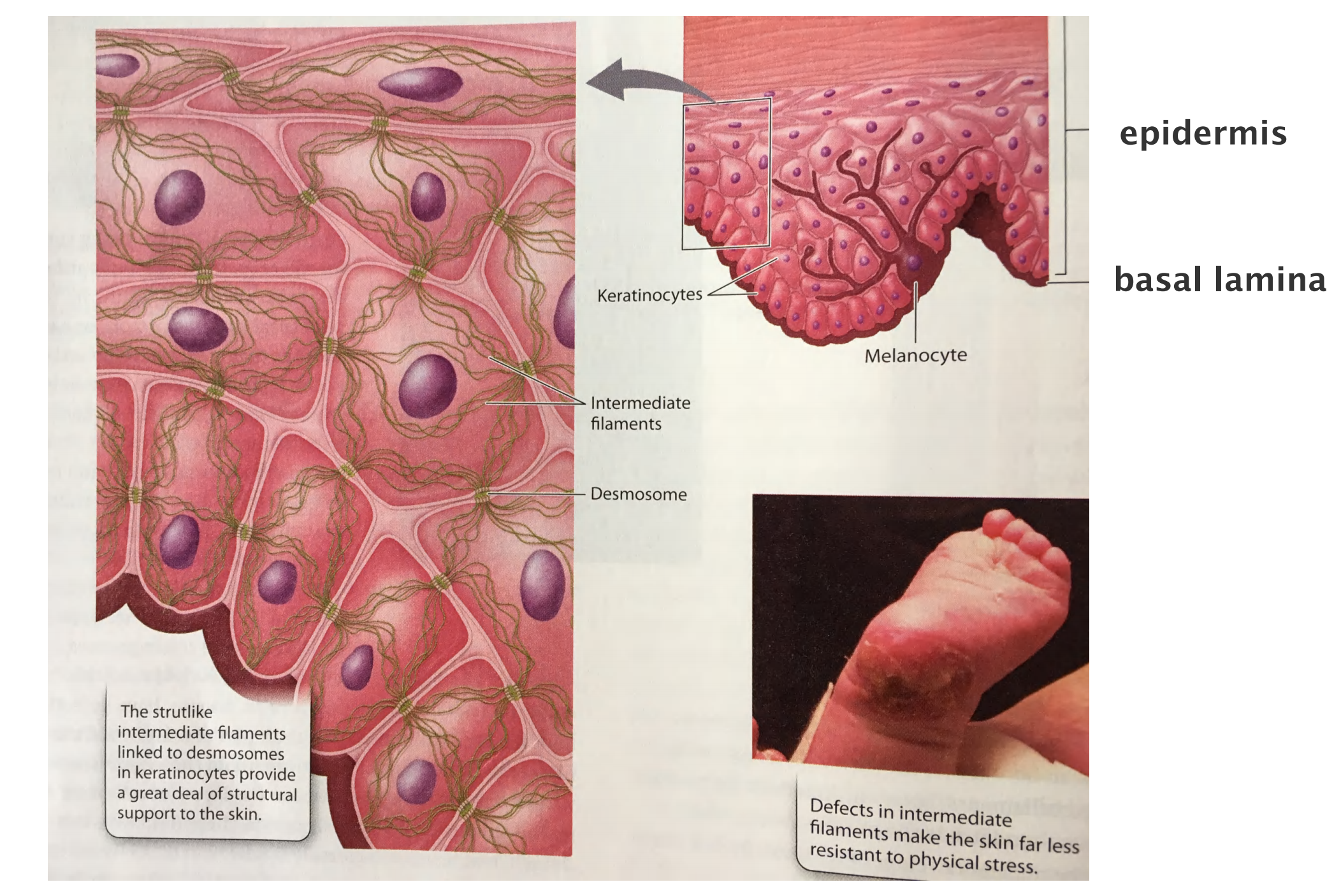
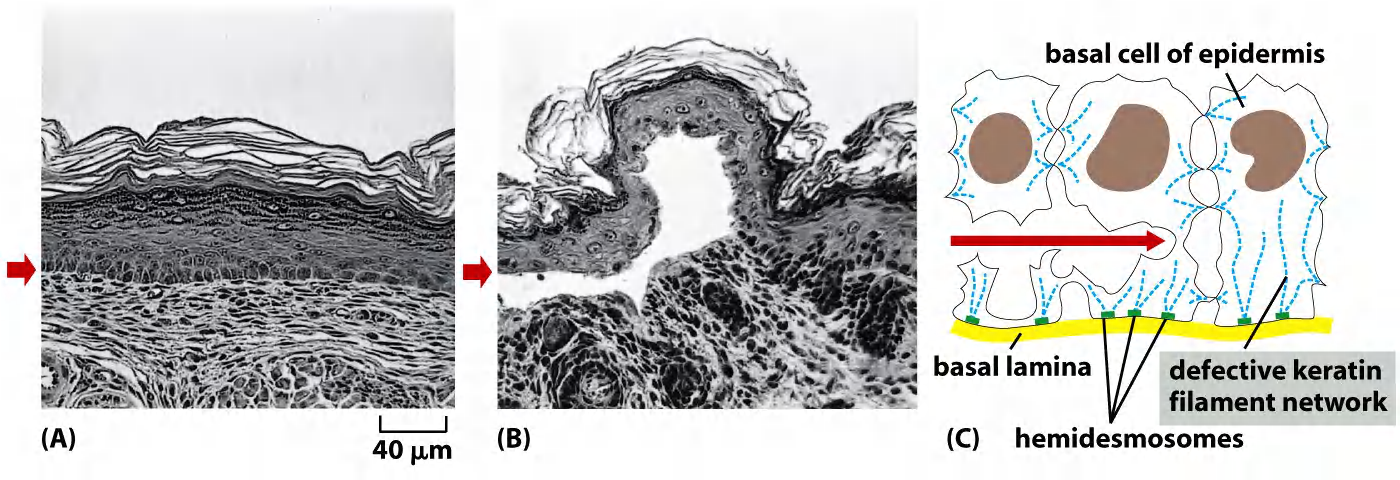
Keratin filaments impart mechanical strength to epithelial tissues in part by anchoring the intermediate filaments at sites of cell–cell contact, called desmosomes (桥粒), or cell–matrix contact, called hemidesmosomes
Accessory proteins, such as filaggrin (丝蛋白), bundle keratin filaments in differentiating cells of the epidermis to give the outermost layers of the skin their special toughness. Individuals with mutations in the gene encoding filaggrin are strongly predisposed to dry skin diseases such as eczema (湿疹).
Mutations in keratin genes cause several human genetic diseases. For example, when defective keratins are expressed in the basal cell layer of the epidermis, they produce a disorder called epidermolysis bullosa simplex, in which the skin blisters in response to even very slight mechanical stress, which ruptures the basal cells
Epidermolysis bullosa simplex
4. Neurofilament
Members of another family of intermediate filaments, called neurofilaments, are found in high concentrations along the axons of vertebrate neurons
Three types of neurofilament proteins (NF-L, NF-M, and NF-H) co-assemble in vivo, forming heteropolymers
- The NF-H and NF-M proteins have lengthy C-terminal tail domains that bind to neighboring filaments, generating aligned arrays with a uniform interfilament spacing.
- During axonal growth, new neurofilament subunits are incorporated all along the axon in a dynamic process that involves the addition of subunits along the filament length as well as the ends.
- After an axon has grown and connected with its target cell, the diameter of the axon may increase as much as fivefold
The level of neurofilament gene expression seems to directly control axonal diameter, which in turn influences how fast electrical signals travel down the axon. In addition, neurofilaments provide strength and stability to the long cell processes of neurons.
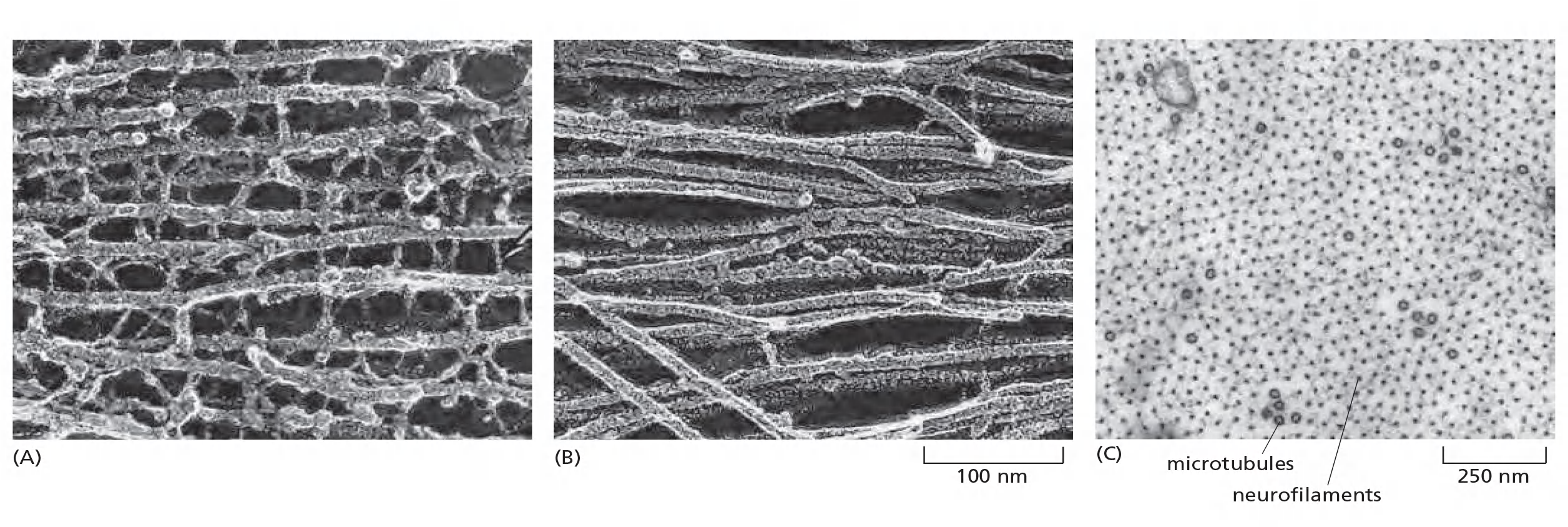
The vimentin-like filaments are a third family of intermediate filaments. Desmin (肌间线蛋白), a member of this family, is expressed in skeletal, cardiac, and smooth muscle, where it forms a scaffold around the Z disc of the sarcomere
Intermediate filaments are crosslinked and bundled into strong arrays
Through lateral contacts
Through proteins such as filaggrin- keratin filaments, family of plakins (e.g. plectin) crosslinks intermediate filaments.
Mutation in plectin results in serious human disease characterized by epidermolysis bullosa, muscular dystrophy and neurodegeneration.
Keratins are further crosslinked by disulfide bonds.
1. Intermediate Plectin cross-linking of diverse cytoskeletal elements
The intermediate filament network is linked to the rest of the cytoskeleton by members of a family of proteins called plakins (血小板溶素).
Plectin (网蛋白) links the intermediate filaments to microtubules, actin filament bundles, and filaments of the motor protein myosin II; it also helps attach intermediate filament bundles to adhesive structures at the plasma membrane
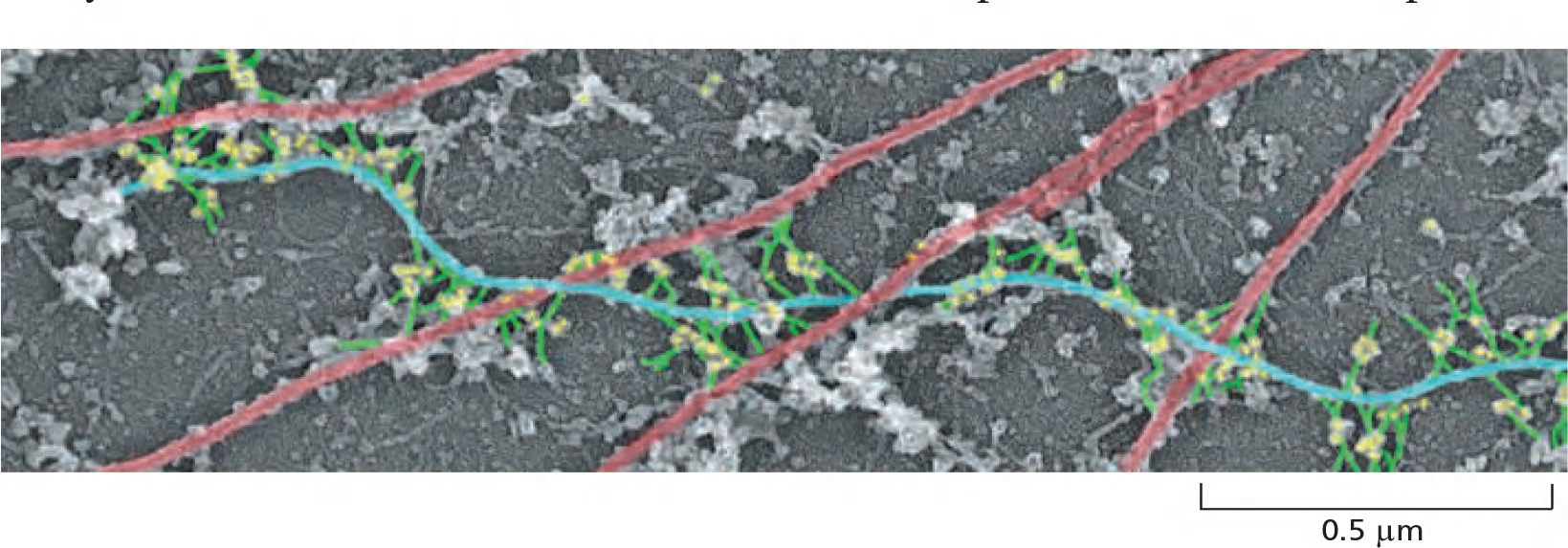
- Microtubule: red
- IFL blue
- Plectin: yellow
2. Connecting cytoskeleton to the nucleus
Cytoplasmic intermediate filaments are closely related to their ancestors, the much more prevalent nuclear lamins, which are found in many eukaryotes but missing from unicellular organisms
The nuclear lamins form a meshwork lining the inner membrane of the nuclear envelope, where they provide anchorage sites for chromosomes and nuclear pores.
Plectin and other plakins can interact with protein complexes that connect the cytoskeleton to the nuclear interior. These complexes consist of SUN proteins of the inner nuclear membrane and KASH proteins (also called nesprins) of the outer nuclear membranes
- SUN and KASH proteins bind to each other within the lumen of the nuclear envelope, forming a bridge that connects the nuclear and cytoplasmic cytoskeletons.
- Inside the nucleus, the SUN proteins bind to the nuclear lamina or chromosomes, whereas in the cytoplasm, KASH proteins can bind directly to actin filaments and indirectly to microtubules and intermediate filaments through association with motor proteins and plakins, respectively.
Mutations in the gene for plectin cause a devastating human disease that combines epidermolysis bullosa (caused by disruption of skin keratin filaments), muscular dystrophy (caused by disruption of desmin filaments), and neurodegeneration (caused by disruption of neurofilaments).
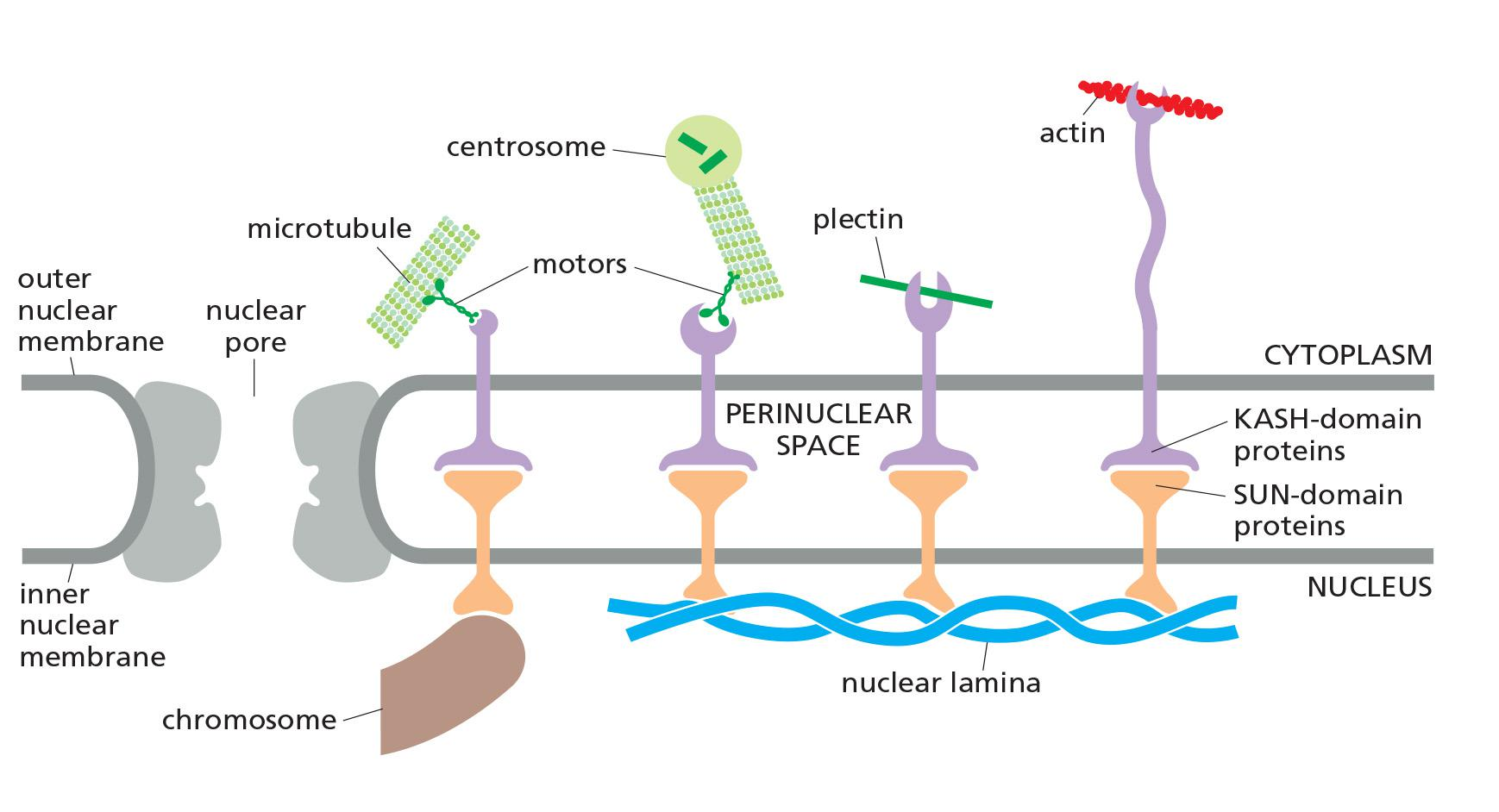
The cytoplasmic cytoskeleton is linked across the nuclear envelope to the nuclear lamina or chromosomes through SUN and KASH proteins
The SUN and KASH domains of these proteins bind within the lumen of the nuclear envelope.
From the inner nuclear envelope, SUN proteins connect to the nuclear lamina or chromosomes.
KASH proteins in the outer nuclear envelope connect to the cytoplasmic cytoskeleton by binding microtubule motor proteins, actin filaments, or plectin.