L06 Lipid Metabolism I Fatty Acids, Triacylglycerols, and Lipoproteins
一、Utilization and Transport of Fat and Cholesterol
Lipids play important roles in:
- Energy metabolism;
- As membrane constitutes;
- Hormones, fat-soluble vitamins, thermal insulators, biological regulators such as prostaglandins(前列腺素类)
Overview of intermediary metabolism with fatty acid and triacylglycerol pathways highlighted:
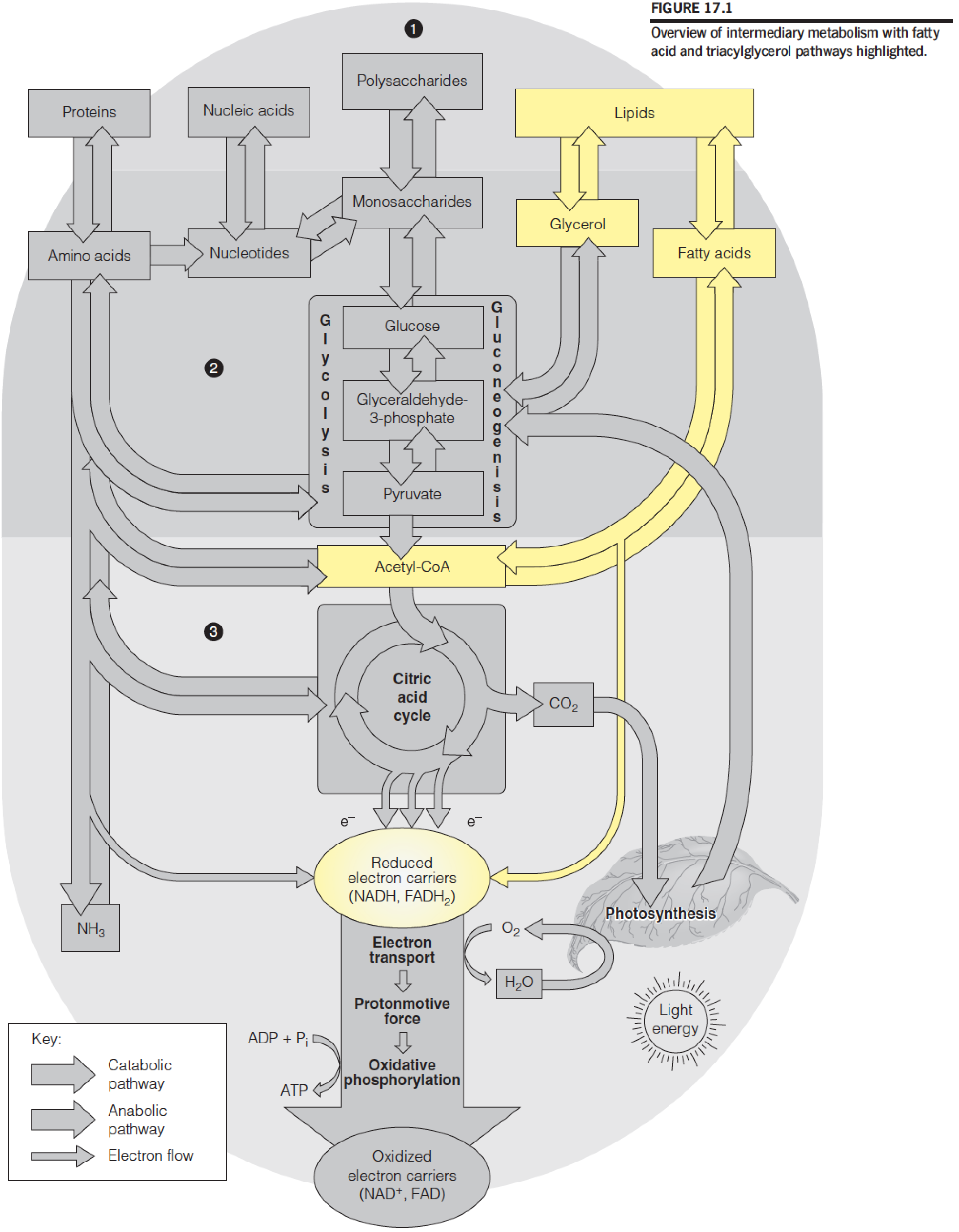
- The catabolism of fat begins with the hydrolysis of TG to yield glycerol and FFA (free fatty acid) 95% energy from FFA, 5% from glycerol
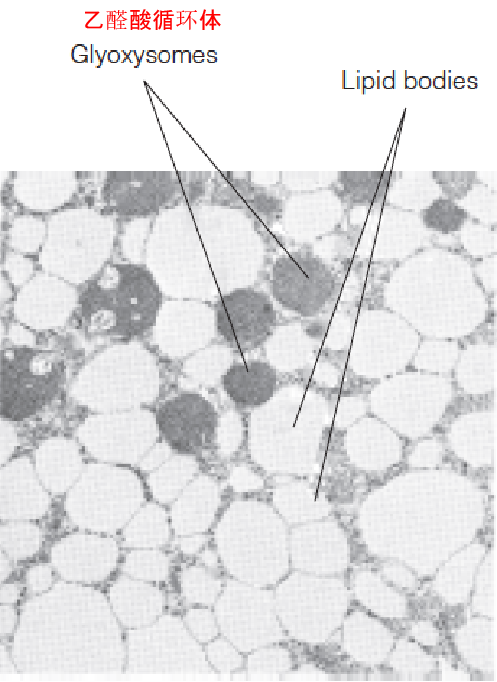
Fat storage in a plant seedling
The electron micrograph shows a cell from a cucumber cotyledon (seed leaf, 黄瓜子叶) a few days after germination.
Fat stored in lipid bodies is degraded, oxidized, and converted to carbohydrate in neighboring glyoxysomes (or microbodies) to support the growth of the plant.
- Fats as energy reserves in plant
The bulk of the lipid in most organisms: triacylglycerol (formerly called triglycerides): TG, TAG, fat or neutral fat
A mammal contains 5-25% body weight as lipids, 90% TAG, stored in adipocyte/adipose tissue, primary energy reserve, plant seed contains great quantities of fat.
- 1 kJ = 0.239 kcal (Cal)
- 1 kcal (Cal)=4.185 kJ
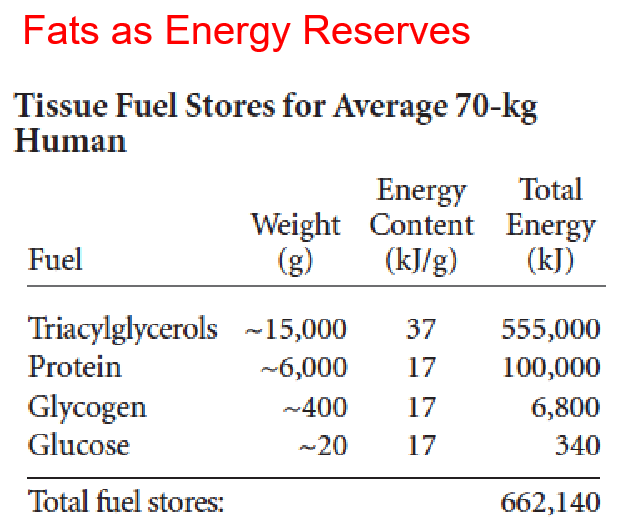
- Fat has six times more caloric content by weight than carbohydrate because fat is more highly reduced and is anhydrous (无水的(尤指结晶水)).
1. Fat Function Examples
Bird Migration
Some small land birds prepare for migration: increases body weight 15% per day, all TG, fly nonstop 60 hrs;
Hibernating animal: store several months’ worth of food;
thermal insulator;
Cushion.
Junk Foods
About 40% of the caloric value of Western diet
Nutritionist recommendation: 25-30% from fat for cardiovascular health
In addition, excess carbohydrate is readily converted to fat,one can of regular coke: about 40 g sugar
Obesity and diabetes (type II diabetes)
Fat Digestion and Absorption
1. Overview of fat digestion, absorption, storage, and mobilization in the human
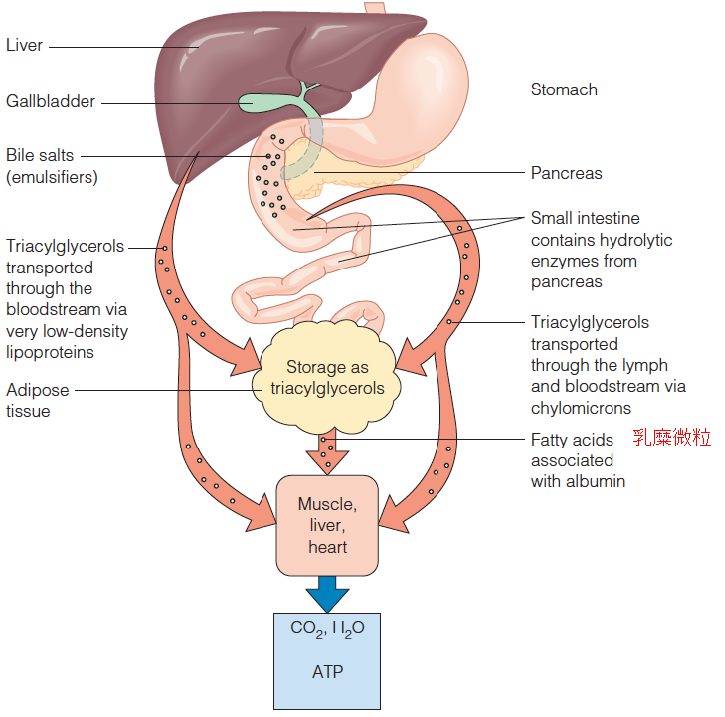
- Pancreatic lipase: Pancreatic lipase, also known as pancreatic triacylglycerol lipase, is an enzyme secreted from the pancreas. As the primary lipase enzyme that hydrolyzes (breaks down) dietary fat molecules in the human digestive system, it is one of the main digestive enzymes, converting triglyceride substrates found in ingested oils to monoglycerides and free fatty acids.
- The products of fat digestion: glycerol, free fatty acids, monoacylglycerols, diacylglycerols
- Less than 10% of the original TAG remains unhydrolyzed
- Through intestinal mucosal cells
- Resynthesis of TG from the digestive products: endoplasmic reticulum and Golgi complex of mucosal cells
- TG emerge into the lymph system, complexed with protein to form chylomicron (CM), transport fat to tissues
TAG three primary sources:
- the diet;
- de novo biosynthesis, particularly in liver;
- storage depots in adipocytes.
2. Utilization and Transport of Fat and Cholesterol
A major problem for the digestion, absorption, and transport of dietary lipids: their insolubility in aqueous media. How to solve this problem?
Action of bile salts in emulsifying (乳化) fats in the intestine
Cholic acid (胆酸), a typical bile acid (胆汁酸), ionizes to give its cognate bile salt.
The hydrophobic surface of the bile salt molecule associates with triacylglycerol (三酰甘油), and several such complexes aggregate to form a micelle (微团).
The polar surface of the bile salts faces outward, allowing the micelle to associate with pancreatic lipase/colipase (辅脂酶).
Hydrolytic action of this enzyme frees the fatty acids to associate in a much smaller micelle that can be absorbed through the intestinal mucosa.
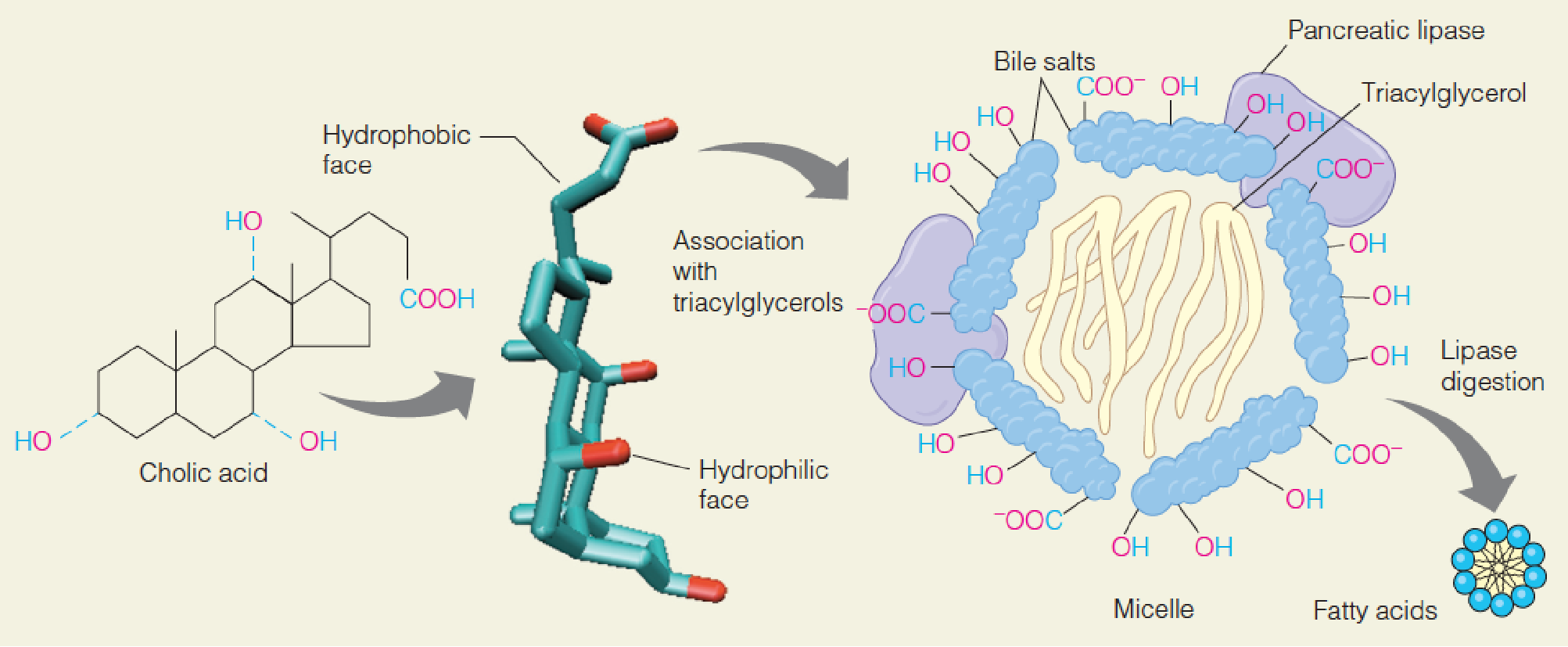
- Bile salts: synthesized in liver and stored in gallbladder(胆囊)
- detergent
- gallstone 胆(结)石
How to transport fat and cholesterol to tissue via blood?
Lipoproteins (脂蛋白) are lipid–protein complexes that allow movement of a polar lipids through aqueous environments.
(1) Generalized structure of a plasma lipoprotein
The spherical particle, part of which is shown, contains neutral lipids in the interior and phospholipids, cholesterol, and protein at the surface.
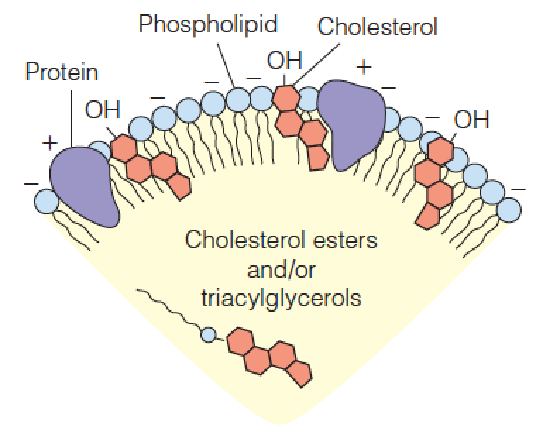
- Apoprotein (载脂蛋白): the polypeptide components of lipoproteins; mainly synthesized in the liver (80%), 20% produced in intestinal mucosal cells
- FFA binds to albumin(清蛋白,白蛋白) in blood
(2) Classification (by centrifugation) and Functions of Lipoproteins
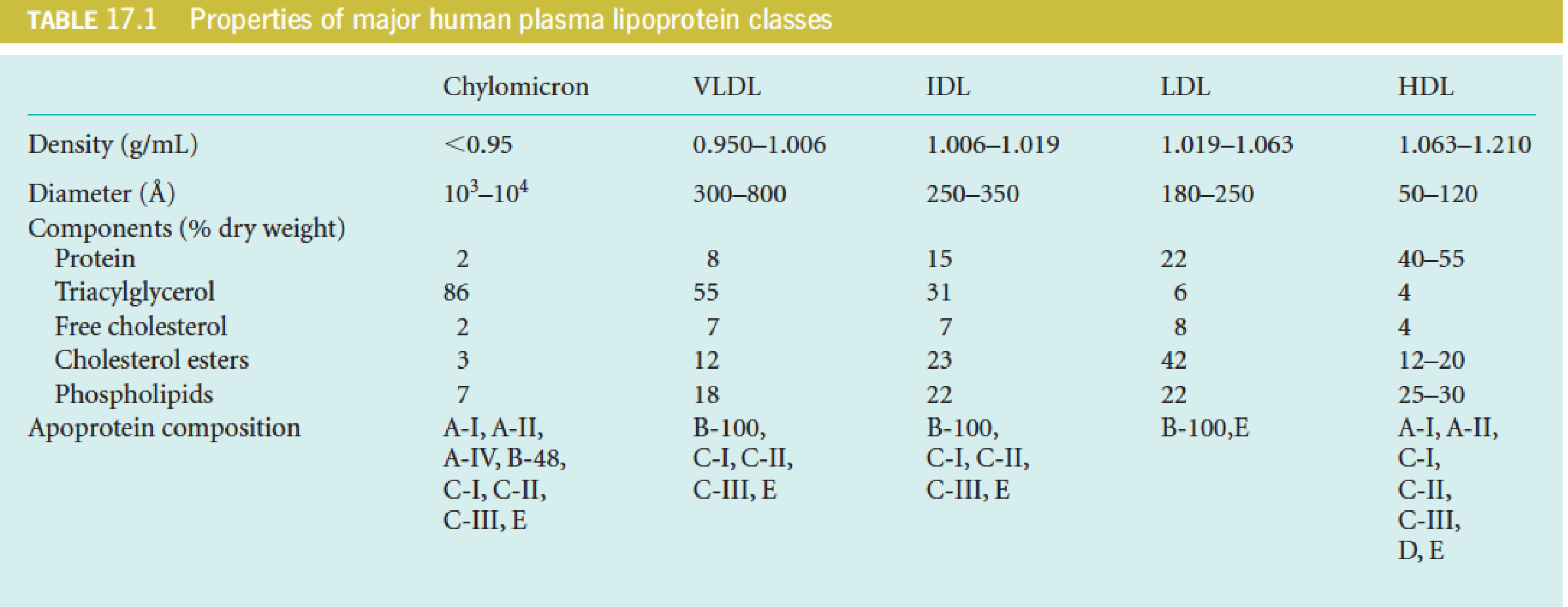
- CM: chylomicron 乳糜微粒
- VLDL: very low-density lipoprotein 极低密度脂蛋白
- IDL: intermediate-density lipoprotein 中等密度脂蛋白
- LDL: low-density lipoprotein 低密度脂蛋白
- HDL: high-density lipoprotein 高密度脂蛋白
A total of 10 major apolipoproteins in human lipoproteins
ApoE4 is associated with Alzheimer’s disease
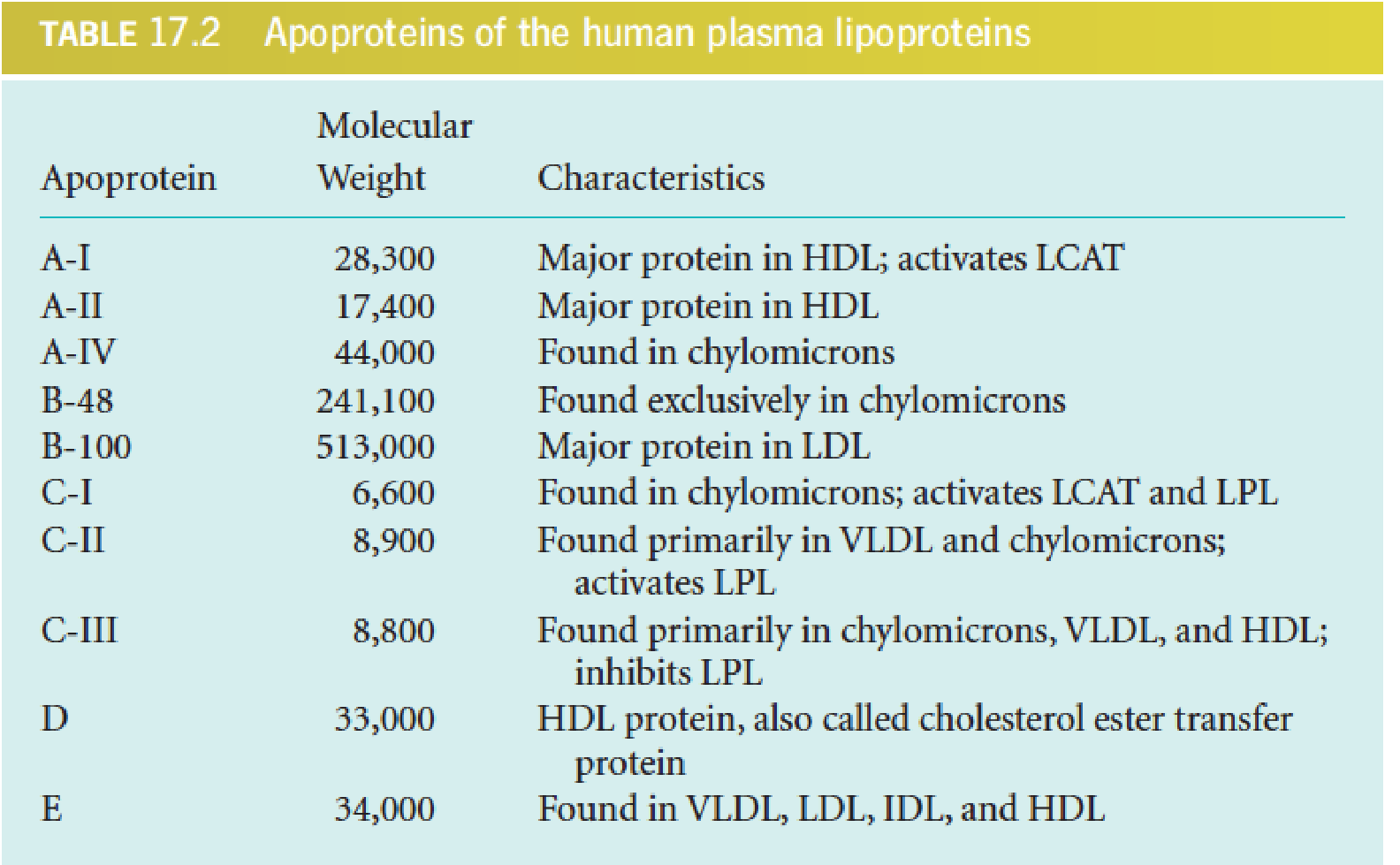
- LCAT (lecithin-cholesterol acyltransferase): 卵磷脂胆固醇酰基转移酶
- Lipoprotein lipase (LPL): 脂蛋白脂酶
- Lipoprotein help maintain 500 mg/100ml human blood in emulsified form: 120 mg TAG, 220 mg cholesterol (2/3 esterified, 1/3 free cholesterol), 160 mg phospholipids
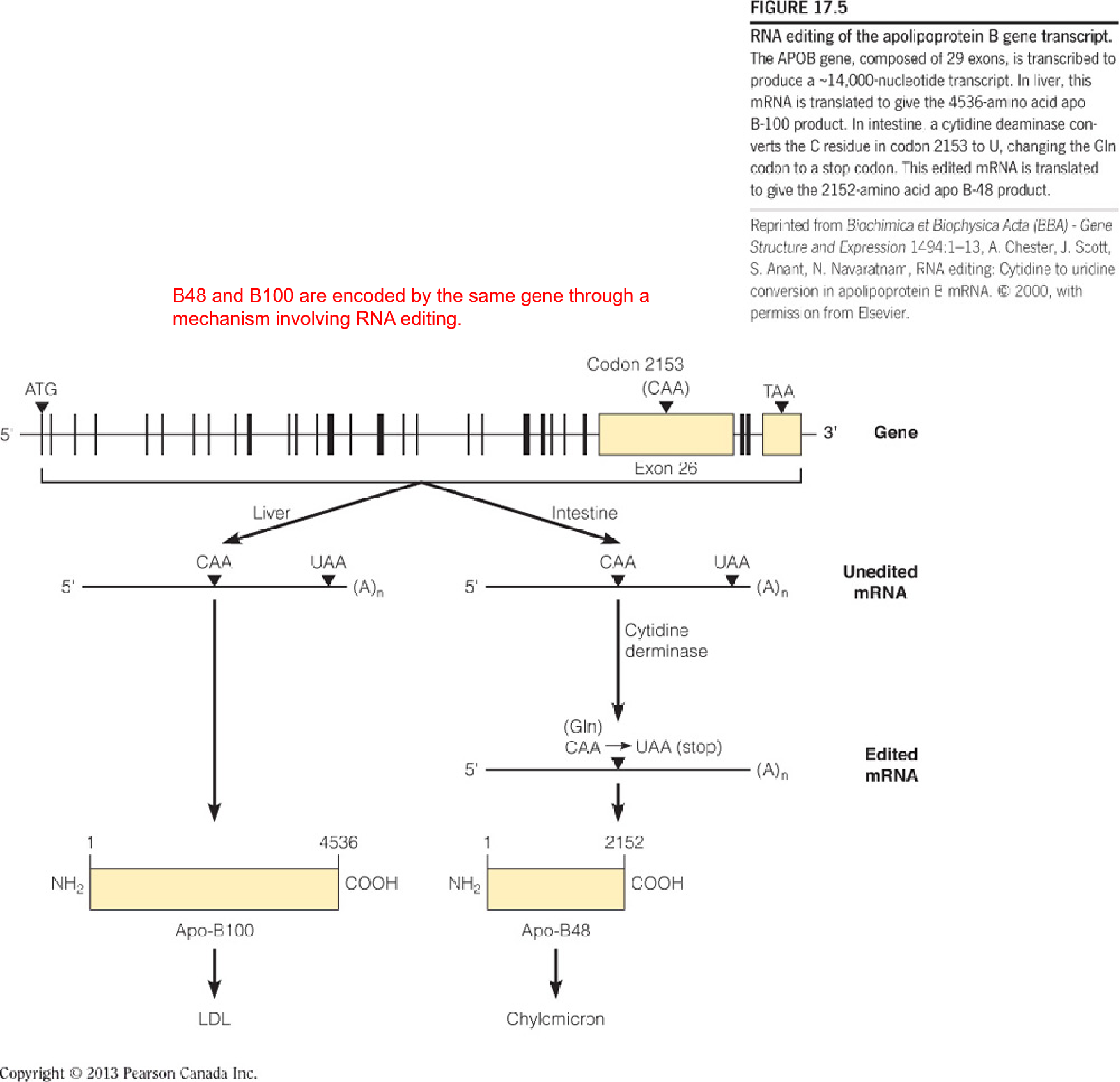
- B48 and B100 are encoded by the same gene through a mechanism involving RNA editing.
- RNA editing: 小肠内的CAA被 Cytidine derminase (胞苷脱氨酶) 修饰之后变为UAA,成为stop codon
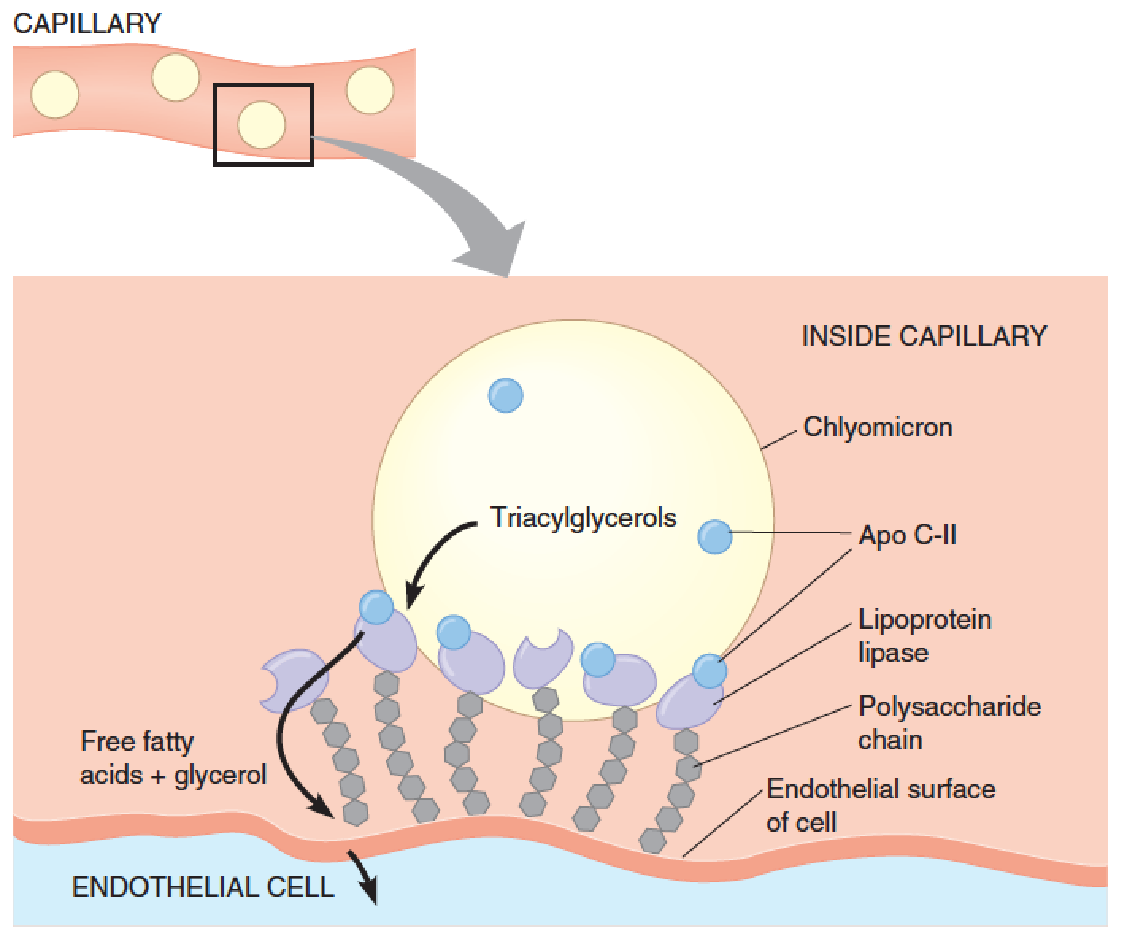
(3) Binding of a chylomicron to lipoprotein lipase on the inner surface of a capillary
The chylomicron is anchored by lipoprotein lipase (LPL), which is linked by a polysaccharide chain to the lumenal surface of the endothelial cell.
When activated by apoprotein C-II, the lipase hydrolyzes the triacylglycerols in the chylomicron, allowing uptake into the cell of the glycerol and the free fatty acids.
- Following a high-fat meal, CM is so abundant in blood that they give the plasma a milky appearance.
- Apo C-II deficiency in human: high CM and high TAG in blood
Overview of lipoprotein transport pathways and fates
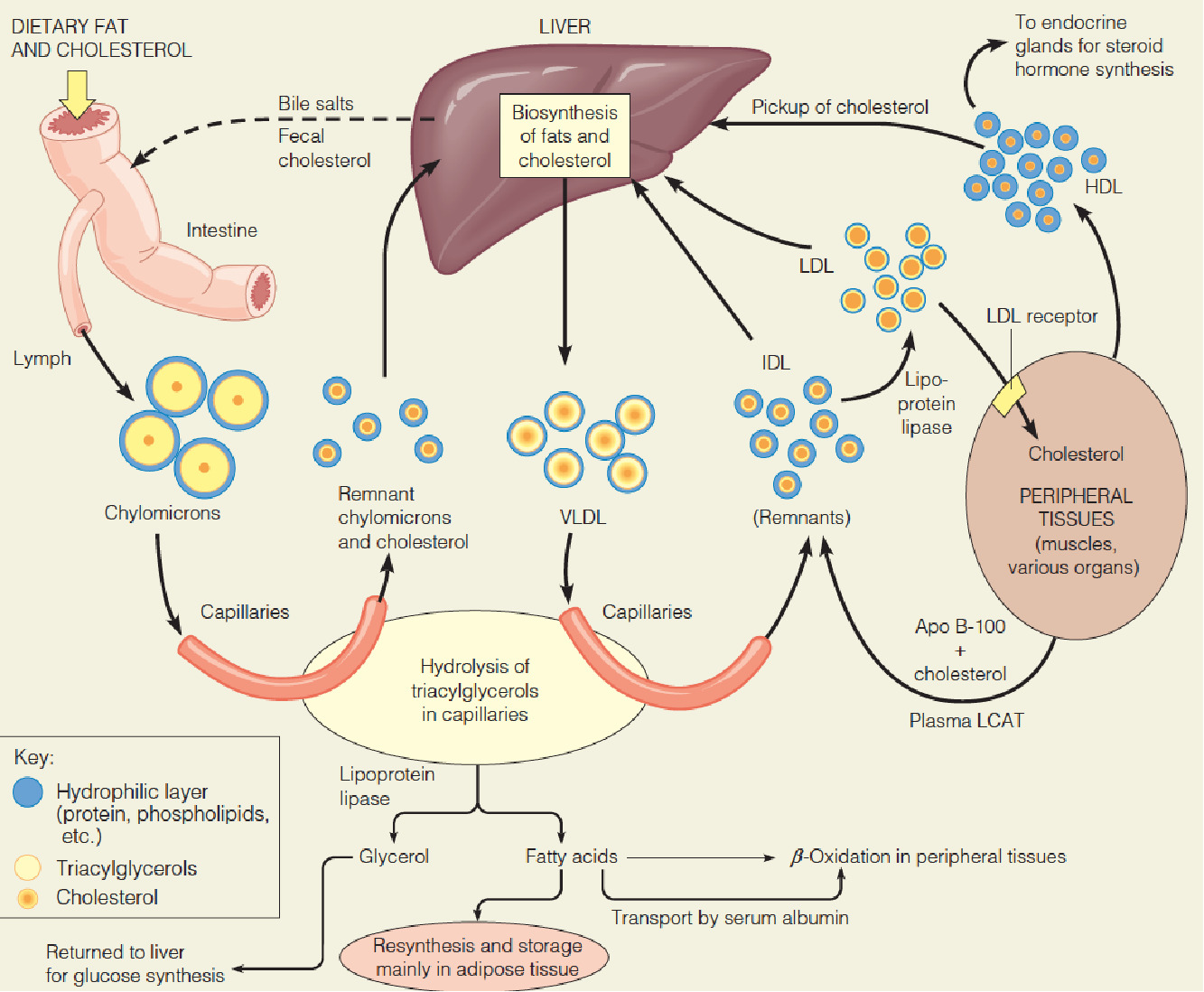
Overall aspects of lipoprotein metabolism and transport
- CM: transport TG from intestine to other tissues (heart, muscle and adipose tissue);
- VLDL: transport TG from liver to other tissues (heart, muscle and adipose tissue);
- LDL: transport cholesterol from liver to other tissues;
- HDL: return excess cholesterol from tissues to the liver for metabolism or excretion.
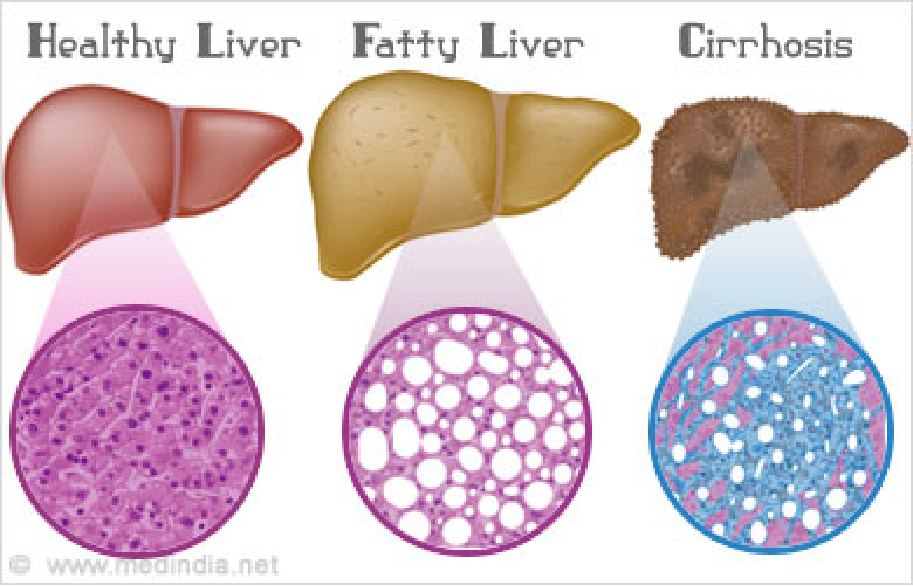
Liver is a major site of apolipoprotein synthesis (80%), damage to this organ causes endogenously synthesized fat to accumulate there because it cannot be transported to peripheral tissues, resulting in fatty liver.
Plasma cholesterol: 3.5-6.7 mM or 130-260 mg/100 mL; Above 200 mg/100 mL is a major factor for heart disease
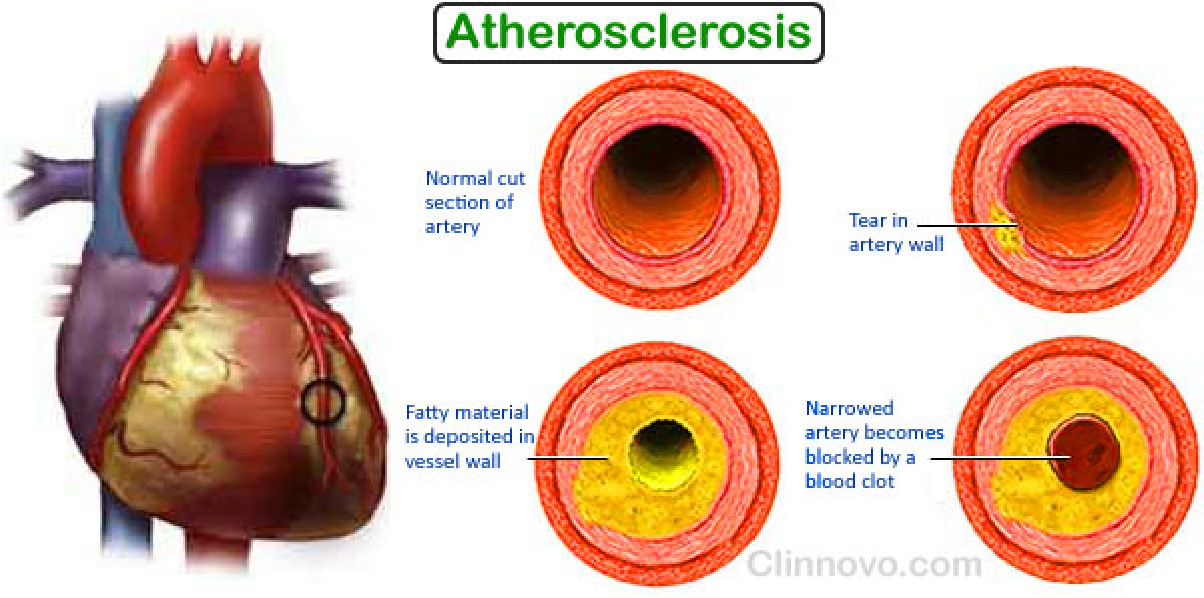
Cholesterol accumulation in the blood is correlated with development of atherosclerotic plaque. (动脉粥样硬化斑块)
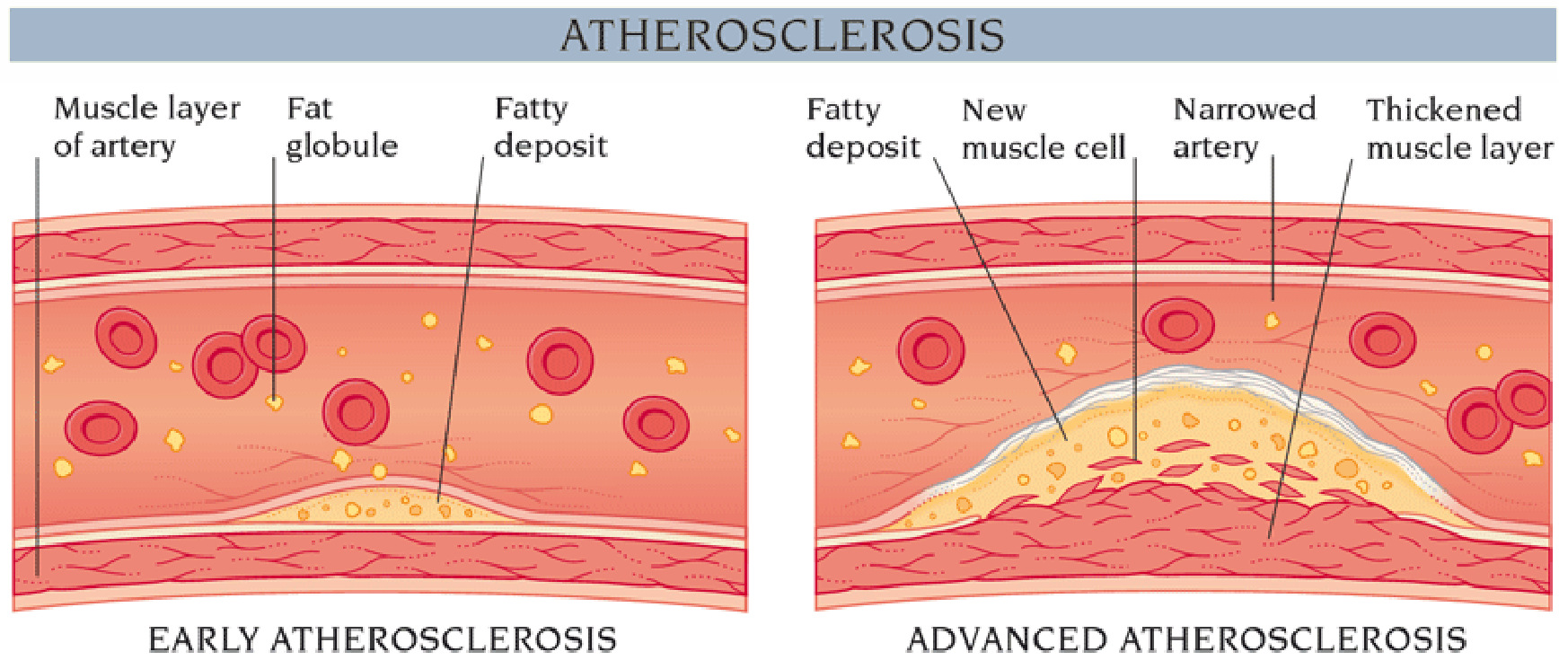
High cholesterol level (insoluble, accumulated in white blood cells (macrophages) ➔deposited at the sites of injury or damage on inner arterial walls ➔ these cells become engorged with fatty deposits, which then harden into plaque; ➔ this situation, called atherosclerosis (动脉粥样硬化), ultimately blocks key blood vessels and causes myocardial infarctions and heart attacks.
Why? must understand how cholesterol is taken up from LDL into cells
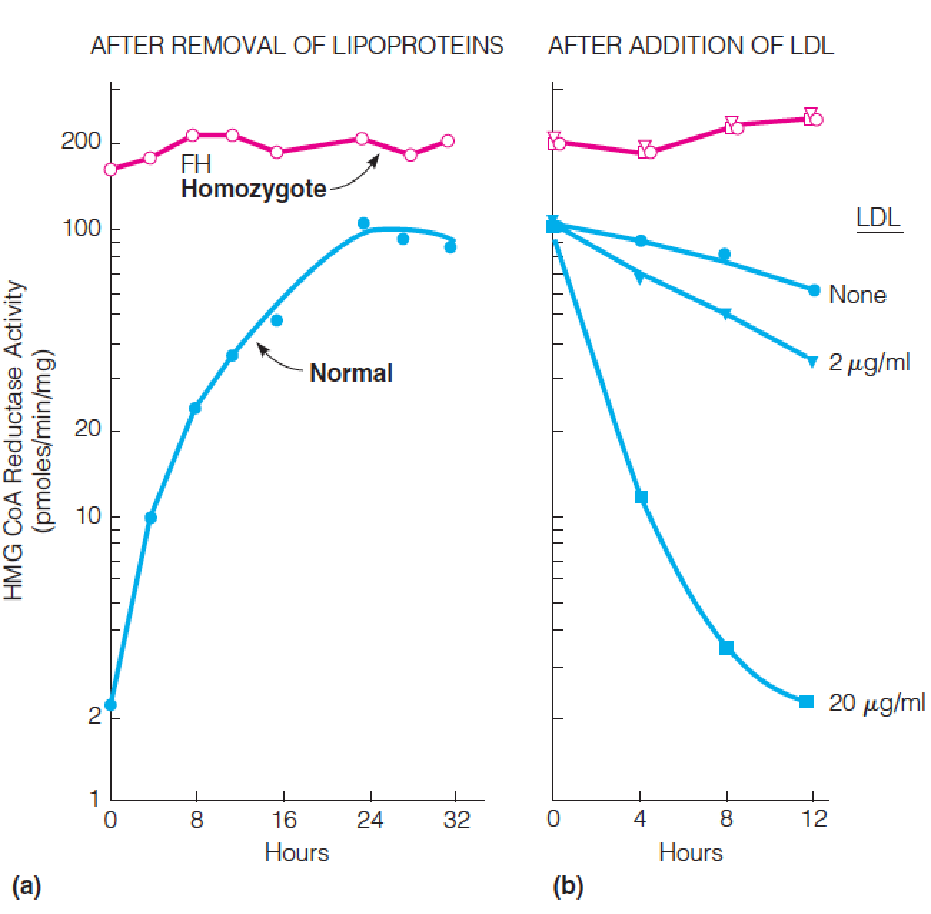
Feedback regulation of HMG-CoA reductase (3-hydroxy-3-methylglutaryl-CoA reductase) activity:
Fibroblasts obtained from a normal subject or from a patient homozygous for familial hypercholesterolemia (FH Homozygote) were grown in monolayer cultures.
- At time zero, the medium was replaced with fresh medium depleted of lipoproteins, and HMG-CoA reductase activity was measured in extracts prepared at the indicated times.
- Twenty-four hours after addition of the lipoprotein-deficient medium, human LDL was added to the cells at the indicated levels, and HMG-CoA reductase activity was measured at the indicated time.
- Familial hypercholesterolemia (FH,家族性高胆固醇血症): which results from mutations in the LDLR gene.
- Individuals with the rare homozygous from of FH (about 1 in 1 million),cholesterol: > 600-1200 mg/100ml plasma
- Develop atherosclerosis early in life and usually die of heart disease before 20.
- Heterozygous : 1 in 500; cholesterol: 250-500 mg/100 ml; high risk to have heart attacks at age 30-40
Results and data interpretation
- In the presence of LDL, normal cells showed low HMA-CoA reductase activity
- In the absence of LDL, the same cells showed 50-100-fold higher activity
- Results showed that cholesterol is transported into the cells and it regulates its own synthesis
In contrast, the FH cells showed high level enzyme activity in the presence or absence of LDL, suggesting they were deficient in ability to take up cholesterol from medium
These results suggest that cholesterol is taken into cells through action of a specific receptor, which is deficient or defective in FH patients
In shorter order, Brown and Goldstein demonstrated the existence of the receptor, the LDL receptor and demonstrated a new mechanism by which cells can interact with their environment-receptor-mediated endocytosis
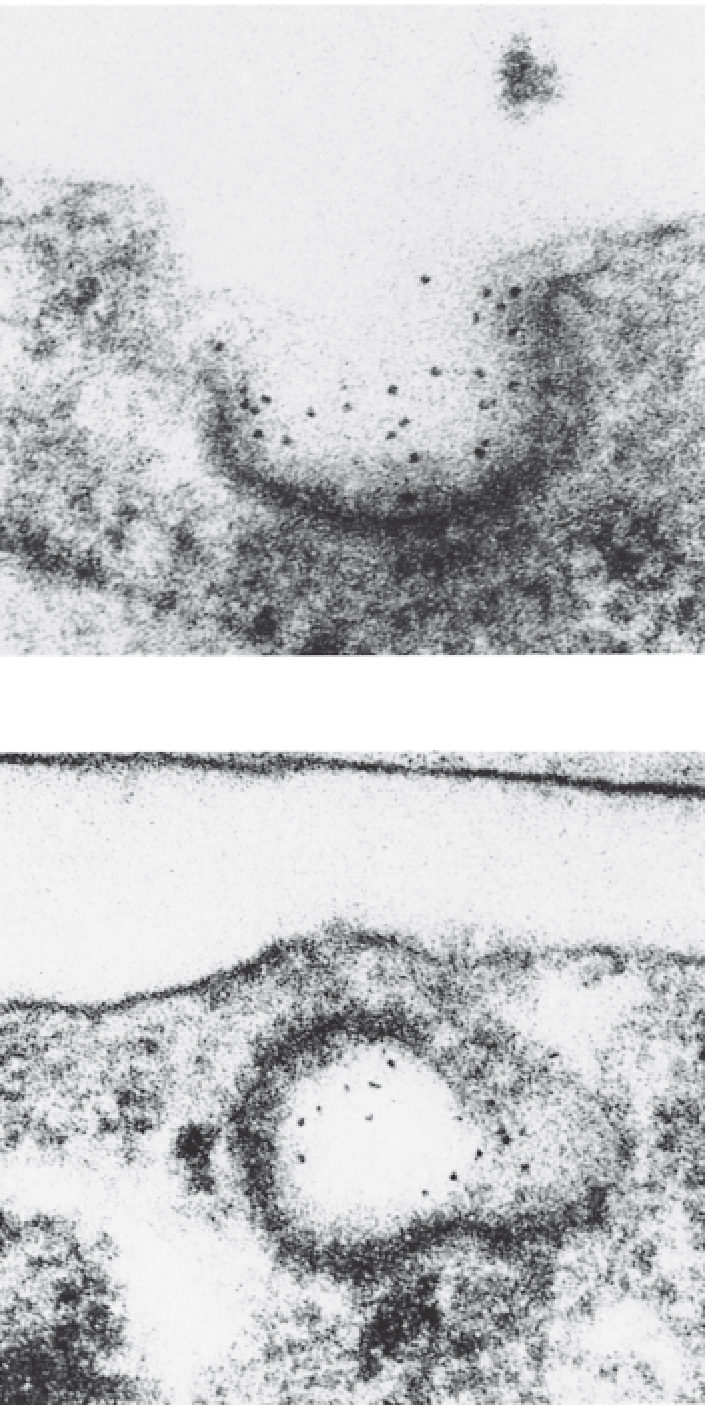
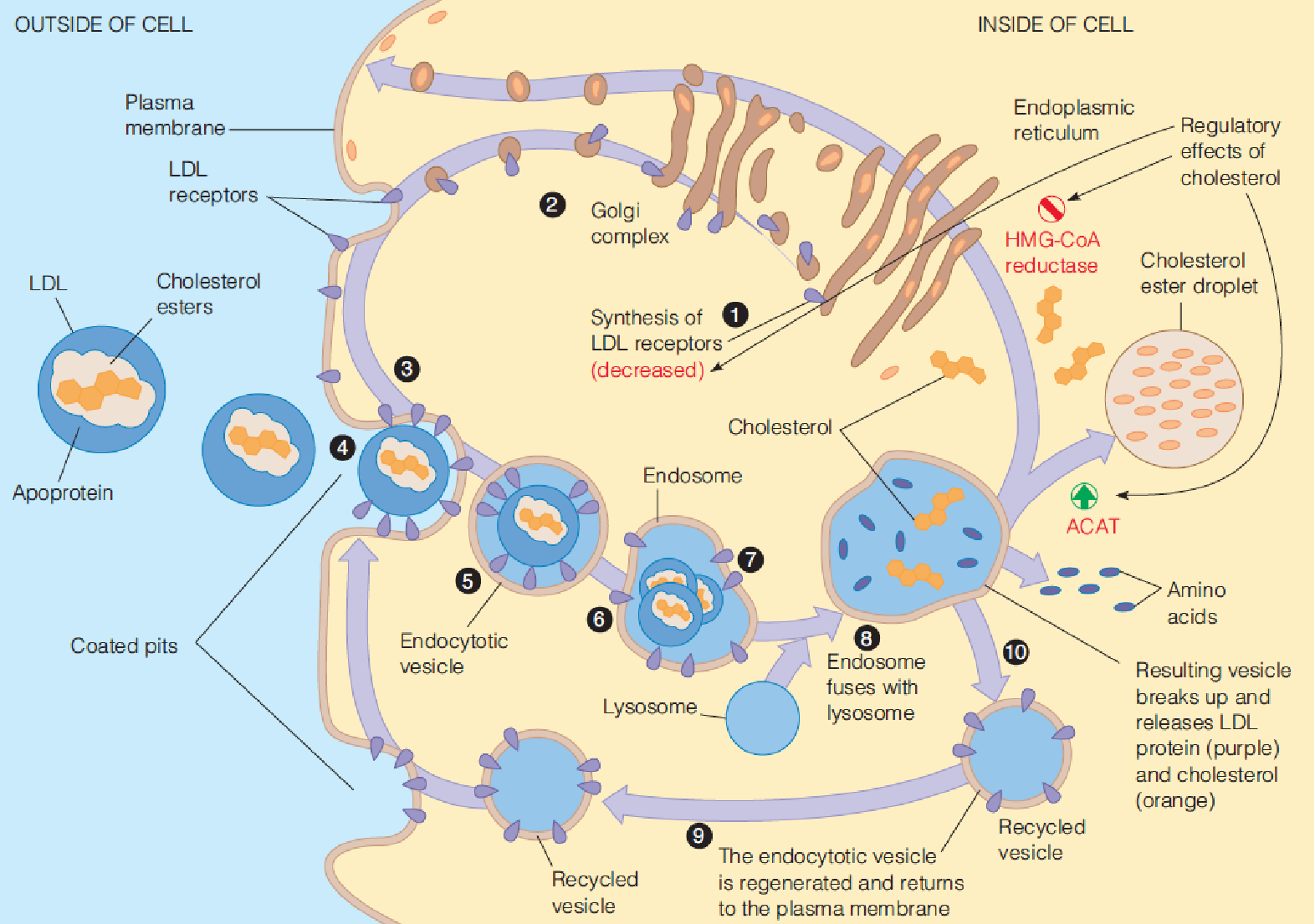
(1) Receptor-mediated endocytosis of LDL
LDL was conjugated with ferritin (铁蛋白) to permit electron microscopic visualization.
The LDL–ferritin (dark dots) binds to a coated pit on the surface of a cultured human fibroblast.
The plasma membrane closes over the coated pit, forming an endocytotic vesicle.
(2) Structure of a clathrin (网格蛋白)-coated pit:
Clathrin, the major protein in coated pits, forms triskelions (三脚蛋白复合体) (named after the symbol of three legs radiating from the center), which assemble into polyhedral lattices composed of hexagons and pentagons, such as the barrel shown in the next panel.
Image reconstruction from electron cryomicroscopy of a clathrin barrel formed from 36 triskelions. A single clathrin triskelion is highlighted in light blue.
A coated pit on the inner surface of the plasma membrane of a cultured mammalian cell is visualized by freeze-fracture electron microscopy.
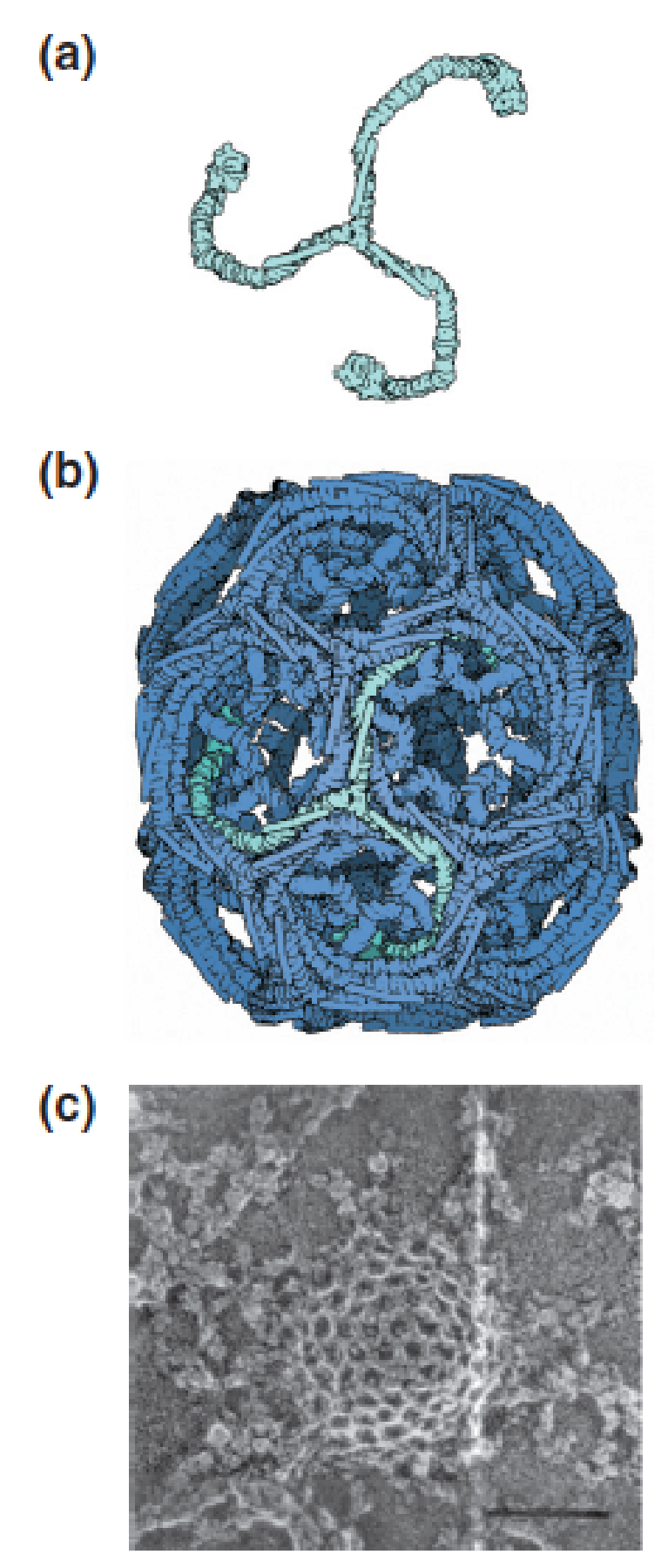
- The cage-like structure of the pit is due to the clathrin lattice.
(3) Involvement of LDL receptors in cholesterol uptake and metabolism
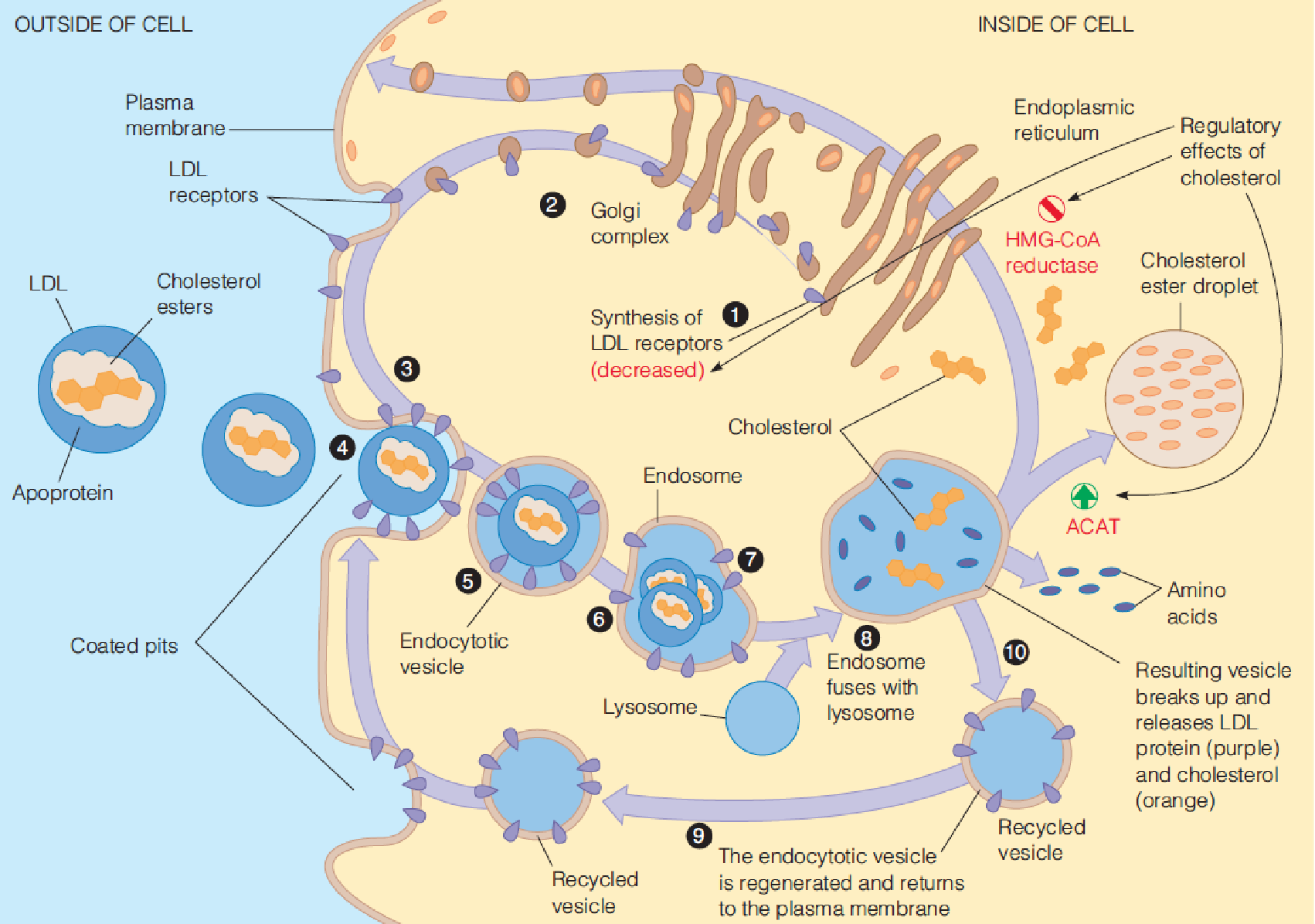
- Cholesterol:
- Down regulate the HMG-CoA reductase (decrease the protein level) $\rarr$ block cholesterol biosynthesis
- Promote ACAT, promote synthesis of cholesterol ester
- Down regulate the synthesis of LDL receptor $\rarr$ down regulate the cholesterol uptake
3. Cholesterol regulatory effects
- It suppresses endogenous cholesterol synthesis by inhibiting HMG-CoA reductase and also suppressing transcription of the gene encoding the enzyme and accelerating degradation of the enzyme protein;
- It activates acyl-CoA:cholesterol acyltransferase (ACAT), an intracellular enzyme that synthesizes cholesterol ester from cholesterol and a long-chain acyl CoA. This promotes the storage of excess cholesterol in the forms of droplets of cholesterol esters;
- It regulates the synthesis of the LDL receptor itself by somehow reducing the content of mRNA for the receptor. High cholesterol level lessen the synthesis of LDL receptor
How do elevated cholesterol levels lead to atherosclerosis (plaque formation)?
How do elevated cholesterol levels lead to atherosclerosis (plaque formation)?
- 在高胆固醇的情况下:LDL is oxidized to oxidized LDL➔
- uptake by scavenger LDL receptor in white blood cells (macrophages) ➔
- oxidized LDL receptor is not down-regulated by cholesterol, so cholesterol uptake into the cells is virtually unlimited ➔
- which converted these cells to a cholesterol-engorged species called a foam cell ➔
- These events have a chemotactic effect(趋化作用), causing more macrophages to migrate to the site ➔
- leading them to accumulate more cholesterol ➔
- plaque formation ➔
- blocks key blood vessels and causes myocardial infarctions and heart attacks.
“Good cholesterol” vs. “bad cholesterol”-inappropriate terms
Cholesterol in LDL is considered “bad”, because prolonged elevation of LDL levels is what leads to atherosclerosis;
Cholesterol in HDL is considered “good” because high level of HDL counteracts atherosclerosis. This is because HDL returns excess cholesterol from peripheral cells to the liver and lower total serum cholesterol.
Smoking ; Physical exercise
STATINS: HMG-CoA reductase inhibitor
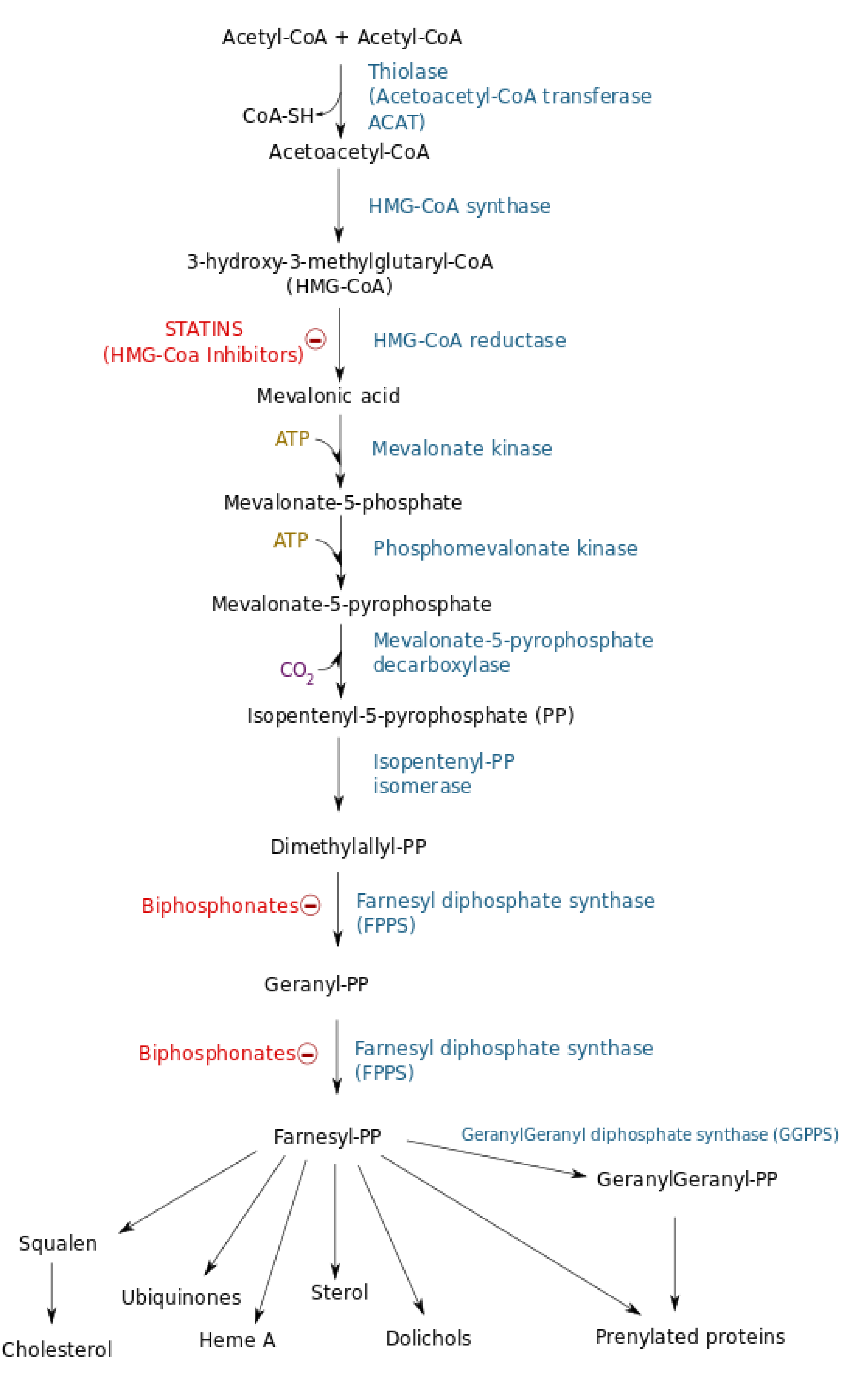
(1) HMG-CoA reductase inhibitors reduce blood cholesterol level
HMG-CoA reductase inhibitor ➔ de novo cholesterol biosynthesis ↓ ➔ intracellular cholesterol level ↓ ➔ LDL receptor ↑ ➔ clearance of extracellular cholesterol ↑ ➔ the level of blood cholesterol ↓ .
One such inhibitor, called lovastatin (洛弗斯塔特因), was approved by FDA in 1987.
Lipitor:
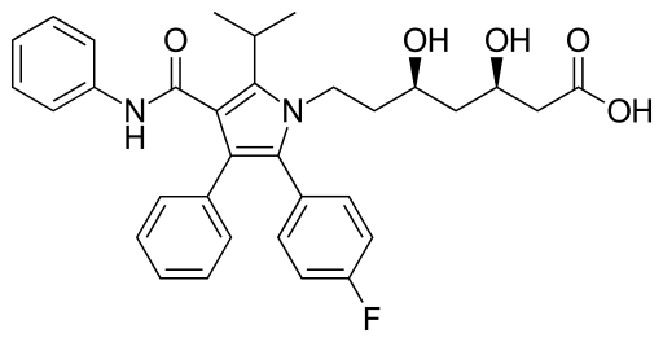
Atorvastatin was first made in August 1985 at Warner-Lambert’s Parke-Davis research facility in Ann Arbor, Michigan by a team led by Bruce Roth. From 1996 to 2012 under the trade name Lipitor, atorvastatin became the world’s best-selling medication to that point, with more than US$125 billion in sales over approximately 14.5 years.
The discovery of the LDL receptor and receptor-mediated endocytosis earned Michael S. Brown (1/2) and Joseph L. Goldstein (1/2) the Nobel Prize in Physiology or Medicine in 1985
Polyunsaturated fatty acid (PUFA) (also known as omega-3 fatty acids) ingestion is correlated with low plasma cholesterol levels.
The mechanisms involved are not completely understood.
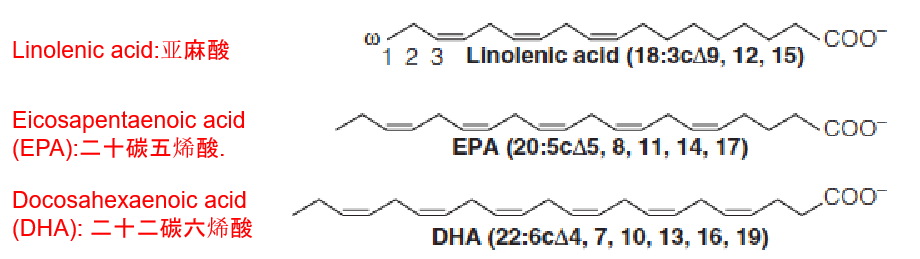
Red meat: rich in both saturated fatty acid and cholesterol;
Fish or fish oils: help reduce cholesterol and TAG
Mobilization of adipose cell triacylglycerols by lipolysis
Three lipases act sequentially to hydrolyze TG to glycerol and FFA.
These enzymes act at the oil–water interface of the lipid droplet.
FFA are exported to the blood plasma, where they are bound to albumin for transport to liver and other tissues for subsequent oxidation.
Glycerol is released to the blood to be taken up by liver cells, where it serves as a gluconeogenic substrate.
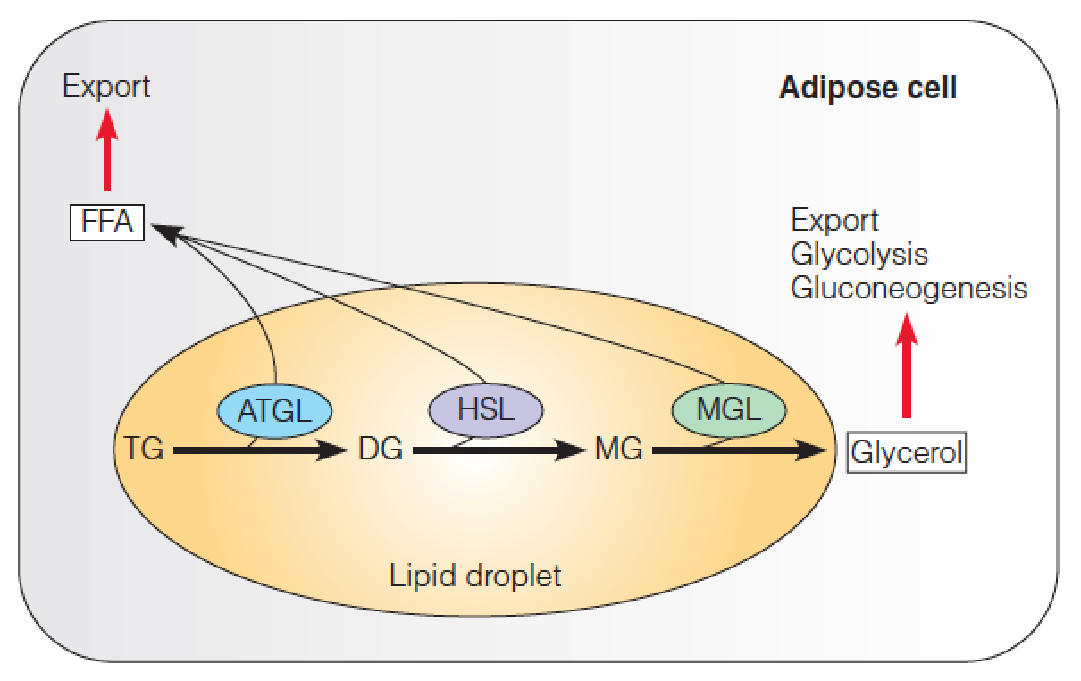
- Adipose triglyceride lipase (ATGL)
- Hormone-sensitive lipase (HSL) (also known as triacylglycerol lipase)
- Monoacylglycerol lipase (MGL)
- FFA: free fatty acid
Control of lipolysis in adipose cells by a cAMP-mediated cascade system
- Hormonal activation of a β–adrenergic G-protein coupled receptor on the plasma membrane leads to elevation of cAMP levels, which in turn, activates protein kinase A (PKA). PKA phosphorylates perilipin (PL) and HSL.
- CGI-58 dissociates from phosphorylated-PL, and binds ATGL.
- Phosphorylated HSL is recruited to the lipid droplet and activated by phosphorylated-PL.
- Phosphorylated-PL also recruits the ATGL/CGI-58 complex to the lipid droplet, activating this lipase.
- Activated ATGL hydrolyzes TG to DG; activated HSL hydrolyzes DG to MG; cytoplasmic MGL hydrolyzes MG to free glycerol + FFA.
Glucagon(胰高血糖素); Epinephrine(肾上腺素); Beta-corticotropin
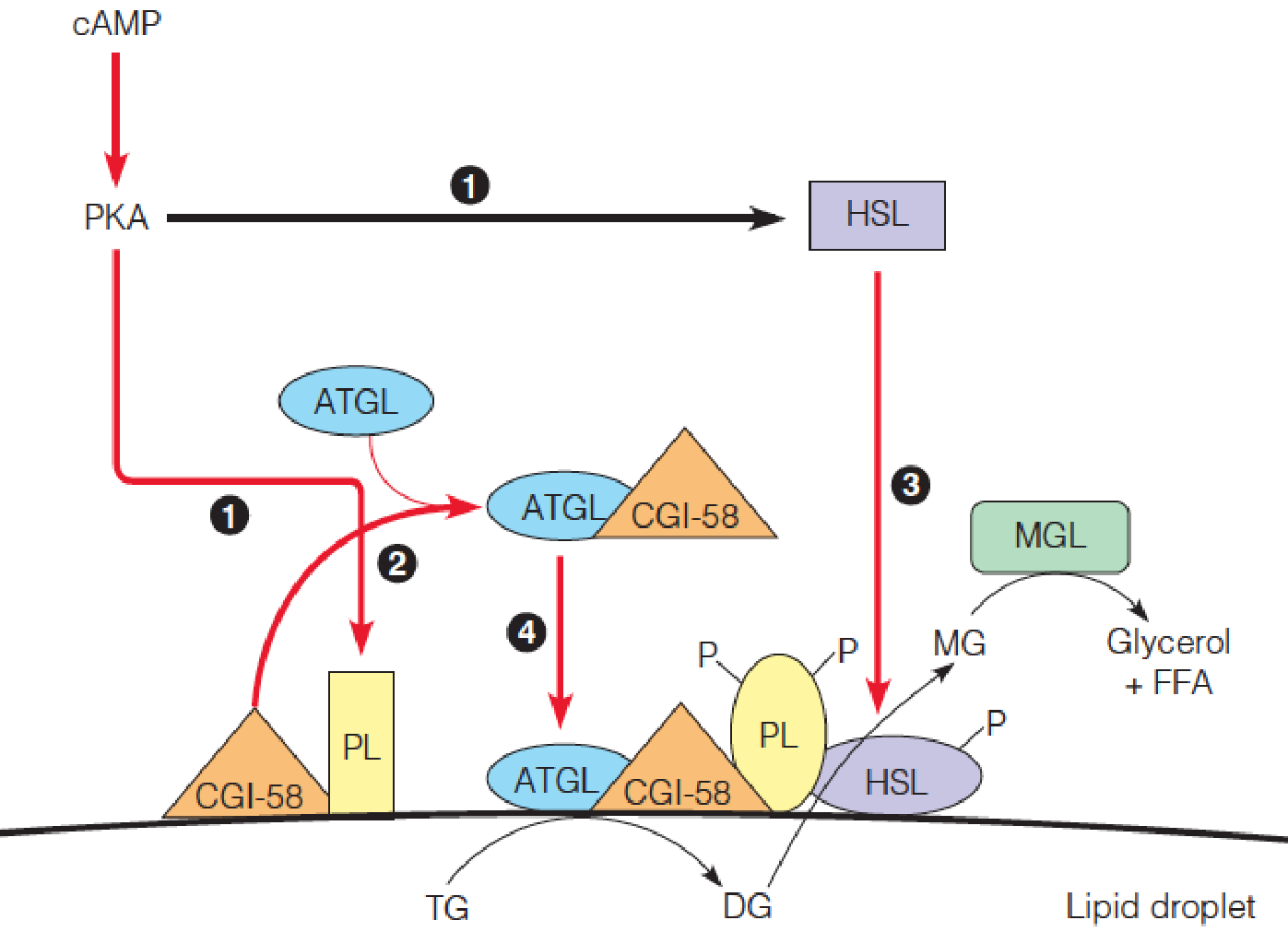
- HSL: hormone-sensitive lipase
- CGI-58: comparative gene identification-CGI58
- ATGL: adipose triglyceride lipase
- DG: diacylglycerol
- MGL: monoglyceride lipase
(2) Knoop’s experiments to determine the β-oxidation of fatty acids
- When dogs fed fatty acids that had an even-numbered carbon chain, the final breakdown product, recovered from urine, was phenylacetic acid (苯乙酸).
- When the fed fatty acid had an odd-numbered chain, the product was benzoic acid (苯甲酸).
- These results led Knoop to propose that fatty acids are oxidized in a stepwise fashion, with initial attack on carbon 3 (the β-carbon with respect to the carboxyl group).
- This attack would release the terminal two carbons, and the remainder of the fatty acid molecule could undergo another oxidation.
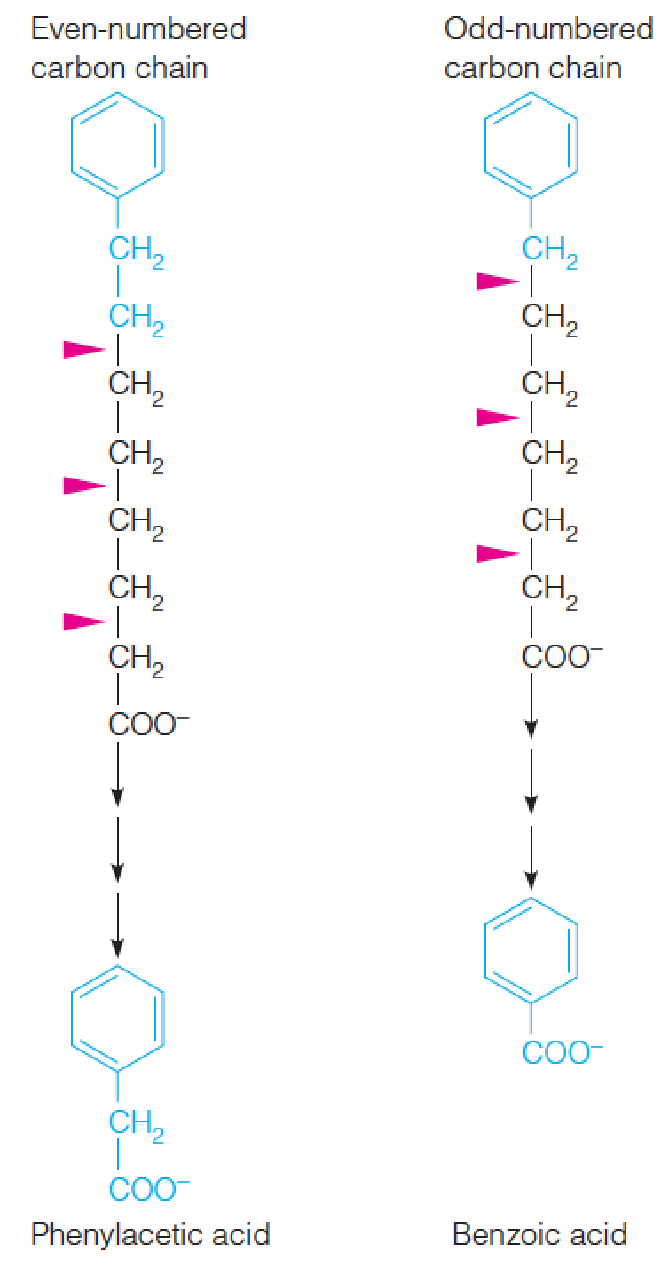
- German chemist Franz Knoop, Metabolic tracers (terminal methyl group of fatty acids was labelled with a phenyl group),1904 (40 years before radioactive tracers); 1940s, Lehninger, ATP is required (activation)…; By the mid-1950s…..
二、Fatty Acid Oxidation
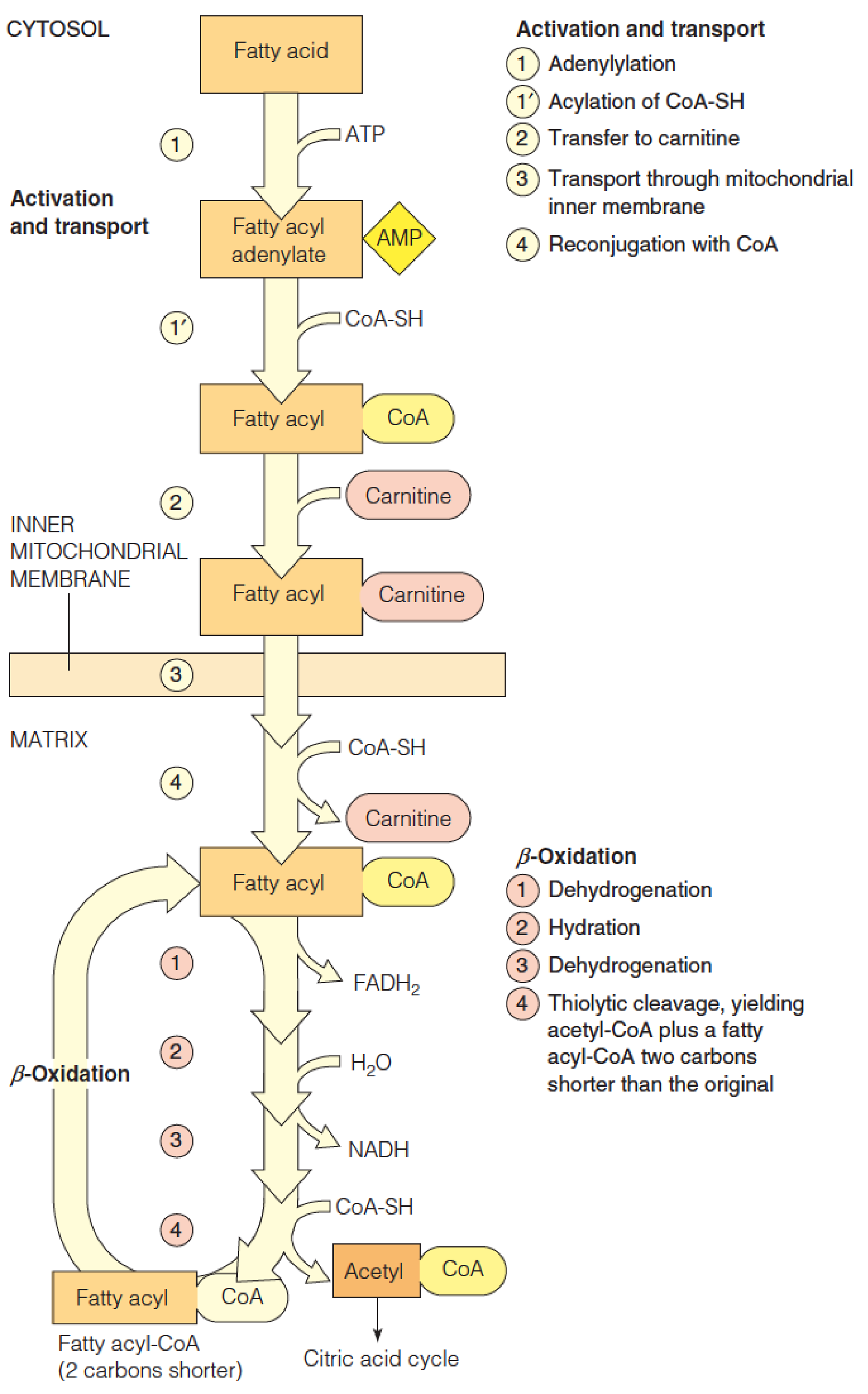
Overview of the fatty acid oxidation pathway
- Fatty acid oxidation and biosynthesis (quite similar in plants, animals and microorganisms).
- Brain cannot use fatty acid, but use glucose, under starvation, use ketone bodies.
Fatty Acid Activation
Fatty acids are activated for oxidation by ATP-dependent acylation of coenzyme A.
The loss of pyrophosphate is equivalent to 2 ATP’s used for activation.
The further hydrolysis of pyrophosphate makes the activation step irreversible.(放出能量很多)
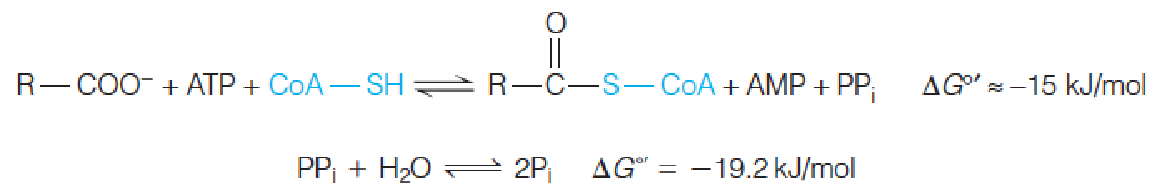
- ER and mitochondrial outside membrane: a series of acyl-CoA synthetases specific for short-chain, medium-chain, or long-chain fatty acids.
1. Mechanism of acyl-CoA synthetase reactions
The figure shows reversible formation of the activated fatty acyl adenylate, nucleophilic attack by the thiol sulfur of CoA-SH on the activated carboxyl group, and the quasi-irreversible pyrophosphatase reaction, which draws the overall reaction toward fatty acyl-CoA.
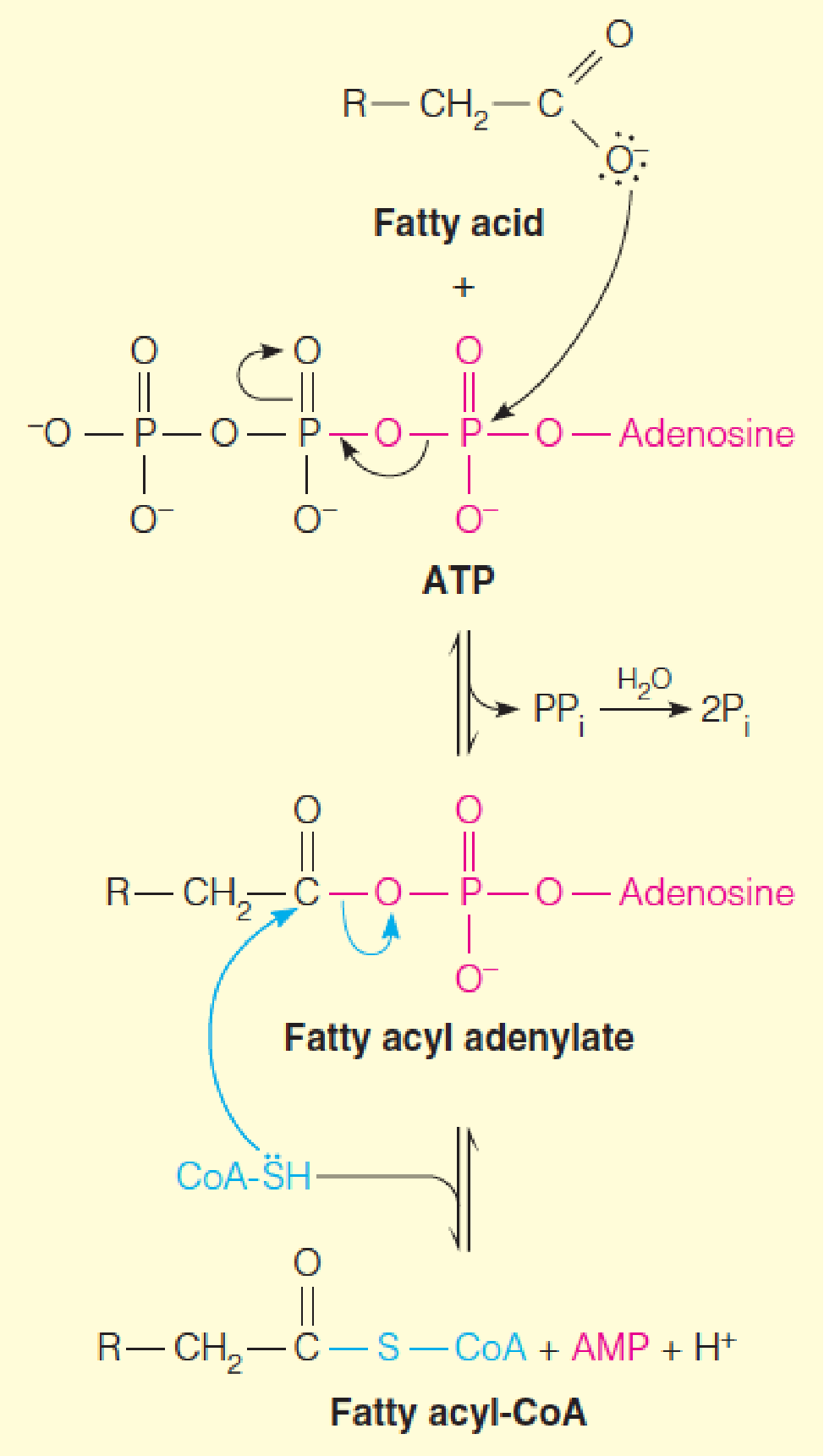
- A two-step mechanism
2. Transport fatty acyl-CoA into mitochondria
Carnitine (肉碱) transports acyl-CoAs into mitochondria for oxidation.
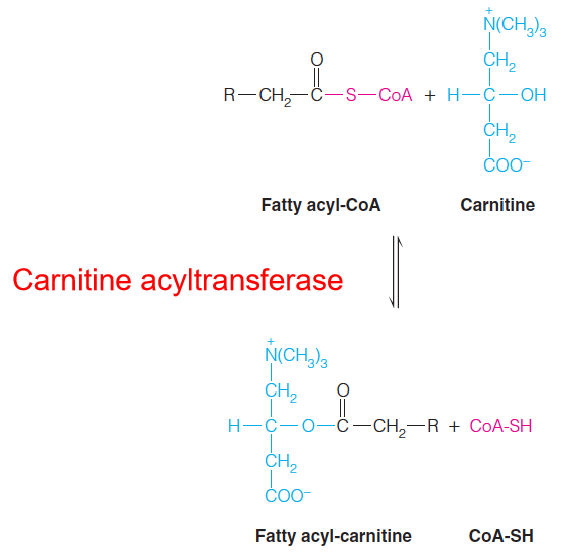
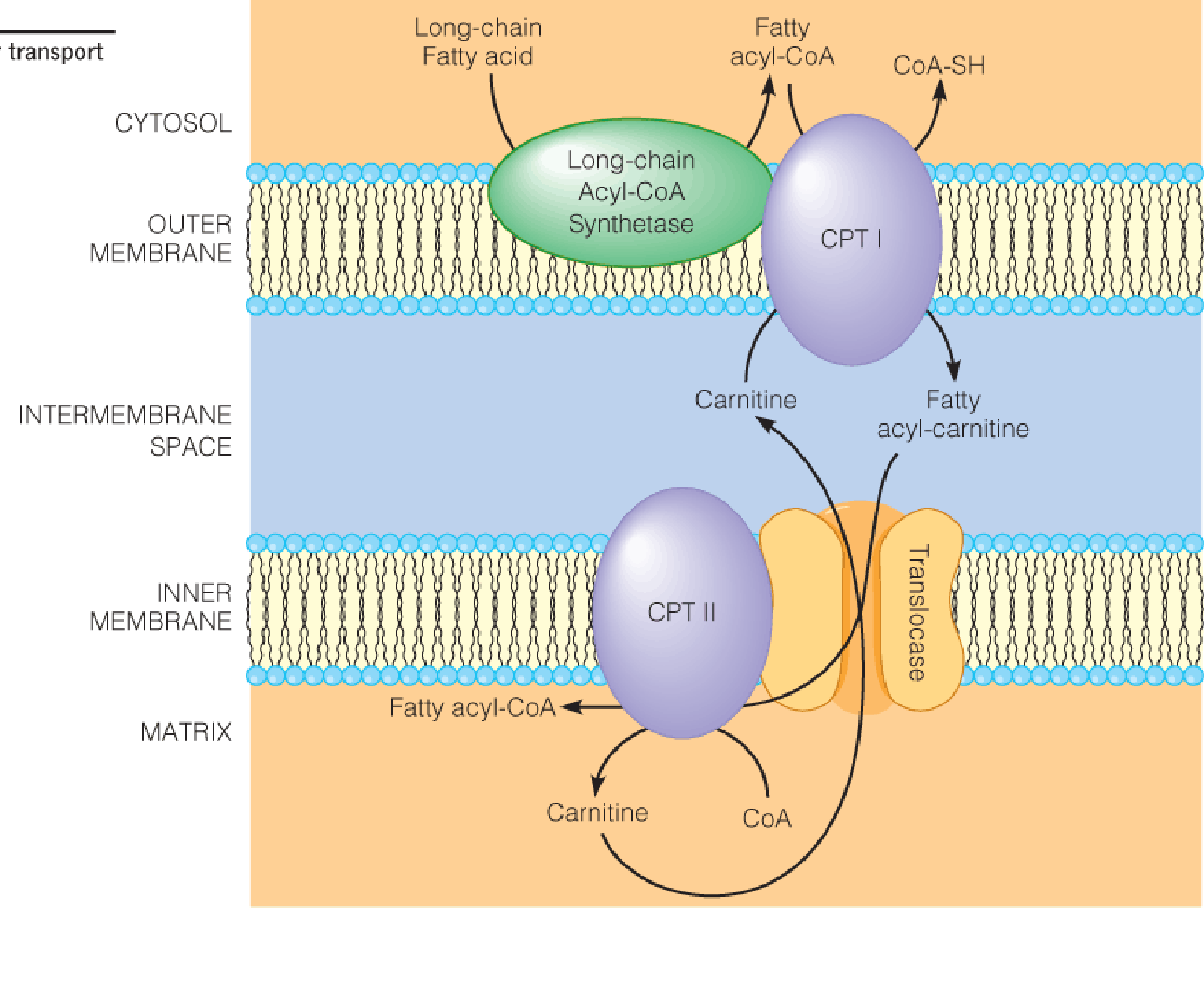
The carnitine acyltransferase system, for transport of fatty acyl-CoAs into mitochondria:
Carnitine acyltransferase I (also called carnitine pamitoyltransferase I (CPT1) ) is strongly inhibited by malonyl-CoA(丙二酰辅酶A), the first committed intermediate in fatty acid synthesis (to avoid “futile cycle”, i.e., oxidation and resynthesis occur at the same time);
CPT II is insensitive to malonyl-CoA;
Entry of fatty acids into mitochondria is rate-limiting for β-oxidation and is the primary point of regulation;
Thus, conditions in the cell that favor fatty acid synthesis prevent the transfer of fatty acyl moieties to their intracellular sites of oxidation and, prevent that oxidation.
3. Outline of the β-oxidation of fatty acids
In the diagram a 16-carbon saturated fatty acyl-CoA (palmitoyl-CoA) undergoes seven cycles of oxidation to yield eight molecules of acetyl-CoA.
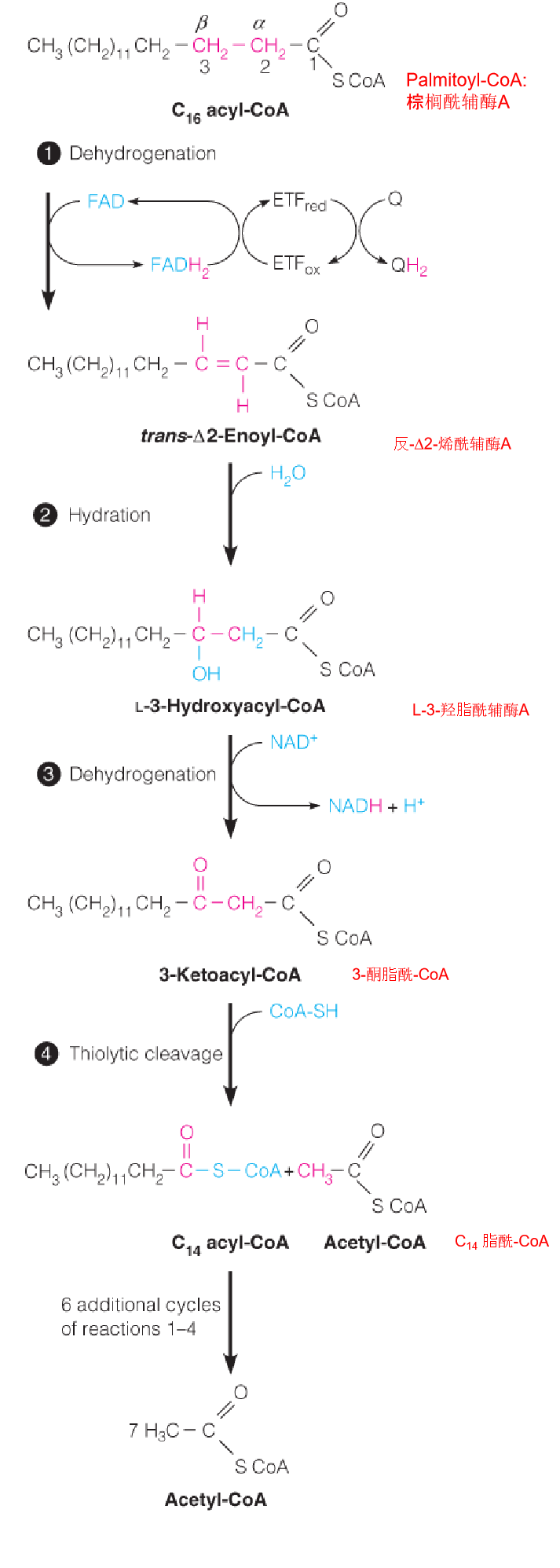
- In mitochondrial matrix
- Four steps;
- The fatty acyl chain is shortened by two carbons at a time;
- Release one acetyl CoA ( go to citric acid cycle)
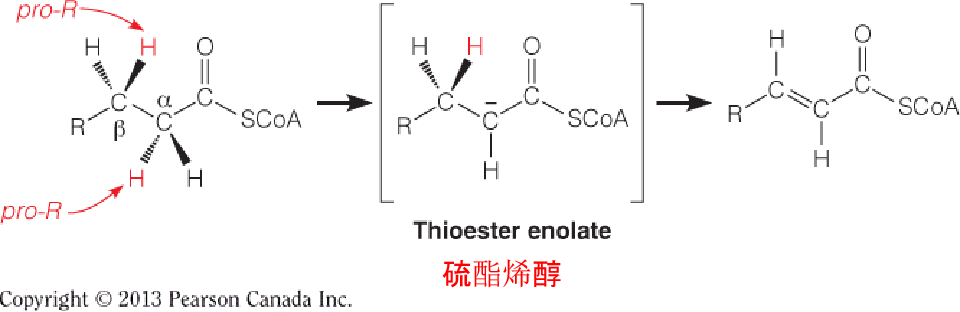
Reaction 1: The Initial Dehydrogenation
The first reaction is catalyzed by an acyl-CoA dehydrogenase, which catalyzes the removal of two hydrogen atoms from the α- and β-carbons to give a trans α, β-unsaturated acyl CoA (trans-D2-enoyl-CoA) as the product.
The pro-R hydrogen on the β-carbon is then transferred as a hydride equivalent to FAD to give the trans double bond and enzyme-bound FADH2.
- 3 acyl-CoA dehydrogenases: for short-, medium-, and long-chain fatty acyl-CoA
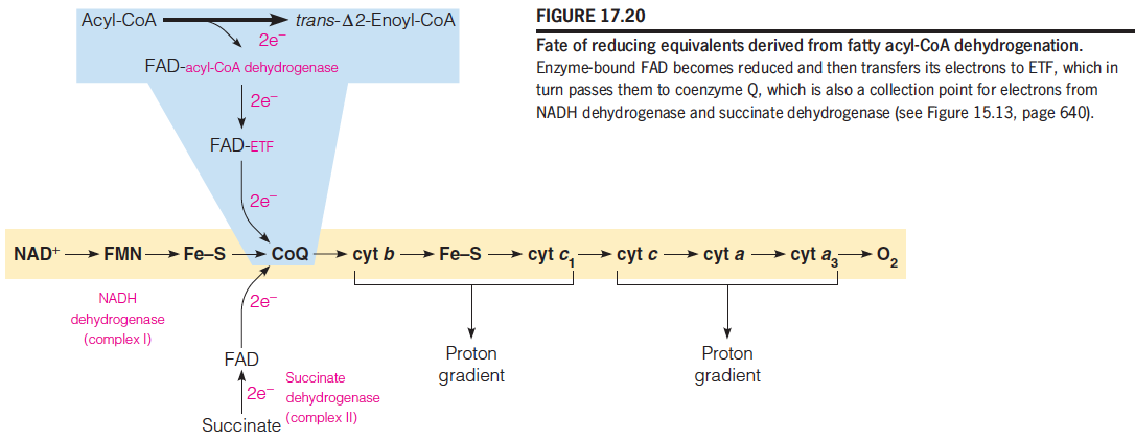
- Prosthetic group: FAD (将电子给FAD)
- Electron transfer flavoprotein (ETF)
- 2H go to CoQ, through respiratory chain, produce 1.5 ATP (like NADH dehydrogenase and succinate dehydrogenase)
Reactions 2 and 3: Hydration and Dehydrogenation
Like succinate oxidation, an initial FAD-dependent fatty acyl-CoA oxidation is followed by hydration and NAD+-dependent dehydrogenation (2.5 ATP);
Enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase, respectively;
Both reactions are stereospecific;
Products: L-β-hydroxyacyl-CoA and b-ketoacyl-CoA, respectively。
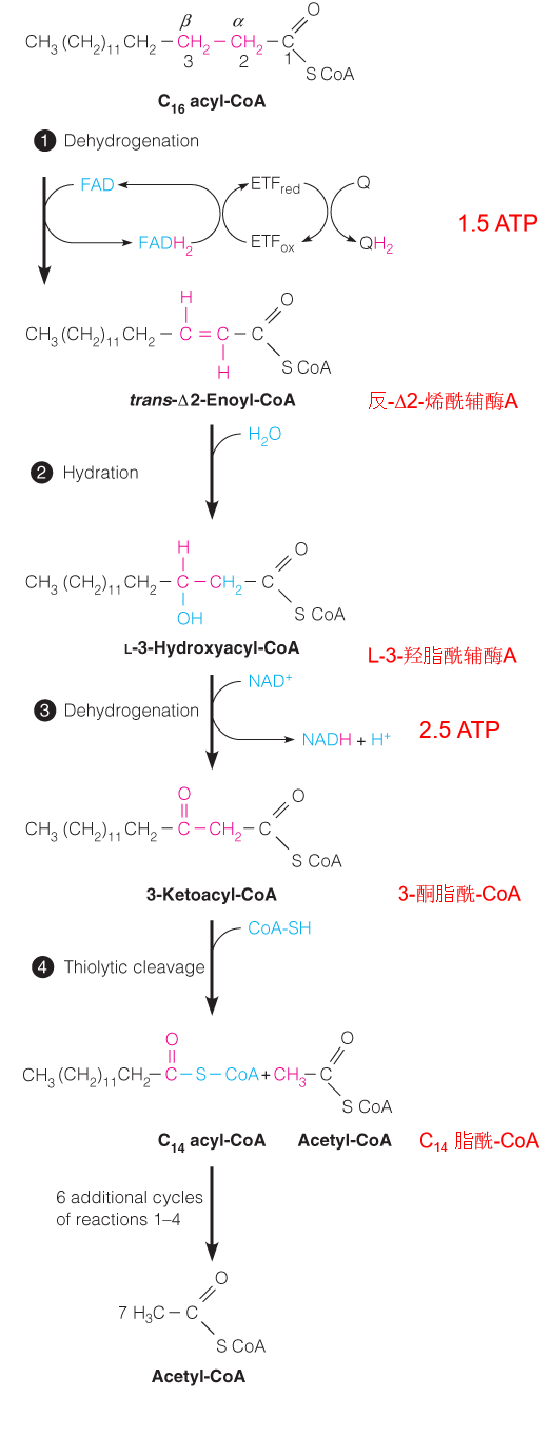
Reaction 4: Thiolytic Cleavage 硫解
The fourth and last reaction in each cycle of the β-oxidation pathway involves attack of the nucleophilic thiol sulfur of coenzyme A on the electron-poor keto carbon of 3-ketoacyl-CoA, with cleavage of the bond and release of acetyl-CoA.
The other product is a shortened fatty acyl-CoA, ready to begin a new cycle of oxidation:
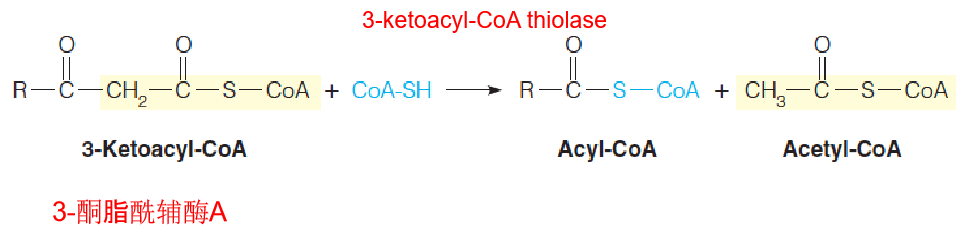
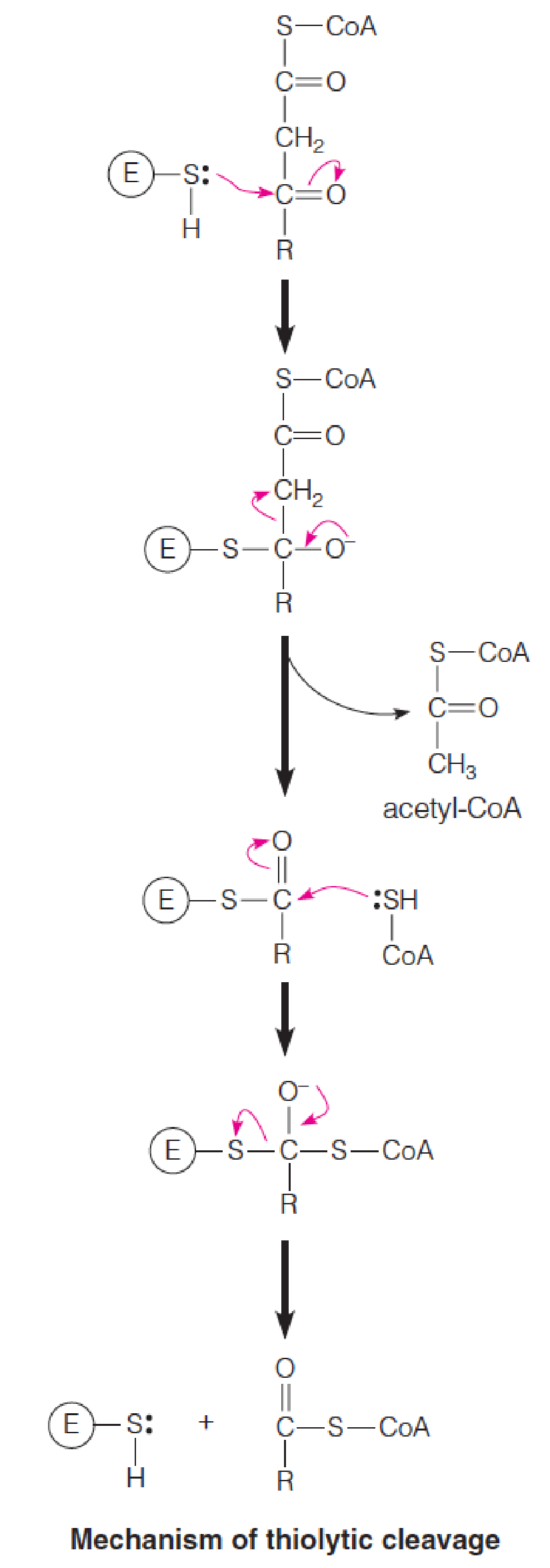
- Nucleophilic thiol sulfur of coenzyme A on the electron-poor keto carbon of 3-ketoacyl-CoA, with cleavage of the α-β bond and release of acetyl-CoA and a shortened fatty acyl-CoA, ready to begin a new cycle of oxidation
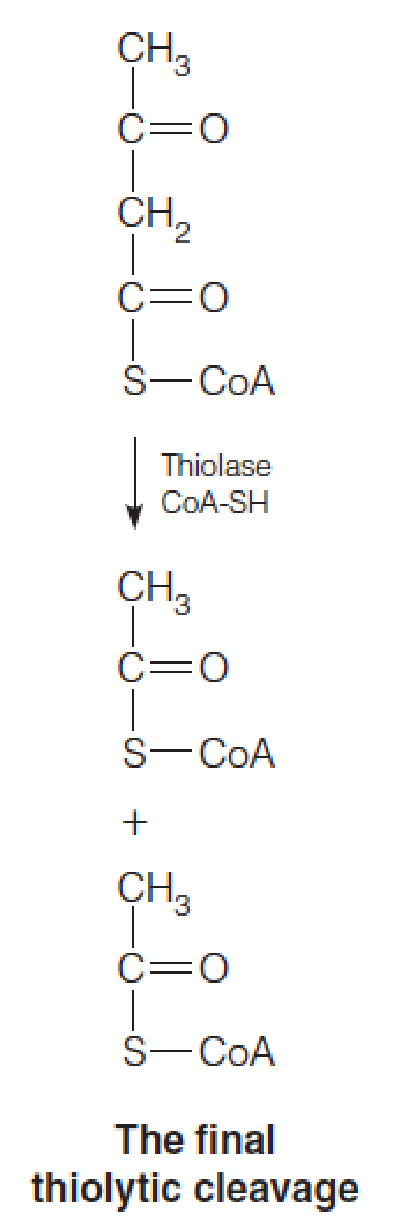
- Fatty acids are oxidized by repeated cycles of dehydrogenation, hydration, dehydrogenation, and thiolytic cleavage, with each cycle yielding acetyl-CoA and a fatty acyl-CoA shorter by two carbons than the input acyl-CoA.
4. Energy Yield from Fatty Acid Oxidation
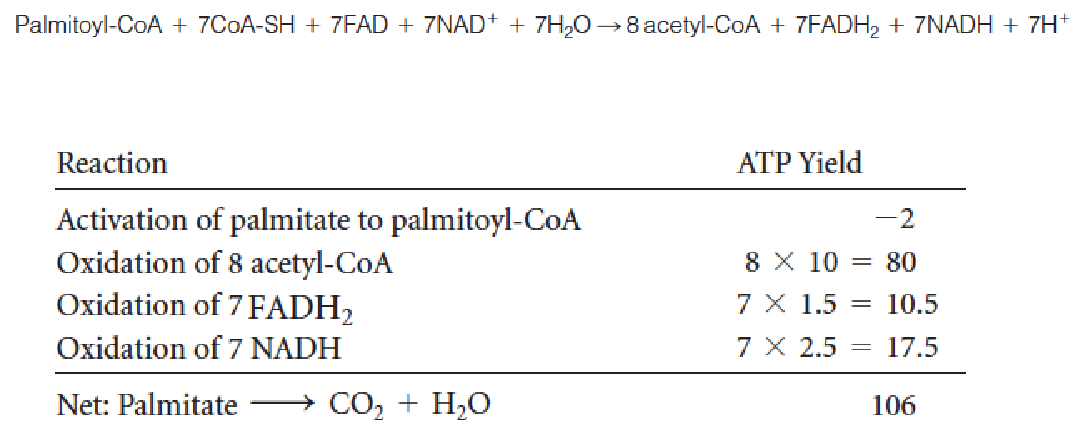
- β-oxidation: even numbers of carbon atoms and fully saturated fatty acids: C16 palmitoyl-CoA: produces 8 acetyl-CoA
β-Oxidation pathway for unsaturated fatty acids
Many natural unsaturated fatty acids contain one or more double bonds: cis configuration
Enoyl-CoA hydratase: acts only on trans compounds
2 additional enzymes, enoyl-CoA isomerase (烯酰-CoA异构酶) and 2,4-dienoyl-CoA reductase (2,4-二烯酰基 - 辅酶A还原酶), must come into play
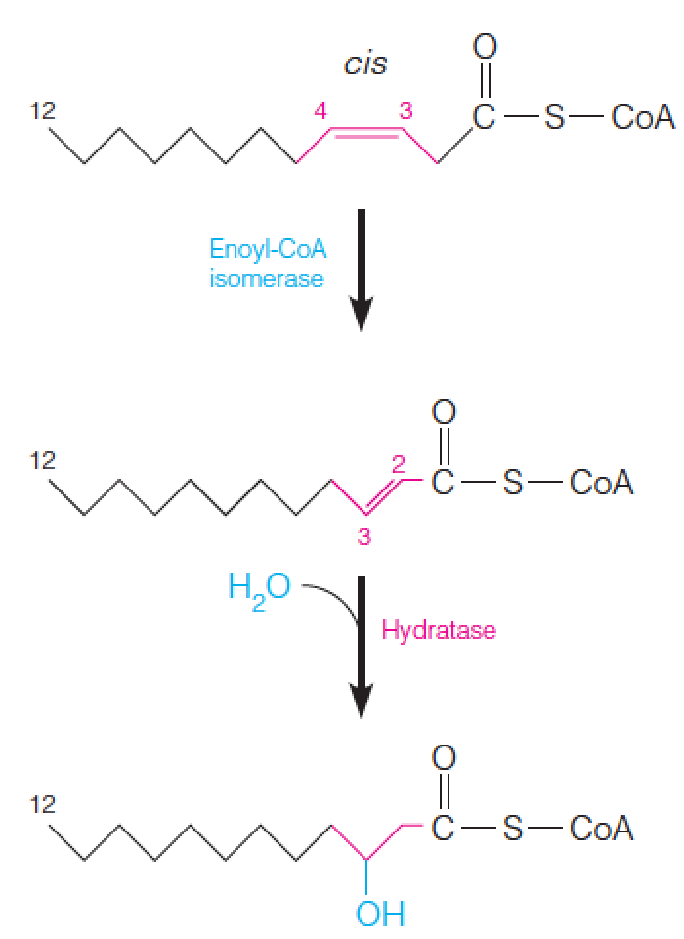
For monounsaturated fatty acid:
- Enoyl-CoA isomerase converts cis-Δ3-enoyl-CoA to the corresponding trans-Δ2-enoyl-CoA
- 需要转换成trans才能进行后续的氧化
- Enoyl-CoA hydratase acts on trans-Δ2-enyol-CoA, all subsequent reactions are identical to those for saturated fatty acids
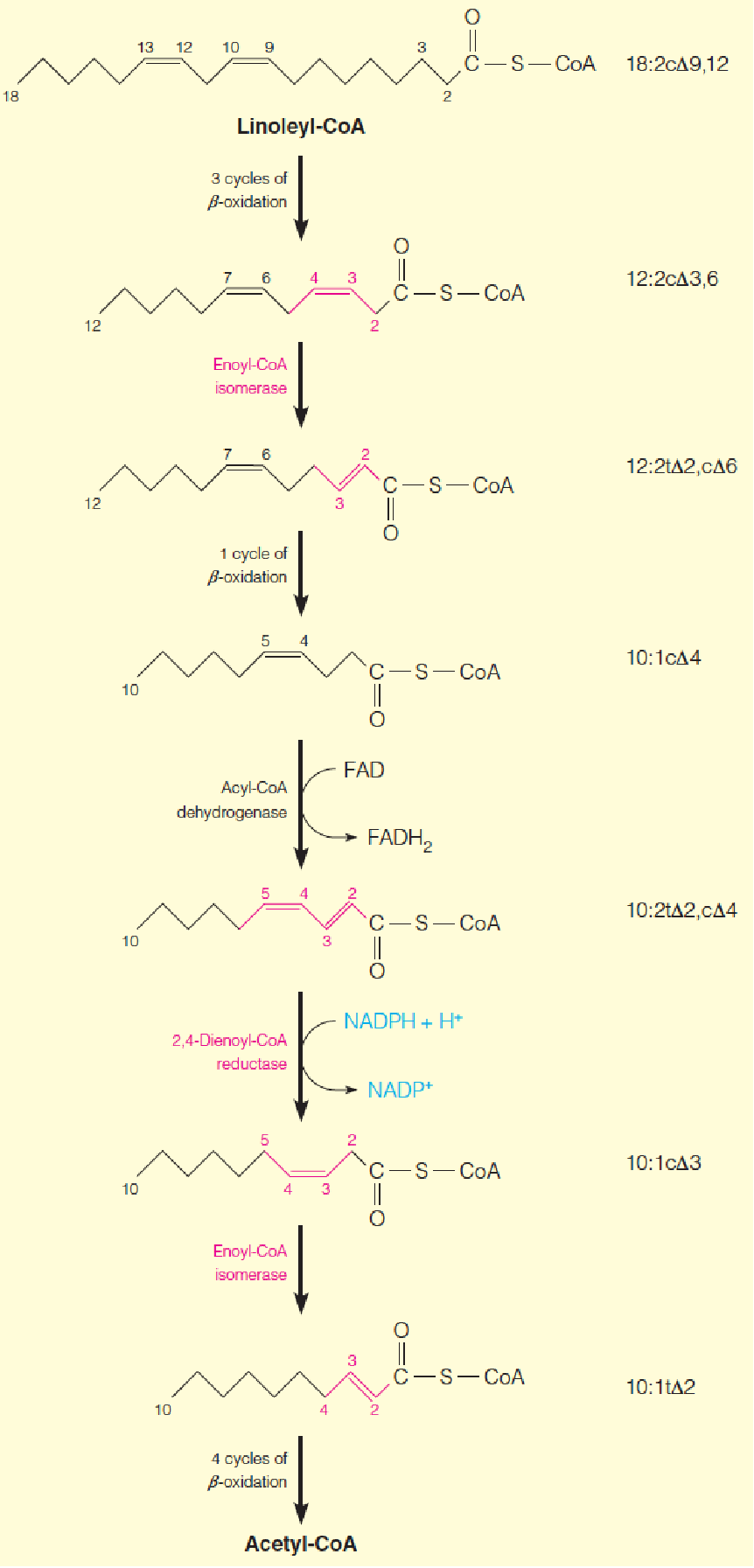
β-Oxidation pathway for polyunsaturated fatty acids
This example, using linoleyl-CoA (亚油酰辅酶A(18C)), shows sites of action of enoyl-CoA isomerase and 2,4-dienoyl-CoA reductase, enzymes specific to unsaturated fatty acid oxidation (identified with red type).
Oxidation of Fatty Acid with Odd-number Carbon Chains
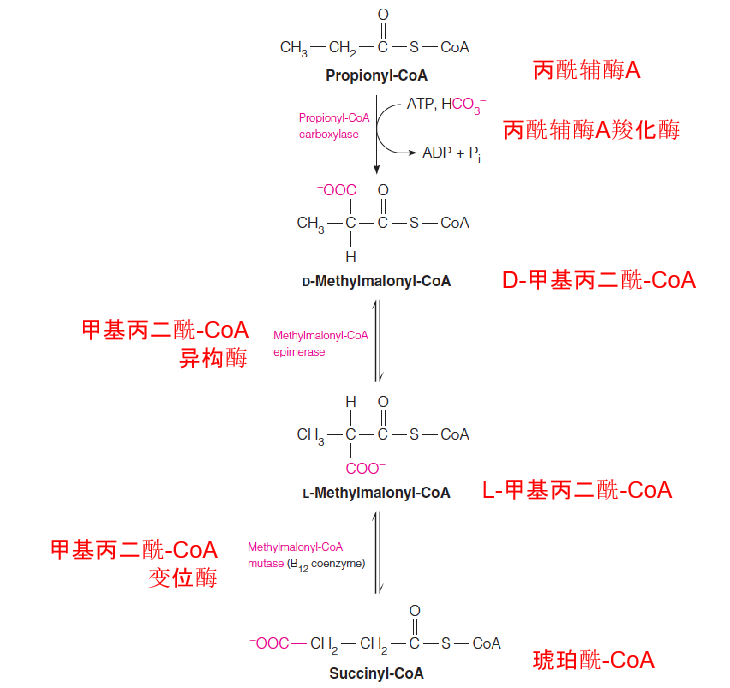
Odd-numbered fatty acid chains yield upon β-oxidation 1 mole of propionyl-CoA, whose conversion to succinyl-CoA involves a biotin-dependent carboxylation and a coenzyme B12–dependent rearrangement.
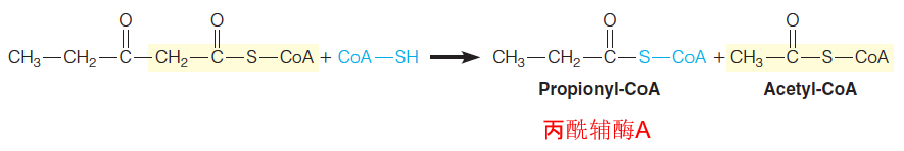
- Most of the fatty acids in natural lipids contain even-numbered carbon chains;
- A small proportion of fatty acids have odd-numbered carbon chains;
- The last step presents a special metabolic problem: five-carbon homolog of acetoactyl-CoA;
- Thiolytic cleavage of this substrate yields 1 molecule of acetyl and propionyl-CoA.
Pathway for catabolism of propionyl-CoA
Control of Fatty Acid Oxidation
In most cells, fatty acid oxidation is controlled by availability of substrates for oxidation, the fatty acids themselves
- Fat mobilization control by hormones (glucagon and epinephrine);
- Malonyl-CoA(丙二酰辅酶A): inhibits fatty acyl-CoA movement into mitochondria by the acylcarnitine shuttle.
- Malonyl-CoA是fatty acid biosyhthesis的重要组分
Ketogenesis 酮体生成
During fasting or starvation, when carbohydrate intake is too low ➔ oxaloacetate (草酰乙酸) levels ⬇ ➔ flux through citrate synthase ⬇ ➔ acetyl-CoA levels ⬆
Under these conditions, 2 moles of acetyl-CoA undergo a reversal of the thiolase reaction to give acetoacetyl-CoA (乙酰乙酰CoA).
Acetoacetyl-CoA can react in turn with a 3rd mole of acetyl-CoA to give 3-hydroxy-3-methylglutaryl-CoA (3-羟基-3-甲基戊二酰-CoA) (HMG-CoA), catalyzed by HMG-CoA synthase.
In the cytosol, HMG-CoA is an early intermediate in cholesterol biosynthesis. In the mitochondria, HMG-CoA is acted on by HMG-CoA lyase (裂解酶) to yield acetoacetate plus acetyl-CoA.
Acetoacetate undergoes NADH-dependent reduction to give D-β-hydroxybutyrate (D-β-羟丁酸) or in very small amounts spontaneous decarboxylation(自动脱羧(不需要酶参与)) to acetone.
Collectively, acetoacetate, acetone, and $\beta$-hydroxybutyrate are called ketone bodies (酮体).
- In cytosol: HMG-CoA ➔ ➔ ➔ ➔ cholesterol (Chapter 19)
- IN mitochondria: HMG-CoA ➔ ➔ ➔ ➔ ketone bodies
1. Biosynthesis of ketone bodies in the liver
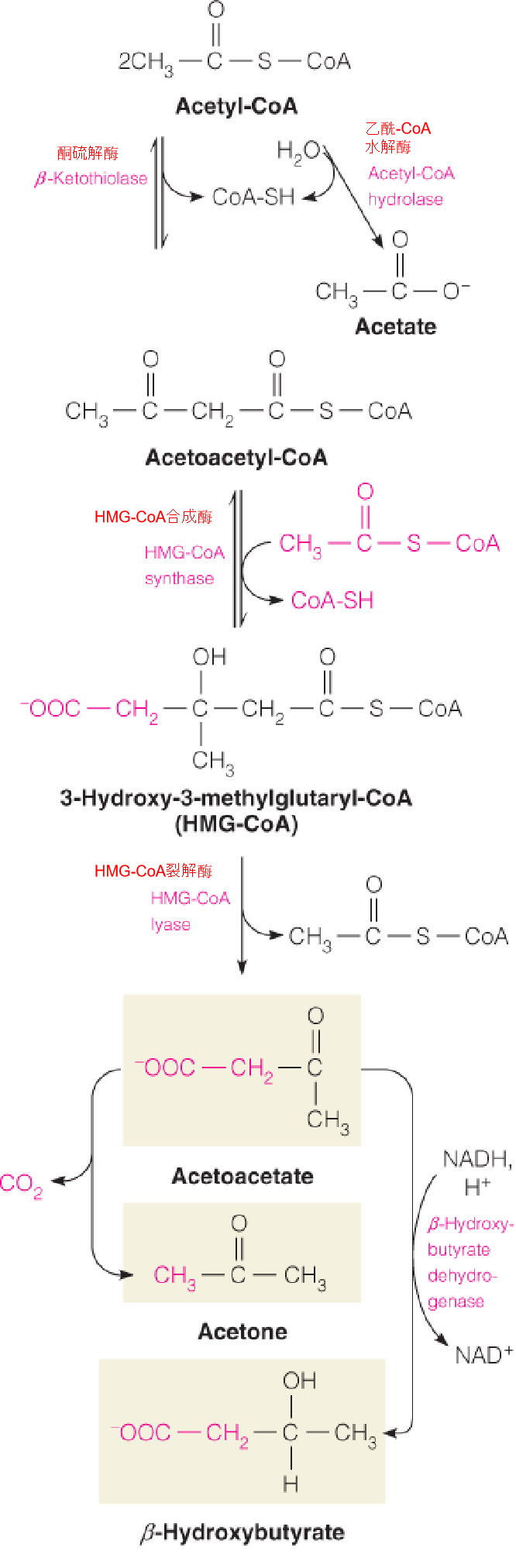
- Ketogenesis occurs primarily in liver, because of the high level of HMG-CoA synthase in that tissue;
2. Use of ketone bodies
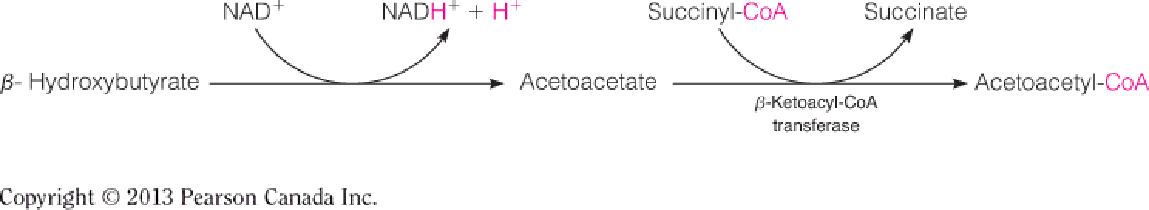
β-Ketoacyl-CoA transferase is present in all tissues except liver so that liver does not compete with the peripheral tissues (brain and heart) for use of ketone bodies;
Acetoacetyl-CoA is converted to acetyl-CoA, which goes to TCA for energy production;
- Ketogenesis becomes extremely important in starvation: brain, heart (Chapter 18);
- Under normal conditions, heart also uses ketone bodies;
- In untreated diabetes, ketone bodies production > the capacity of peripheral tissues to use ➔ ketosis, acidosis and ketoacidosis.
三、Fatty Acid Biosynthesis
Relationship of Fatty Acid Synthesis to Carbohydrate Metabolism
The majority of the stored fuel in most animal cells: fat
A large proportion of the caloric intake of many animal diets, certainly most human diets: carbohydrate
Convert carbohydrate to fat (fatty acid synthesis)
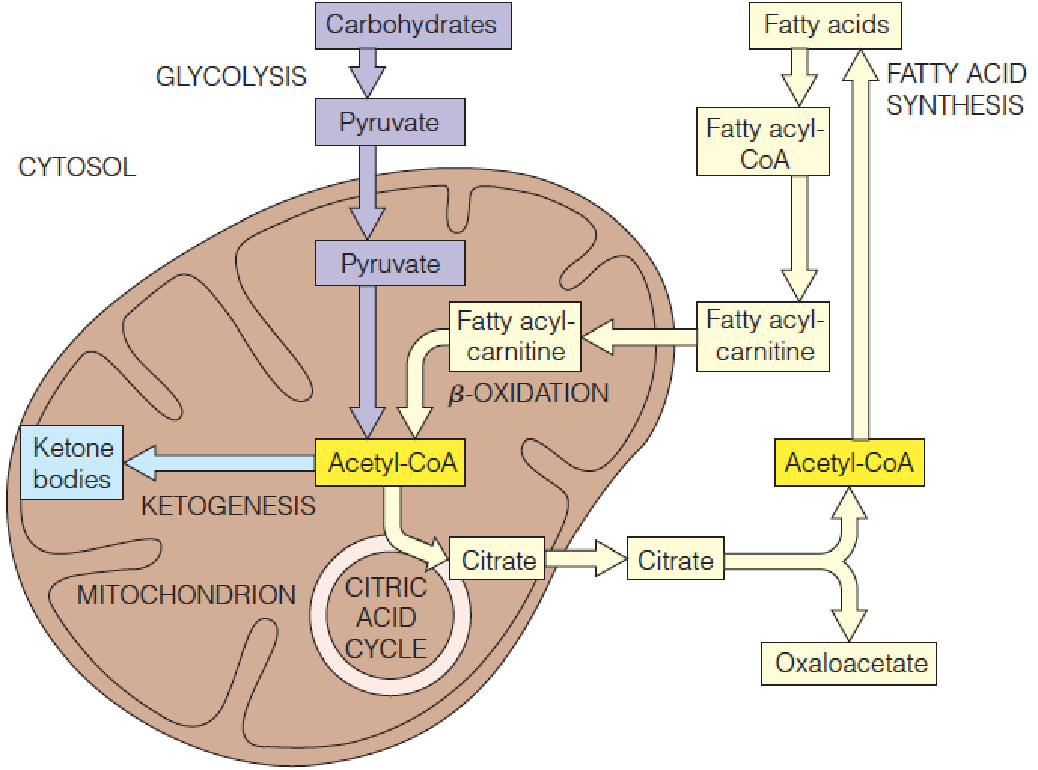
Acetyl-CoA: central metabolite can be from pyruvate and fatty acid oxidation (from carbohydrate and fat breakdown)
In animals, acetyl-CoA cannot undergo net conversion to carbohydrate (because the virtual irreversibility of the pyruvate dehydrogenase reaction)
As noted in Chapter14, the glyoxylate cycle in plants and some microorganisms permits a bypass of this step, with net conversion of acetyl-CoA to gluconeogenic precursors.
- However, in animals the conversion of carbohydrate to fat is unidirectional.
Although fatty acids synthesis is regulated, the total capacity for fat storage is not.
1. Acetyl-CoA as a key intermediate between fat and carbohydrate metabolism
Citrate serves as a carrier to transport acetyl units from the mitochondrion to the cytosol for fatty acid synthesis.
Early Studies of Fatty Acid Synthesis
Early in the twentieth century, most fatty acids in lipids contain even-numbered chains; it is reasonable to expect that the biosynthetic process involves some stepwise addition of activated two-carbon fragments;
This process was demonstrated experimentally in the 1940s using isotopic tracers;
David Rittenberg and Konrad Bloch (1940s): fed to mice with acetate labeled with the stable isotopes13C and deuterium (^13^C^2^H3C^13^COO-) and found both isotopes incorporated into fatty acids;
Once the β-oxidation pathway had been discovered, it was generally thought that fatty acid synthesis would simply by a reversal of its degradation pathway;
In the late 1950s, FA synthesis absolutely requires bicarbonate, which was not in the final product. This led to the discovery of malonyl-CoA (丙二酰-CoA), the first committed intermediate;
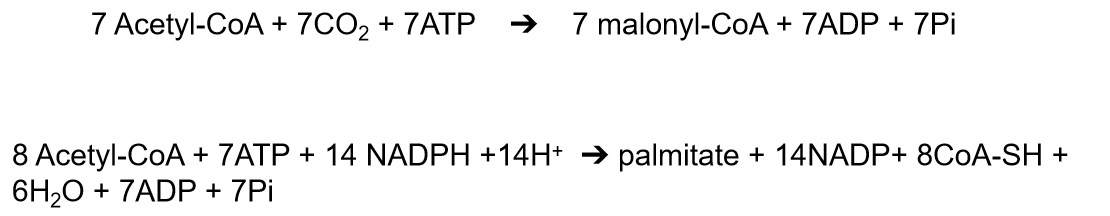
Biosynthesis of Palmitate (棕榈酸) from Acetyl-CoA
Malonyl-CoA to Palmitate
Successive addition of two-carbon units. Each cycle contains 7 steps:
- acetyl-CoA carboxylase (ACC)
- malonyl-CoA-ACP transferase (MAT)
- acetyl-CoA-ACP transferase
- condensation (KS)
- reduction
- dehydration
- reduction
- ACP is the acyl carrier for synthesis
- NADPH is the electron for both reduction reactions
7 Acetyl-CoA + 7CO2 + 7ATP ➔ 7 malonyl-CoA + 7ADP + 7Pi
8 Acetyl-CoA + 7ATP + 14 NADPH +14H+ ➔ palmitate + 14NADP+ 8CoA-SH + 6H2O + 7ADP + 7Pi
- Needs energy (ATP)
- Reducing equivalent (NADPH)
Although the chemistries of fatty acid synthesis and degradation are similar, the two pathways differ in:
- the enzymes involved
- acyl group carriers: Acetyl-CoA vs ACP (acyl carrier protein)
- stereochemistry of the intermediates: L vs D configuration
- electron carriers: FAD/NAD+ vs NADPH
- intracellular location: cytosol ➔ mitochondria vs cytosol
- regulation.
1. Synthesis of malonyl-CoA (丙二酰-CoA)
Synthesis of malonyl-CoA is the first committed step in fatty acid biosynthesis from acetyl-CoA and bicarbonate, catalyzed by acetyl-CoA carboxylase (ACC).
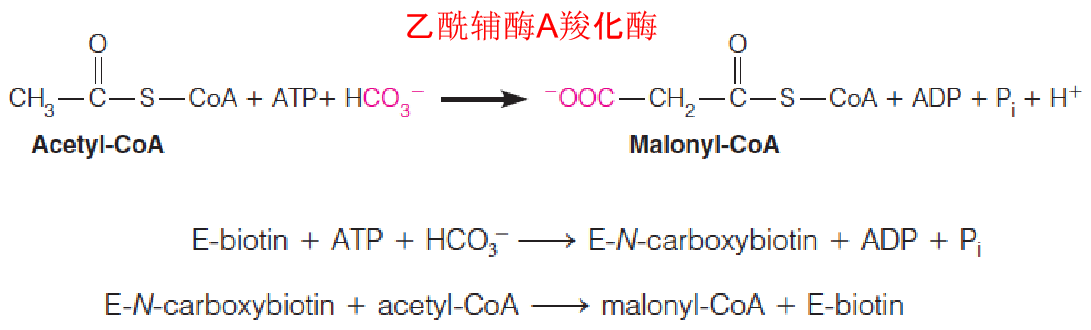
2. Acetyl-CoA carboxylase (ACC)
Biotin cofactor: bound a lysine epsila-amino group, covalently bound N-carboxybiotin intermediate;
The first committed step in the synthetic pathway;
This reaction is so exergonic (放能) as to be virtually irreversible;
Prokaryotic E.coli: three separate proteins:
- a small carrier protein that contains the bound biotin;
- a biotin carboxylase, which catalyzes the formation of N-carboxybiotin;
- a transcarboxylase, that transfers the activated carboxyl group from N-carboxybiotin to acetyl-CoA;
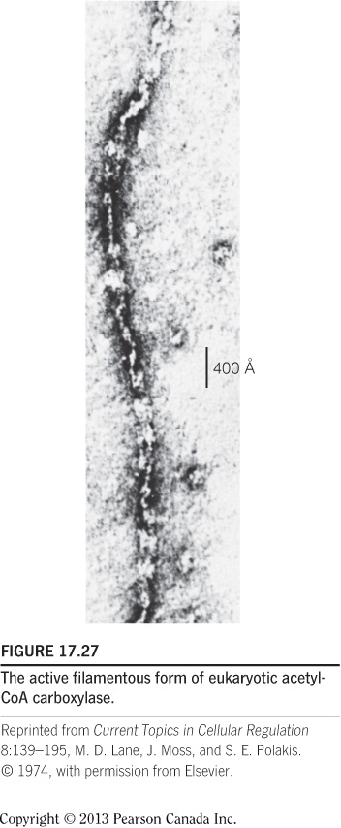
Eukaryotes: a single protein containing two identical polypeptide chains. Inactive dimers; Active filamentous form (can be seen under electron microscope): in the presence of citrate;
- The balance between the two forms: regulation of fatty acids synthesis;
Primary regulator: long-chain fatty acyl-CoAs (not citrate).
3. Chemical similarities between oxidation and synthesis of a fatty acid
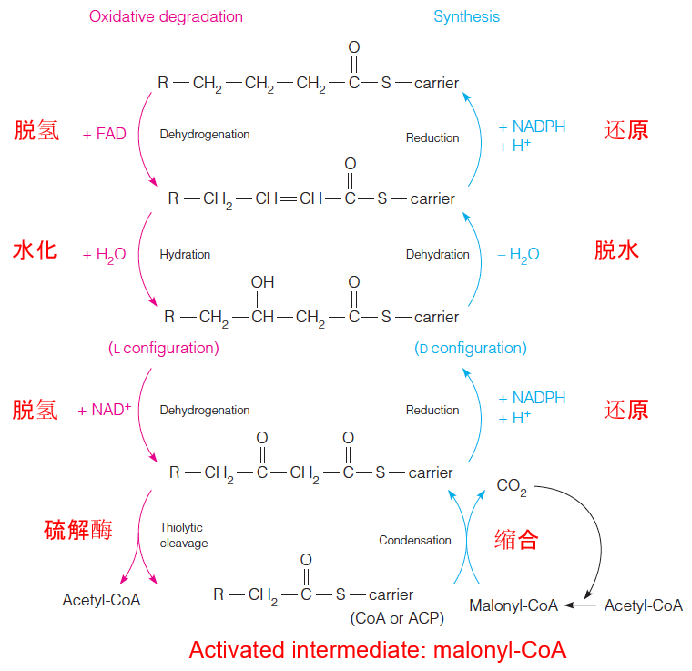
- This is one of the best examples of the statement that anabolic pathways are never the simple reversal of catabolic pathways.
4. Acyl Carrier Proteins(ACP)
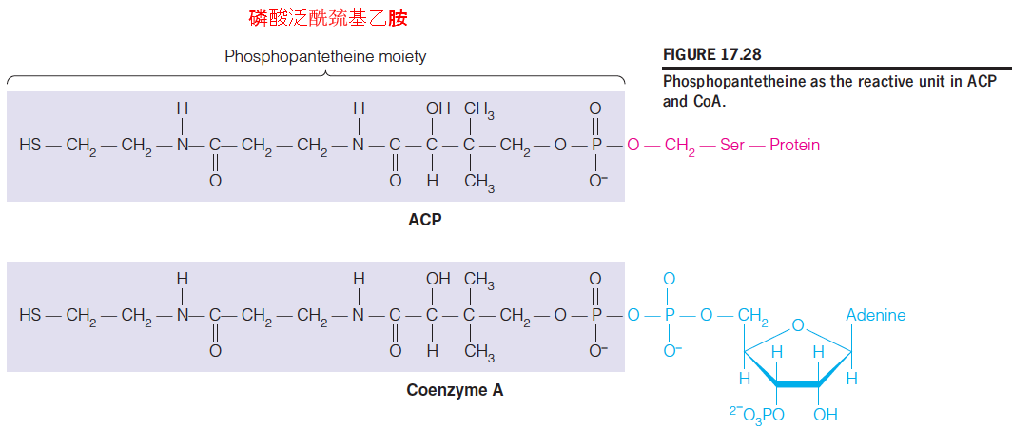
All of the intermediates in fatty acid oxidation are activated via their linkage to a carrier molecule, coenzyme A;
In fatty acid synthesis, a similar activation, the carrier is a small protein called acyl carrier protein (ACP)
The chemistry of activation is identical to that in acyl-CoAs;
A phosphopantetheine moiety, derived from pantothenic acid (泛酸), is linked to a serine group in the polypeptide.
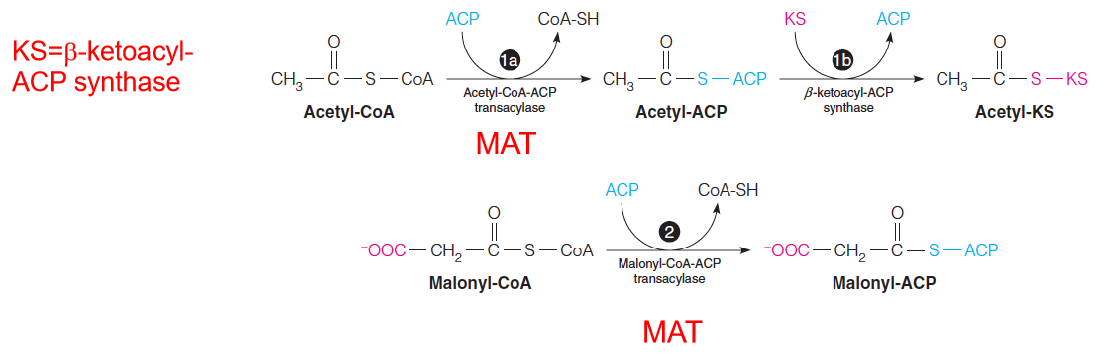
- Malonyl/acetyl-CoA-ACP transacylase (MAT) loads fatty acid synthase with substrates.
- The acyl groups (acetyl or malonyl) are transferred from the SH group of CoA to the SH group of the phosphopantetheine moiety of acyl carrier protein (ACP).
- The reactions shown here produce acetyl-KS and malonyl-ACP, which are used in the remaining reactions of the cycle.
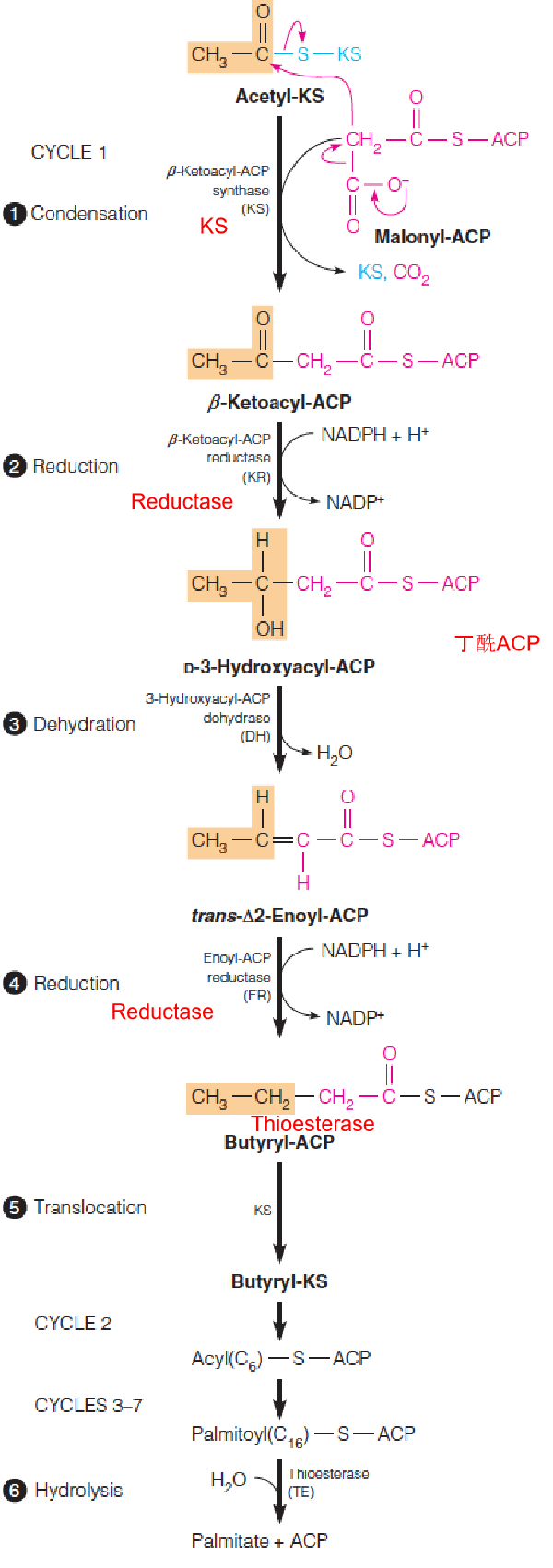
Synthesis of palmitate, starting with malonyl-ACP and acetyl-KS
The first cycle of four reactions generates butyryl-ACP (丁酰ACP).
Following translocation from ACP, butyryl-KS reacts with a second molecule of malonyl-ACP, leading to a second cycle of two-carbon addition.
A total of seven such cycles generates palmitoyl-ACP.
Hydrolysis of this product releases palmitate by thioesterase (TE).
- NADPH provides the electron for both reduction reactions; NADPH 来自PPP pathway
- D-3-hydroxyacyl-CoA–synthesis
- L-3-hydroxyacyl-CoA–fatty acid oxidation
Malonyl-CoA to Palmitate
Successive addition of two-carbon units. Each cycle contains 7 steps:
- acetyl-CoA carboxylase (ACC)
- malonyl-CoA-ACP transferase (MAT)
- acetyl-CoA-ACP transferase
- condensation (KS)
- reduction
- dehydration
- reduction
- ACP is the acyl carrier for synthesis
- NADPH is the electron for both reduction reactions
- Needs energy (ATP)
- Reducing equivalent (NADPH)
Although the chemistries of fatty acid synthesis and degradation are similar, the two pathways differ in:
- the enzymes involved
- acyl group carriers: Acetyl-CoA vs ACP (acyl carrier protein)
- stereochemistry of the intermediates: L vs D configuration
- electron carriers: FAD/NAD+ vs NADPH
- intracellular location: cytosol ➔ mitochondria vs cytosol
- regulation.
5. Structure and swinging arm mechanism in the mammalian fatty acid synthase complex
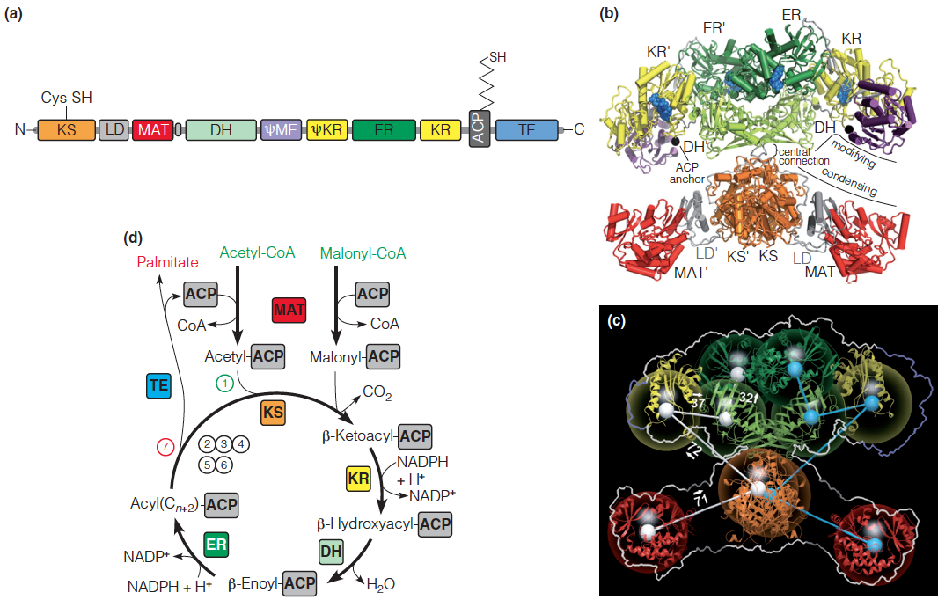
Malonyl-CoA represents an activated source of two-carbon fragments for fatty acid biosynthesis, with the loss of CO2 driving C-C bond formation.
In eukaryotes, fatty acid synthesis is carried out by a megasynthase, an organized multienzyme complex that contains multifunctional proteins.
6. Transport of acetyl units and reducing equivalents used in fatty acid synthesis
Citrate serves as a carrier of two-carbon fragments from mitochondria to cytosol for fatty acid biosynthesis.
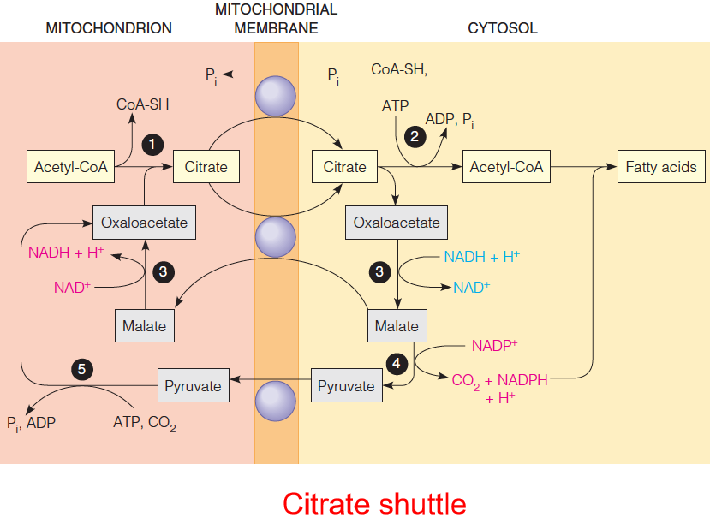
- 1 = citrate synthase
- 2 = citrate lyase
- 3 = malate dehydrogenase
- 4 = malic enzyme
- 5 = pyruvate carboxylase (使得Pyruvate → OA)
Because acetyl-CoA is generated in the mitochondrial matrix, it must be transported to the cytosol for use in fatty acid synthesis.
Like longer-chain acyl-CoA, acetyl-CoA cannot penetrate the inner membrane
Elongation of Fatty Acid Chains
Fatty acid synthase actions lead primarily to palmitate;
In eukaryotic cells: elongation occurs in both mitochondria and endoplasmic reticulum (microsomal elongation system).
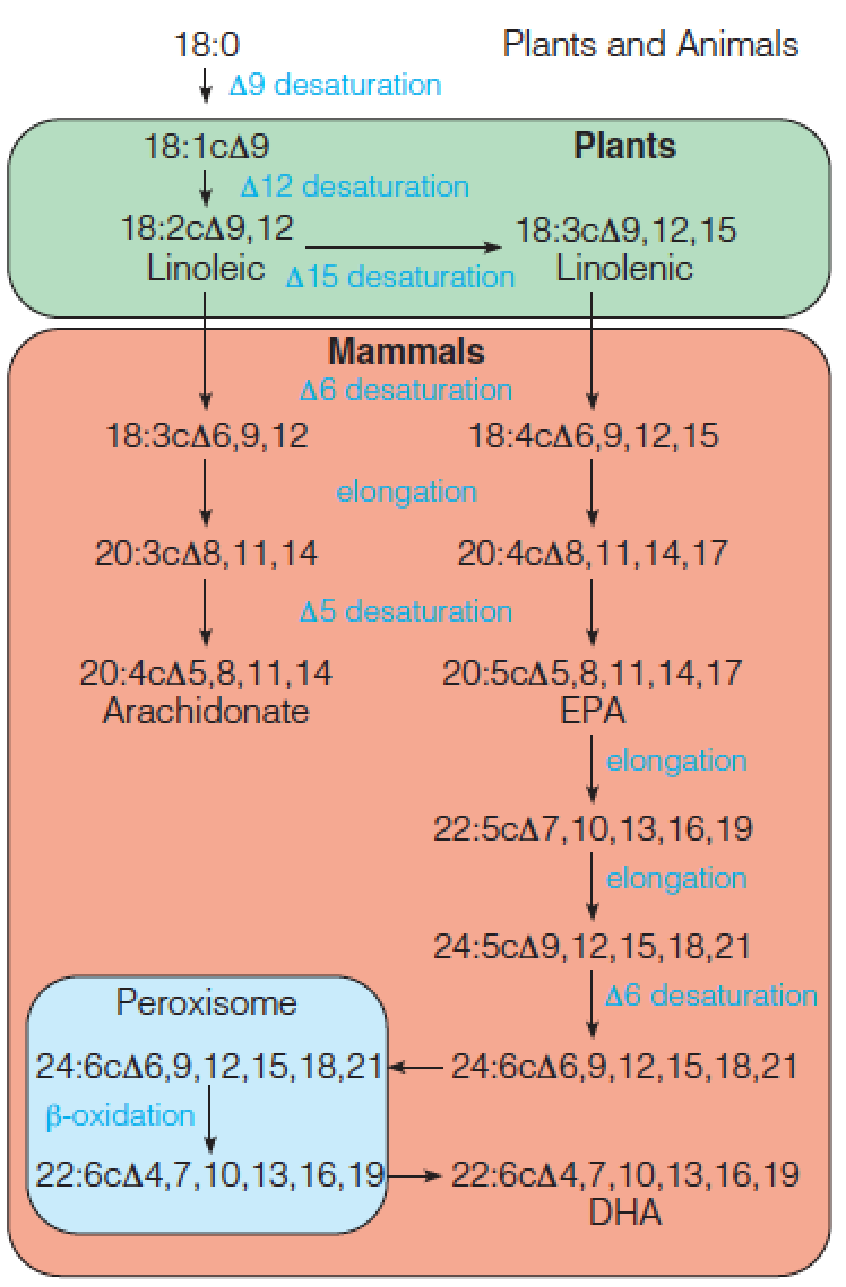
Fatty Acid Desaturation 去饱和
The most common monounsaturated fatty acids in animal lipids are oleic acid (油酸) (18:1cΔ9 acid) and palmitoleic acid (棕榈油酸) (16:1cΔ9 acid), synthesized from stearate and palmitate by a microsomal system called fatty acyl-CoA desaturase;
Mammals are unable to introduce double bonds beyond 9 in the fatty acid chain. They cannot synthesize either linoleic acid (亚油酸) (18:2cΔ9, 12) and linolenic acid (亚麻酸) (18:3cΔ9,12,15). There are called essential fatty acids: must be provided in the diet;
Arachidonate acid (花生四烯酸酸): precursors of eicosanoids (类花生酸) (prostaglandins (前列腺素类) and thromboxanes (血栓烷)).(Chapter 19)
Pathway for synthesis of polyunsaturated fatty acids (PUFAs) in plants and animals
Regulation of fatty acid synthesis in animal cells
The rate-limiting enzyme, acetyl-CoA carboxylase (ACC), is controlled by both allosteric (citrate and long-chain fatty acids) and covalent modification mechanisms.
- Phosphorylation by AMP-activated protein kinase (AMPK, AMP活化的蛋白激酶) or cyclic AMP–dependent protein kinase (PKA) inactivates ACC.
- Insulin stimulates fatty acid synthesis by increasing glucose uptake and increasing flux through pyruvate dehydrogenase (PDH) to produce acetyl-CoA.
- The dephosphorylated form of PDH is the enzymatically active form.
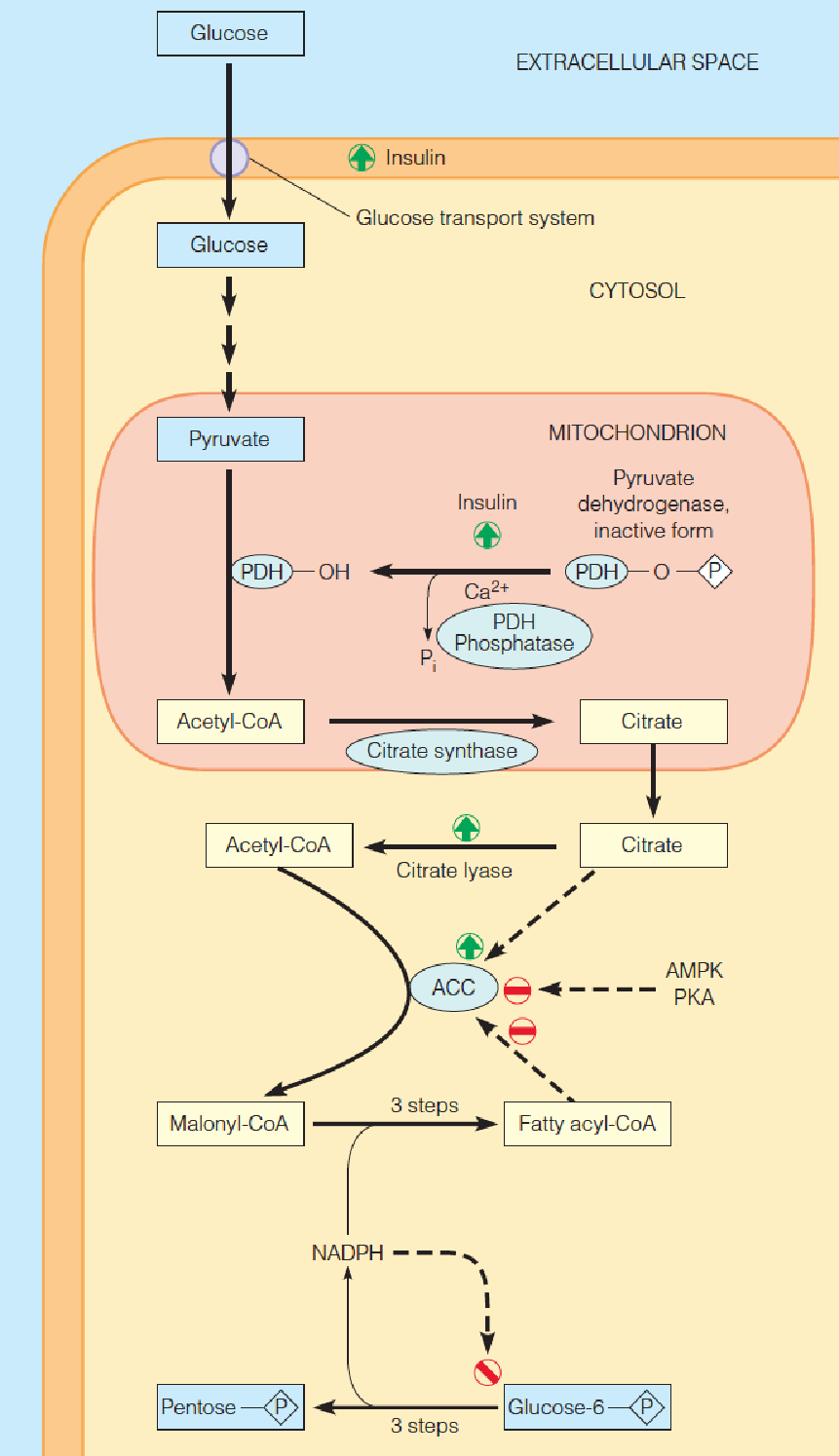
Control of Fatty Acid Synthesis
Much of the fatty acid synthesis in animals takes places in adipose tissues;
Hormonal mechanism: major;
Insulin:
- Insulin ➜ glucose entry into cells ↑➜ acetyl-CoA↑;
- Insulin ➜ activates pyruvate dehydrogenase (PDH) ↑➜ acetyl-CoA↑;
- Insulin ➜ activates citrate lyase ↑➜ the level of citrate in mitochondrial matrix↑ ➜ acetyl-CoA in cytosol↑;
Fasting/starvation ➜ AMPK ↑ PKA ↑ ➜ inactivation of Acetyl-CoA carboxylase (ACC) (covalent modification);
Long-chain fatty acyl-CoA➜ ACC polymerization↓➜ ACC activity ↓(allosteric regulation);
Citrate➜ ACC polymerization ↑ ➜ ACC activity ↑(allosteric regulation)
Availability of NADPH: from both the transport of citrate out of mitochondria and the pentose phosphate pathway (PPP) (60%).
- AMPK (AMP-activated protein kinase): activated by increased cellular AMP:ATP ratio (fasting/starvation);
- AMPK acts as a sensor of cellular energy status;
- AMPK and PKA phosphorylate and inhibits ACC
四、Biosynthesis of Triacylglycerols
Glycerolipid/free fatty acid cycle and glyceroneogenesis 甘油再生
Mammals hydrolyze and resynthesize TG in a glycerolipid/free fatty acid (GL/FFA) cycle.
FFA are released from TG by lipases acting on lipid droplets.
- ATGL, adipose triglyceride lipase;
- HSL, hormone-sensitive lipase;
- MGL, monoacylglycerol lipase.
Some of the FFAs are released into the blood for transport and oxidation, but ~75% are re-esterified back to TG.
ATP hydrolysis in this futile cycle is shown in red.
- GPAT: glycerolphosphate acyltransferase;
- LPA: lysophosphatidic acid
- PA: phosphatidic acid
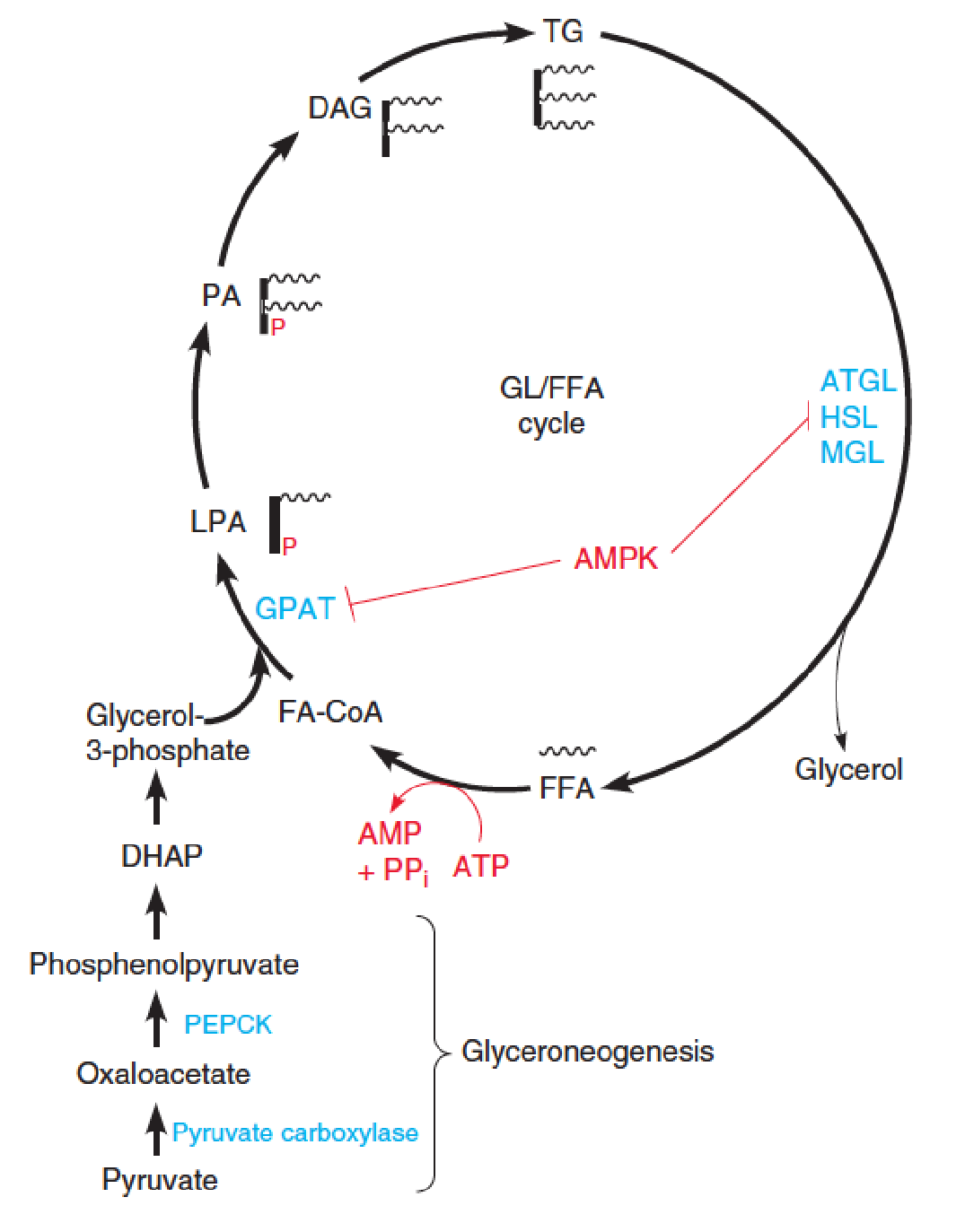
- Major precursors (TG): fatty acyl-CoAs and glycerol-3-phosphate (limiting substrate)
- Adipose tissue, liver, heart
- 需要一个GAP,它由DHAP转化而来,是糖代谢(糖异生)的产物
Glycerolipid/free fatty acid cycle and glyceroneogenesis
The glycerol backbone is produced via glyceroneogenesis, involving reactions catalyzed by pyruvate carboxylase and phosphoenolpyruvate carboxykinase (PEPCK), and reversal of glycolytic steps to give DHAP. 磷酸烯醇丙酮酸羧激酶
DHAP is reduced to glycerol-3-phosphate.
AMP-activated protein kinase (AMPK)-dependent phosphorylation of GPAT and HSL inhibits these steps of the cycle.
The lysophosphatidic acid (LPA, 溶血磷脂酸), phosphatidic acid (PA, 磷脂酸), and sn-1,2-diacylglycerol (DAG) intermediates in the TG resynthesis pathway are also important lipid signaling molecules.
- Heat production (thermogenesis) and body temperature maintenance;
- Elevated FFA level is associated with Type 2 diabetes and insulin resistance.