L5 Cell membranes lipids and lipid modification
The cell membrane defines the outer limit of cells
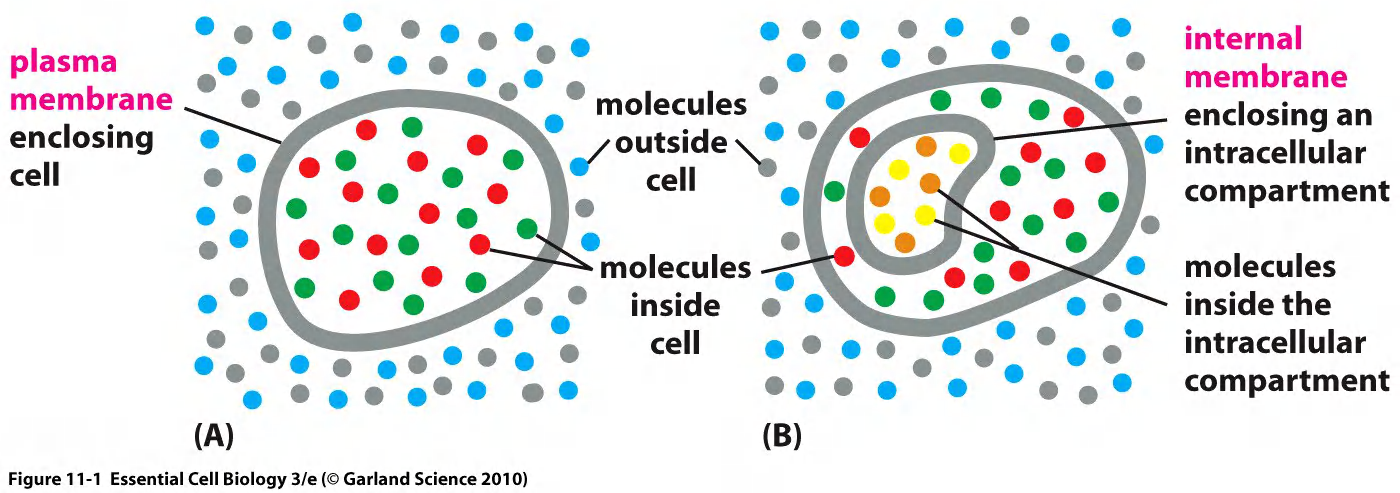
The limiting membrane of the cell is the plasma membrane (PM).
The PM separates the content of the cell from the environment.
Selective barrier
- Import and export molecules
- Receive information
- Has a role in moving and expanding cell
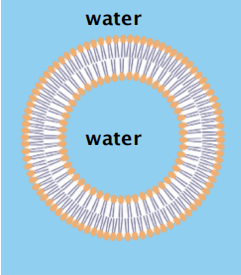
1. Cell Membranes contain hydrophilic outer surfaces and a hydrophobic core
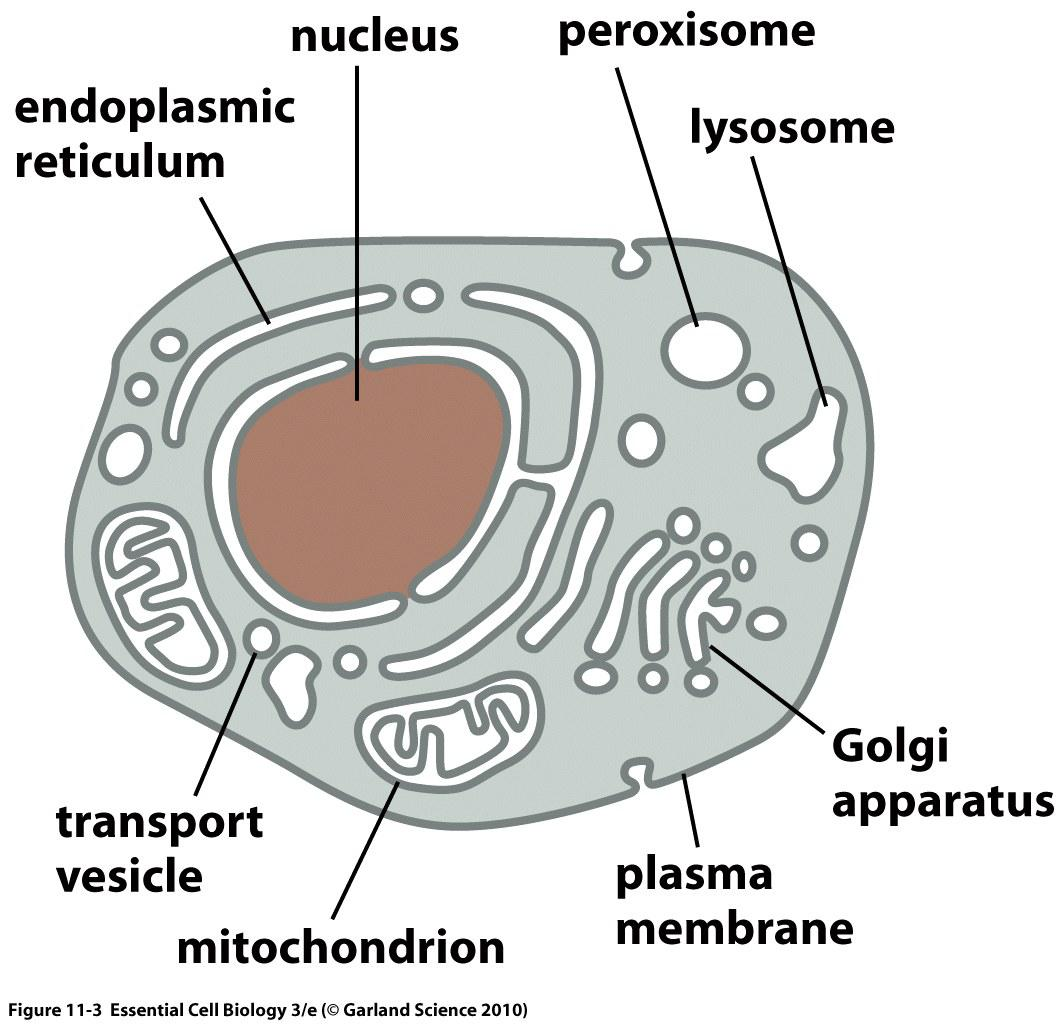
Eukaryote cellular compartments
Prokaryotes are one-membrane cells
Eukaryotes have multiple closed and open one- and double-membrane compartments
Membranes are not always the same.
Nucleus (double membrane)
Mitochondria (double membrane)
Plastids (chloroplasts, etc.) (double membrane)
Endoplasmic reticulum (single membrane)
Golgi apparatus (single membrane)
Peroxisome, lysosomes, endosomes, vesicles… (single membrane)
一、Membranes
Lipid Assembly
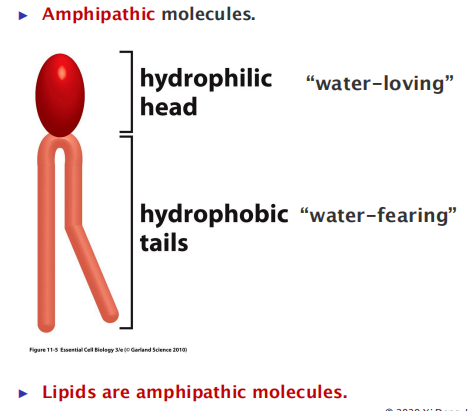
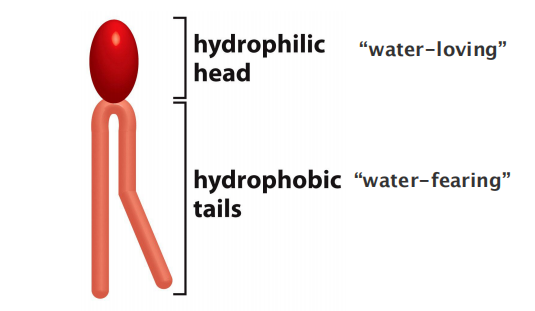
All of the lipid molecules in cell membranes are amphiphilic—that is, they have a hydrophilic (“water-loving”) or polar end and a hydrophobic (“water-fearing”) or nonpolar end.
Acetone (丙酮) is polar and forms electrostatic interaction
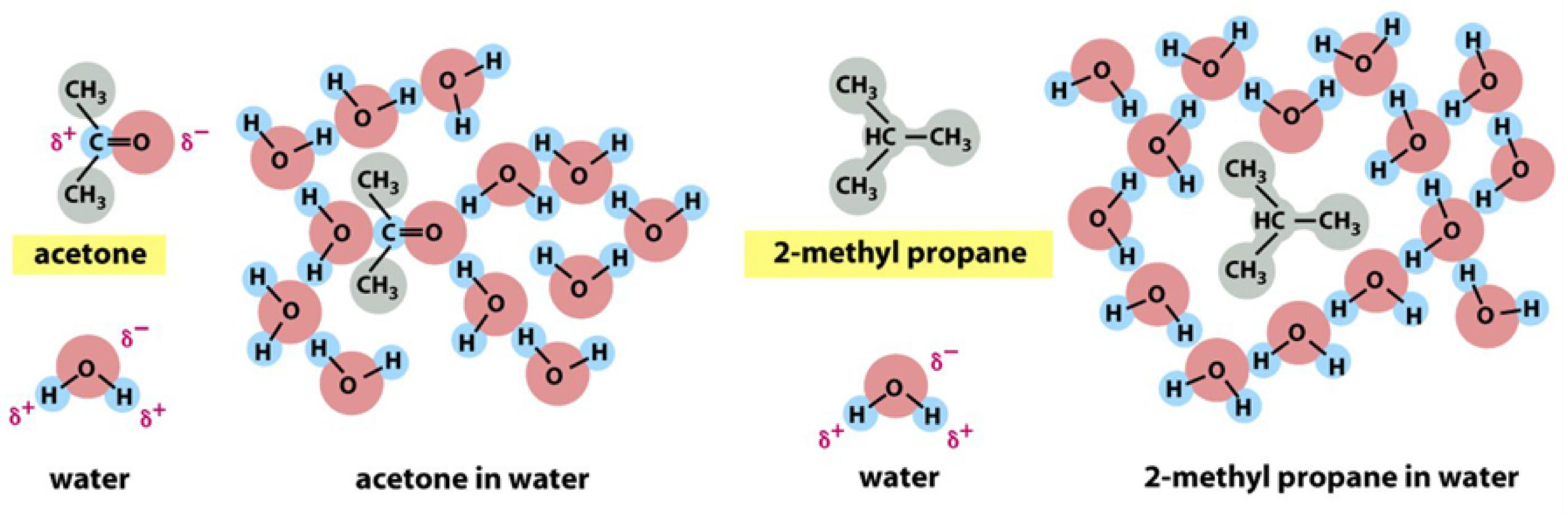
Water molecules are forced into ice-like cages:
- This causes increase in order, so multiple hydrophobic molecules stay together to minimized the increase in free energy
1. Micelle And Lipid Bilayer
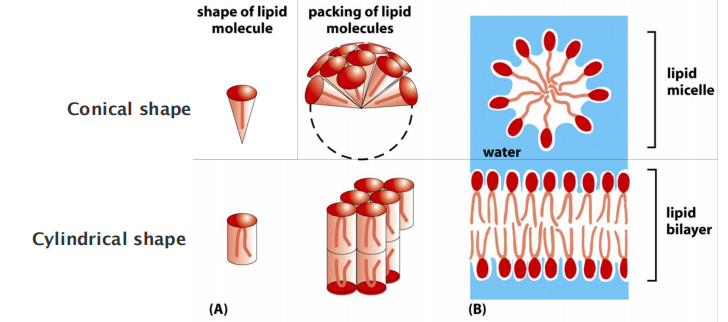
Micelle or bilayer depends on the shape of the molecule: conical versus cylindrical.
Remember the difference between a liposome (lipid bilayer) and a micelle
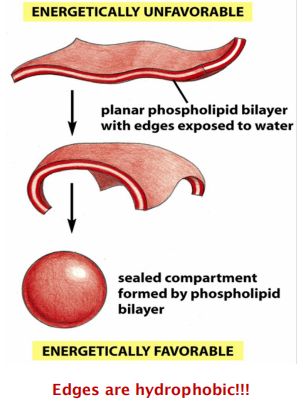
How and why does a spherical lipid bilayer form
hydrophilic molecules dissolve readily in water because they contain charged groups or uncharged polar groups that can form either favorable electrostatic interactions or hydrogen bonds with water molecules
- Edges are hydrophobic, and they tend to stay together, which is free energy favorable.
- If dispersed in water, they force the adjacent water molecules to reorganize into ice-like cages (Hydration Shell) that surround the hydrophobic molecule
- Because these cage structures are more ordered than the surrounding water, their formation increases the free energy. This free-energy cost is minimized
- the hydration shell of the polar head groups of the lipids needs to be removed before fusion
The prohibition of free edges has a profound consequence: the only way for a bilayer to avoid having edges is by closing in on itself and forming a sealed compartment
- This process undergo Spontaneously!!
2. Black membranes and Liposomes
Black membranes are planar lipid bilayers (they appear black when they separate two aqueous compartments)
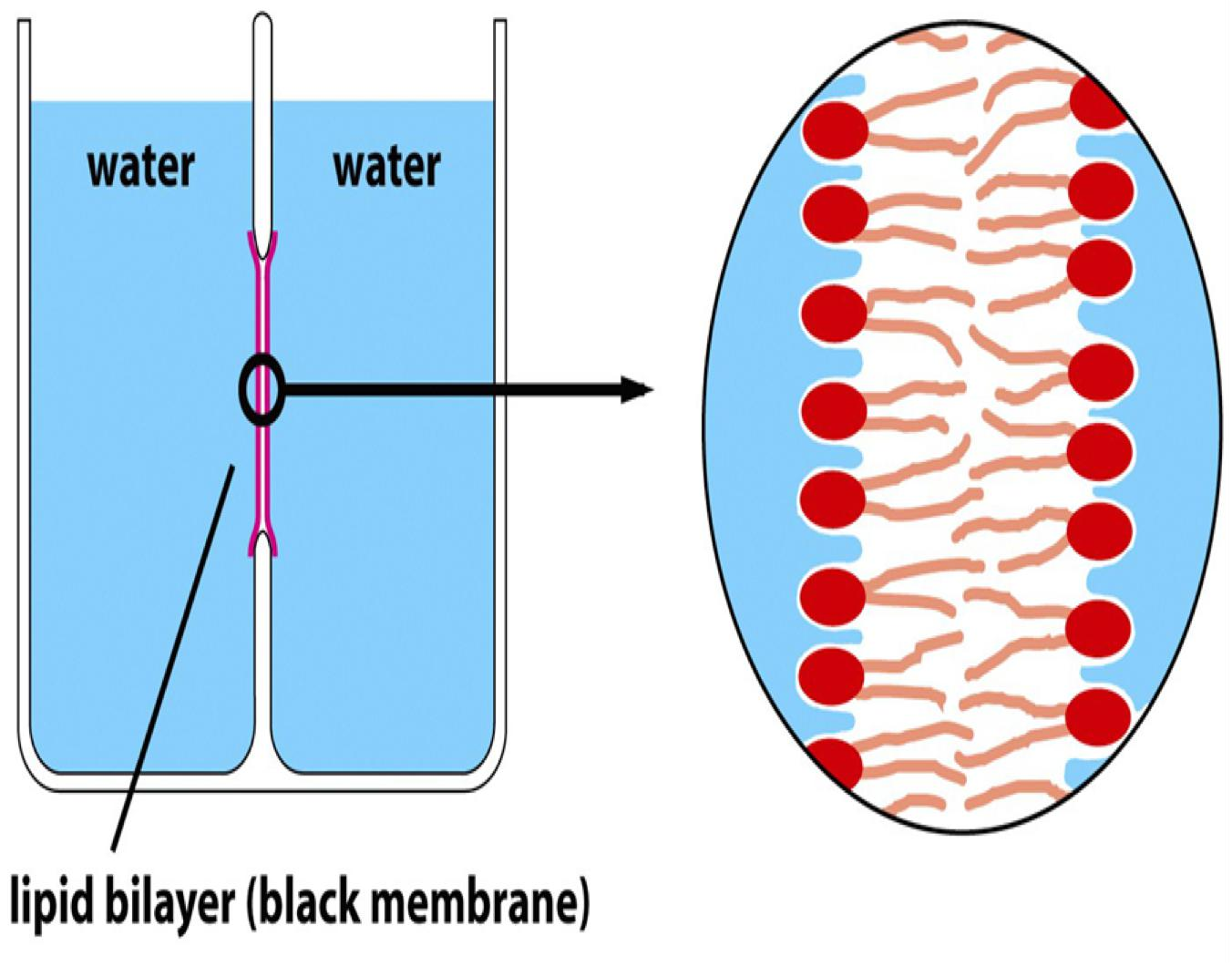
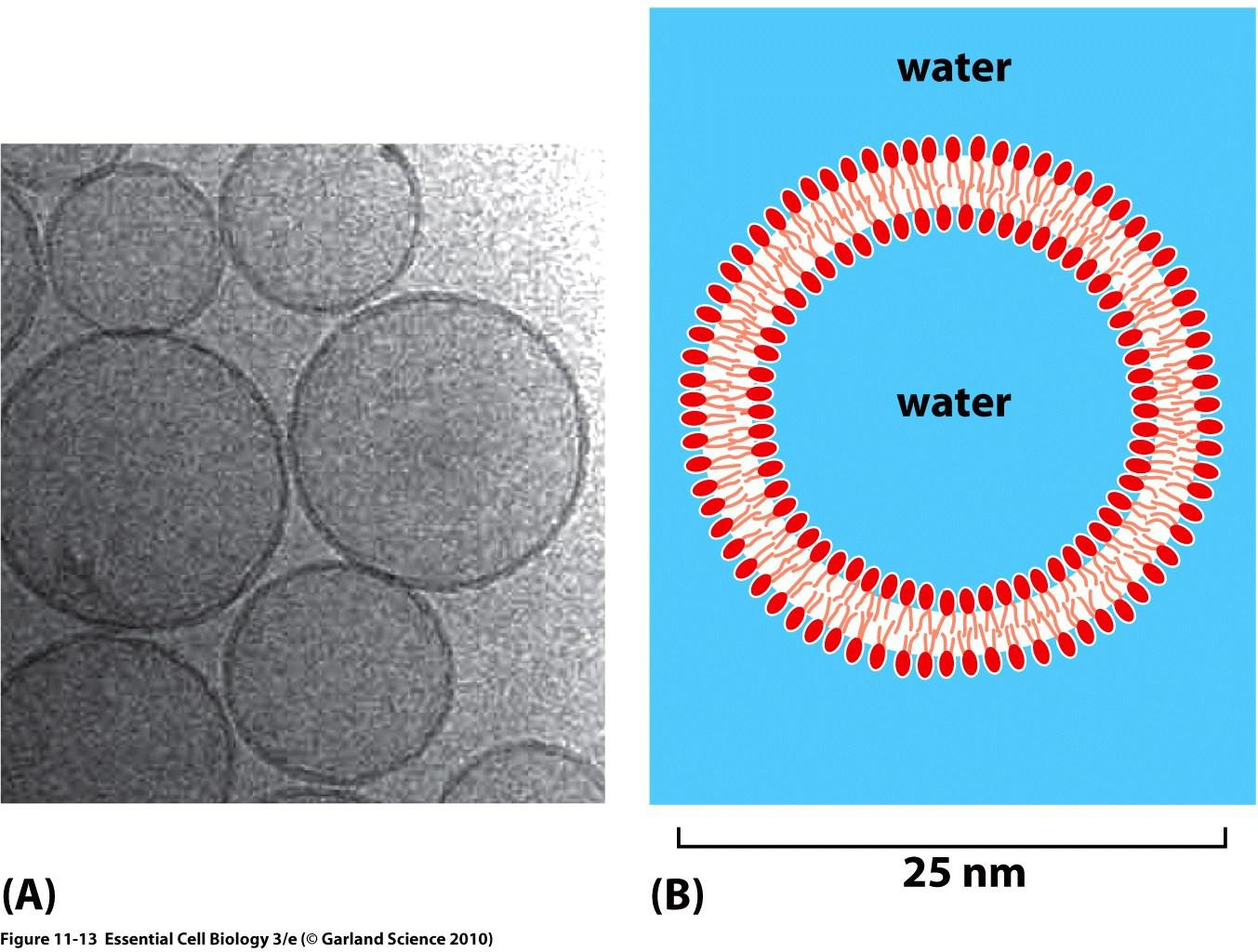
Liposomes are spherical lipid bilayers
Membrane Lipid
1. The Cell Membrane
Characteristics:
- Two layers of lipids
- Scattered proteins
- Carbohydrates attached to outer surface (cortex)
2. Lipid Molecular
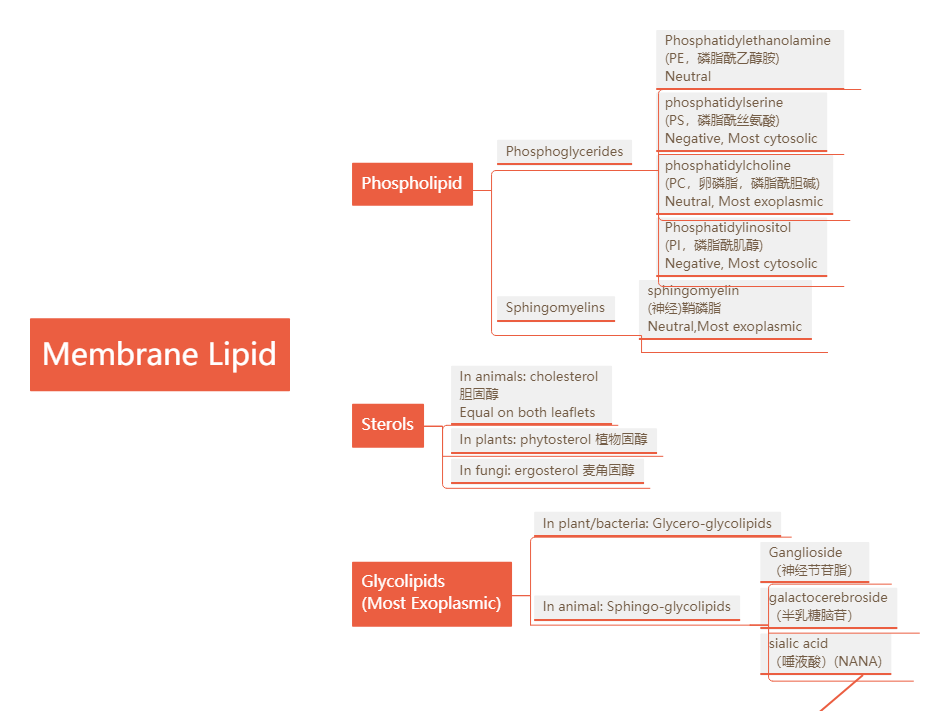
膜上的lipid可以大致被分为以下三类:
- Phospholipids 磷脂
- Sterols 固醇类
- Glycolipids 糖脂
Fluidity
The fluidity of a lipid bilayer depends on both its composition and its temperature, as is readily demonstrated in studies of synthetic lipid bilayers
A shorter chain length reduces the tendency of the hydrocarbon tails to interact with one another, in both the same and opposite monolayer, and cis-double bonds produce kinks in the chains that make them more difficult to pack together, so that the membrane remains fluid at lower temperatures
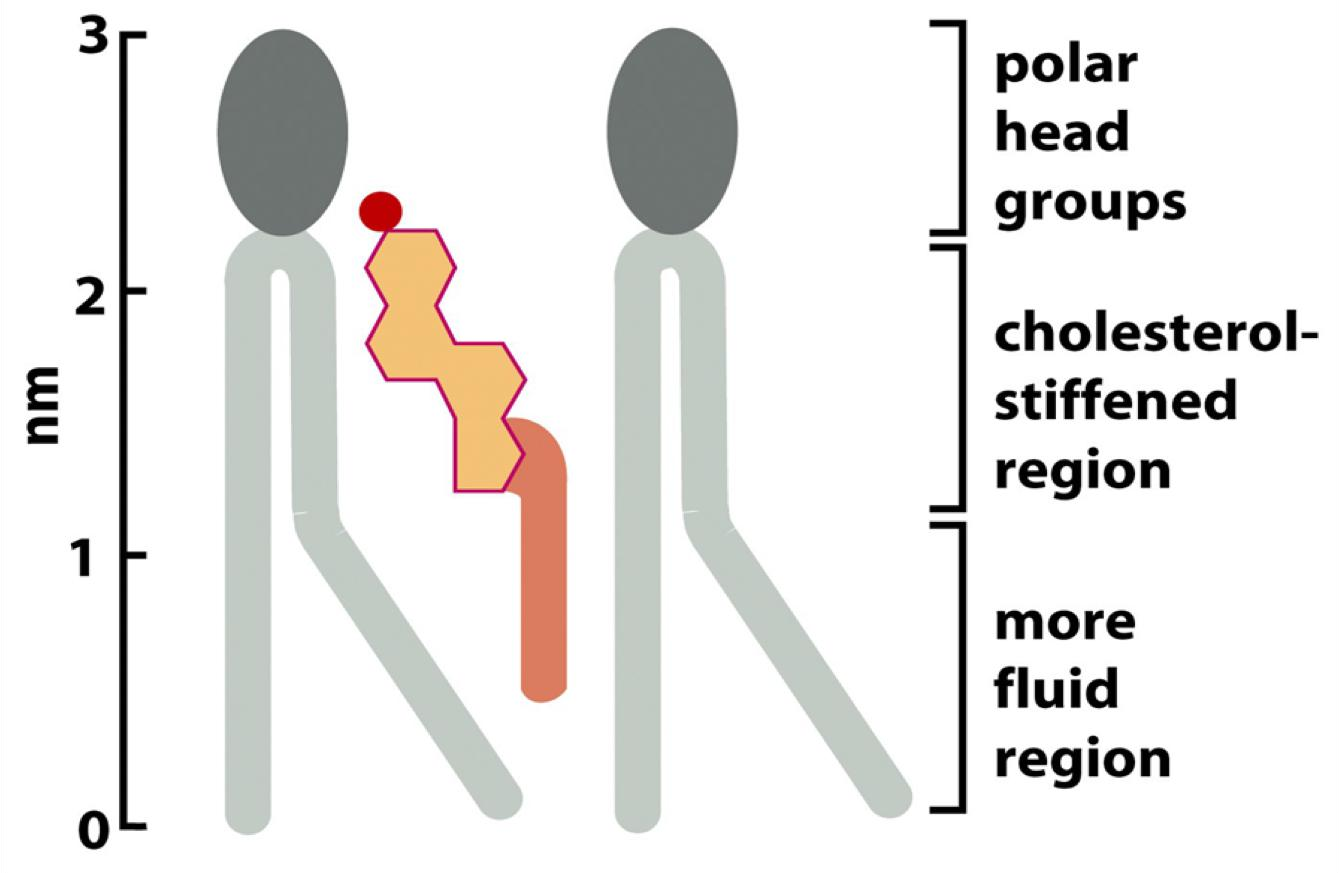
Cholesterol modulates the properties of lipid bilayers. When mixed with phospholipids, it enhances the permeability-barrier properties of the lipid bilayer.
- By decreasing the mobility of the first few CH2 groups of the chains of the phospholipid molecules, cholesterol makes the lipid bilayer less deformable (更少形变的) in this region and thereby decreases the permeability of the bilayer to small water-soluble molecules.
- Although cholesterol tightens the packing of the lipids in a bilayer, it does not make membranes any less fluid. At the high concentrations found in most eukaryotic plasma membranes, cholesterol also prevents the hydrocarbon chains from coming together and crystallizing.
- Bacterial plasma membranes are often composed of one main type of phospholipid and contain no cholesterol
- In archaea, lipids usually contain 20–25-carbon-long prenyl chains(异戊二烯链) instead of fatty acids
Thus, lipid bilayers can be built from molecules with similar features but different molecular designs.
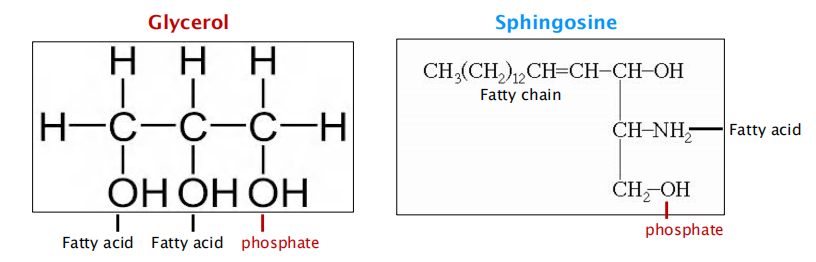
Phospholipids: Phosphoglycerides and Sphingomyelins
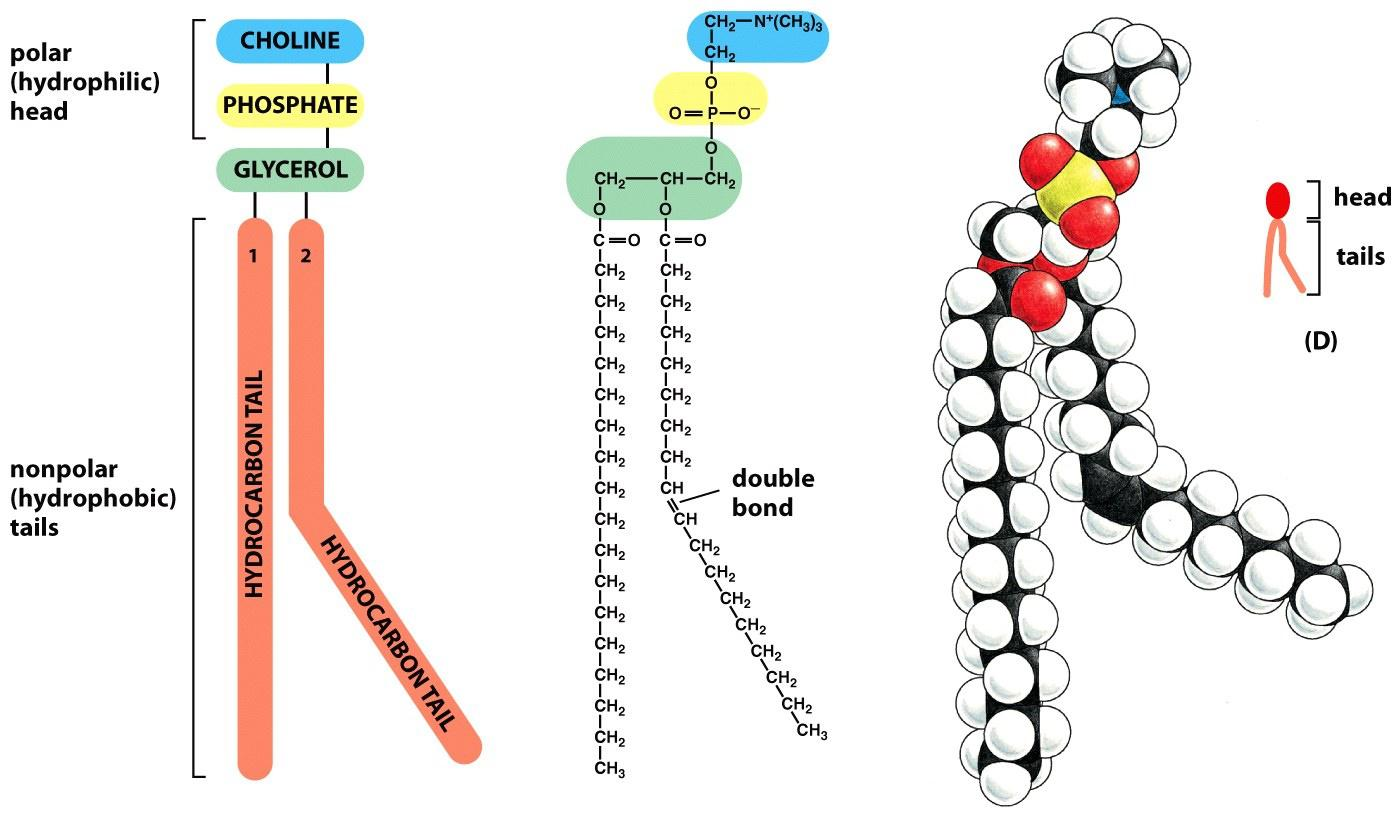
The main phospholipids in most animal cell membranes are the phosphoglycerides, which have a three-carbon glycerol backbone
- Two long-chain fatty acids are linked through ester bonds to adjacent carbon atoms of the glycerol, and the third carbon atom of the glycerol is attached to a phosphate group, which in turn is linked to one of several types of head group.
Two major groups of phospholipids, based on the backbone:
- Phosphoglycerides(甘油磷脂): glycerol(甘油) as backbone; main phospholipids
- Sphingomyelins (鞘磷脂): sphingosine as backbone
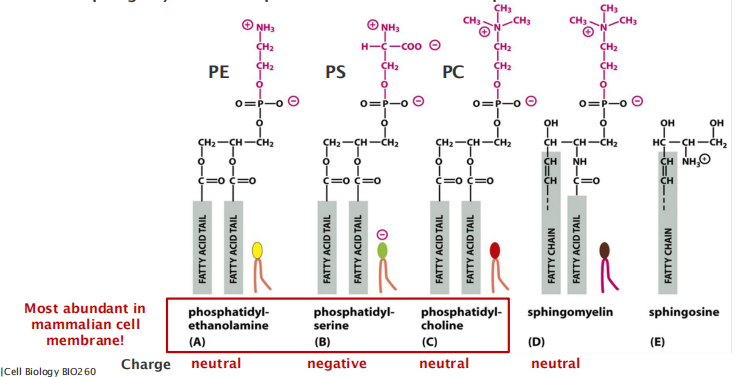
Together, the phospholipids phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin constitute more than half the mass of lipid in most mammalian cell membranes
1 | |
(1) Phosphoglycerides 甘油磷脂
Two fatty acids linked by ester bonds with glycerol, differ in length 14-24 carbon atoms
Usually one fatty acid tail contains one or more cis-double bonds (unsaturated), while the other tail is saturated.
The cis-double bonds create kinks in the tail, and make the lipid more fluid.
(2) Phospholipids can have a variety of head groups
Four major types of phospholipids of the PM:
Phosphatidylethanolamine (PE,磷脂酰乙醇胺), phosphatidylserine (PS,磷脂酰丝氨酸), phosphatidylcholine (PC,卵磷脂,磷脂酰胆碱) and sphingomyelin make up to 50% of the mass of all lipids in a mammalian cell! (The most abundant)
Sterols
All sterols have the similar 4-ring isoprenoid(类异戊二烯的) structure (Ring structure reduces the membrane fluidity)
- In animals: cholesterol 胆固醇
- In plants: phytosterol 植物固醇
- In fungi: ergosterol 麦角固醇
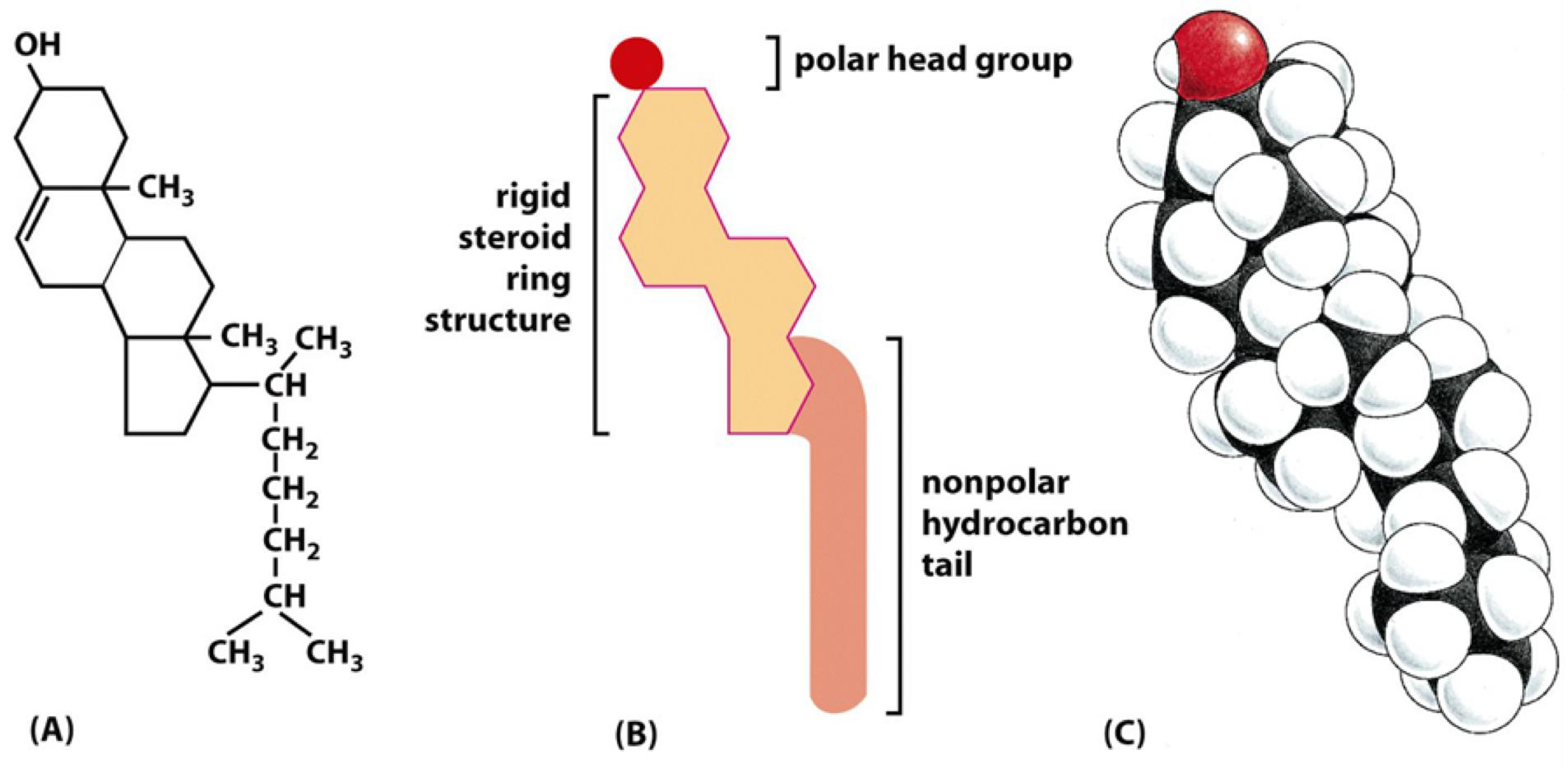
Cholesterol can stiff membranes, which depends on cholesterol concentration and temperature.
Glycolipids: sugar-containing lipids
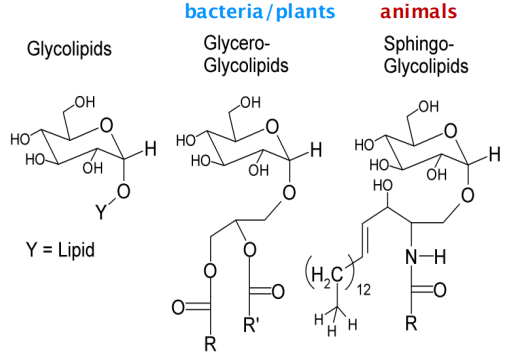
In plant/bacteria: Glycero-glycolipids
In animal: Sphingo-glycolipids
Glycolipids probably occur in all eukaryotic cell plasma membranes, where they generally constitute about 5% of the lipid molecules in the outer monolayer. They are also found in some intracellular membranes.
- Present in relatively small amounts (less than 5%)
- Present mainly in nerve cells (animals).
- They are present on the surface of all plasma membranes (PM)
- They locate in the outer monolayer (non-cytosolic) of the PM
Sugar part projects always outside on the cell surface
- Why?
(1) Sphingo-glycolipids in animal cells
Almost all glycolipids are based on sphingosine in animal cells.
Present mainly in nerve cells (animals).
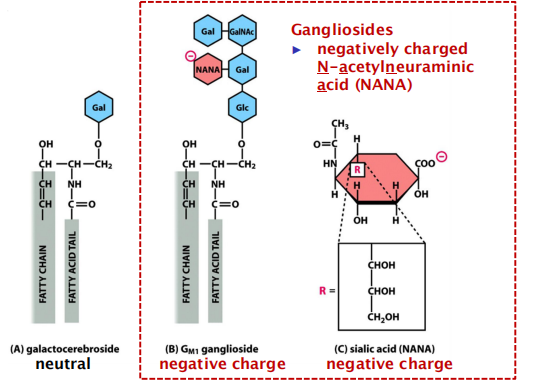
Example: Ganglioside(神经节苷脂), galactocerebroside(半乳糖脑苷), sialic acid (唾液酸)(NANA)
Gangliosides
- May contain oligosaccharide chains with negatively charged residues.
- The most complex of the glycolipids, the gangliosides, contain oligosaccharides with one or more sialic acid moieties (唾液酸部分), which give gangliosides a net negative charge
- Are found to constitute about 5-10% of the total lipid mass in the plasma membrane of neurons,
- Are found in the extracellular leaflet (facing away from the cytosol) in the cellular membranes,
- glycolipids are confined to the exposed apical surface, where they may help to protect the membrane against the harsh conditions frequently found there (such as low pH and high concentrations of degradative enzymes)
- Affect the electrical environment of the membrane.
- Charged glycolipids, such as gangliosides, may be important because of their electrical effects: their presence alters the electrical field across the membrane and the concentrations of ions—especially Ca2+—at the membrane surface.
Polyomaviruses also enter the cell after binding initially to gangliosides.
(2) Functions of glycolipids in the cell
Help to protect the cell surface/membrane against harsh conditions (e.g. pH, proteolytic enzymes)
Function in cell recognition processes (lectin-binding)
Charged glycolipids (gangliosides) influence electrical field of the membrane
Provide entry points for toxins, e.g. cholera toxin
- Cholera toxin binds to and enters only cells that have the ganglioside G
M1 on the surface (e.g. intestinal epithelial cells) - Cholera toxin causes prolonged increase of cyclic AMP (cAMP), which causes efflux of Cl-, Na+ and water into the intestine
- Cholera toxin binds to and enters only cells that have the ganglioside G
Membrane Bilayer
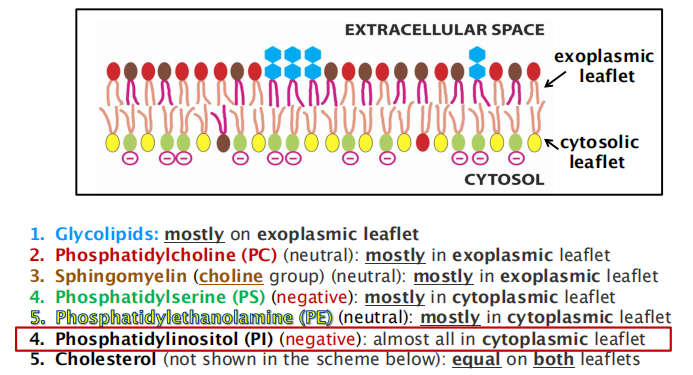
Membranes contain a mix of phospholipids and each leaflet has a different phospholipid composition
- Membrane contains many different types of phospholipids
- Different types of phospholipids located unevenly between two layers
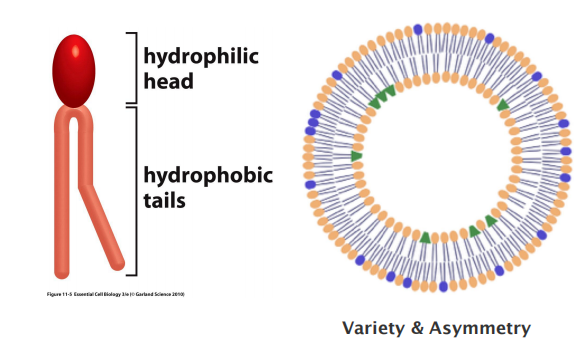
1. Asymmetric lipid distribution in membrane leaflets
Knowledge about the distribution of lipids is crucial to understand cellular functions !!!
Phospholipids: Phosphatidylinositol (PI,磷脂酰肌醇)
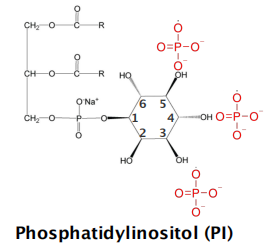
Phosphoinositides (PI) is present at the inner/cytosolic leaflet of plasma membrane.
- Phosphatidylinositol is present in very small quantities in the plasma membranes of mammalian cells
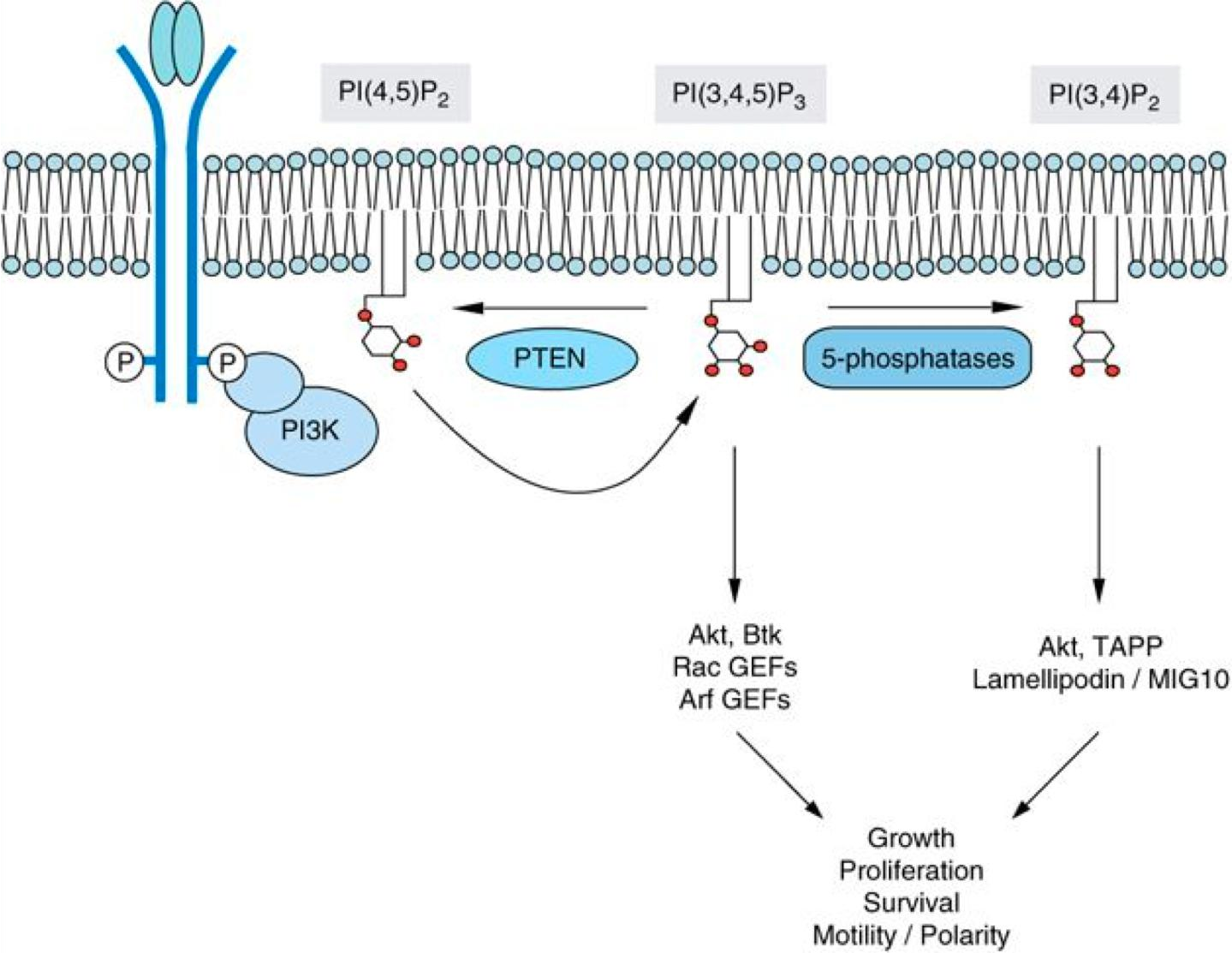
Serving as signal molecules, but how?
(1) Modification of phosphatidylinositol (PI): phosphorylation
Phosphatidylinositol (PI) at the inner/cytosolic leaflet
- Phosphorylation of PI by Phosphoinositide 3-kinase (PI3K) creates PI3P that binds other proteins that relay the signal…..
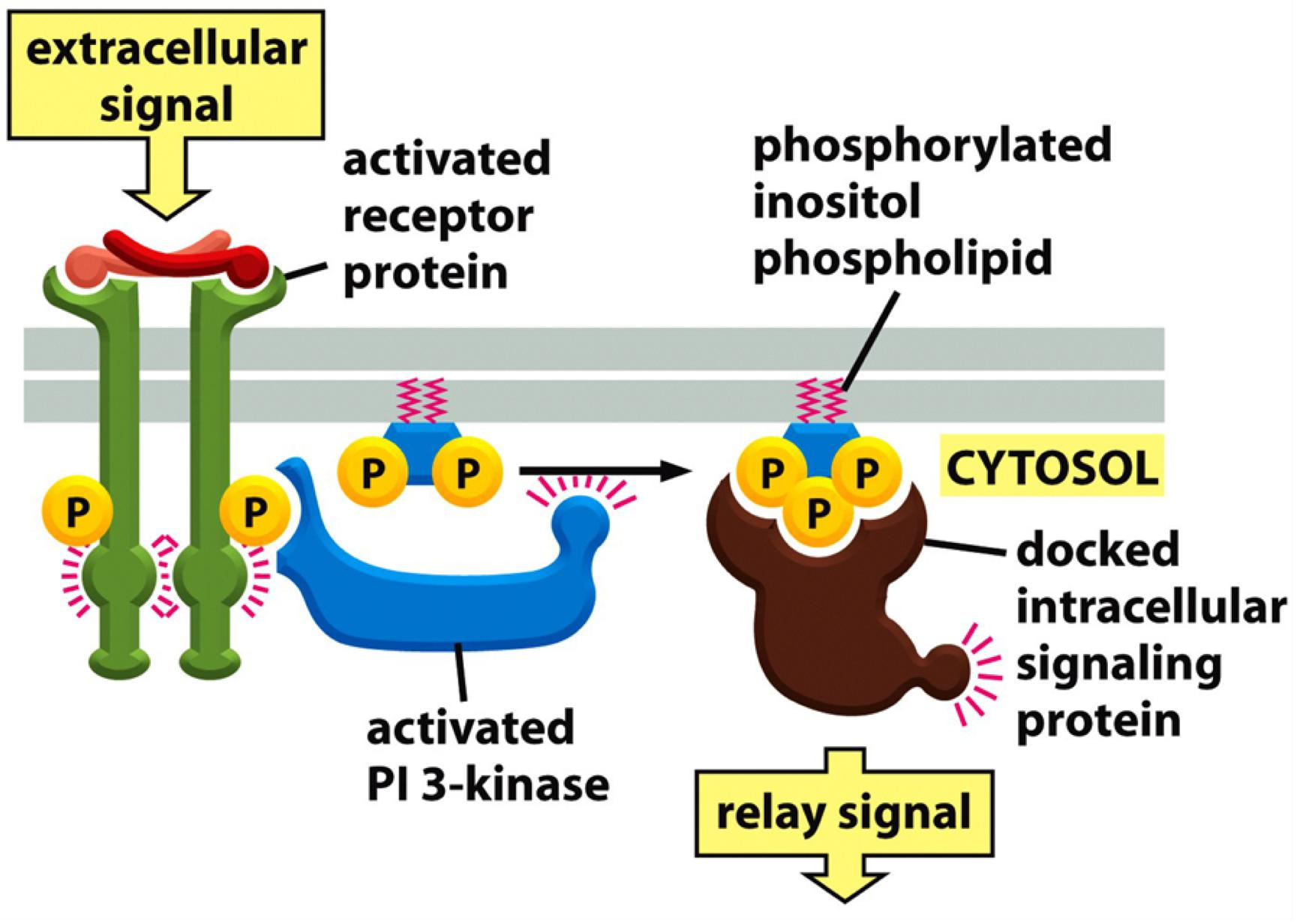
Phosphorylation of PI
- creates membrane binding sites for other proteins that relay the signal
- can induce curvature and induce transport events
(2) Modification of phosphatidylinositol (PI): cleavage
Phosphatidylinositol (PI) at the inner/cytosolic leaflet
- Cleavage by lipase(脂肪酶) results in signaling molecules that relays the signal
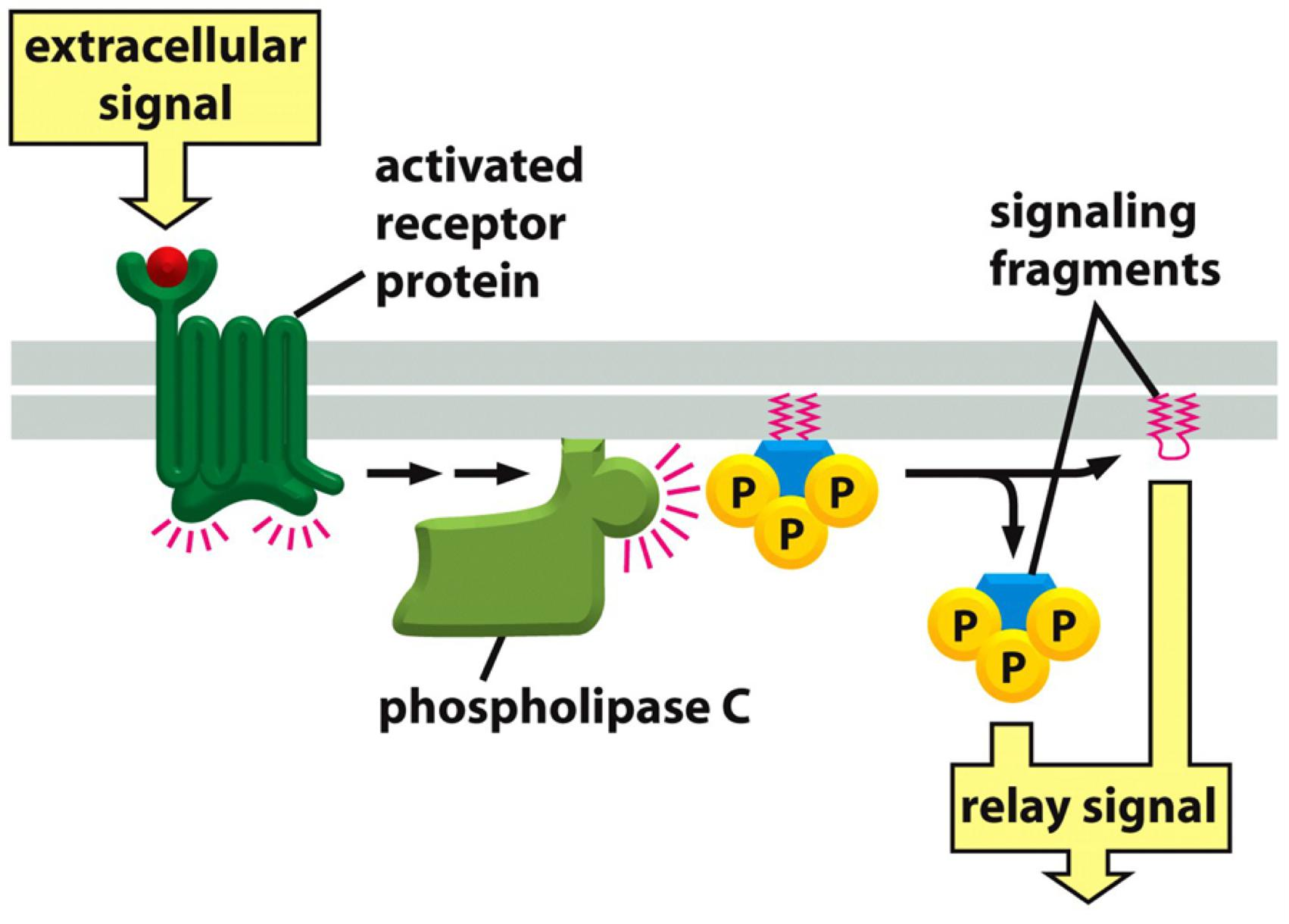
An activated receptor recruits phospholipase C to the membrane, which cleaves phosphoinsoitides and cleaved (free) phosphoinositides relay the signal.
Phosphatidylserine (PS,磷脂酰丝氨酸)
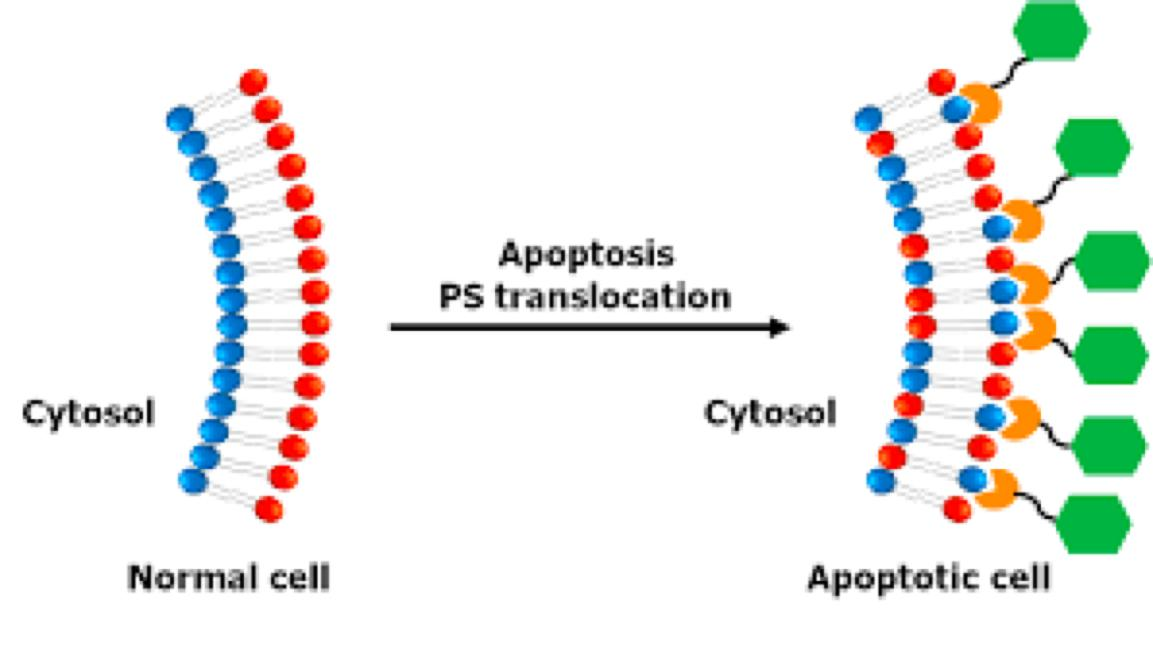
Phosphatidylserine (PS) locates only on the cytosolic leaflet, but when cells undergo programmed cell death (apoptosis), it is translocated to the exoplasmic side.
Therefore, it can be detected by Annexin V (膜联蛋白 Ⅴ) labeling.
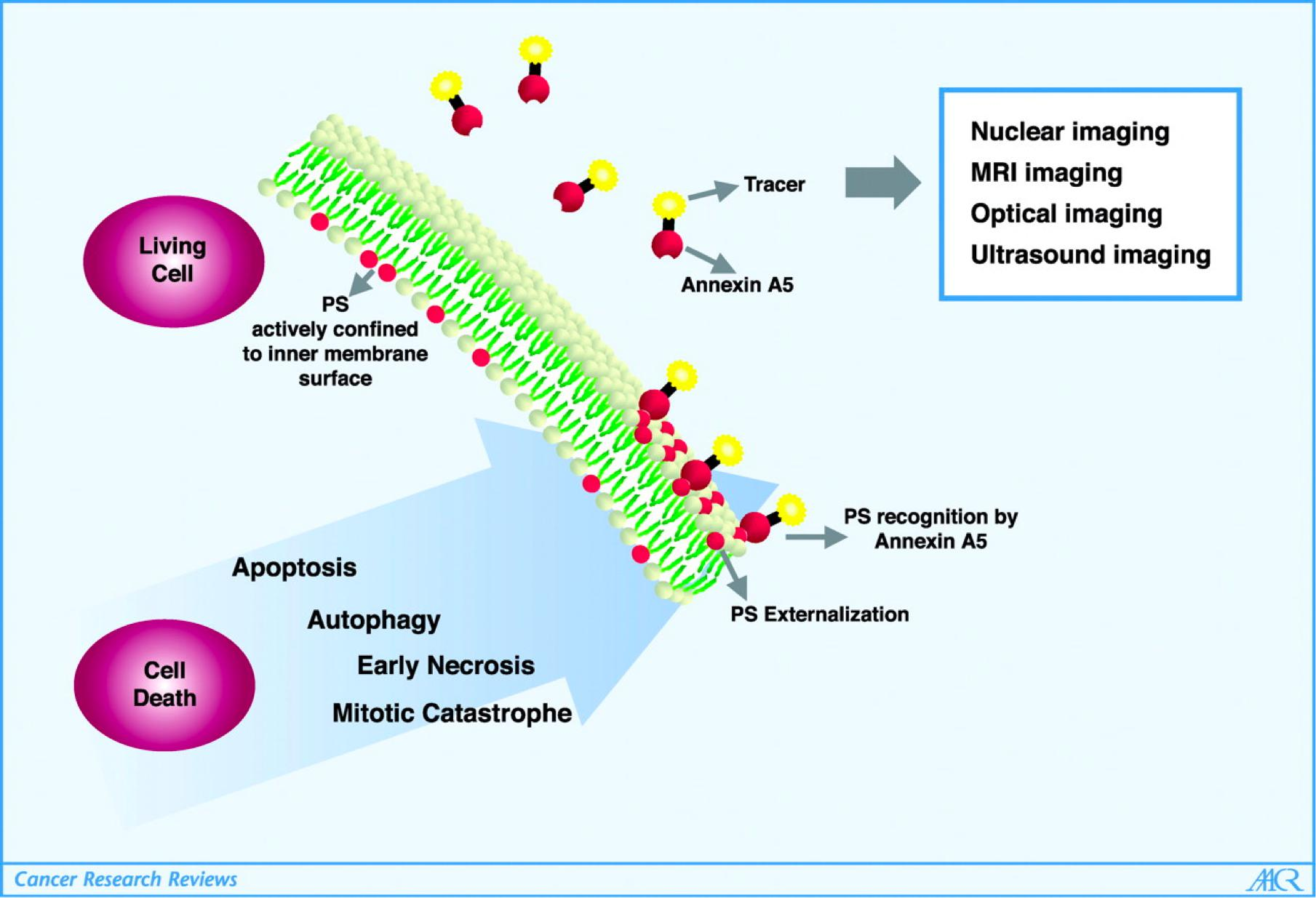
Method to identify early stages of programmed cell death (apoptosis)
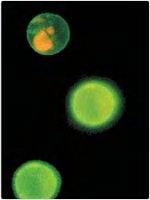
Saving membrane asymmetry
Lipid asymmetry is functionally important, especially in converting extracellular signals into intracellular ones
In other cases, specific lipid head groups must first be modified to create protein-binding sites at a particular time and place
- Animals exploit the phospholipid asymmetry of their plasma membranes to distinguish between live and dead cells.
Sugar-containing lipid molecules called glycolipids have the most extreme asymmetry in their membrane distribution
- These molecules, whether in the plasma membrane or in intracellular membranes, are found exclusively in the monolayer facing away from the cytosol
- In animal cells, they are made from sphingosine, just like sphingomyelin
These intriguing molecules tend to self-associate, partly through hydrogen bonds between their sugars and partly through van der Waals forces between their long and straight hydrocarbon chains, which causes them to partition preferentially into lipid raft phases
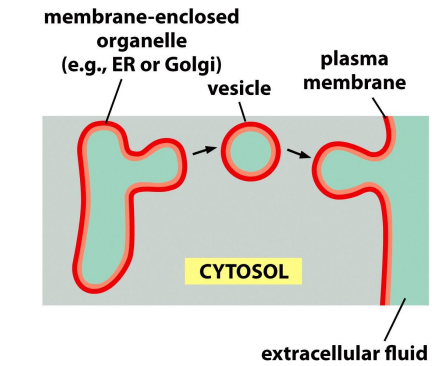
The asymmetry is maintained by vesicle fusion.
Membrane Fluidity And Microdomains
1. Membrane fluidity
Physically, membranes are lipid suspension in water.
The Fluidity of lipid bilayer depends on its composition
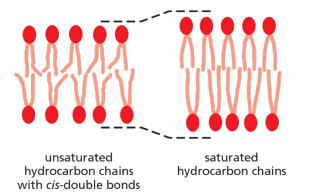
Double bonds lower T
m.Short hydrocarbon chains lower T
m.
Lower organisms or simple organisms can adjust lipid composition to keep membrane fluid at different environmental temperatures.
How about cholesterol?
- At lower temperature/lower concentration: it inserts into lipid molecules and prevents the tightening.
- At higher temperature/higher concentration: it tightens the packing of the lipids.
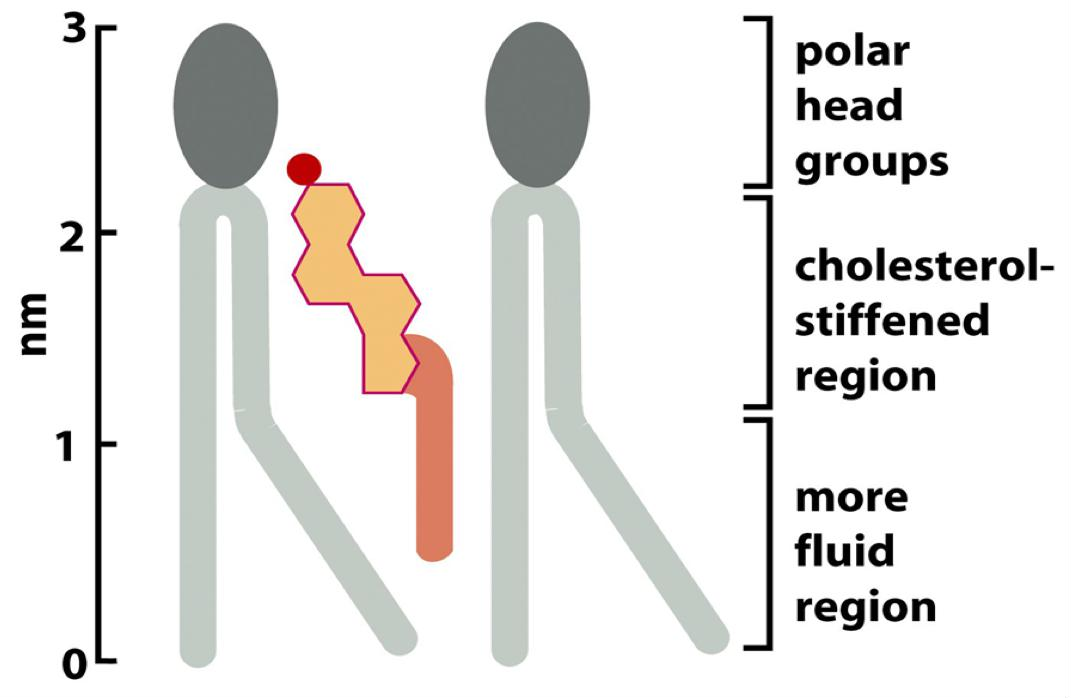
2. Phase transition of lipid membrane
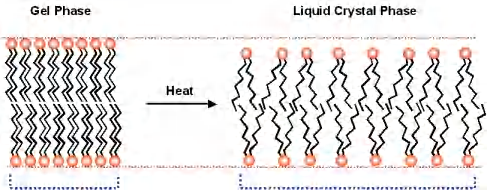
Depending on the temperature, membranes exist in different phases
- gel phase
- lipid crystal phase
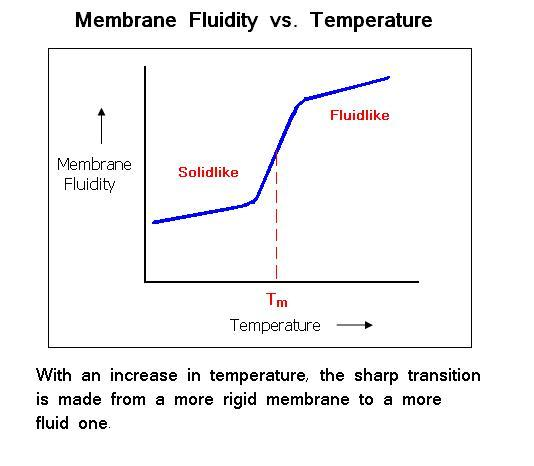
3. The two-dimensional lipid bilayer is a viscous fluid
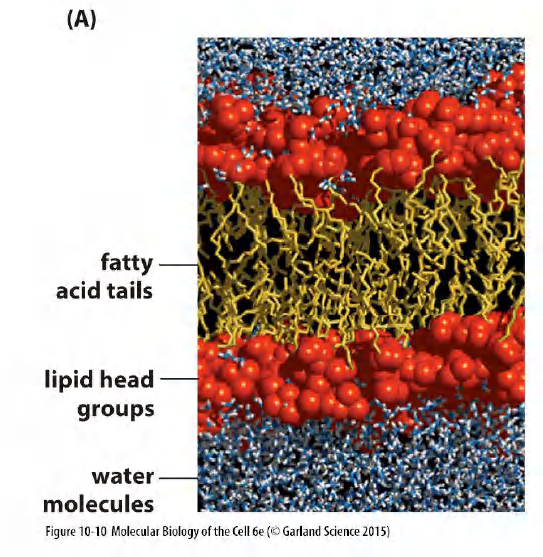
Theoretic calculation
- 100 phosphatidylcholine(PC)molecules arranged in a regular bilayer.
- Computer calculates the position of every atom after 300 picoseconds of simulated time.
- Build a model of the lipid bilayer that accounts for almost all of the measurable properties of a synthetic lipid bilayer
- Thickness
- number of lipid molecules per membrane area
- depth of water penetration
- unevenness of the two surfaces.
4. Motion of the lipid molecules: modes and directions
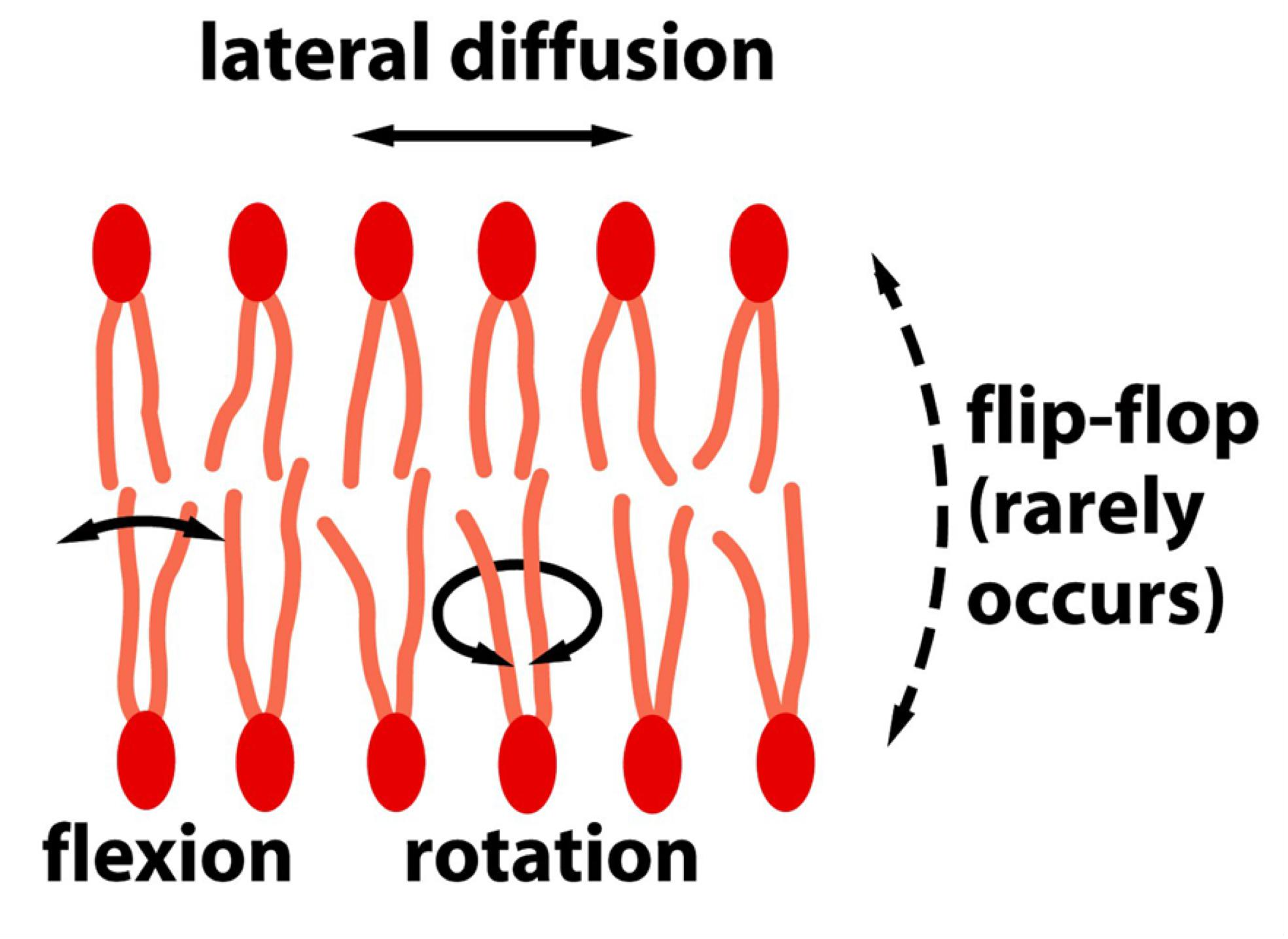
(How can lipid motion be measured?)
- Lipids are highly dynamic
Mode of motion of lipid:
- Lateral diffusion: Rapidly change their lateral position in the leaflet. 径向扩散
- an average lipid molecule diffuses the length of a large bacterial cell (~2 μm) in about 1 second
- Rotate very rapidly along their long axis. (Hydrophobic tail is highly flexible.) 旋转
- Flexion 弯曲
- Flip-flop (moving from one leaflet to the other leaflet) only rarely. (Intertransport between out-layer and inner-layer,在不同leaflets之间翻转)
- Flip-flop requires a special enzyme on membrane.
- Phospholipid translocators can catalyze the rapid flip-flop.
- The flip-flops are very rare for phospholipids but cholesterol molecules flip-flop more often.
5. Membranes can possess different microdomains: “lipid rafts”
Is plasma membrane a homogeneous structure or is it compartmentalized?
- Lipids are NOT always homogeneously distributed
- “Lipid rafts” mainly consists of a mixture of sphingomyelin and cholesterol, they are thought to also concentrate specific membrane proteins
Liposome (artificial bilayer) with mixture of:
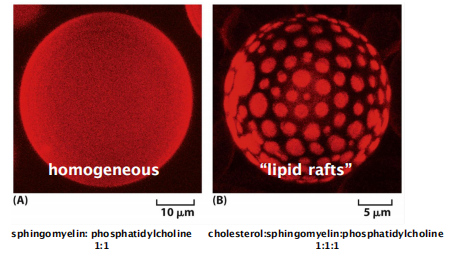
“Lipid rafts” microdomain
1 | |
(Which lipids form “lipid rafts”? (GM1: cholera toxin binding)
The characteristics of the lipid:
- Lipid rafts can concentrate specific membrane domains)
- Are thought to help stabilize many membrane proteins.
- may enhance the crystallization of the bound membrane proteins.
- interact specifically with the protein.
- can have head groups of various sizes and charges depending on the protein.
二、Detergents In Membrane Studies
Detergents
- Small amphiphilic molecules of variable structure (Small lipid-like molecules).
- Better solubility in water than that of lipids.
- Solubilize phospholipids, leaving proteins intact
Detergents in membrane studies: ionic and non-ionic
Categories
Categories: divided into two major groups:
- ionic detergents
- non-ionic detergents
The hydrophobic part intercalate(设置,插入) into hydrophobic parts of lipids and of transmembrane proteins.
The polar group brings lipids or proteins into aqueous face and make them soluble.
The structure of some common detergents
Sodium dodecyl sulfate (SDS) (ionic detergent)
Triton-X-100 (non-ionic detergent)
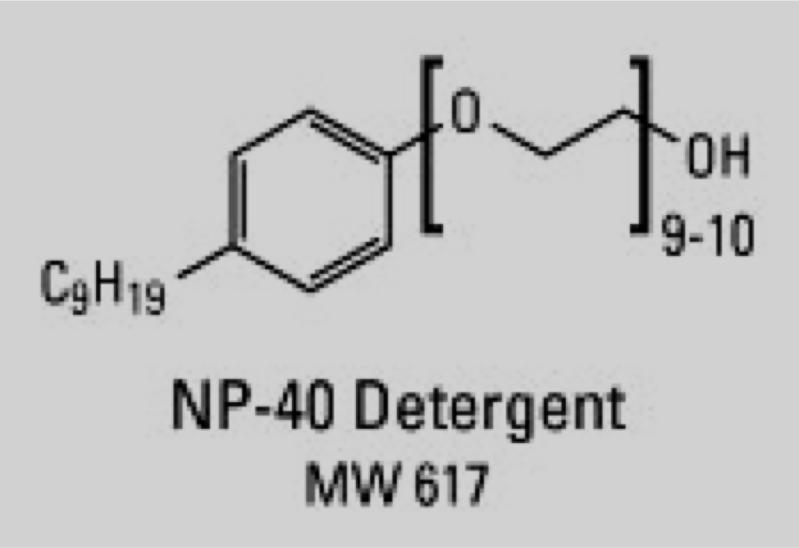
NP-40 (nonidet-P-40) (non-inonic detergent)
β-octylglucoside 辛烷基葡糖苷
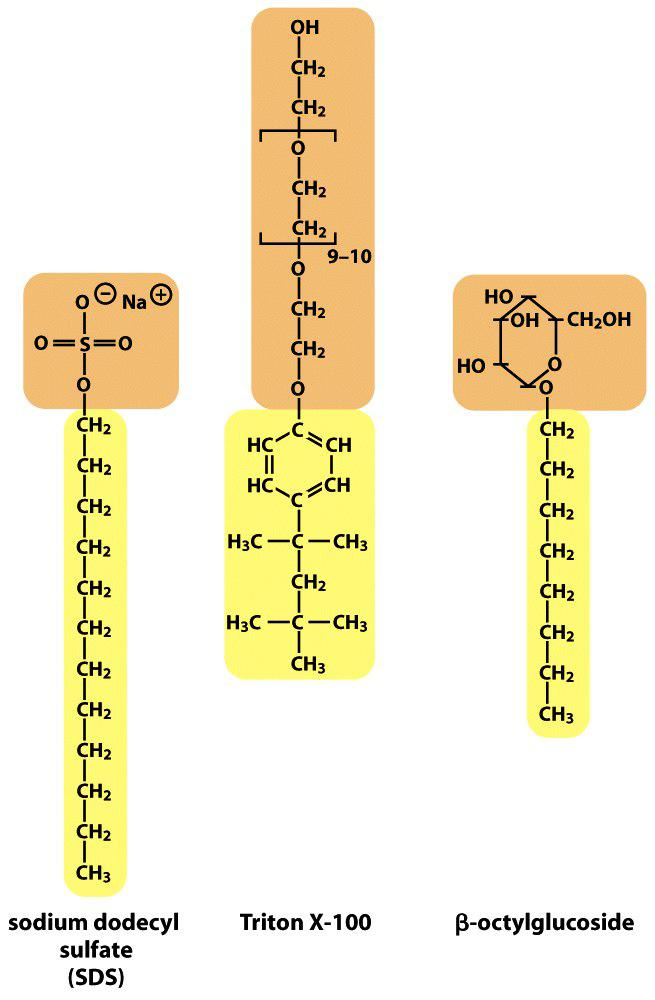
Detergents are absolute essential to solubilize membrane proteins.
1. SDS is an ionic detergent that denature proteins
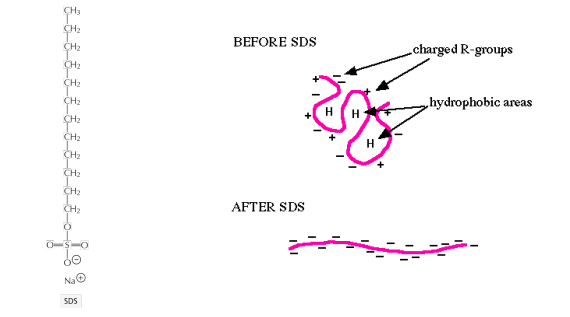
SDS fully denatures proteins
SDS covers protein molecules with negative charges
Charging the protein is essential for electrophoretic separation via SDS-polyacrylamide gel electrophoresis (PAGE)
2. Non-ionic detergents do NOT denature proteins
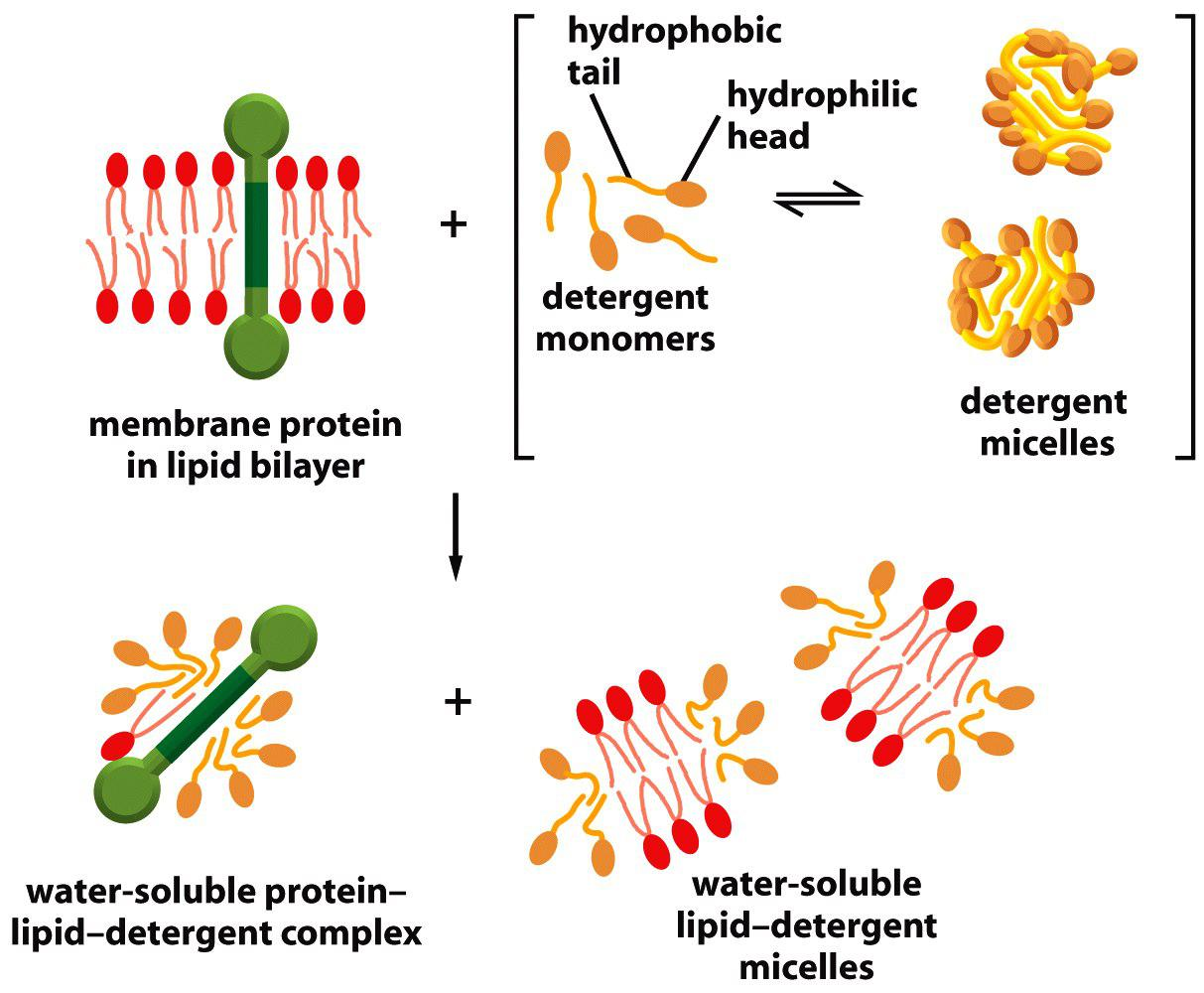
Non-ionic detergents only solubilize membrane components
Remember the differences between ionic and nonionic detergents!
3. Detergents form micelles
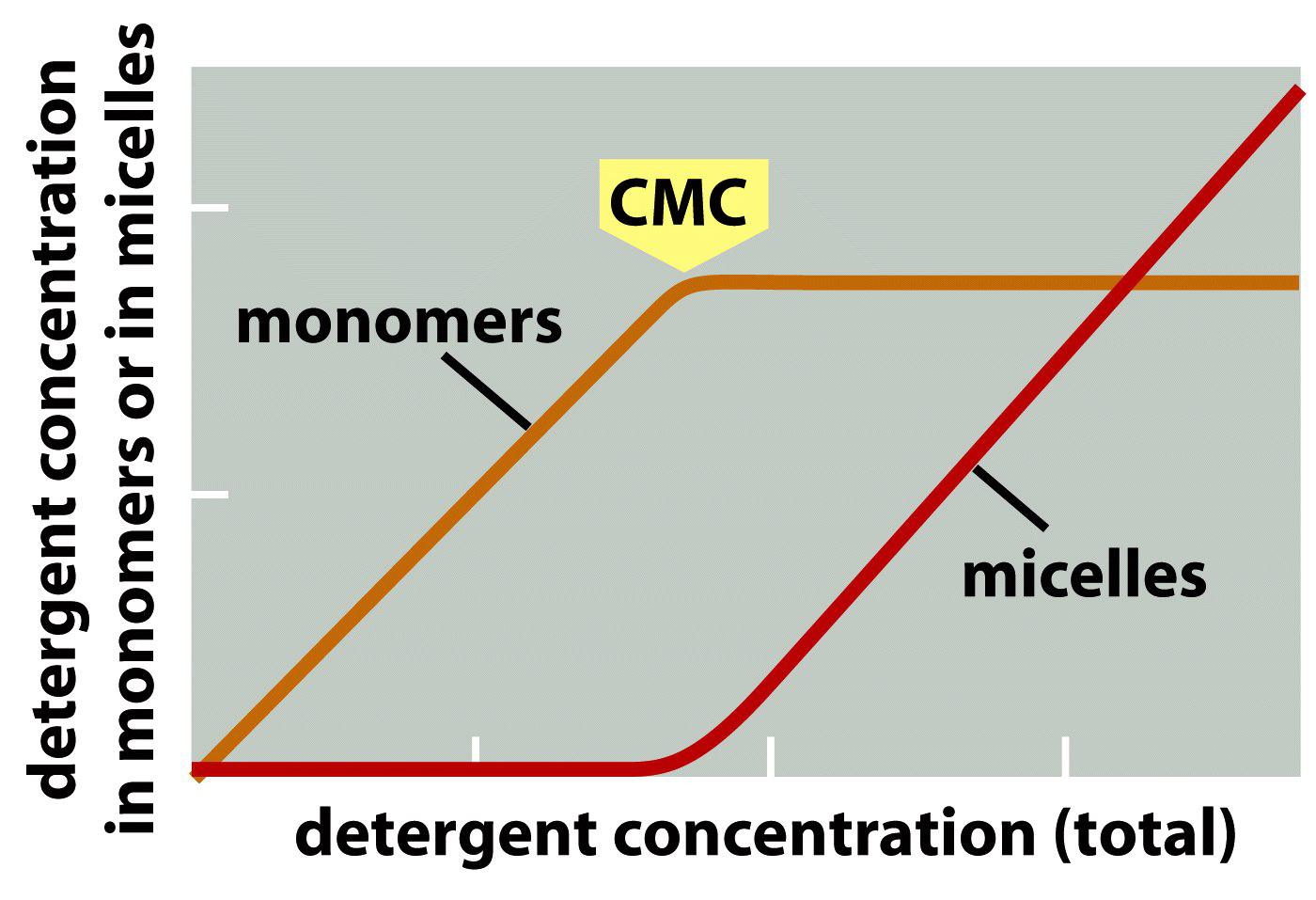
Critical micelle concentration (CMC) is the concentrations at which detergents aggregate to form micelles.
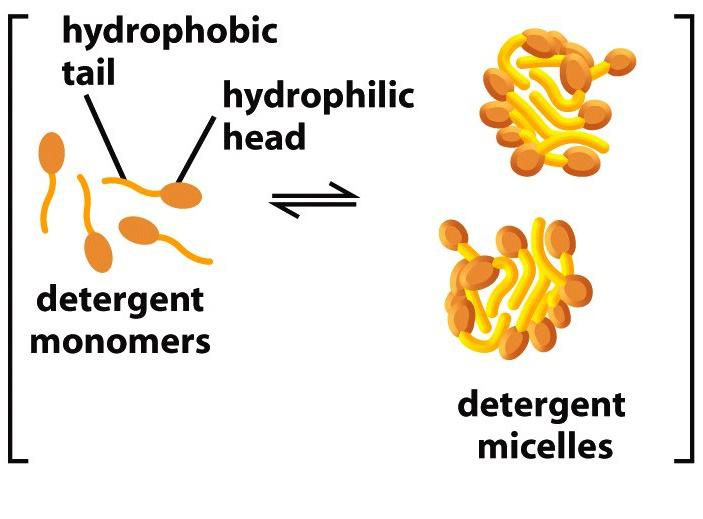
What is the critical micelle concentration and why is it important?
三、Lipid Droplets
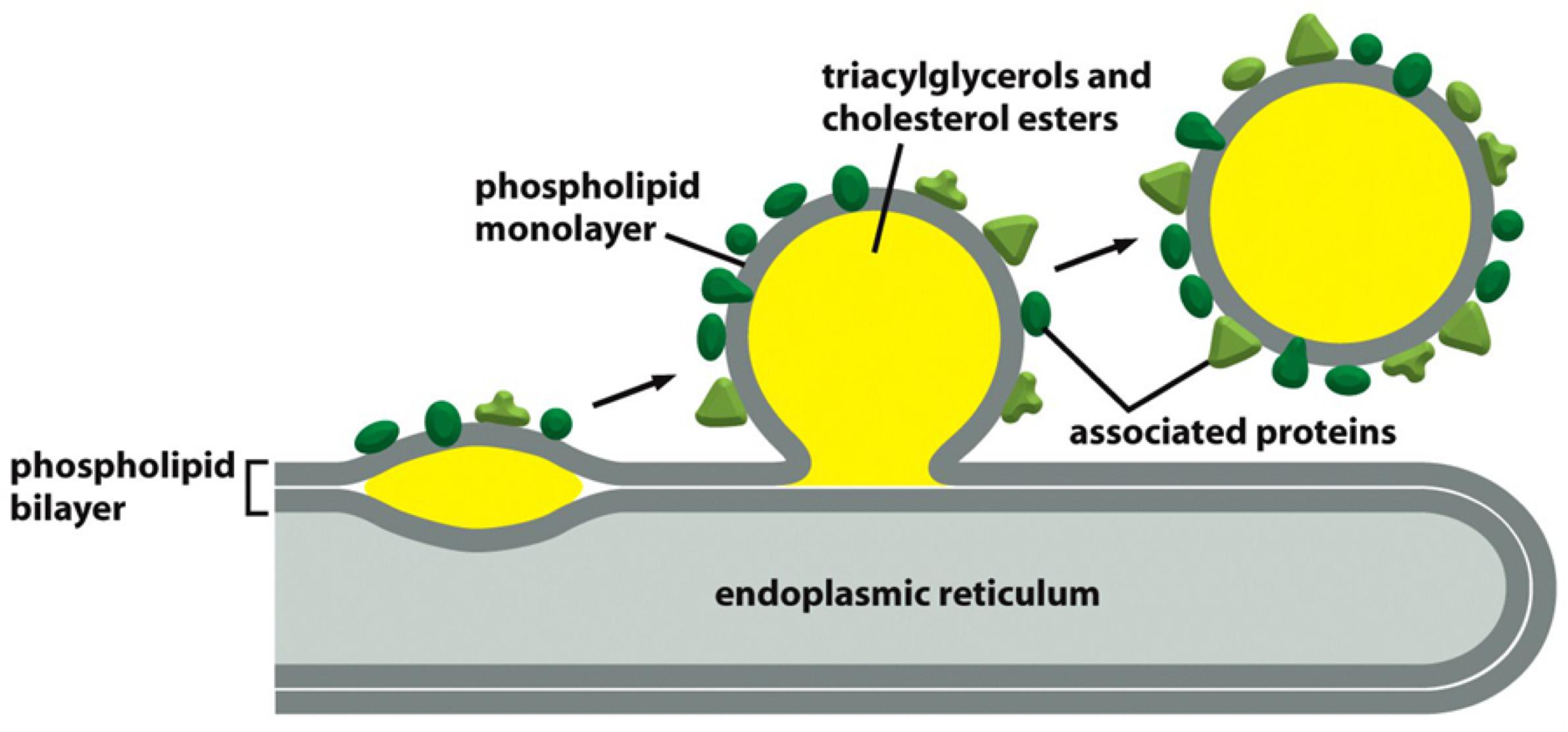
Most cells store an excess of lipids in lipid droplets, from where they can be retrieved as building blocks for membrane synthesis or as a food source
Lipid droplets store neutral lipids, such as triacylglycerols and cholesterol esters, which are synthesized from fatty acids and cholesterol by enzymes in the endoplasmic reticulum membrane
- Because these lipids do not contain hydrophilic head groups, they are exclusively hydrophobic molecules, and therefore aggregate into three-dimensional droplets rather than into bilayers
Lipid droplets form rapidly when cells are exposed to high concentrations of fatty acids.
Lipid droplets are surrounded by a lipid monolayer.
- store neutral triacylglycerides(三酰基甘油酯) and cholesterol esters.
- They are surrounded by monolayer of phospholipids and a variety of proteins, some of which are important for lipid metabolism.
- Made in the Endoplasmic Reticulum(ER)