L09 Metabolism of Nitrogenous Compounds I Principles of Biosynthesis, Utilization, and Turnover
Metabolism of Nitrogenous Compounds I: Principles of Biosynthesis, Utilization, and Turnover
- 含氮化合物代谢Ⅰ:生物合成,利用与周转
一、Utilization of Ammonia: Biogenesis of Organic Nitrogen
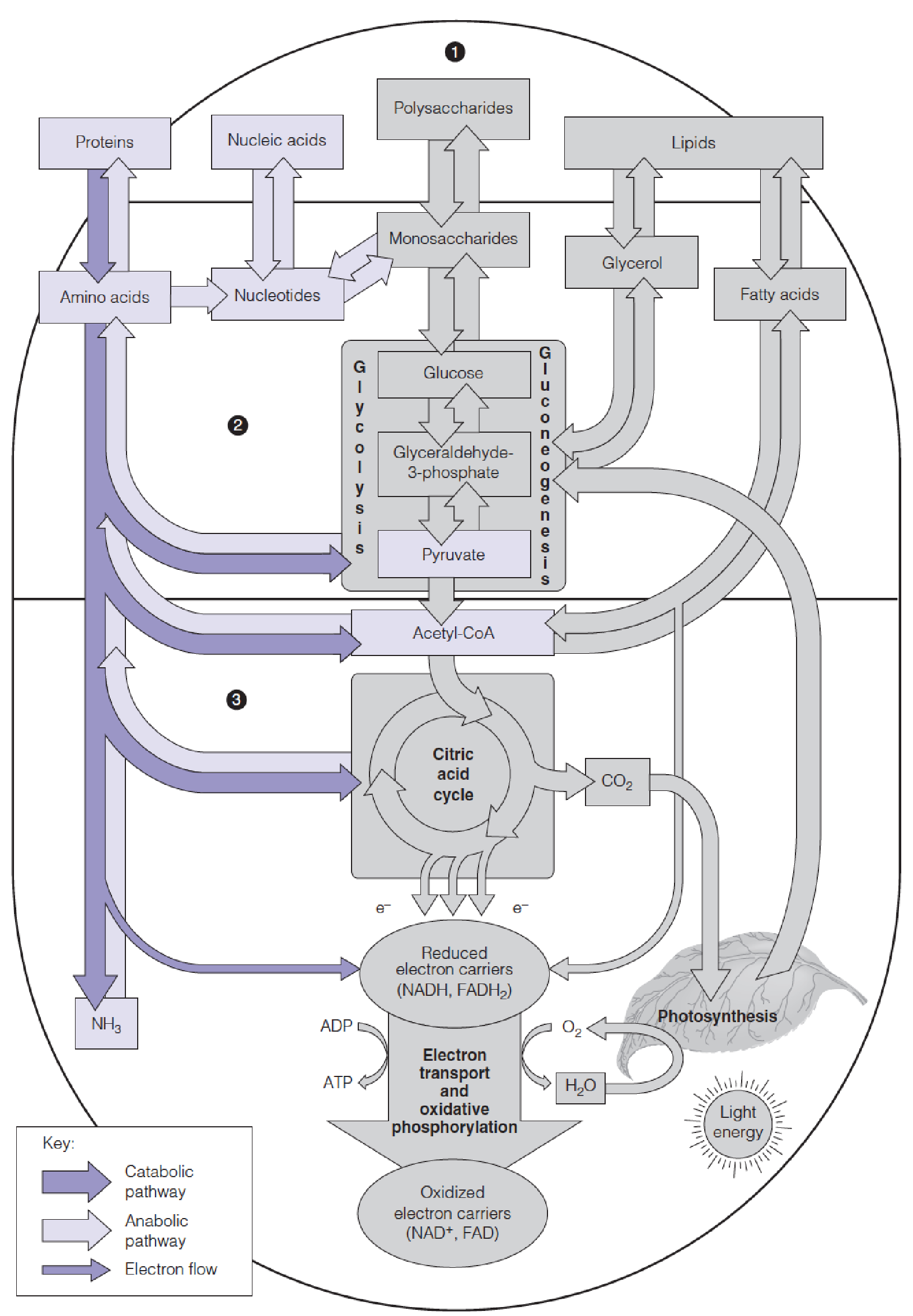
- Pathways of nitrogen metabolism (purple) in the general pattern of intermediary metabolism
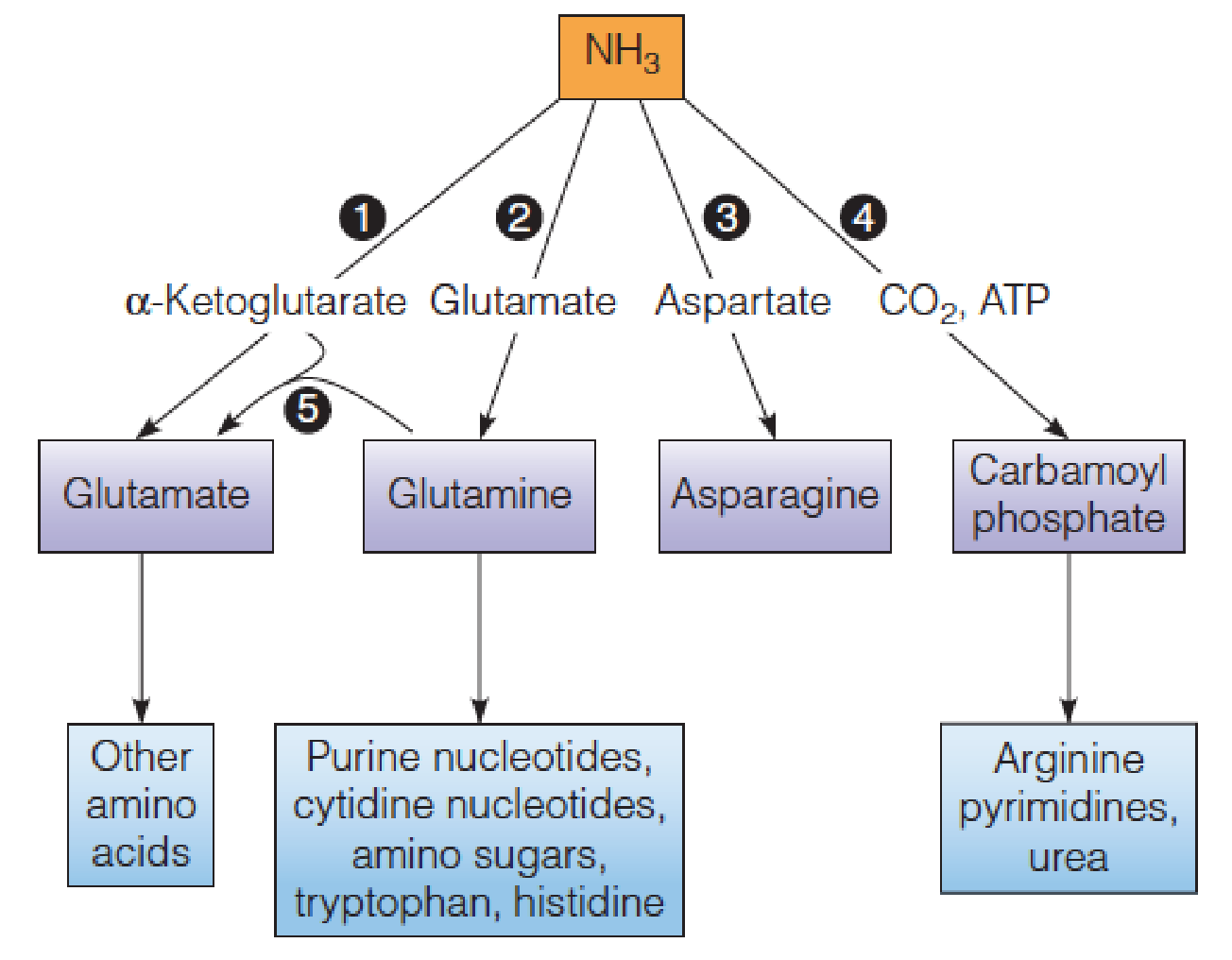
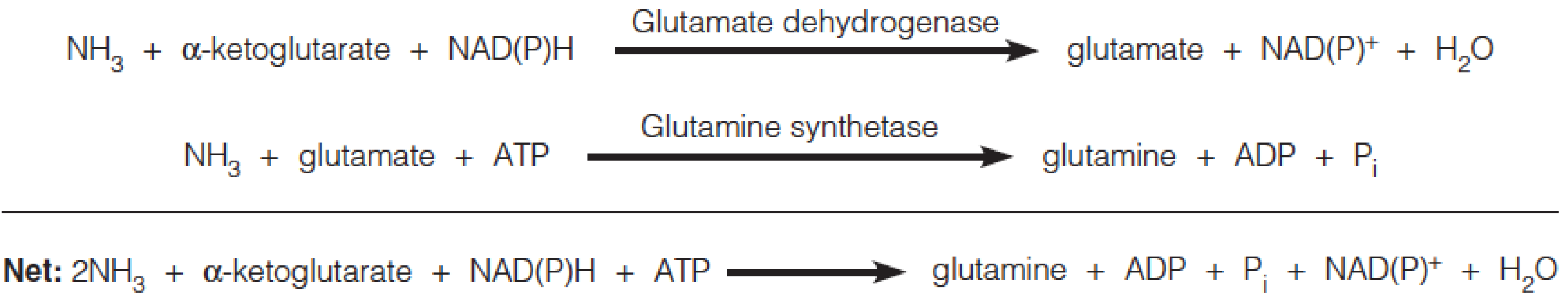
- Reactions in assimilation of ammonia and major fates of the fixed nitrogen:
- Glutamate dehydrogenase
- Glutamine synthetase
- Asparagine synthetase
- Carbamoyl phosphate synthetase (氨甲酰磷酸合成酶)
- Glutamate synthase.
- In animals, glutamine, rather than NH3, is the chief nitrogen source for pyrimidines (嘧啶).
Ammonia in high concentrations is quite toxic;
- At lower concentrations, it is a central metabolite, serving as substrate for four enzymes that convert it to various organic compounds;
All organisms assimilate ammonia via reactions leading to glutamate, glutamine, asparagine, or carbamoyl phosphate;
- Carbamoyl phosphate is used only in the biosynthesis of arginine, urea, and the pyrimidine nucleotides;
- Most of the nitrogen that finds its way from ammonia to amino acids glutamate and glutamine;
The α-amino nitrogen of glutamate and the side chain amide nitrogen of glutamine are both primary source of N in biosynthetic pathways.

Glutamate dehydrogenase
Glutamate dehydrogenase catalyzes the reductive amination of α-ketoglutarate(α-酮戊二酸)
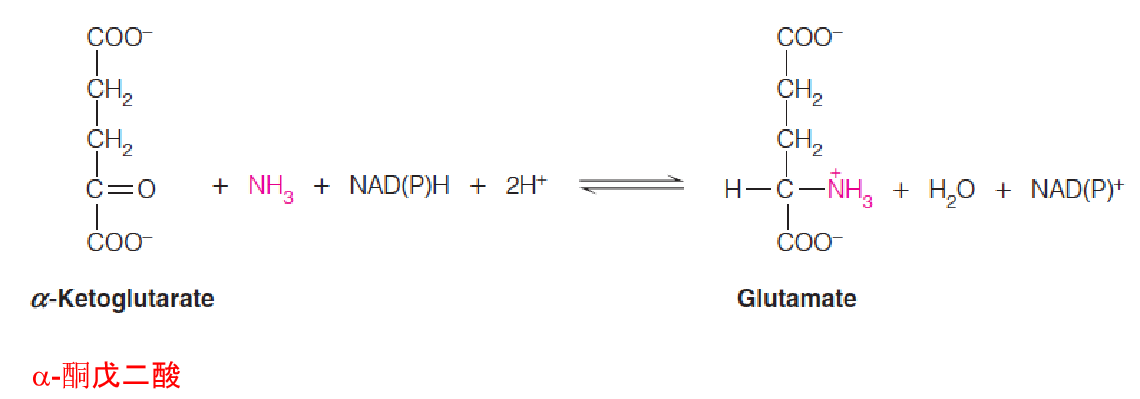
This reaction is reversible;
In bacteria
- Use NADPH;
- this enzyme has a high K
mfor ammonia (1 mM) - primarily the direction of glutamate formation;
- Bacteria growing with ammonia as their sole nitrogen source use this reaction as the primary route for nitrogen assimilation;
In animal cells
- the reaction occurs in both directions, primarily the formation of α-ketoglutarate ➔citric acid cycle ➔ATP production ;
- Animal cells use both NAD+ and NADP+ as cofactor;
- The animal enzyme is allosterically controlled; α-ketoglutarate synthesis is inhibited by GTP and ATP and stimulated by ADP. Thus the enzyme is activated under conditions of low energy charge.
Glutamate synthase

- A related enzyme that occurs only in microorganisms, plants, and lower eukaryotes, glutamate synthase, catalyzes a reaction comparable to that catalyzed by glutamate dehydrogenase but functions primarily in glutamate biosynthesis:
- The amide N of glutamine replaces NH3 as the N donor
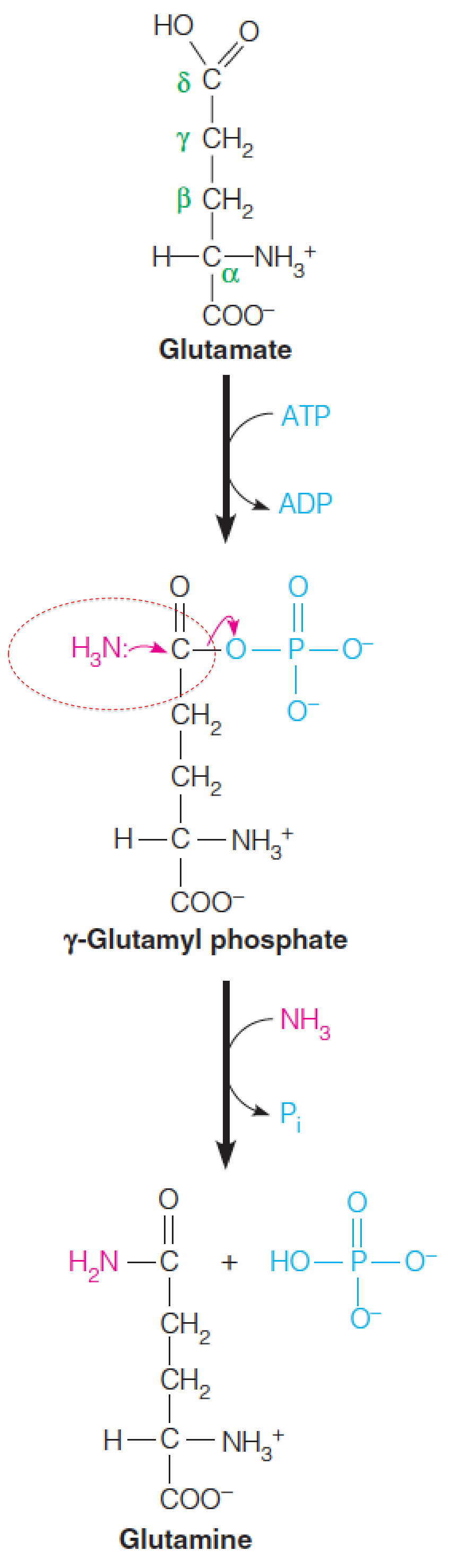
Glutamine synthetase (GS)
Whether formed by action of glutamate dehydrogenase or glutamate synthase, or by transamination, glutamate can accept a second ammonia moiety to form glutamine in the reaction catalyzed by glutamine synthetase (谷氨酰胺合成酶).
Mn2+ is required.
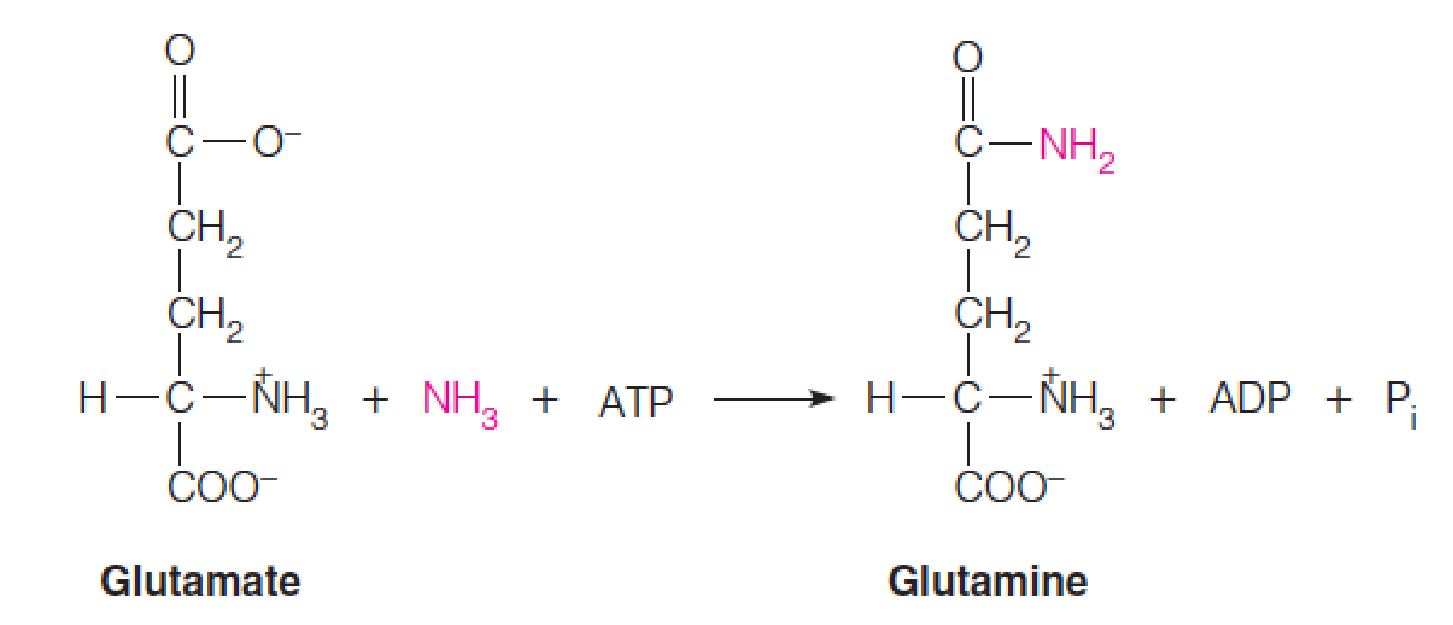
The glutamine synthetase reaction occurs via an acyl phosphate intermediate (酰基磷酸中间物).
ATP phosphorylates the δ-carboxylate of glutamate to give a carboxylic-phosphoric acid anhydride (γ-glutamyl phosphate), which undergoes nucleophilic attack by the nitrogen of ammonia to give the amide product, glutamine.
1. Structure of GS
Structure of Salmonella typhimurium (鼠伤寒沙门氏菌) glutamine synthetase (GS):
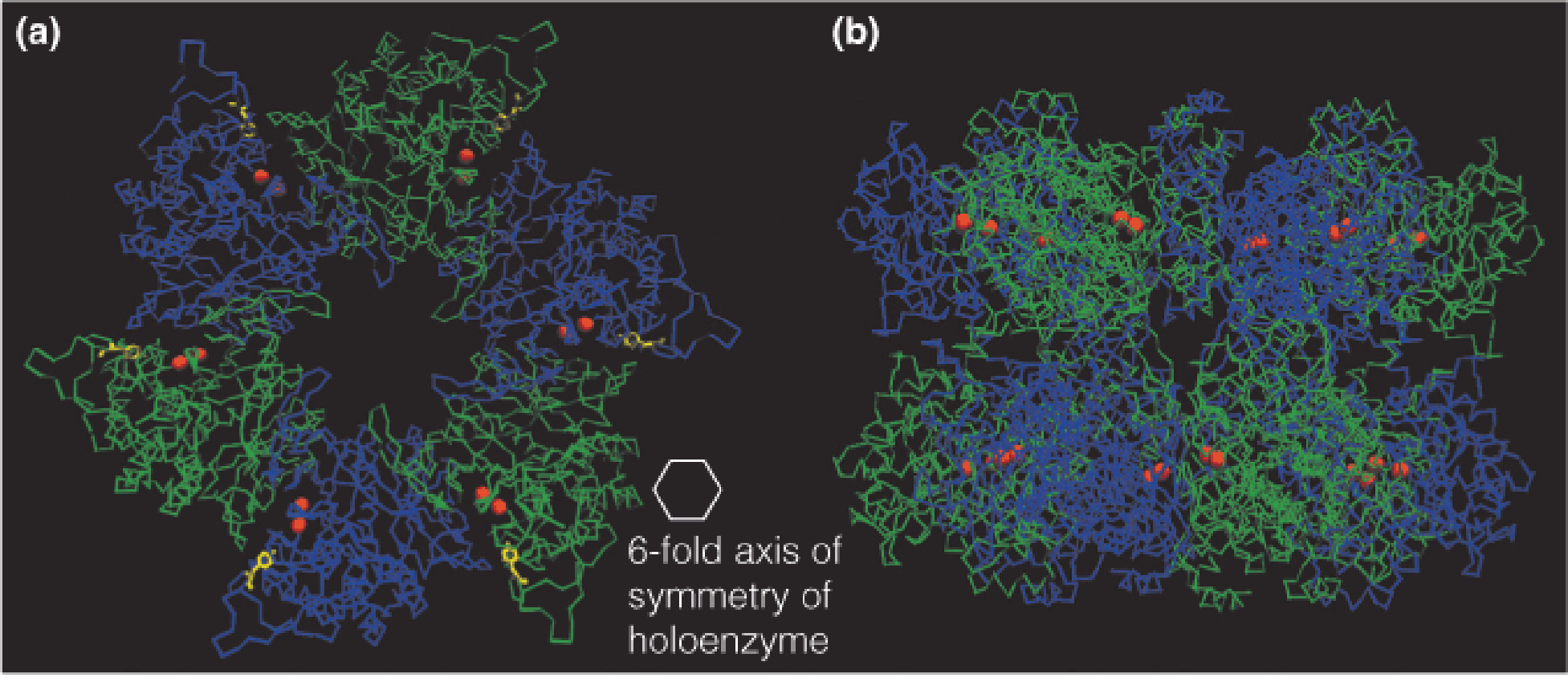
- Bacterial glutamine synthetase is a dodecamer, with 12 identical subunits forming two facing hexagonal arrays.
2. Regulation of Glutamine Synthetase (GS)
Prokaryotic
Prokaryotic glutamine synthetase is controlled by:
Cumulative feedback inhibition
- Involves the action of eight specific feedback inhibitors.
- Metabolic end products of glutamine (trp, his, glucosamine-6-phosphate (葡糖胺-6-磷酸), carbamoyl phosphate, CTP, and AMP)
- Indicators of the general status of amino acid metabolism (ala, gly)
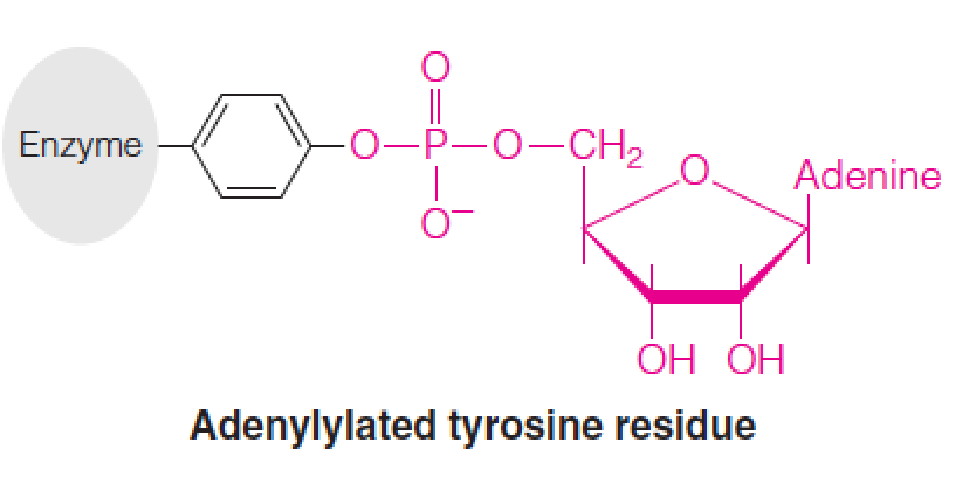
Covalent modification
- Adenylylation (腺苷酰化) of a specific tyrosine residue (Tyr 397)
Regulation of the activity of bacterial glutamine synthetase (GS):
- The complex of AT (adenylyltransferase, 腺苷转移酶) and (a regulatory protein, P
II) catalyzes both the adenylylation and deadenylylation of glutamine synthetase (GS), depending on whether PIIis deuridylylated (脱脲基) (left) or uridylylated (尿酰化) (right).
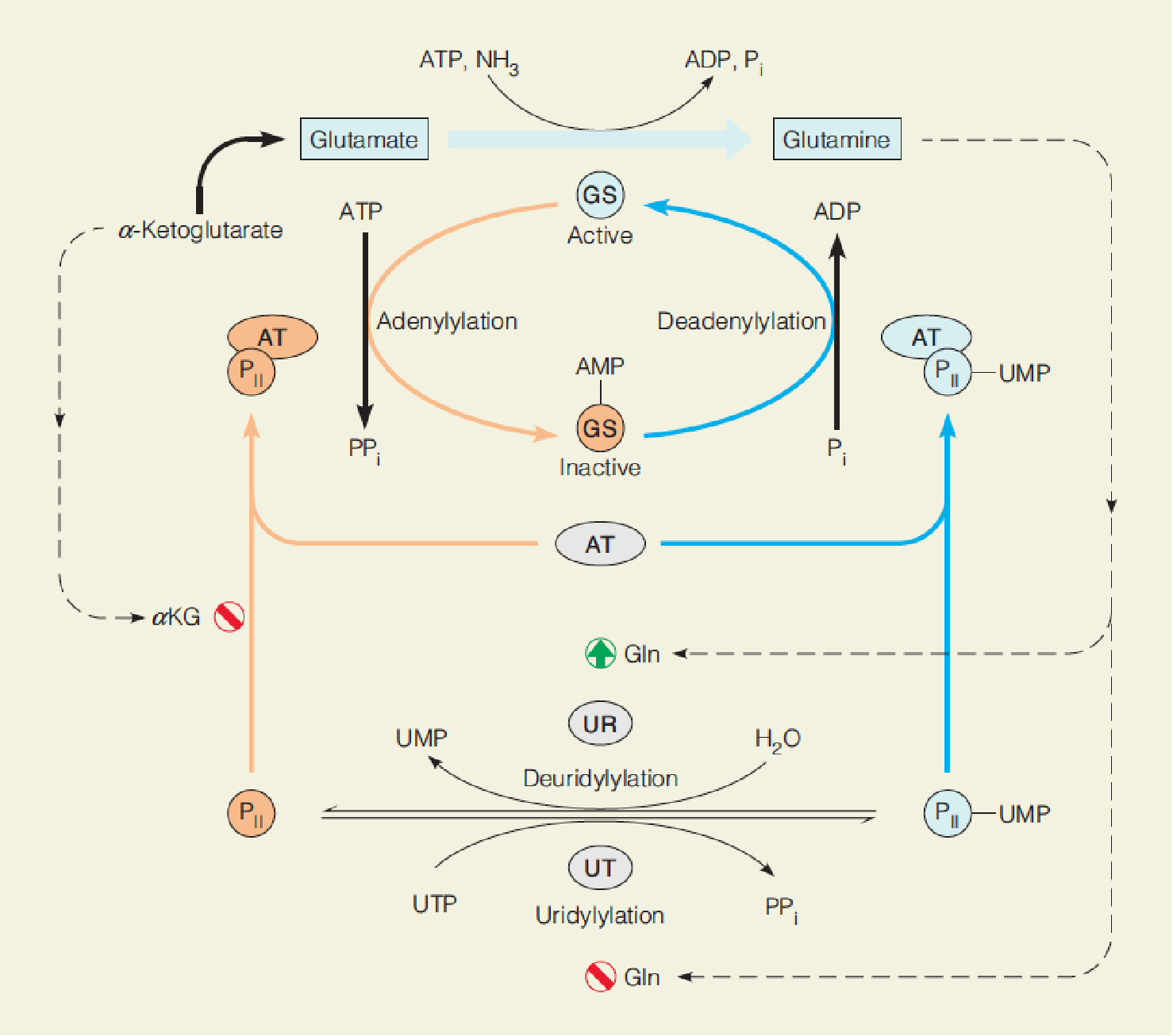
- Uridylylation of P
IIis catalyzed by uridylyltransferase (尿酸转移酶, UT), and deuridylylation is catalyzed by uridylyl-removing enzyme (UR), both activities part of a bifunctional enzyme.
- Adenylation (腺苷酸化): Tyr 397 react with ATP to form an ester, inactivates the enzyme;
- Adenylylation and deadenylylation of GS are catalyzed by the same enzyme: complex of adenylyltransferase (腺苷酸转移酶, AT) and a small regulatory protein, P
II; - UMP-PII and P
IIcontrols adenylation or deadenylylation, respectively;- Reversible uridylylation of PII is catalyzed by another enzyme, a bifunctional uridylyltransferase (UT)/uridylyl-removing enzyme (UR), in response to intracellular glutamate concentrations.
- UT: PII ➔ UMP-PII ➔ GS adenylylation ↓ ➔ GS activity ↑ ;
- UR: UMP-PII ➔ PII ➔ GS adenylylation ↑ ➔ GS activity ↓;
- PII-UMP is deuridylylated by hydrolysis of the UMP residues, catalyzed by uridylyl-removing activity of the bifunctional UT/UR;
- Glutamine ↑ ➔ UT↓ UR↑ ➔ PII ↑ ➔ PII -AT ↑ ➔ GS adenylylation ↑ ➔ GS activity ↓ ➔ glutamine↓;
- Glutamine ↓ ➔ UT ↑ UR↓ ➔ AMP-PII ↑ ➔ AMP-PII -AT ↑ ➔ GS adenylylation ↓ ➔ GS activity ↑ ➔ glutamine ↑;
- α-ketoglutarate ↑ ➔ PII -AT ↓ ➔ GS activity ↑ ➔ glutamine ↑.
- Reversible uridylylation of PII is catalyzed by another enzyme, a bifunctional uridylyltransferase (UT)/uridylyl-removing enzyme (UR), in response to intracellular glutamate concentrations.
- Adenylylation and deadenylylation of GS are catalyzed by the same enzyme: complex of adenylyltransferase (腺苷酸转移酶, AT) and a small regulatory protein, P
Glutamine plays a central role in nitrogen metabolism
The amide nitrogen is used in biosynthesis of several amino acids (including glutamic acid, tryptophan (trp), histidine), purine and pyrimidine nucleotides, and amino sugars.
In animals, glutamine synthetase is a key participant in detoxifying ammonia formed from amino acid catabolism, particularly in brain cells. Glutamate and glutamine are two of the most abundant free amino acids in brain cells; accumulation of these amino acids can deplete α-ketoglutarate, their principal precursors, thereby interfering with the citric acid cycle and energy generation.
Glutamine participates in ammonia excretion in the kidney
3. glutamate dehydrogenase/glutamine synthetase
If NH4+ levels are high, the glutamate dehydrogenase/glutamine synthetase pathway operates:
- To assimilate NH4+ in bacteria, glutamine synthetase functions together with glutamate dehydrogenase or glutamate synthase, depending on ammonia levels.
Asparagine Synthetase
Asparagine Synthetase: A similar amidation Reaction
- Catalyzes a reaction comparable to that of glutamine synthetase.
- Although widespread, asparagine synthetase accounts for much less ammonia assimilation.
- This enzyme uses ammonia or glutamine in catalyzing the conversion of aspartate to asparagine.

- Glutamine synthetase: ATP to ADP;
- Asparagine synthetase: ATP to AMP
Carbamoyl phosphate synthetase
The final route for assimilating ammonia first forms carbamoyl phosphate.
- The enzyme responsible is carbamoyl phosphate synthetase (氨甲酰磷酸合成酶).
Either ammonia or glutamine can serve as the nitrogen donor:
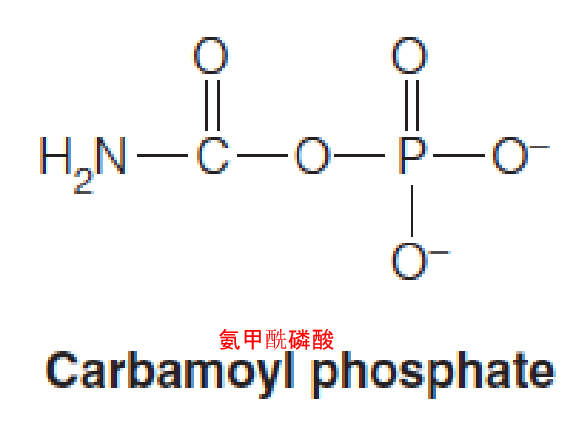

- Carbamoyl phosphate: generation of an intermediate for arginine (urea) (CPSI, mitochondria) and pyrimidine synthesis (CPSII, cytosol)
二、The Nitrogen Economy: Aspects of Amino Acid Synthesis and Degradation
Overview
- Nitrogen equilibrium:
- Normal nitrogen balance: the daily intake of nitrogen throughout diet is equal to that lost through urinary excretion and other processes, such as ammonia in sweat;
- Positive nitrogen balance: pregnancy, growth of a juvenile, or recovery from starvation;
- Negative nitrogen balance: senescence(衰老), starvation, and certain diseases states.
Although mammals can synthesize Arg and Met, the use of these amino acids for the production of urea and methyl groups, respectively, is greater than the capacity of their biosynthetic pathways;
- 50-100 grams per day;
- Nutritional quality of proteins.
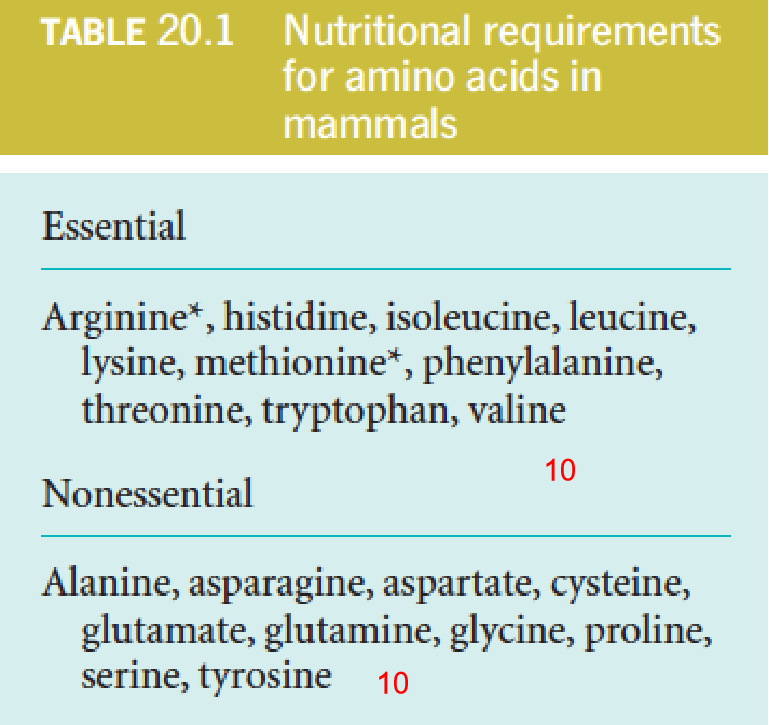
Essential and nonessential amino acids:
Transamination
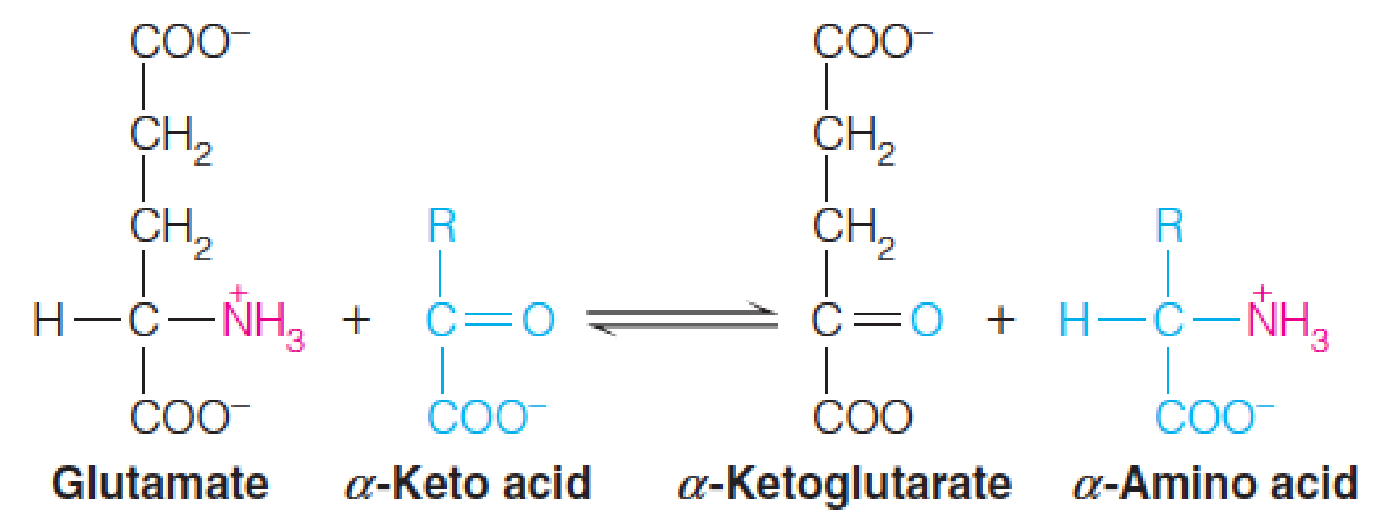
1. The Process
Transamination (转氨基) is the reversible transfer of an amino group from an α-amino acid to an α-keto acid, with pyridoxal phosphate (磷酸吡哆醛, Vitamin B6) as a coenzyme.
- Therefore transamination needs Vitamin B
6
2. The functions of transamination
- A process whereby amino acids can replenish citric acid cycle intermediates; Funnel excess amino acids towards catabolism and energy generation
- Redistribution of amino acid nitrogen;
- Bridge the amino acids composition into in line with the organism’s needs;
Because of the key role of glutamate in ammonia assimilation, it is a star player in transamination. Transamination uses glutamate nitrogen to synthesize other amino acids.
3. Aminotransferase 转氨酶
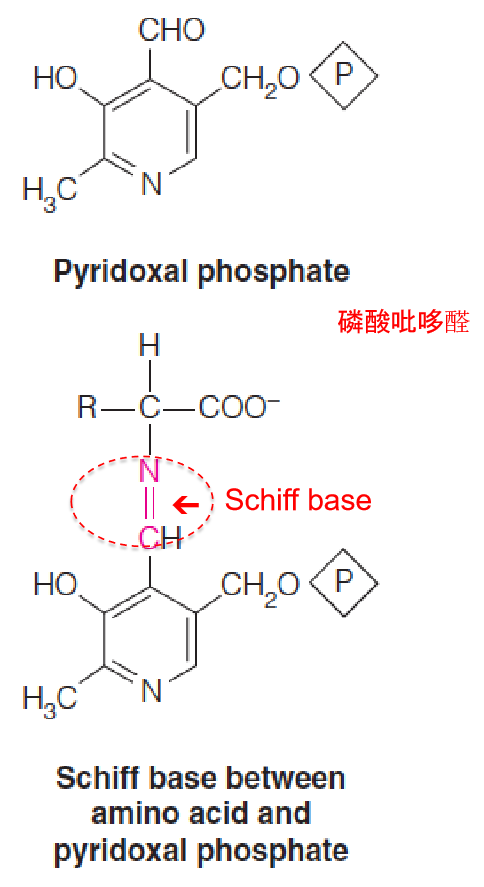
Aminotransferases utilize a coenzyme, pyridoxal phosphate (磷酸吡哆醛), that is derived from vitamin B6.
The functional part of the cofactor is an aldehyde functional group, CHO, attached to a pyridine ring (吡啶环).
The catalytic cycle begins with condensation of this aldehyde with the α-amino group of an amino acid, to give a Schiff base (席夫碱), or aldimine (醛亚胺), intermediate.
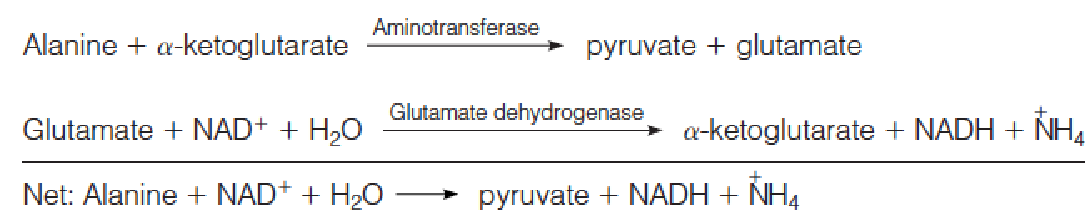
4. The bi-directional feature of transamination
Transamination can be used for amino acid synthesis and also for degradation of amino acids that accumulate in excess of need.
In degradation the transaminase works in concert with glutamate dehydrogenase, as exemplified by the degradation of alanine:
Most aminotransferases use glutamate/α-ketoglutarate as one of the two α-amino/α-keto acid pairs involved.
Two such enzymes are important in the clinical diagnosis of human disease —serum glutamate-oxaloacetate transaminase (SGOT, 谷氨酸 - 草酰乙酸转氨酶) and serum glutamate-pyruvate transaminase (SGPT, 谷氨酸 - 丙酮酸转氨酶):
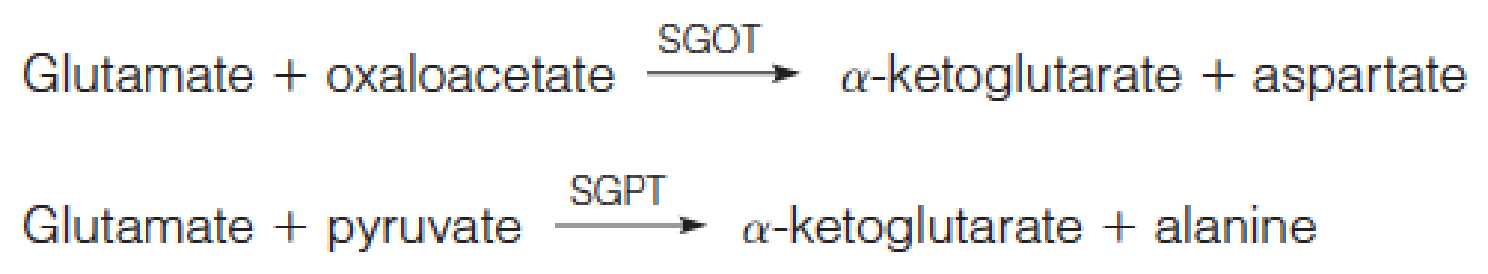
- S指的是serum,正常情况下GOT和GPT一般存在与肝脏
- SGOT和SGPT是检测肝脏功能的重要指标
These enzymes, abundant in heart and in liver, are released from cells as part of the cell injury that occurs in myocardial infarction (心肌梗死), infectious hepatitis (传染性肝炎), or other damage to either organ.
Assays of these enzyme activities in blood serum can be used both in diagnosis and in monitoring the progress of a patient.
三、Protein Turnover
- 蛋白质周转
Proteins are subject to continuous biosynthesis and degradation, a process called protein turnover.
- For an intracellular protein whose total concentration does not change with time, the steady state level is maintained by synthesis of the protein at a rate just sufficient to replenish protein lost by degradation.
Many of the amino acids released during protein turnover are reutilized in the synthesis of new proteins.
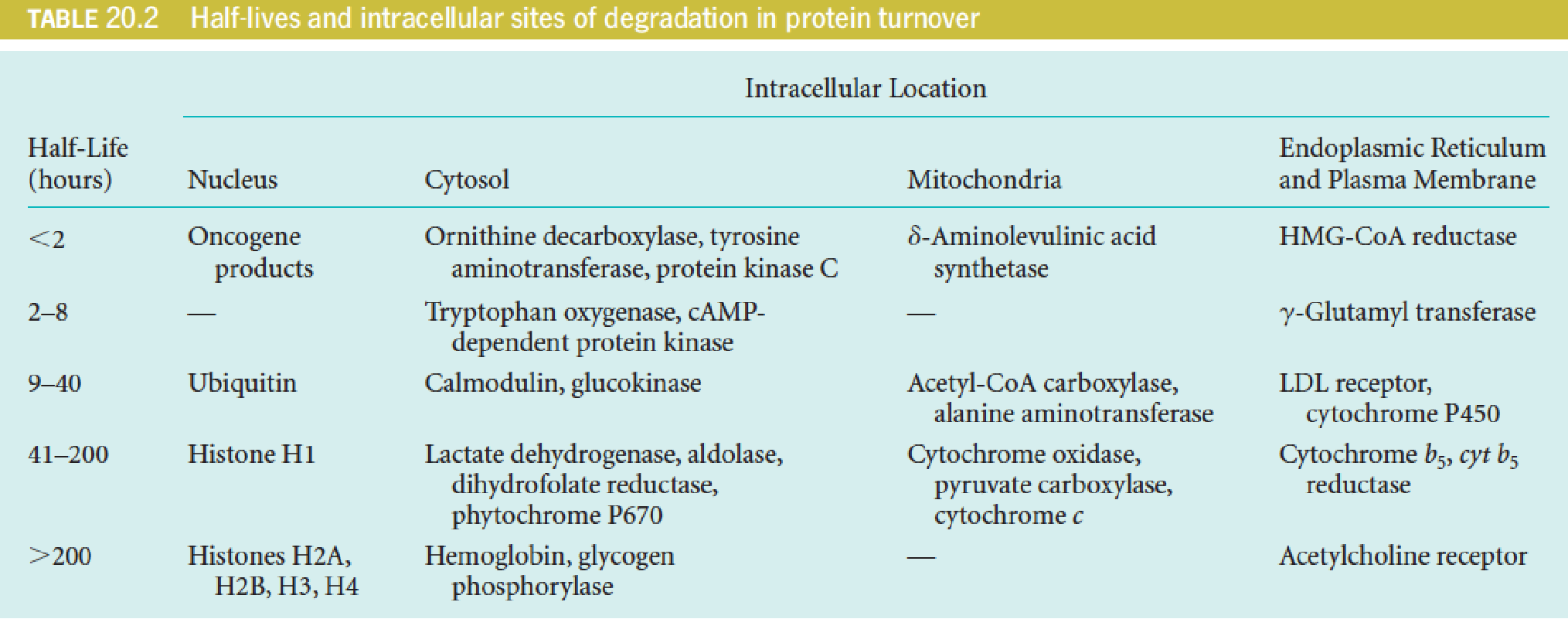
Chemical Signals for Turnover
The turnover rates for different proteins vary by as much as 1000-fold, whereas differences in protein stability, as measured by denaturation in vitro, may be much less.
Chemical Signals for Turnover:
- Ubiquitination (泛素化)
- Metal-catalyzed oxidation of particular residues
- PEST sequences (12-60 aa)
- particular N-terminal residues
1. Ubiquitination
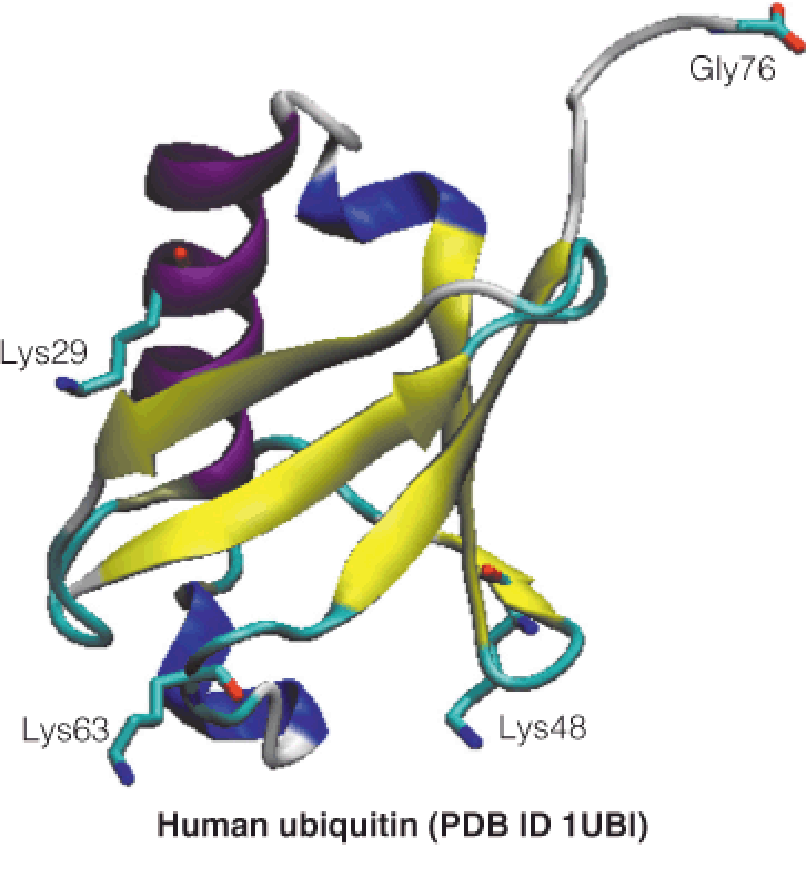
Ubiquitin is a small (76-residue) protein expressed in all eukaryotic cells—it derives its name from its widespread (ubiquitous) distribution.
Ubiquitin is covalently conjugated to specific cellular proteins in an ATP-dependent reaction, which condenses the terminal carboxyl group of ubiquitin with lysine amino groups on target proteins.
- In the first reaction, catalyzed by the ubiquitin-activating enzyme E1, a thioester is formed between the C-terminal Gly residue of ubiquitin and an internal Cys residue of E1 in a two-step process.
- Formation of this high-energy covalent linkage is driven by ATP hydrolysis, via a ubiquitin-adenylate intermediate.
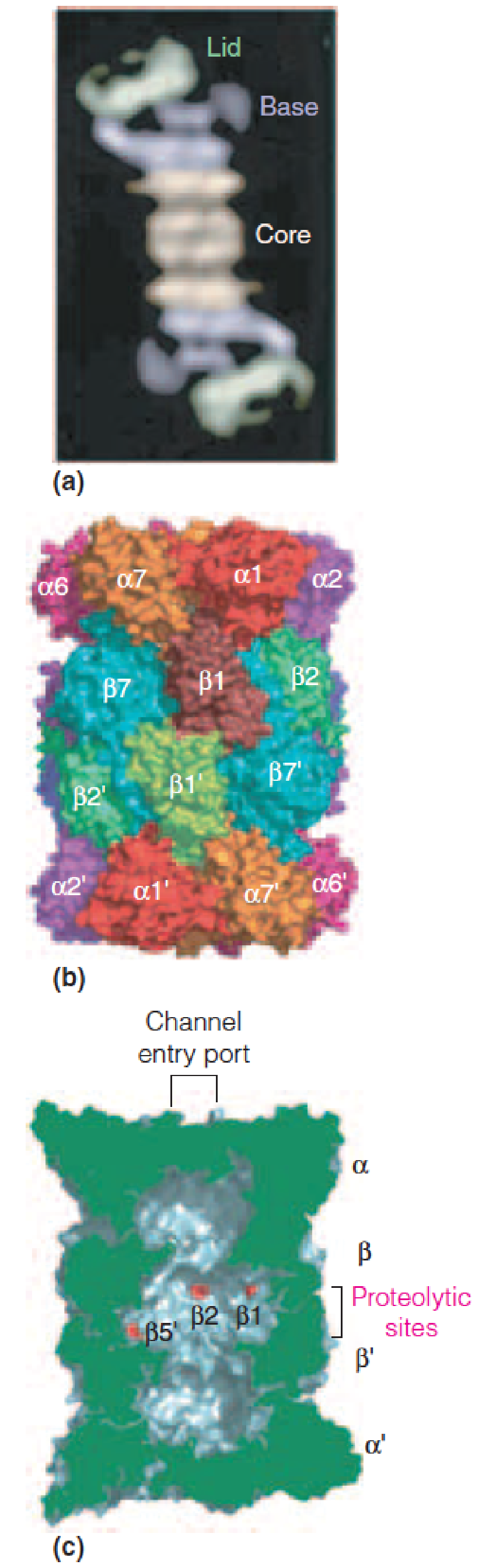
2. Structure of the proteasome (26S) (蛋白酶体)
Structure of the proteasome (26S):
The proteasome contains two major assemblies, a 28-subunit core particle (aka the 20S particle) and a 19-subunit regulatory particle (aka the 19S particle), which forms the base and lid assemblies.
- The proteolytic active sites are located within the large internal space (~100 x 60Å) of the 20S core particle.
- The lid and base assemblies of the 19S regulatory particle control substrate entry into the 20S core particle.
Proteins tagged with the ubiquitin pass through the tube in an ATP-dependent fashion.
3. The process of polyubiquitination
Enzymatic pathway for attachment of ubiquitin to target proteins:
E1, E2, and E3 are proteins involved in the ubiquitin transfer to lysine residues on target proteins.

In the first reaction, catalyzed by the ubiquitin-activating enzyme E1, a thioester is formed between the C-terminal Gly residue of ubiquitin and an internal Cys residue of E1 in a two-step process;
- Formation of this high energy covalent linkage is driven by ATP hydrolysis, via a ubiquitin-adenylate intermediate. AMP is released upon nucleophilic attack by the Cys-SH of E1;
The activated ubiquitin is then transferred to a Cys-SH of one of several E2 enzymes (ubiquitin-conjugating enzymes) in a transthiolation reaction;
A ubiquitin-protein ligase, E3, is required for step 3—covalent attachment of ubiquitin of the $\varepsilon$-NH3+ group of one or more lysine residues on the target protein via an isopeptide bond;
Expenditure of the equivalent of two ATPs per ubiquitin added ensures that the reaction is both irreversible and specific;
E3 can catalyze the successive addition of a ubiquitin moiety to a previously conjugated ubiquitin, forming a polyubiquitin chain in which a Lys of ubiquitin forms an isopeptide bond with the C-terminal Gly –COOH of the succeeding ubiquitin;
Ubiquitin contains seven lysine residues, and polyubiquitination chains can involve one or more of these residues;
Two major families of E3 ubiquitin-protein ligases have been described:
- the HECT domain-containing E3s
- the RING-finger domain-containing E3s.
- These two E3 families differ in structure and in mechanism;
E3 ligase plays a central role in determining the specificity and selectivity of the proteasome system, these proteins are implicated in a number of disease states, including neurodegenerative disorders, inflammatory diseases, muscle wasting disorders, and cancers.
- Only 2 E1 in mammals
- About 28 E2 in humans
- Maybe 1000 E3 in humans
- Deubiquiting enzymes(Dubs)
- SUMO
四、Amino Acid Degradation and Metabolism of Nitrogenous End Products
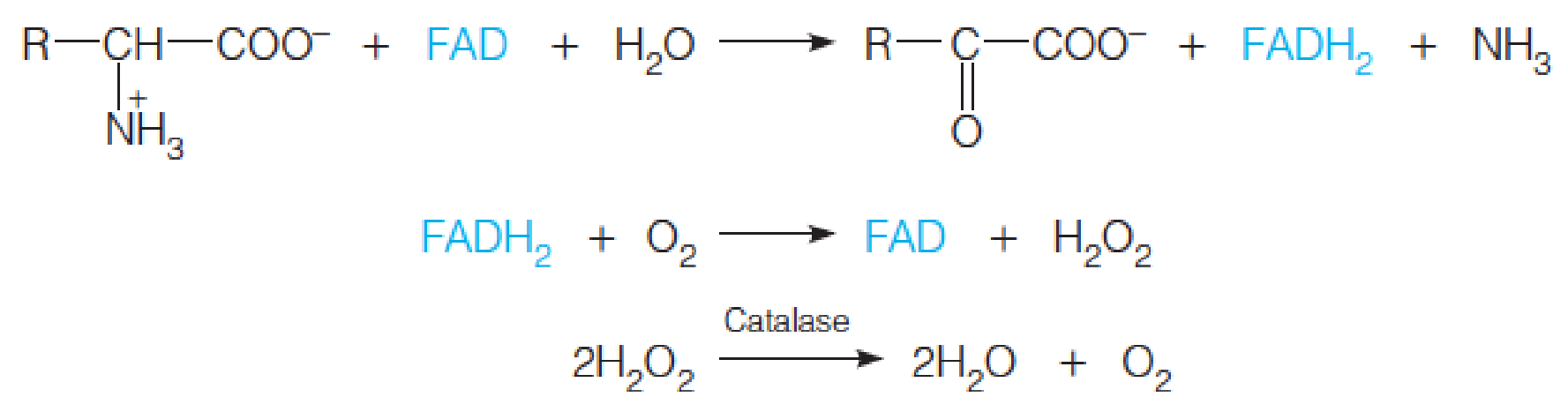
- Amino acid degradation usually begins with conversion to the corresponding α-keto acid by transamination or oxidative deamination.
- α-keto acid can enter the carbohydrate metabolism
Fates of the amino acid carbon skeletons
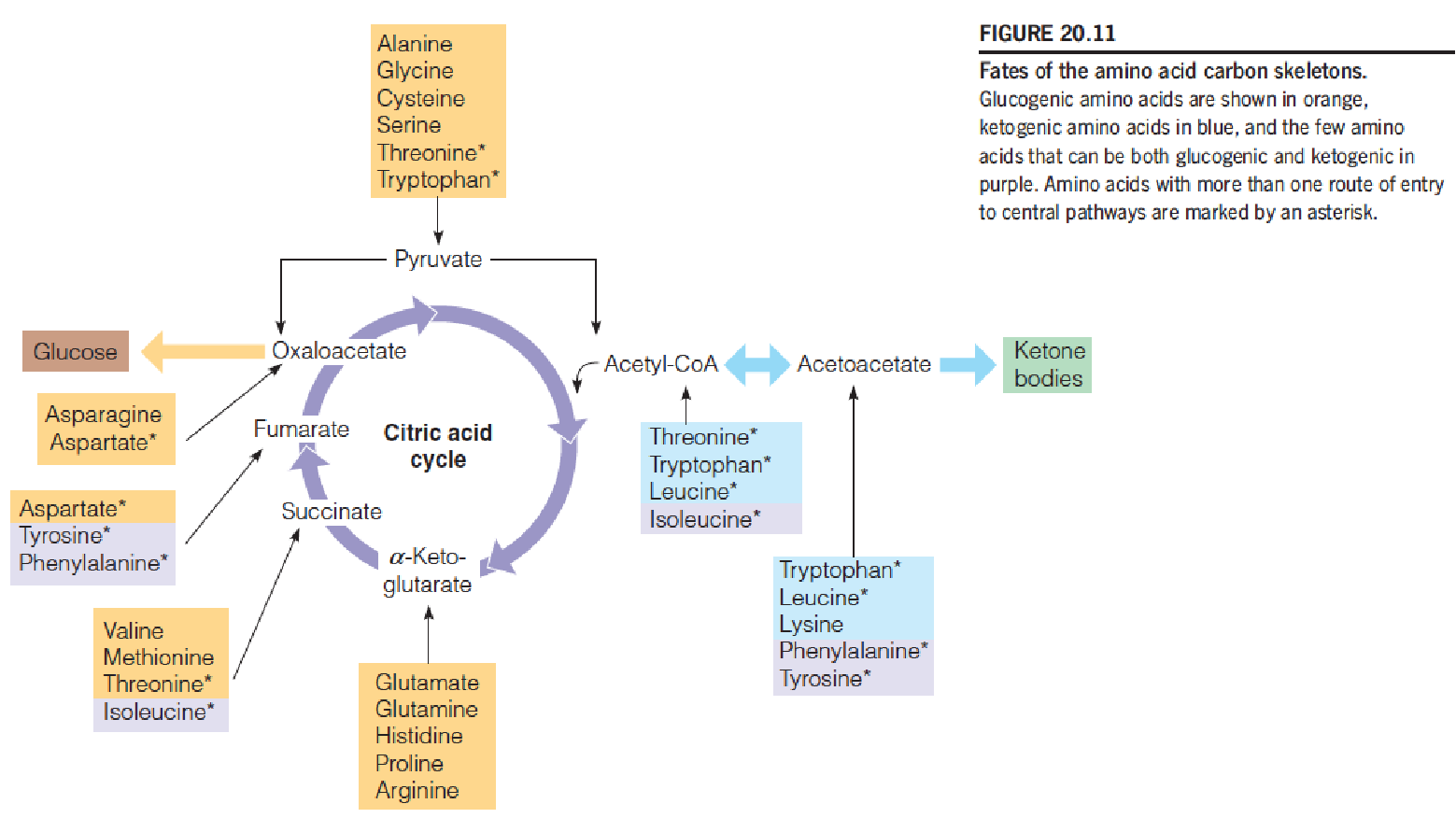
- Once the nitrogen has been removed, the carbon skeleton: citric acid cycle or converted to carbohydrates;
- Amino acids leading to acetyl-CoA or acetoacetyl-CoA, such as leucine, contribute heavily toward ketogenesis;
- Glucogenic(上图黄色标识amino acids) and ketogenic(上图蓝色标识amino acids),
- Ketogenic: 在动物体内Acetyl-CoA不能反向生成糖
Detoxification and Excretion of Ammonia
Although ammonia is a universal participant in amino acid synthesis and degradation, its accumulation in abnormal concentration has toxic consequences.
Animals have evolved pathways, adapted to their lifestyles, for excretion of ammonia, uric acid(尿酸), or urea as the major nitrogenous end product.
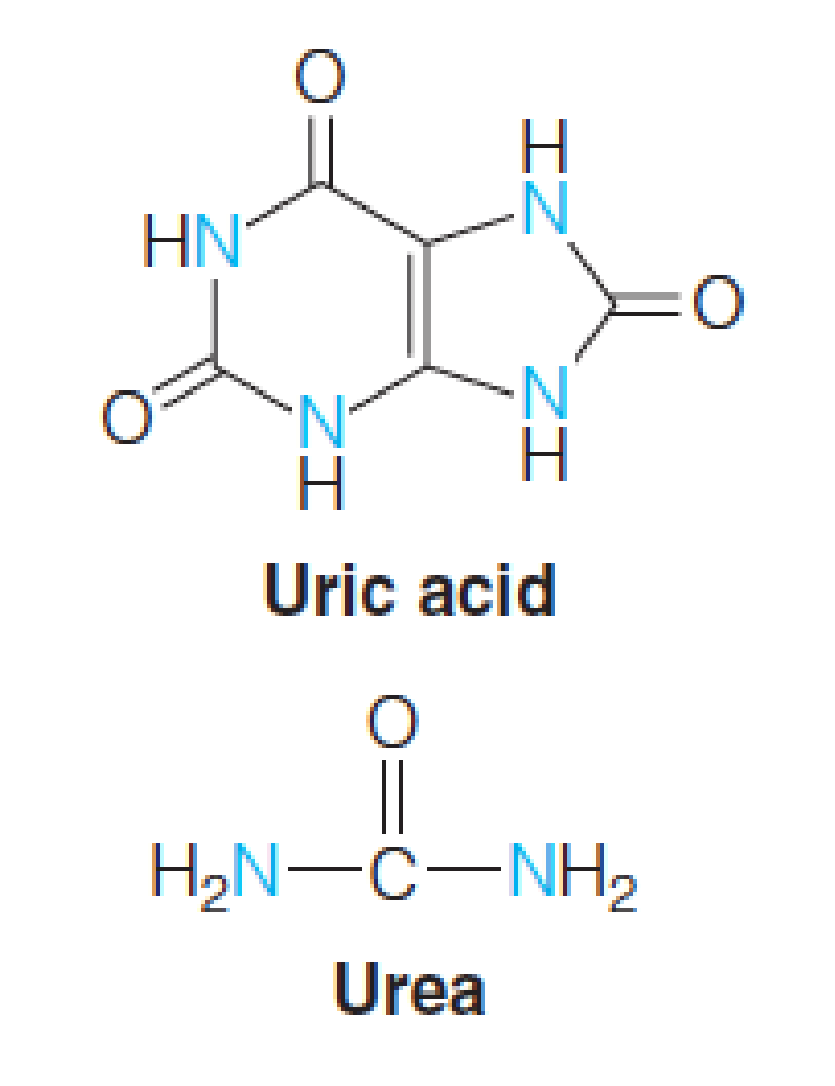
- 排氨的方式:
- Most aquatic animals: ammonia
- Terrestrial animals, birds and insects: uric acid 尿酸
- Mammals (except Dalmatian dog): urea 尿素
The Krebs-Henseleit Urea Cycle
Urea is synthesized almost exclusively in the liver and then transported to the kidneys for excretion.
The synthetic pathway, which is cyclic, was discovered by Hans Krebs and Kurt Henseleit in 1932.
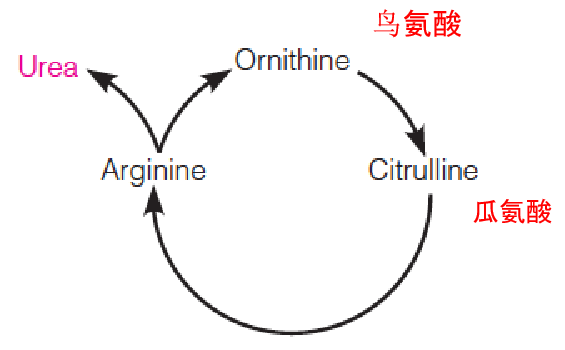
- Liver slices:
- Arginine: 30 fold more urea
- Ornithine(鸟氨酸) or citrulline(瓜氨酸)
The proposal was correct: isolation of the enzymes
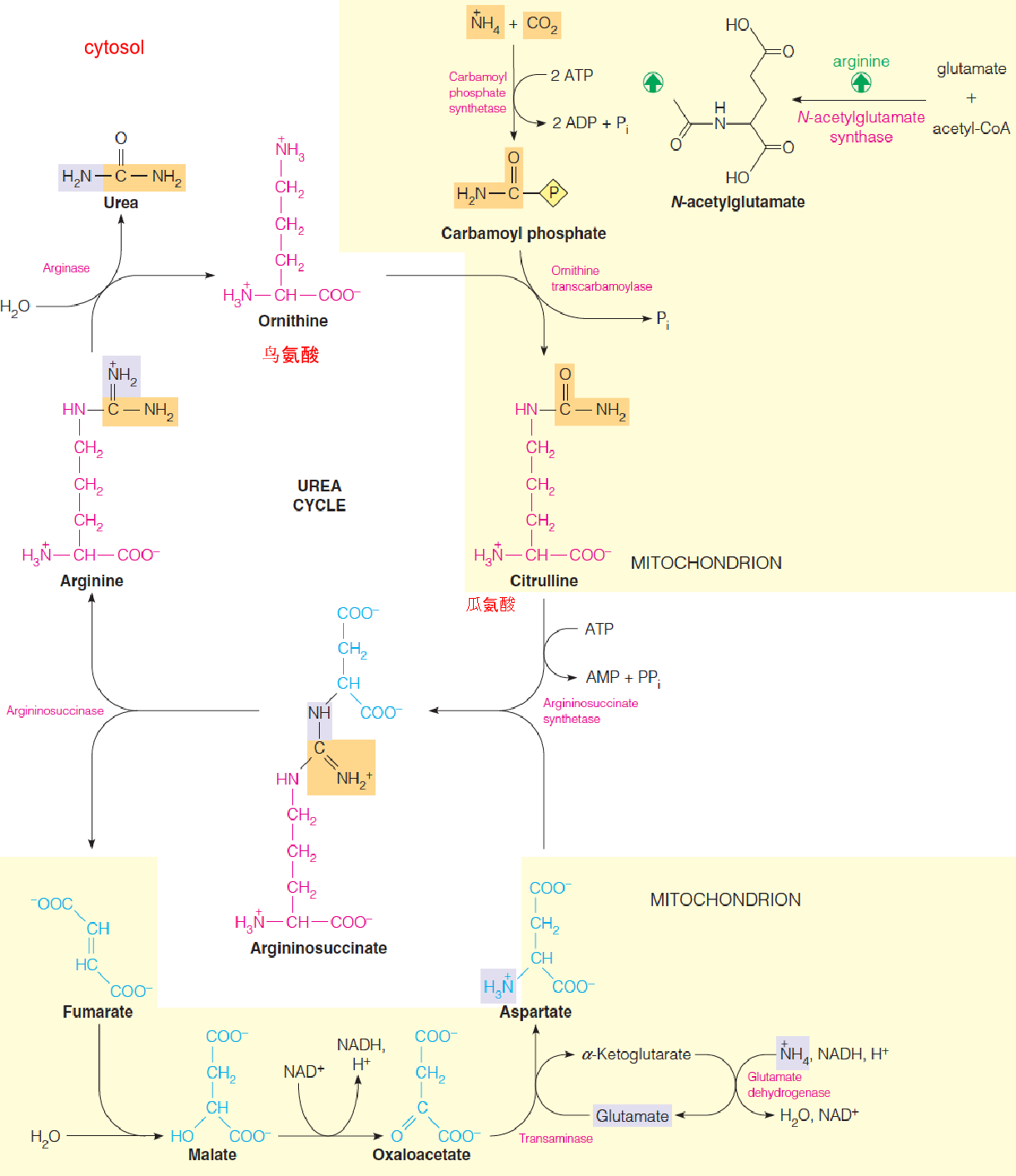
- In mitochondrion and cytosol of liver cells
- The carbon and one nitrogen atom in urea is carbamoyl phosphate, synthesized from NH4+ and CO2 by carbamoyl phosphate synthase (CPS I)
- The second nitrogen comes from aspartate, which reacts with citrulline to form argininosuccinate, through the action of argininosuccinate synthetase
- Transamination: aspartate, glutamate
Argininosuccinate synthetase mechanism
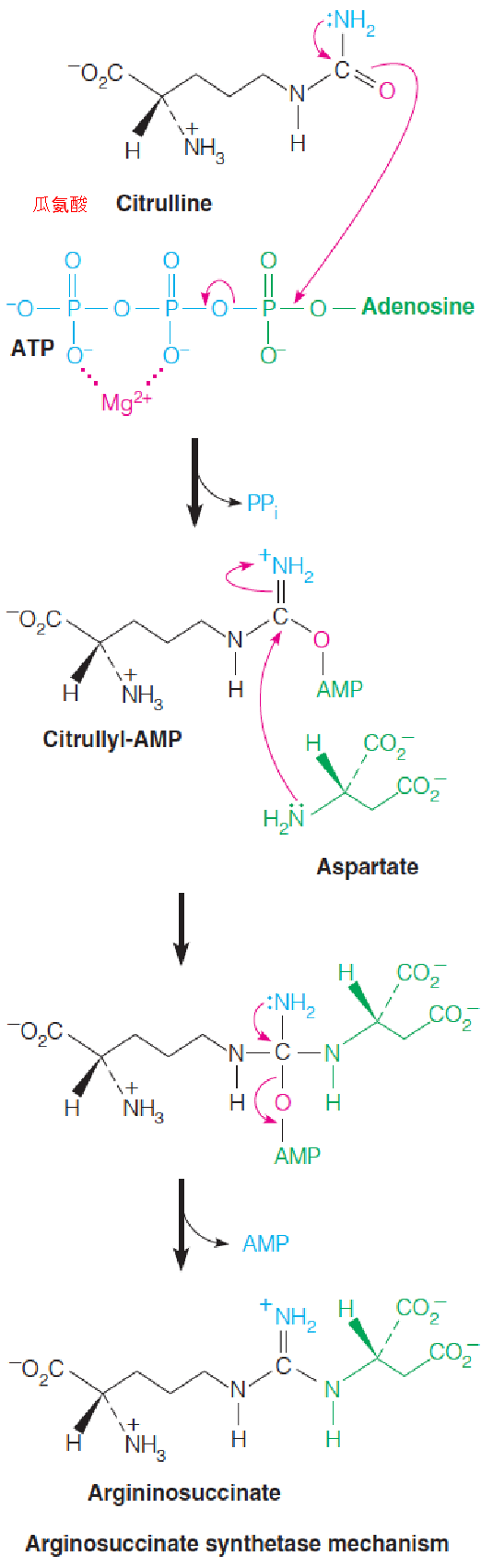
- The second nitrogen comes from aspartate, which reacts with citrulline to form argininosuccinate, through the action of argininosuccinate synthetase
- Argininosucciase cleaves argininosuccinate in a nonhydrolytic, nonoxidative reacton to give arginine and fumarate.
- Arginine is cleaved hydrolytically by arginase, to regenerate ornithine and yield one molecule of urea.
Urea is synthesized by an energy-requiring cyclic pathway that begins and ends with ornithine (鸟氨酸).
The net reaction for one turn of the urea cycle is as follows:
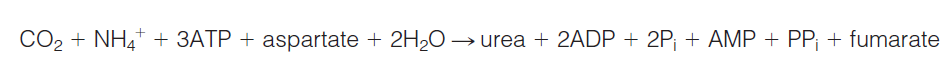
Four (not three) high-energy phosphates are consumed in each turn of the cycle
Regulation of Urea Synthesis
Long-term mechanism: high-protein diets, high enzymes levels; protein-free diet, enzymes reduced;
Short-term mechanism: Allosteric activation of CPS I by N-acetylglutamate, which is synthesized from glutamate by N-acetylglutamate synthase;
- amino acids degradation↑ ➔ glutamate↑ ➔ N-acetylglutamate↑ ➔ CPS I↑ ➔ urea synthesis↑;
CPS I is also regulated by covalent modification—acetylation of specific lysine residies inactivates the enzyme. SIRT 5 deacetylates CPS I and activates it.
Hyperammonemia
A defect in one of the five enzymes in the urea cycle ➔ hyperammonemia (high blood NH4+ levels);
Why is ammonia toxic?
- Ammonia depletes citric acid cycle intermediates (α-ketoglutarate) and NADH by overloading the glutamate dehydrogenase reaction ➔ an ATP deficiency;
The brain is especially susceptible to ammonia toxicity;
Urea is transported in the bloodstream to the kidneys for excretion. Measurement of blood urea nitrogen (BUN) levels provide a sensitive clinical test of kidney malfunction.
Blood ammonia measurements are a sensitive test of liver function. Liver damage, whether (hepatitis (肝炎), poisoning), reduces urea synthesis.
The accumulation of ammonia is toxic to the brain and thus is related to the comatose condition(昏迷状态) seen in advanced cases of chronic alcoholism.
1. Transport of Ammonia to the Liver
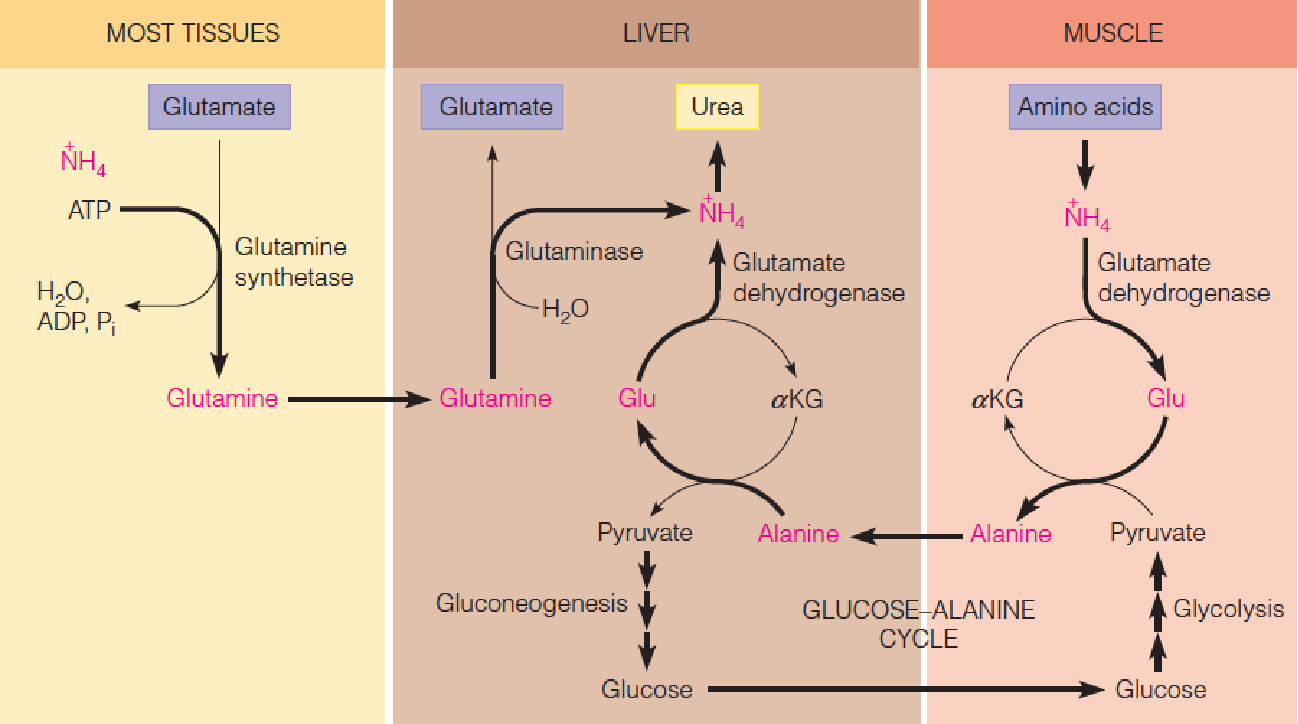
- Transport of ammonia to the liver for urea synthesis.
- The carrier is glutamine in most tissues but is alanine in muscle.
All organs degrade amino acids and produce ammonia. Two mechanisms are involved in transporting this ammonia from other tissues to liver for its eventual conversion to urea.
Most tissues use glutamine synthetase to convert ammonia to the nontoxic, and electrically neutral, glutamine. The glutamine is transported in the blood to the liver, where it is cleaved hydrolytically by glutaminase.
Muscle, which derives most of its energy from glycolysis, uses a different route, the glucose-alanine cycle. This cyclic process helps muscle get rid of ammonia, with the carbon from the pyruvate being returned to the liver for gluconeogenesis.
Transport of ammonia to the liver for urea synthesis. The carrier is glutamine in most tissues but is alanine in muscle
五、Coenzymes Involved in Nitrogen Metabolism
- Pyridoxal phosphate (containing B
6), the cofactor for transamination; - Folic acid coenzymes, which transfer single-carbon functional group in synthesizing nucleotides and certain amino acids;
- B
12or cobalamin, which participates in the synthesis of methionine and the catabolism of methylmalonyl-CoA (D-甲基丙二酰-CoA).
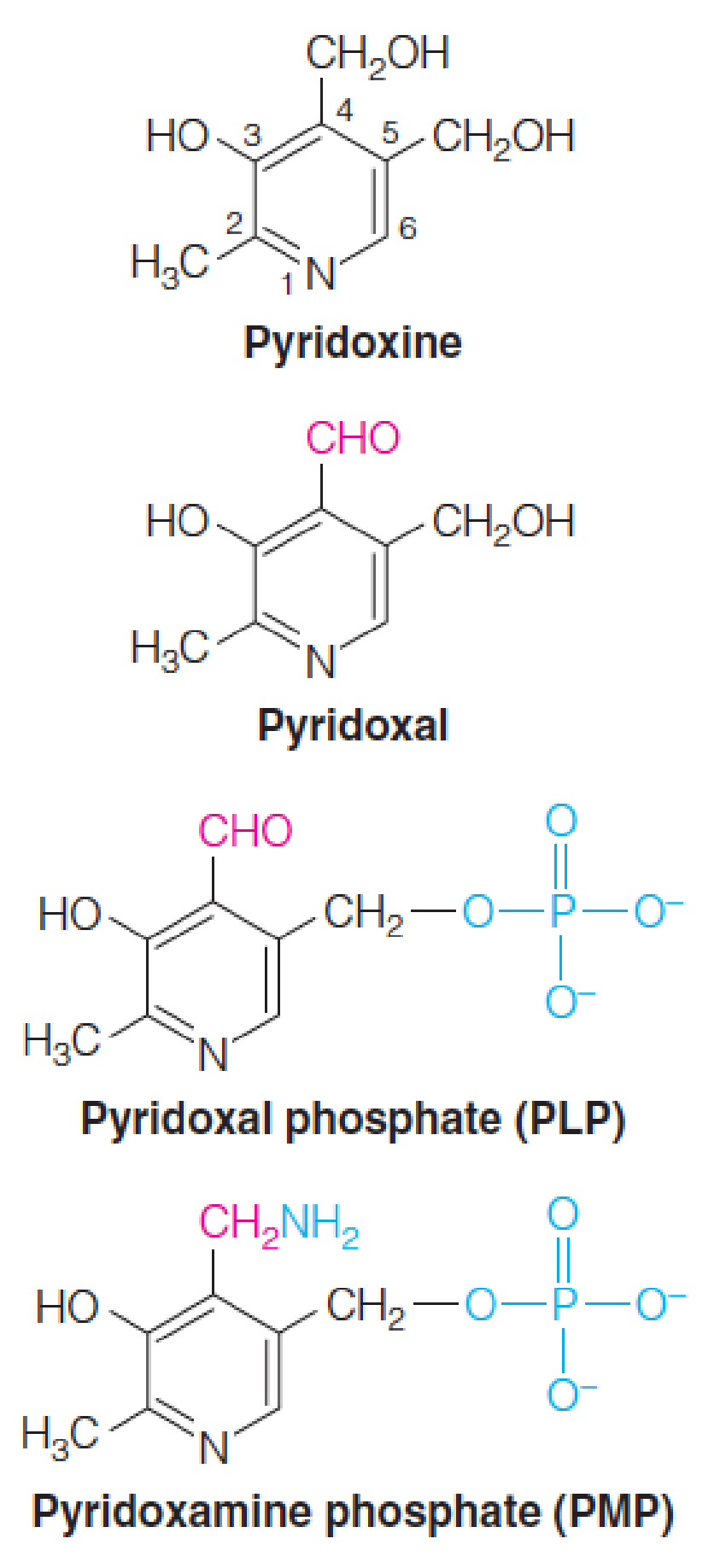
Pyridoxal phosphate
Vitamin B6 is also called pyridoxine (吡哆醇).
The active coenzyme has been oxidized to an aldehyde and the hydroxymethyl group at position 5 is phosphorylated.
Pyridoxal phosphate (PLP, 磷酸吡哆醛) is the predominant coenzyme form
Pyridoxamine phosphate (PMP, 磷酸吡哆胺) is an intermediate form in transamination reactions.
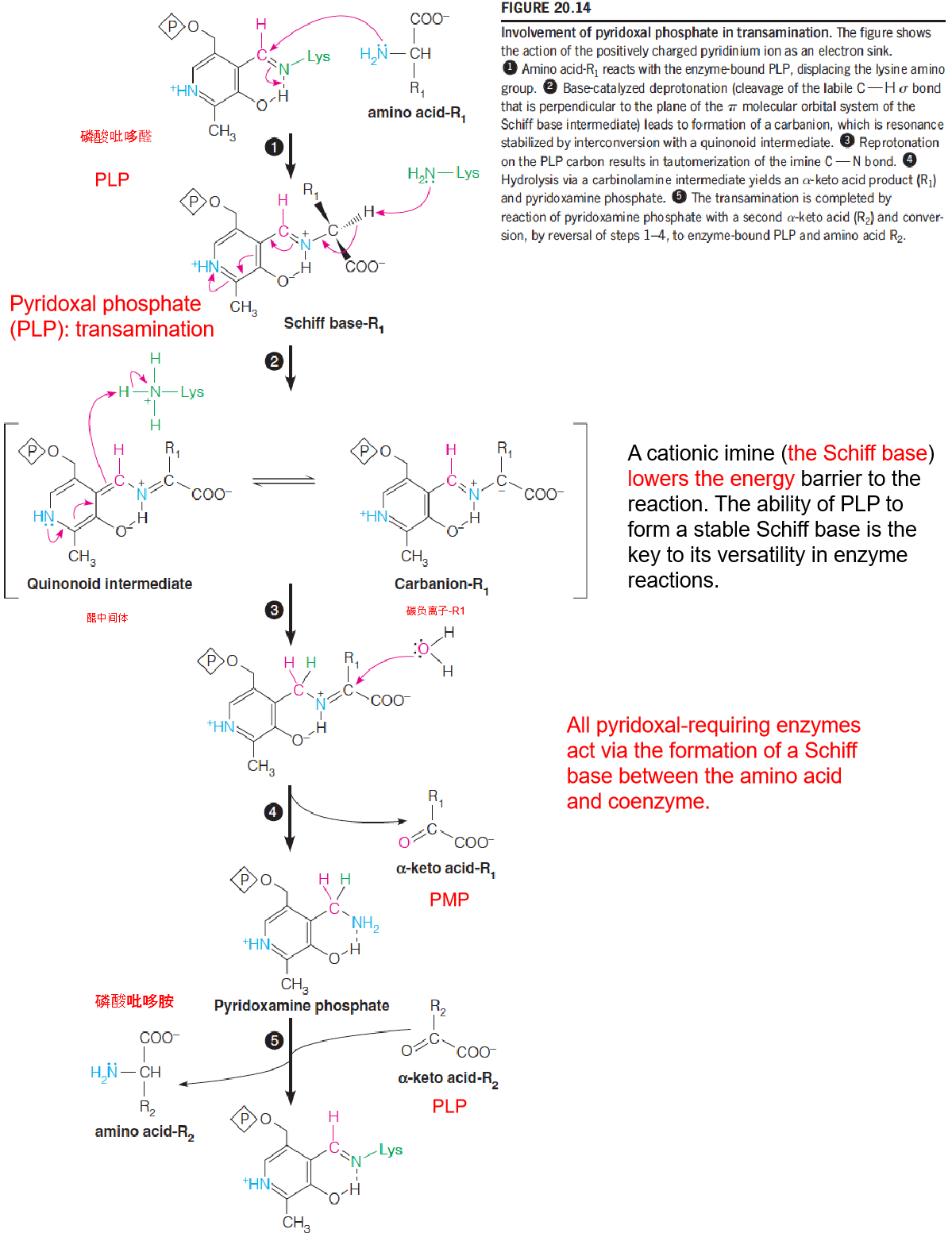
- A cationic imine (the Schiff base) lowers the energy barrier to the reaction. The ability of PLP to form a stable Schiff base is the key to its versatility in enzyme reactions.
- All pyridoxal-requiring enzymes act via the formation of a Schiff base between the amino acid and coenzyme.
Folic Acid
1. Tetrahydrofolate (THF) Coenzymes and One-Carbon Metabolism
Coenzymes derived from the vitamin folic acid: single-carbon functional groups—methyl, methylene, and formyl;
British physician Lucy Wills in the 1930s found that people with a certain type of megaloblastic anemia (巨成红细胞性贫血) can be cured by treatment with yeast or liver extracts……
Four tons of spinach —a few hundred mg of the active component;
- Leaf green vegetable
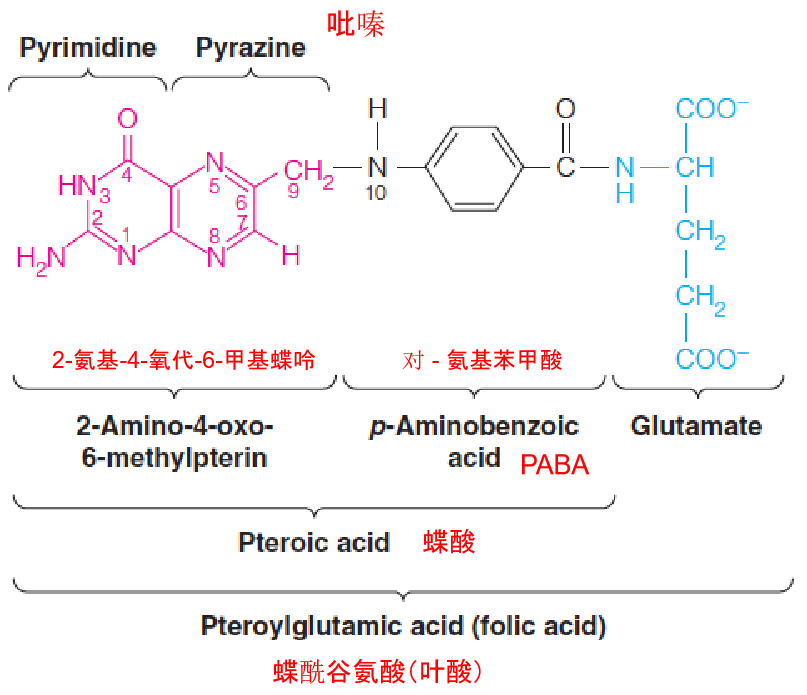
2. Conversion of Folic Acid to Tetrahydrofloate
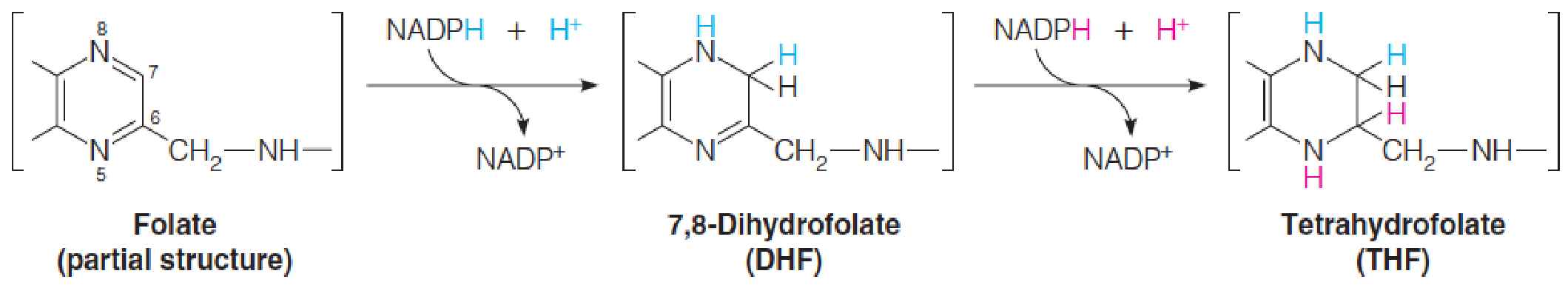
- Once inside a cell, folic acid is converted to active forms by two successive reductions of the pyrazine (吡嗪) part of the pteridine ring (蝶啶环).
- Both reactions are catalyzed by the NADPH-specific enzyme dihydrofolate reductase (二氢叶酸还原酶).
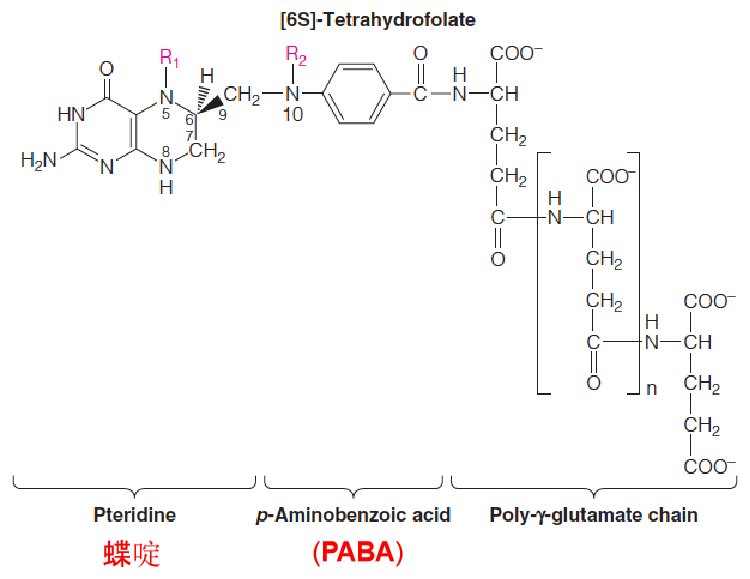
- Folate coenzymes contain multiple glutamate residues, which help them be retained within cells and bind more tightly to enzymes.
- the N-5 and N-10 positions can carry single carbon units—methyl, methylene, and formal.
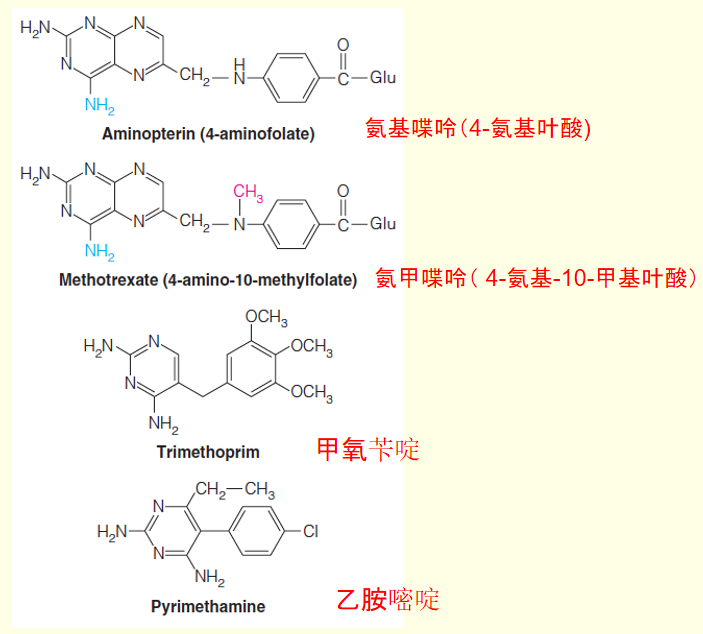
- Dihydrofolate reductase is the target for a number of useful anticancer, antibacterial, and antiparasitic drugs.
- Folate analogs such as methotrexate: leukemia
3. Human dihydrofolate reductase complexed with ligands
This figure shows the X-ray crystal structure of the human enzyme with bound NADPH (yellow) and methotrexate (氨甲喋呤) (red).
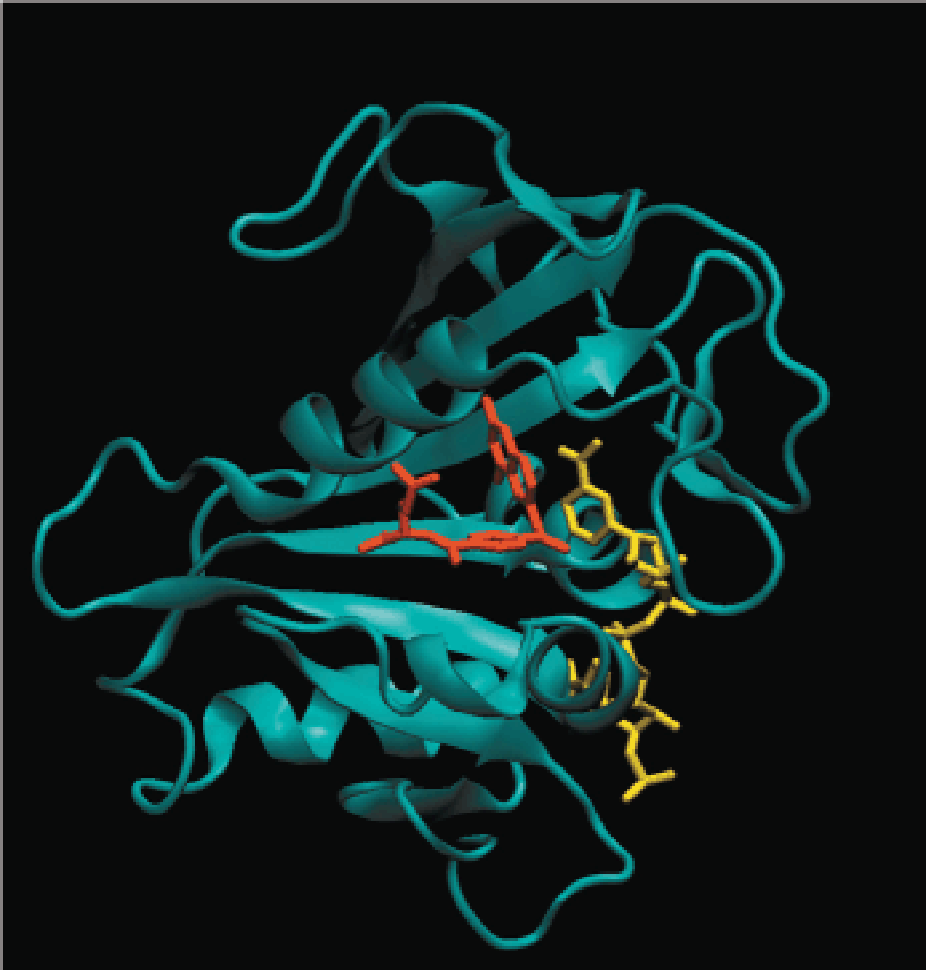
The additional amino group on methotrexate allows formation of an additional hydrogen bond to the enzyme, which increases its binding affinity to the folate binding site.
These two ligands bind such that the pyridine ring of NADPH is in close proximity to the pteridine ring of the folate ligand, as required for the hydride transfer catalyzed by this enzyme.
Notice that the folate binds in a bent, rather than linear, form.
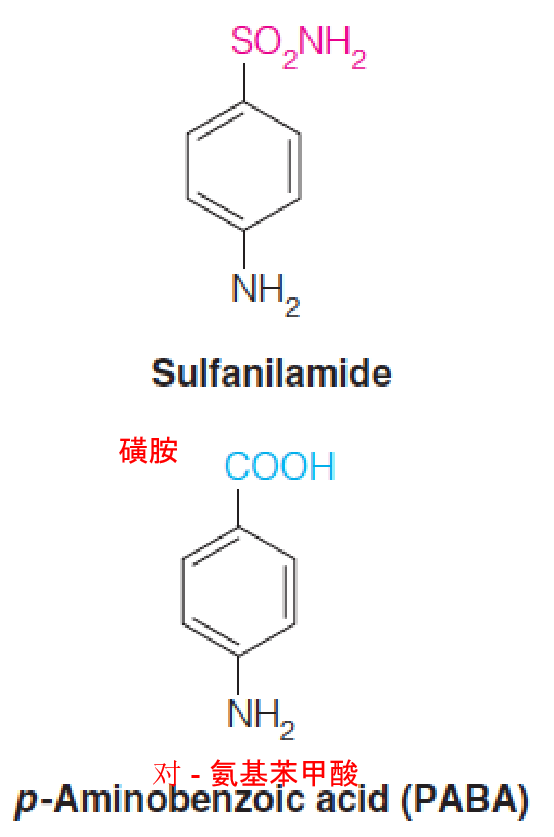
Before World War II, one of the few effective antibacterial drugs available was sulfanilamide, one of the class of sulfonamide drugs (“sulfa drugs”).
D. D. Woods, noted a structural similarity between sulfanilamide and p-aminobenzoate (PABA), which was known to be essential for bacterial growth.
Woods proposed that sulfanilamide acts by blocking the normal utilization of PABA, and he coined the term antimetabolite (抗代谢药).
PABA is not required for growth of animal cells, so the drug is not toxic to human cells.
The enzyme incorporating PABA into folic acid is inhibited by sulfonamides.
Because animal cells do not carry out the synthetic pathway but instead take up fully formed folate from the diet, they are not harmed by the drug
4. Tetrahydrofolate (THF) in the Metabolism of One-Carbon Units
Tetrahydrofolate (四氢叶酸) coenzymes transfer and interconvert one-carbon units at the methyl (甲基), methylene (亚甲基), and formyl (甲酰) oxidation levels.
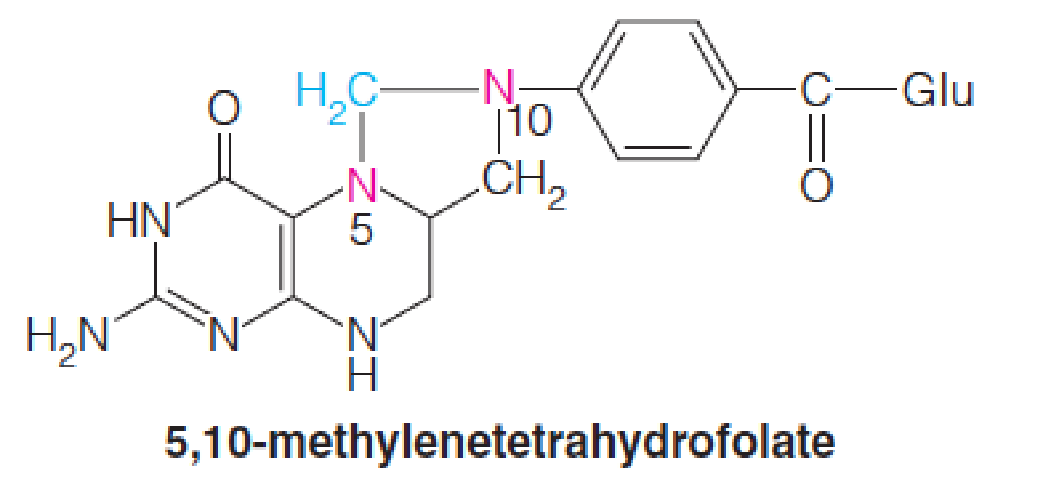
- Biosynthesis of purine nucleotides and the methyl group of thymidine
Most organisms derive the majority of their activated one-carbon units from the β-carbon of serine and the subsequent oxidation of glycine.
The first of these reactions is catalyzed by serine hydroxymethyltransferase (丝氨酸羟甲基转移酶):

Metabolic reactions involving synthesis, interconversion, and utilization of one-carbon adducts of tetrahydrofolate:
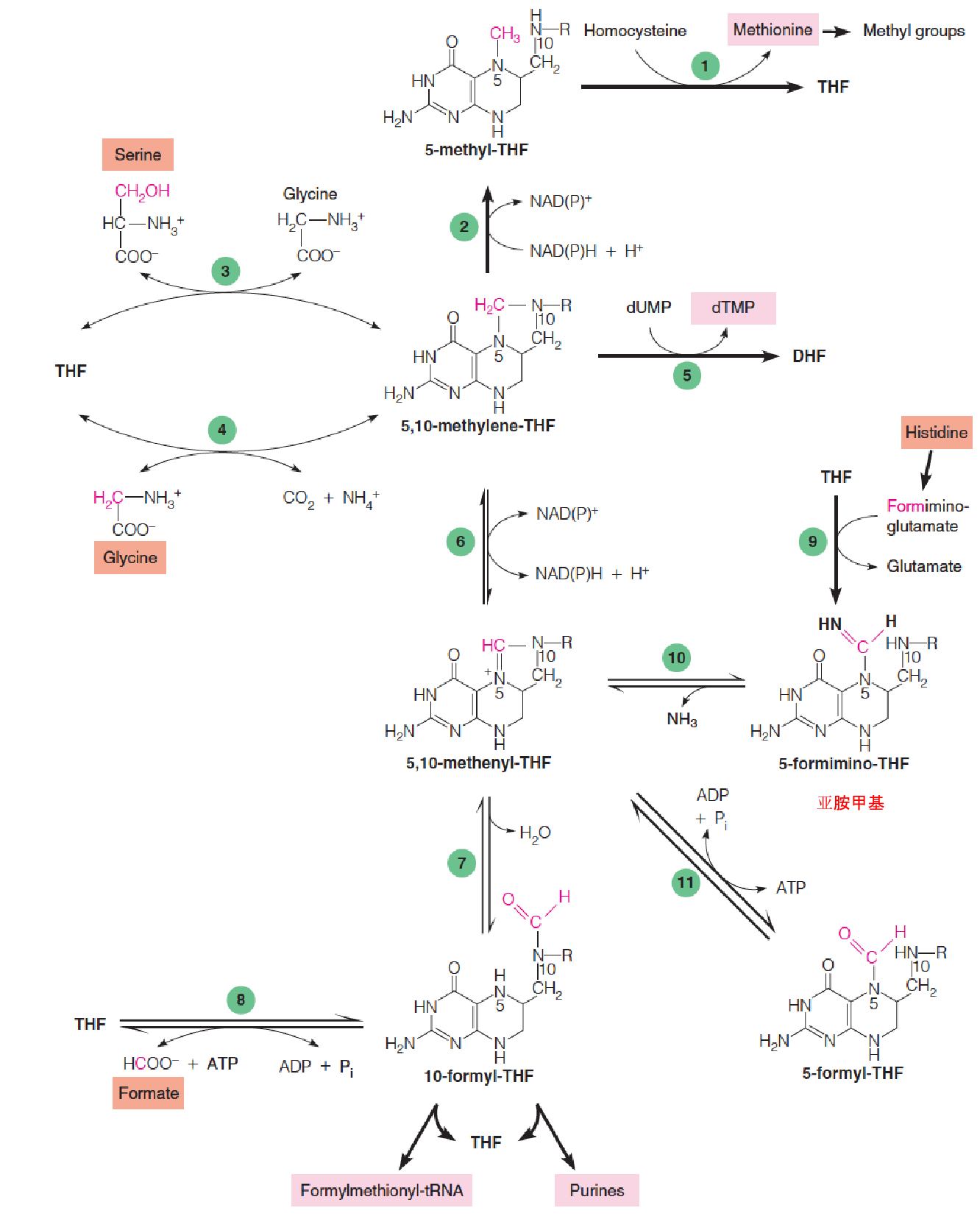
- Serine, glycine, methionine, and histidine
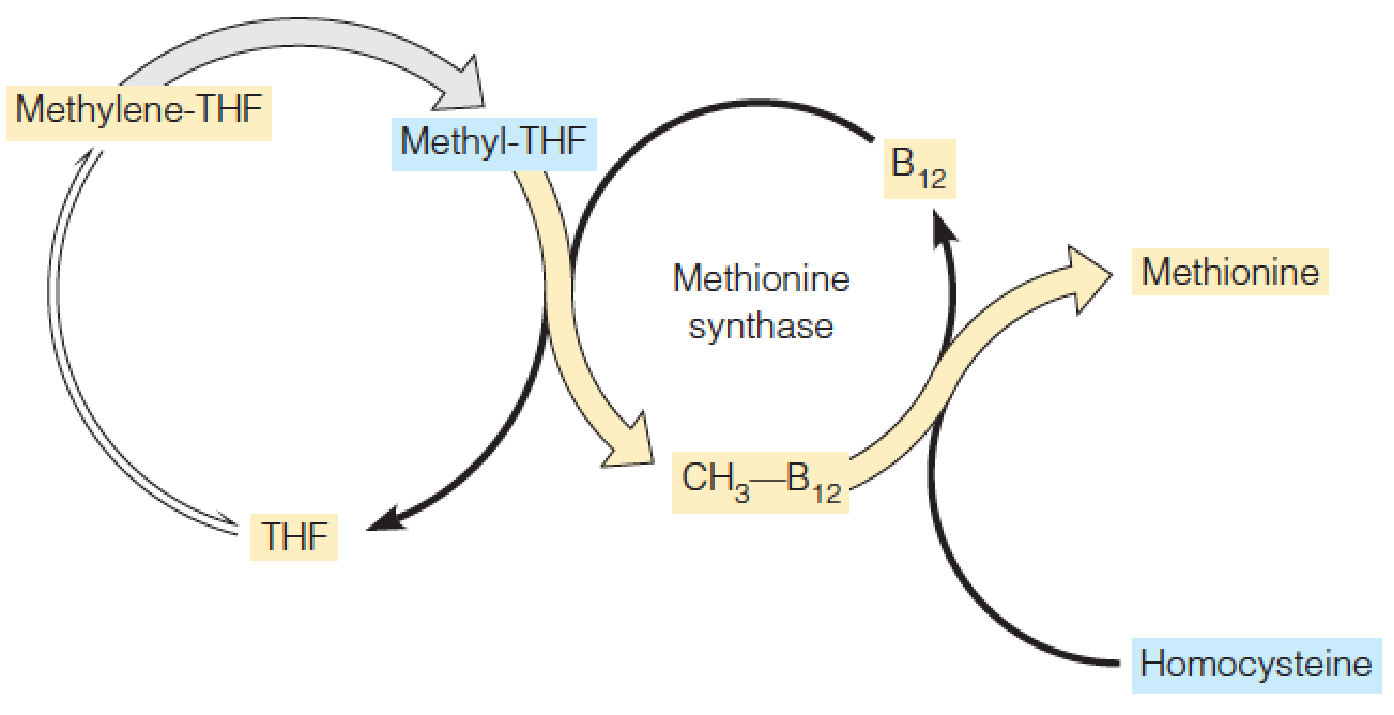
- Homocysteine methytransferase (also called methionine synthase;
- Methylene-THF reductase;
- Serine hydroxymethyltransferase;
- Glycine cleavage system;
- Thymidylate synthase;
- Methylene-THF dehydrogenase;
- Methylene-THF cyclohydrolase;
- 10-formyl-THF synthetase;
- Glutamate formiminotransferase;
- 5-formimino-THF cyclodeaminase;
- 5-formal-THF cycloligase
Glycine can yield an additional molecule of 5,10-methylenetetrahydrofolate (亚甲基四氢叶酸) through action of the glycine cleavage system, a multienzyme complex located in mitochondria:
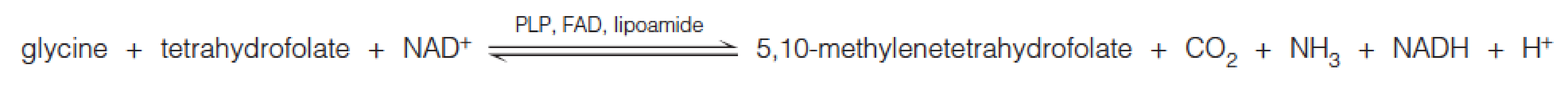
B12 Coenzyme
A formerly incurable disease, pernicious anemia (恶性贫血), begins with a megaloblastic anemia, leads to irreversible degeneration of the nervous system if untreated;
In 1926 two Harvard physicians, George Minot and William Murphy, found that symptoms of this disease could be alleviated by feeding patients with large amounts of raw liver. The active material in the liver was named vitamin B12
In England in 1956, Dorothy Hodgkin and her colleague used X-ray crystallography to complete the structure determination for this active substrate. Hodgkin was awarded the Nobel Prize for this work.
- Steroids, B12, penicillin and insulin structures
1. Coenzyme Forms of B12
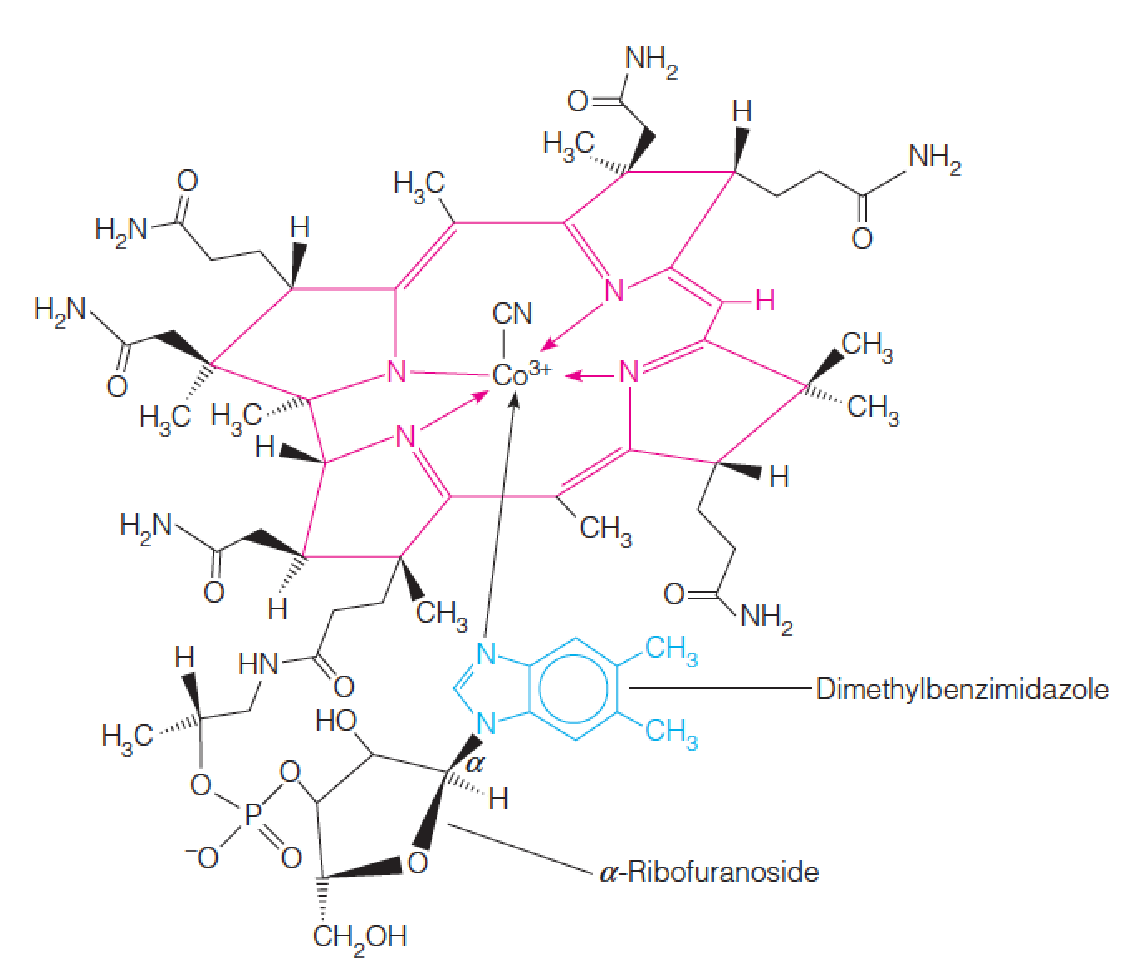
Structure of vitamin B12:
The molecule shown here is the cyanide-containing form originally isolated (cyanocobalamin (氰钴胺)).
In cells, a water molecule or hydroxyl group takes the place of CN, forming the precursor to the coenzyme forms of B12.
The corrin ring (咕啉环) is shown in red.
5,6-Dimethylbenzimidazole (DMB, 5,6-二甲基苯并咪唑), which is linked to the cobalt, is shown in blue.
- The metal cobalt is coordinated with a tetrapyrrole ring system, called a corrin ring, which is similar to porphyrin ring in heme compounds.
Three classes of B12-dependent enzymes are now known—isomerases, methyltransferases, and reductive dehalogenases (脱卤酶)
In mammals, only two reactions:
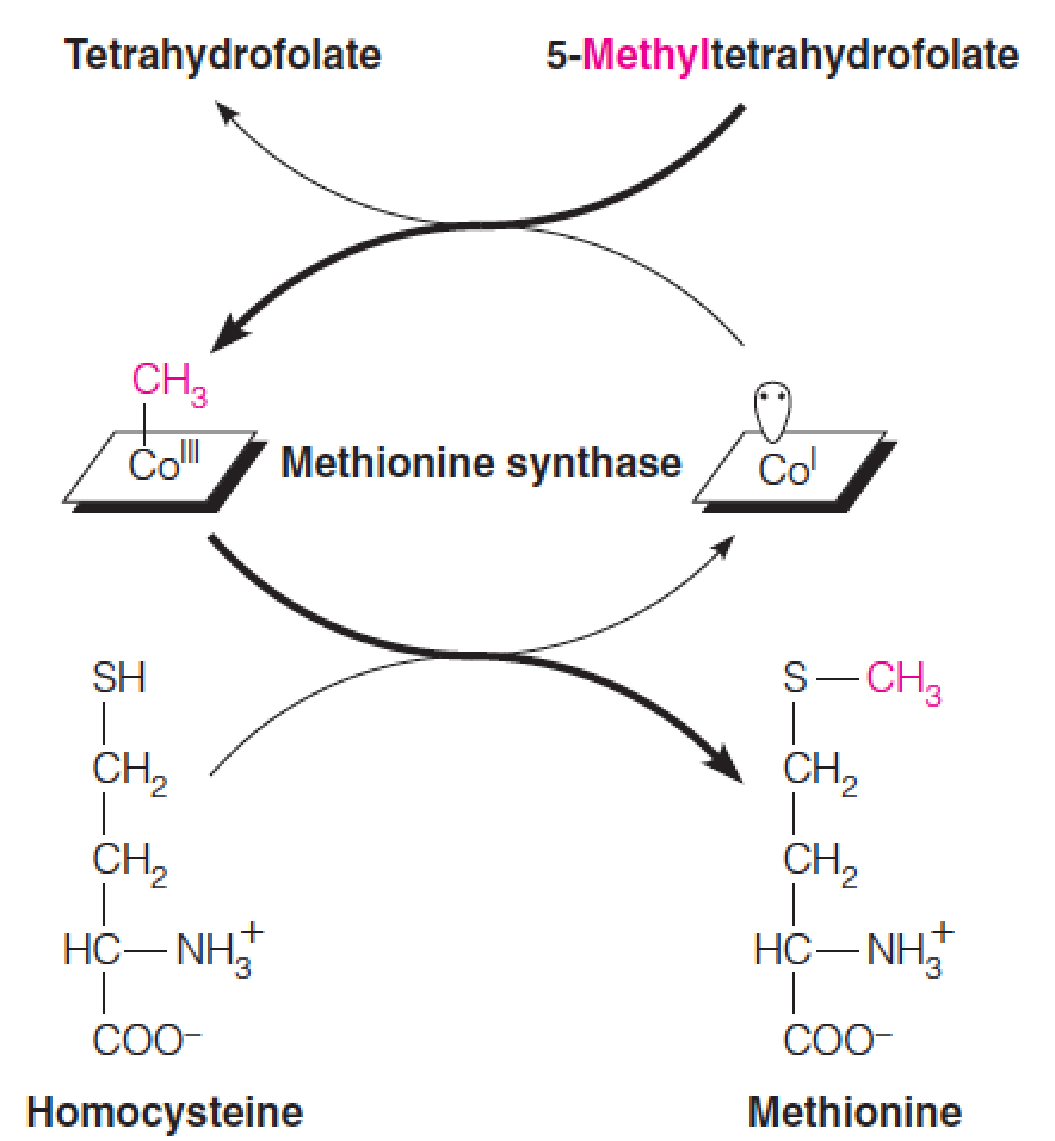
- The role of B12 as a methyl transferase in Met biosynthesis, the synthesis of methionine from homocysteine
- The isomerization of methylmalonyl-CoA to succinyl-CoA (oxidation of odd-chain fatty acids).
- Two forms:
- methylcobalamin;
- 5’-deoxyadenosylcobalamin
The role of B12 as a methyl transferase in Met biosynthesis:
- B12 coenzymes have either a methyl group or a 5’-deoxyadenosyl moiety linked to cobalt, making them the first known organometallics (金属有机化合物) in metabolism.
2. Fatty Acid Oxidation
Pathway for catabolism of propionyl-CoA:
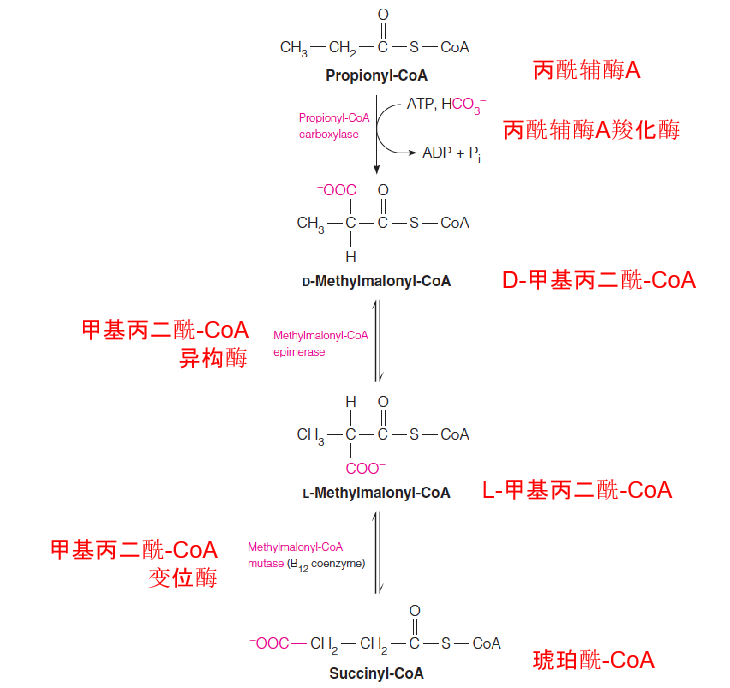
The intramolecular isomerization catalyzed by methylmalonyl-CoA mutase
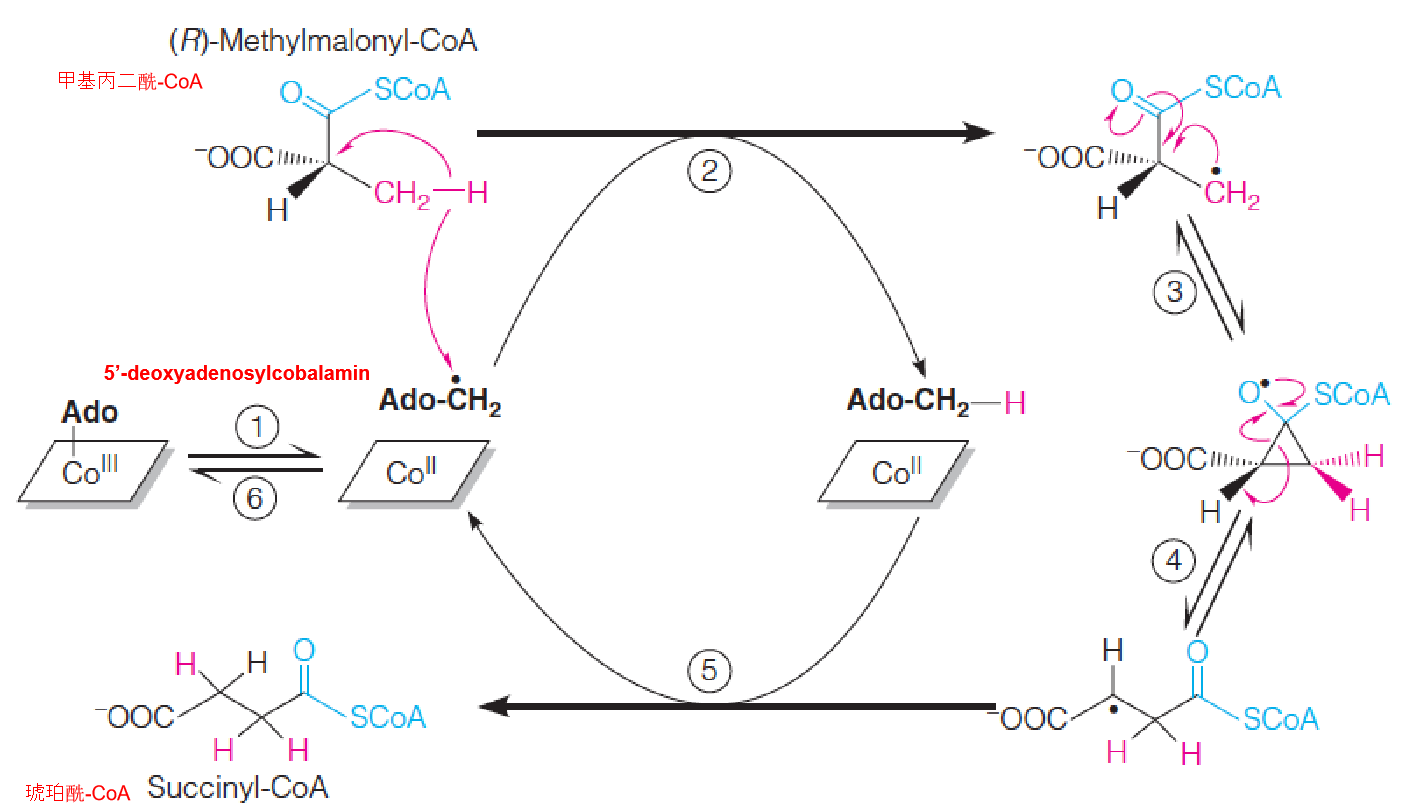
- Step 1: Homolytic cleavage of carbon-cobalt bond and formation of 5’-deoxyadenosyl radical.
- Step 2: Hydrogen abstraction and formation of substitute radical;
- Step 3 and 4: 1.2 rearrangement involving a cyclopropyloxy radical;
- Step 5: Hydrogen abstraction from 5’-deoxyadenosine to give succinyl-CoA and 5’-deoxyadenosyl radical;
- Step 6: Reformation of carbon-cobalt bond.
3. A relationship between folate and B12 metabolism
This scheme is based on the apparent folate deficiency seen in early stages of B12 deficiency as a result of decreased flux through the methionine synthase reaction.
4. Folic Acid in the Prevention of Heart Disease and Birth Defects
In the mid-1990s, correlation of folate deficiency and increased risk of myocardial infarction; individuals at risk for heart attacks also showed high levels of serum homocysteine (homocysteinemia);
Homocysteinemia (高半胱氨酸血症): cardiovascular disease, including coronary artery disease, stroke…
Folate deficiency: birth defects, including craniofacial (cleft palate (腭裂)), the neural tube (anencephaly and spina bifida (无脑畸形和脊柱裂));
Neural tube defects (NTDs): 1/1000; folate acid supplementation prevents 50-75% of NTDs!!!!; Take folic acids in pregnancy, but especially in the early stage (28 d for neural tube); Women of childbearing age: adequate folic acid;
Fortification (flour and cereal grain): mandatory in USA since 1998, ….declined