L05 Amino Acids
Amino Acids
$\alpha$-amino acid
All proteins are polymers, and the monomers that combine to make them are $\alpha$-amino acids.
Chirality 手性
When a carbon atom has four different substituent(取代基) attached to it, it is said to be chiral, or a stereocenter (立构/归正中心,指通过交换连在该原子上的两个基团的位置,就会得到原化合物的立体异构体), or, preferably, an asymmetric carbon.
If a molecule contains one asymmetric carbon, two distinguishable stereoisomers(立体异构体) exist; these are nonsuperimposable(不可叠加) mirror images of one another, or enantiomers (映射异构体,又称对掌异构物、光学异构物、镜像异构物或对映异构体,不能与彼此立体异构体镜像完全重叠。)
在amino acid中,因为手性原因,可以分为L和D-氨基酸:手性及手性物质只有两类:“左手性”和“右手性”。有时为了对比,另外加上一种无手性(也称“中性手性”)。左手性用<leavus>或者<L>表示,右手性用<dexter>或者<D>表示,中性手性用M表示。
The L and D enantiomers can be distinguished from one another experimentally because their solutions rotate plane polarized light in opposite directions. For this reason, enantiomers are sometimes called optical isomers.
All amino acids except glycine can exist in D and L forms because in each case the $\alpha$-carbon is asymmetric Chemical analysis of naturally occurring proteins shows that nearly all of the amino acids found in proteins are of the L form.
在实验室中使用D-氨基酸合成的蛋白质酶只能水解D-氨基酸所组成的蛋白质,虽然现今几乎所有生物蛋白质中的氨基酸都是L-氨基酸,D-氨基酸同样可以承担生命功能
L-氨基酸以外的氨基酸同样具有重要的作用:
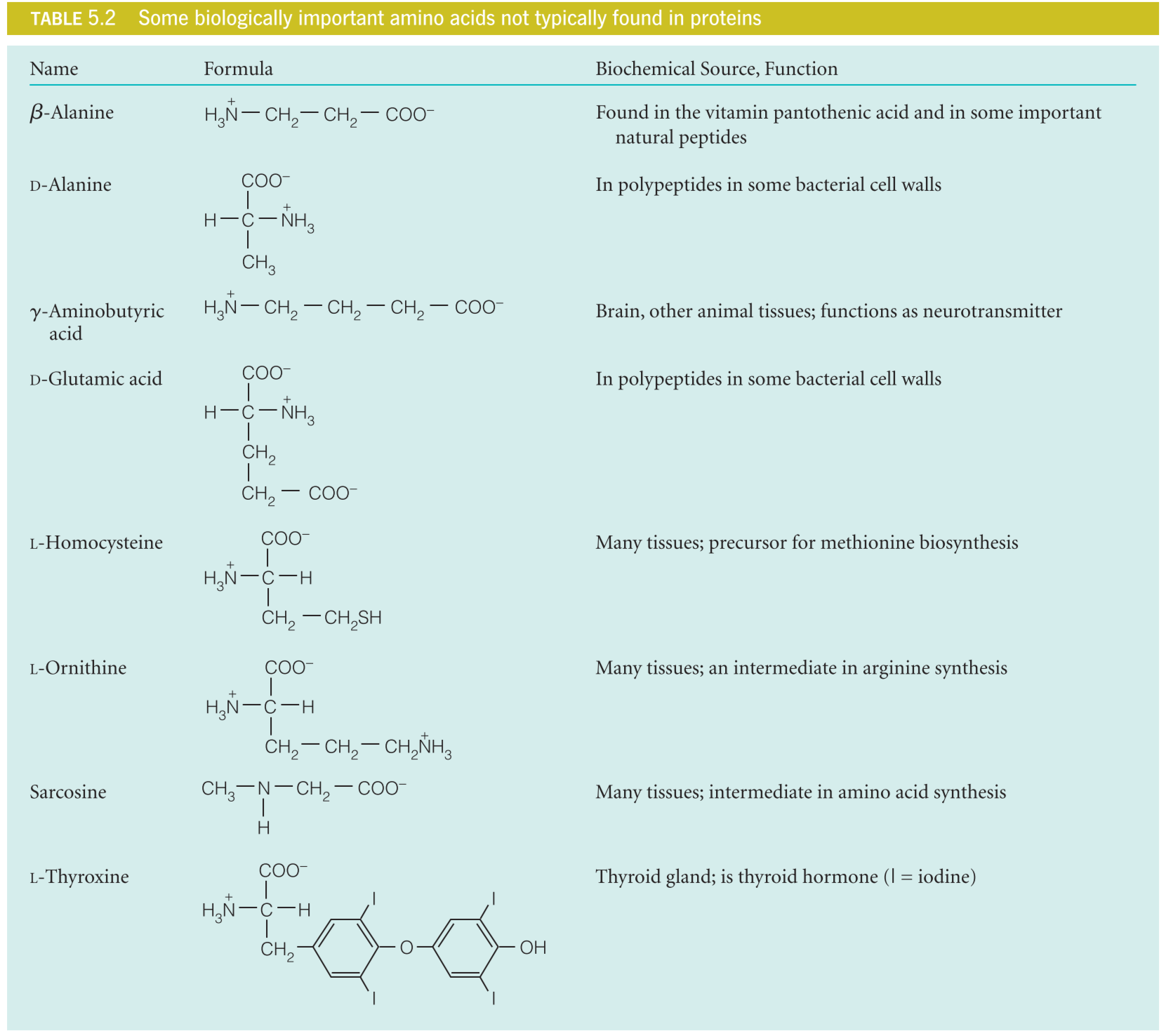
The consequence of the preference of L-amino acid
The surface of any given protein, which is where the interesting biochemistry occurs, is asymmetric. This asymmetry is the basis for the highly specific molecular recognition of binding targets by proteins.
The stereochemistry of the amino acids plays an important role in the formation of so-called “secondary structure” (i.e., helices and strands) and thereby the overall structure of proteins.
Properties of amino acid side chains
1. Aliphatic(脂肪族的) Side Chains and Cyclic amino Acid
脂肪族化合物,又称脂肪烃,是指结构上不含苯环或其它芳香环的烃类,和芳香族化合物相对。
脂肪族化合物中,碳原子以直链、支链或环状排列,分别称为直链脂肪烃、支链脂肪烃及脂环烃。脂肪族化合物可以是烷烃、烯烃或炔烃。除氢之外,其它的原子也可存在,比如氧、氮、硫和氯。
Aliphatic side chains
Glycine, alanine, valine, leucine, and isoleucine have aliphatic side chains.
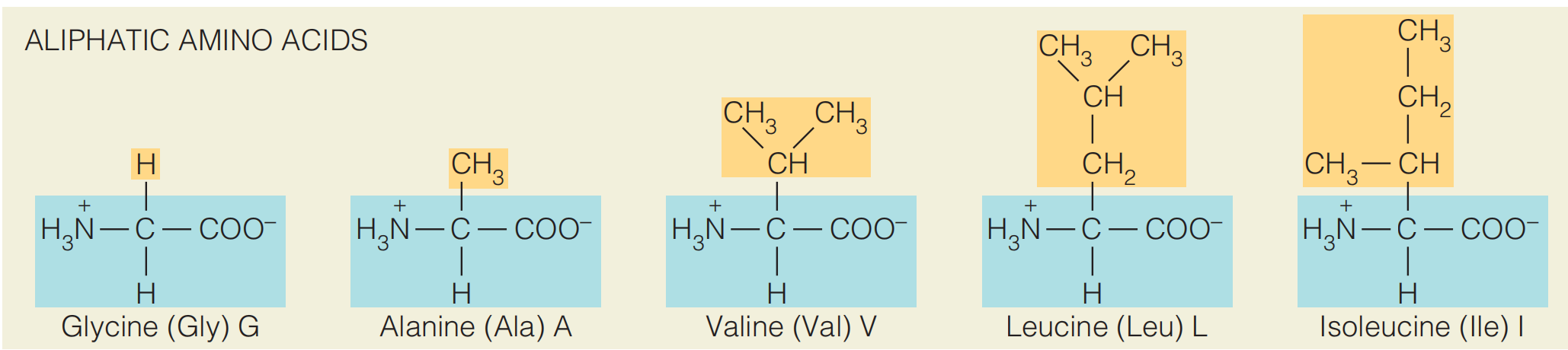
The more extended aliphatic side chains, the more hydrophobic.
Isoleucine, for example, has a much greater tendency to transfer from water to a hydrocarbon solvent than does alanine. The more hydrophobic amino acids such as isoleucine are usually found within the core of a protein molecule, where they are shielded from water.
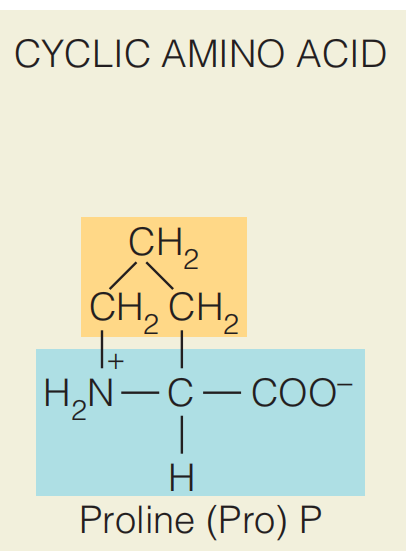
cyclic side chains
Proline, which has a secondary α–amino group, is difficult to fit into any category. It is the only amino acid in this group in which the side chain forms a covalent bond with the α–amino group.
The proline side chain has a primarily aliphatic character; however, it is frequently found on the surfaces of proteins due to its unique structural constraints. The rigid ring of proline is well-suited to those sites in a protein structure where the protein must fold back on itself
2. Amino Acids with Hydroxyl- or Sulfur-Containing Side Chains
In this category we can place serine, cysteine, threonine, methionine, and tyrosine.
Although methionine and tyrosine are fairly hydrophobic, these amino acids, because of their more polar side chains, display more hydrophilic character than their aliphatic analogs.
The -OH group of serine and -SH group of cysteine are good nucleophiles(亲核基) and often play key roles in enzyme activity.所以DNA和RNA分子的五碳糖链可以具有催化活性,因为糖环上具有-OH
3. Aromatic(芳香族的) Amino Acids
Three amino acids, phenylalanine, tyrosine, and tryptophan, carry aromatic side chains.
Phenylalanine, together with the aliphatic amino acids valine, leucine, and isoleucine, is ,one of the most hydrophobic amino acids. Tyrosine and tryptophan have some hydrophobic character as well, but it is tempered(调和的) by the polar groups in their side chains.
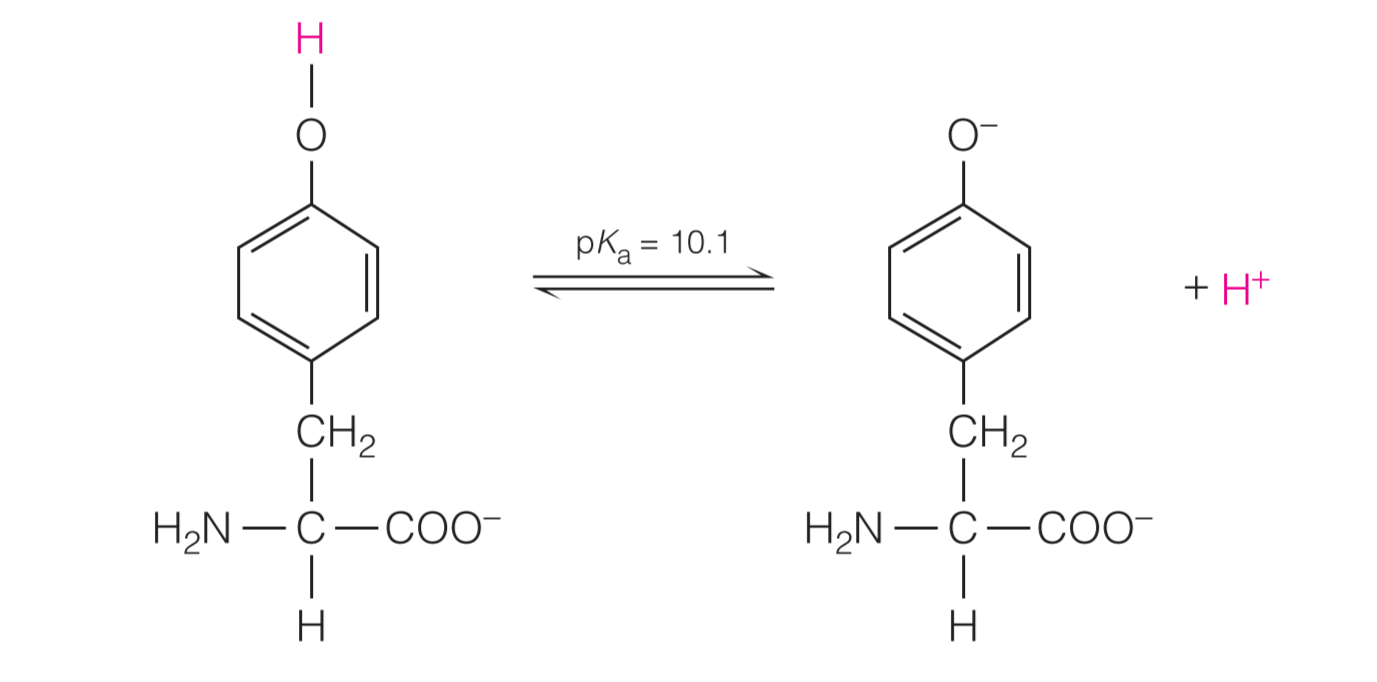
In addition, tyrosine can ionize at high pH:
The aromatic amino acids, like most highly conjugated compounds, absorb light in the near-ultraviolet region of the spectrum
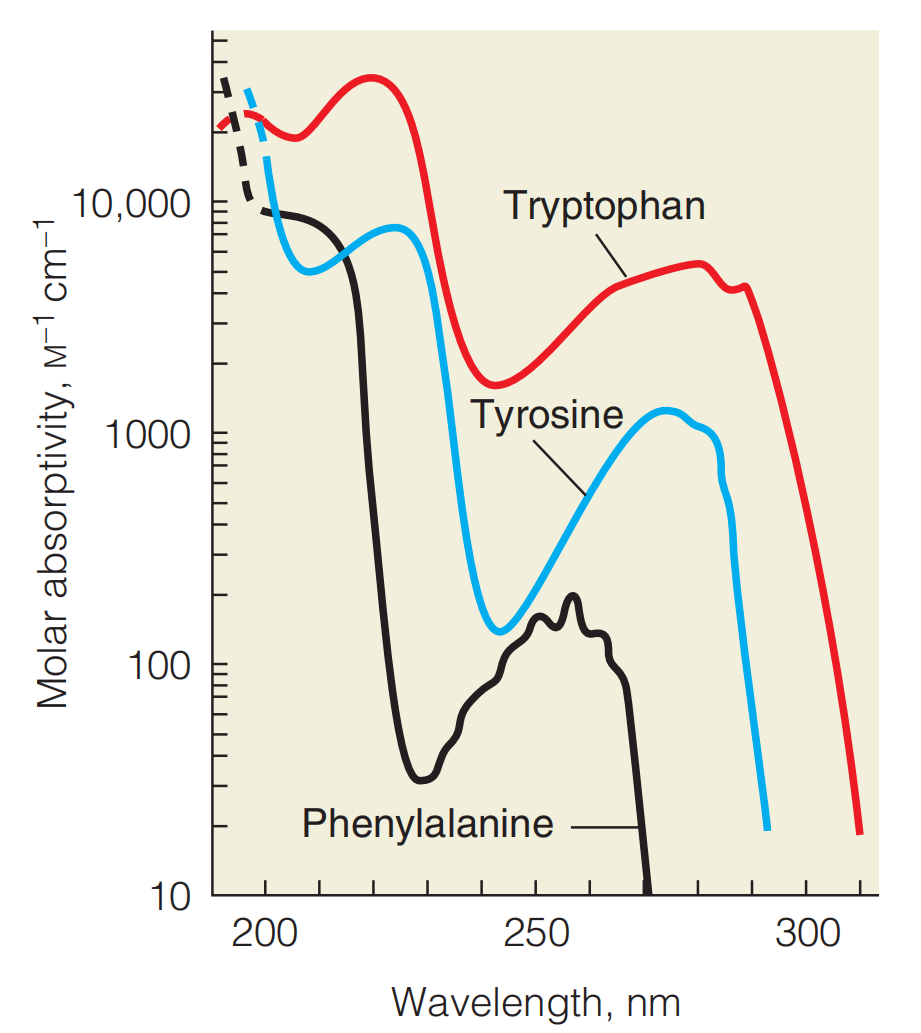
Tryptophan (red; max $\lambda_{max} $ = 278 nm) and tyrosine (blue; max $\lambda_{max} $ = 274 nm) account for most of the UV absorbance by proteins in the region around 280 nm. Phenylalanine (black; max $\lambda_{max} $ = 258 nm) does not absorb at 280 nm. Note that the absorptivity scale is logarithmic. Compared with nucleic acids, amino acids absorb only weakly in the UV.
This characteristic is frequently used for the detection and/or quantitation of proteins, by measuring absorption at 280 nm.
4. Basic Amino Acids
Histidine, lysine, and arginine carry basic groups in their side chains.
Histidine is the least basic of the three, and as its titration curve shows, the imidazole ring(咪唑环) in the side chain of the free amino acid loses its proton at about pH 6
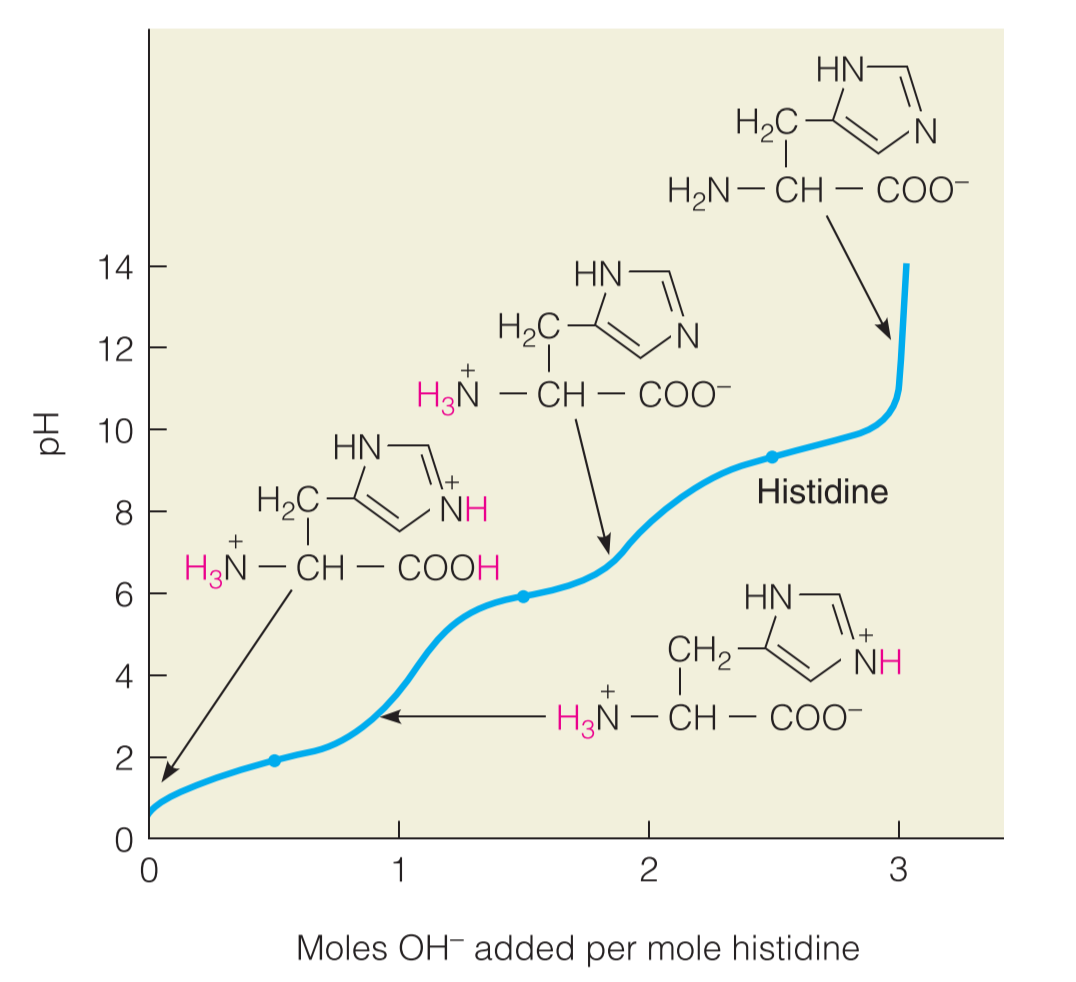
Lysine and arginine are more basic amino acids.
Their side chains are almost always positively charged under physiological (生理学的) conditions. The guanidino group(胍基) of arginine is a particularly strong base due to the resonance stabilization of the protonated side chain.
The basic amino acids are strongly polar, and as a consequence they are usually found on the exterior surfaces of proteins, where they can be hydrated by the surrounding aqueous environment.
5. Acidic Amino Acids and Their Amides
Aspartic acid and glutamic acid typically carry negative charges at pH 7
The $pK_{a}$ values of the acidic amino acids are so low that even when the amino acids are incorporated into proteins, the negative charge on the side chain is typically retained under physiological conditions. Hence, these amino acid residues are often referred to as aspartate and glutamate (i.e., the conjugate bases rather than the acids).
Companions to aspartic and glutamic acids are their amides(酰胺), asparagine and glutamine. Unlike their acidic analogs, asparagine and glutamine have uncharged polar side chains. Like the basic and acidic amino acids, they are hydrophilic and tend to be on the surface of a protein molecule, in contact with the surrounding water.