L20 Regulation of stem cell self-renewal and growth
一、Stem cells and stem cell renewal
The Intestinal Epithelia Cells
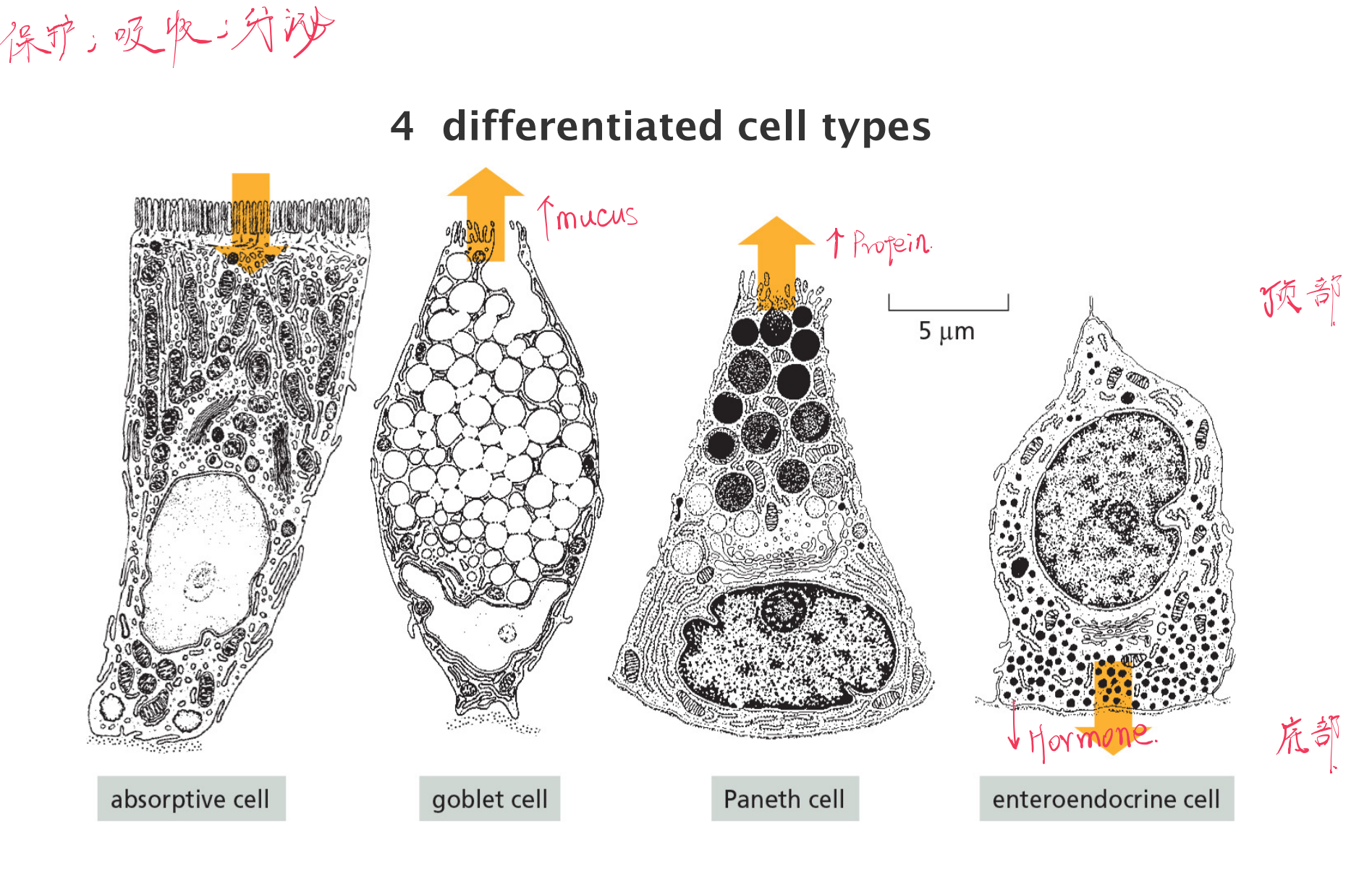
Intestinal epithelial stem cells resides in crypts at the base
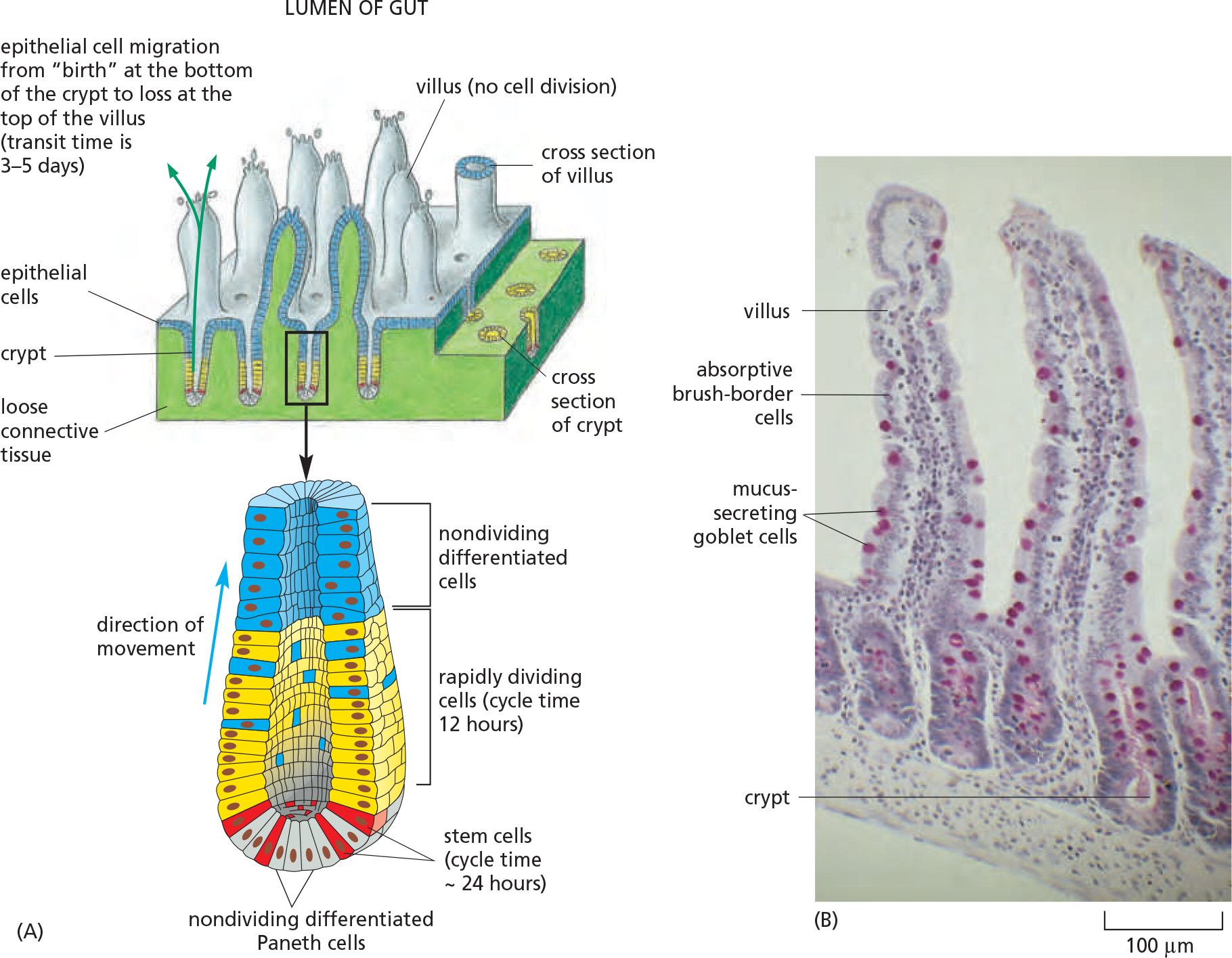
Intestinal differentiated cells are derived from stem cells at the crypt
Stem cells definition
All tissues need to be renewed, but specialization do not allow for cell division
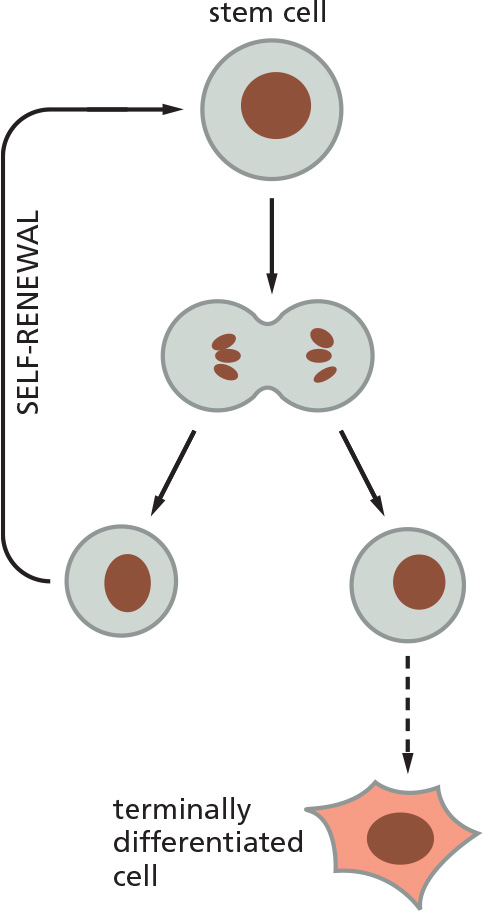
Stem cells are undifferentiated cells that have the potential for self-renewal and differentiation
- Self-renewal: at least one of the daughter cells is identical to the parent!
1. Wnt maintains stem cell niche in intestinal epithelia
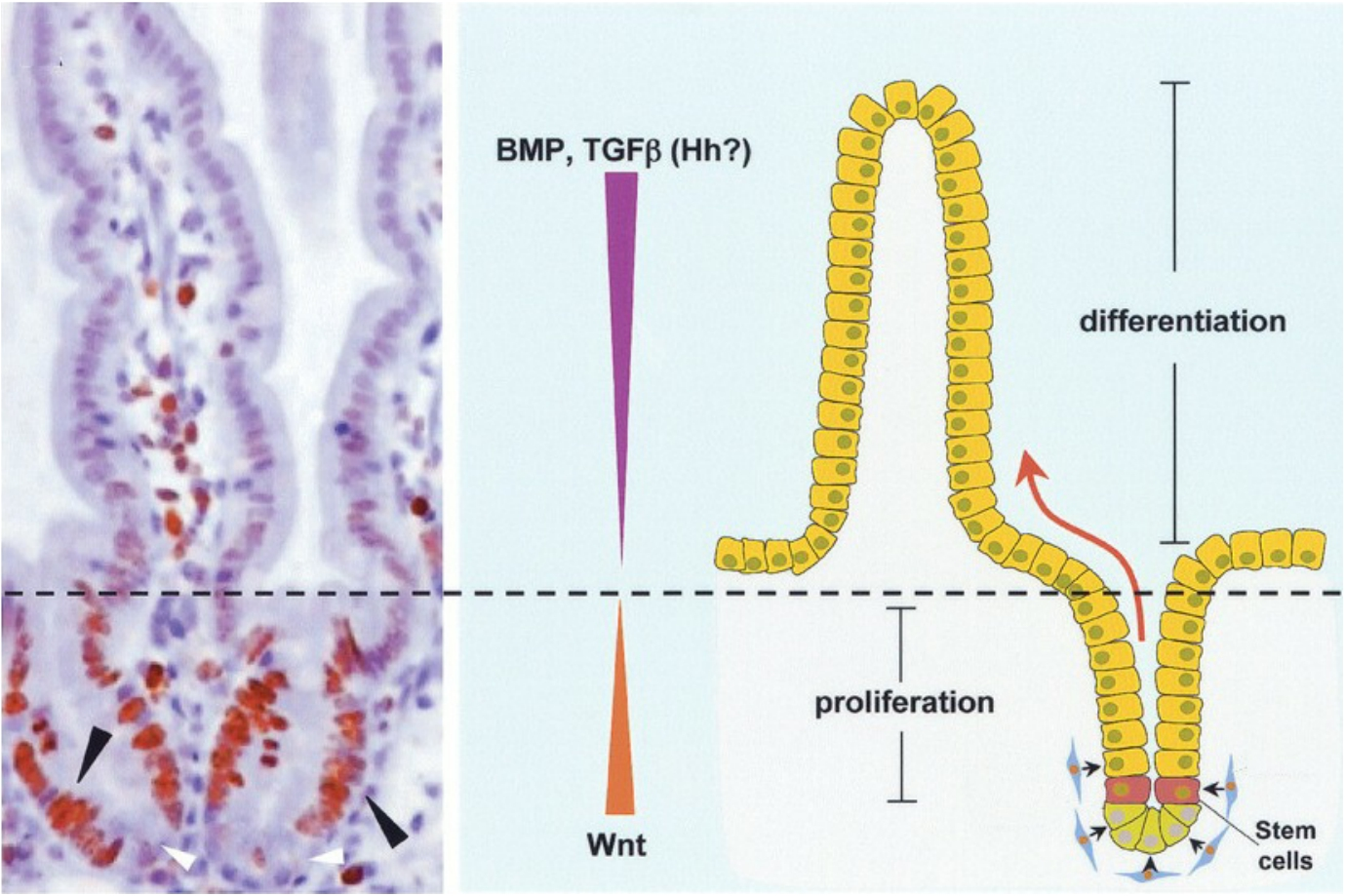
The cell division of the stem cells and differentiated cells in the intestine is regulated by mitogens (有丝分裂原) and anti-mitogens.
Wnt stimulates cell proliferation in crypts of intestinal epithelium, where as anti-mitogens (BMP, TGF-β) inhibit cell proliferation further up the intestinal epithelium
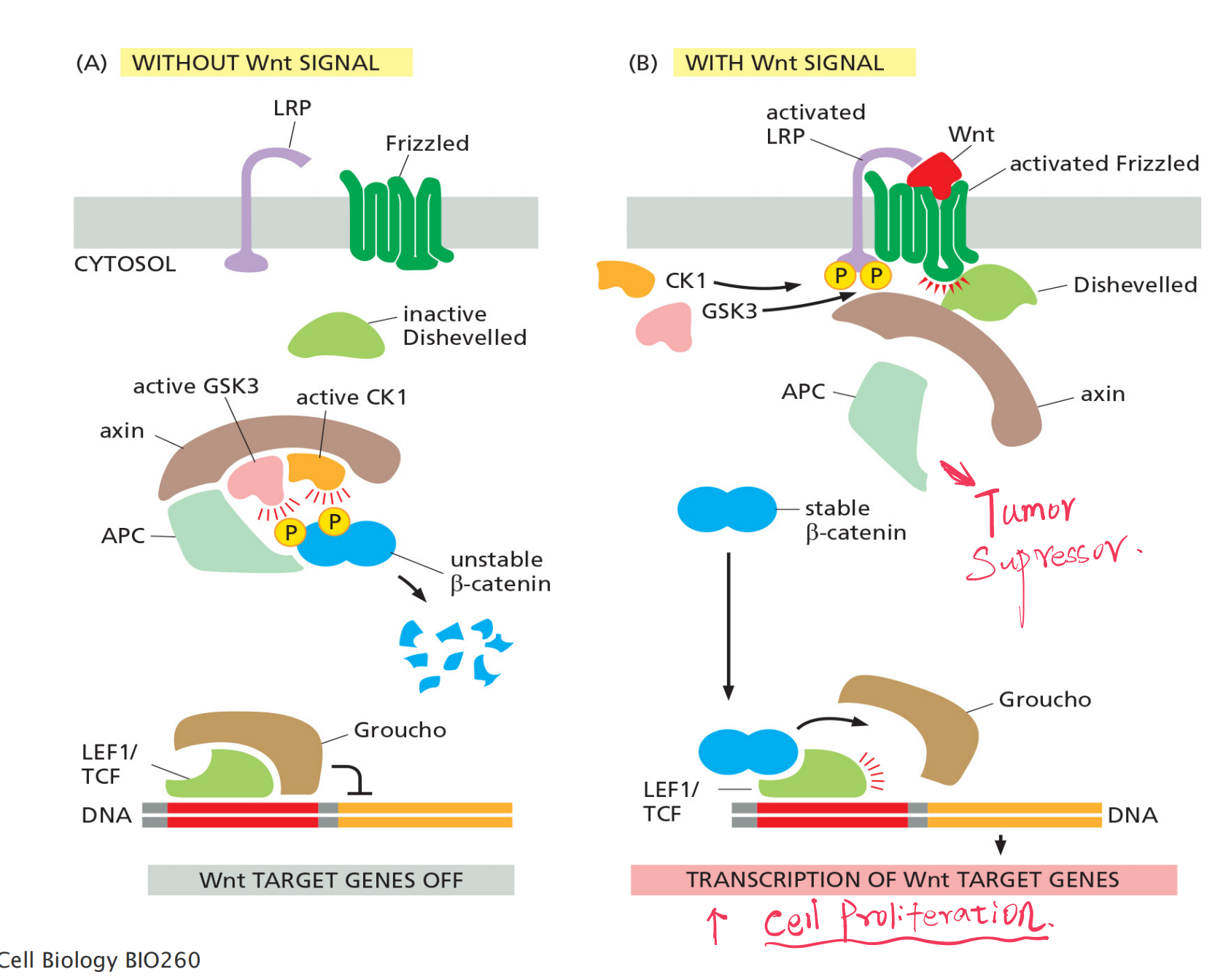
Co-receptor: LRP ( LDL-receptor-related protein) and Frizzled
- Latent transcription regulators: β-catenin
- Scaffold protein:
- Adenomatous polyposis coli (APC)
- axin
Mutated APC leads to elevated beta-catenin in nucleus and cell proliferation:
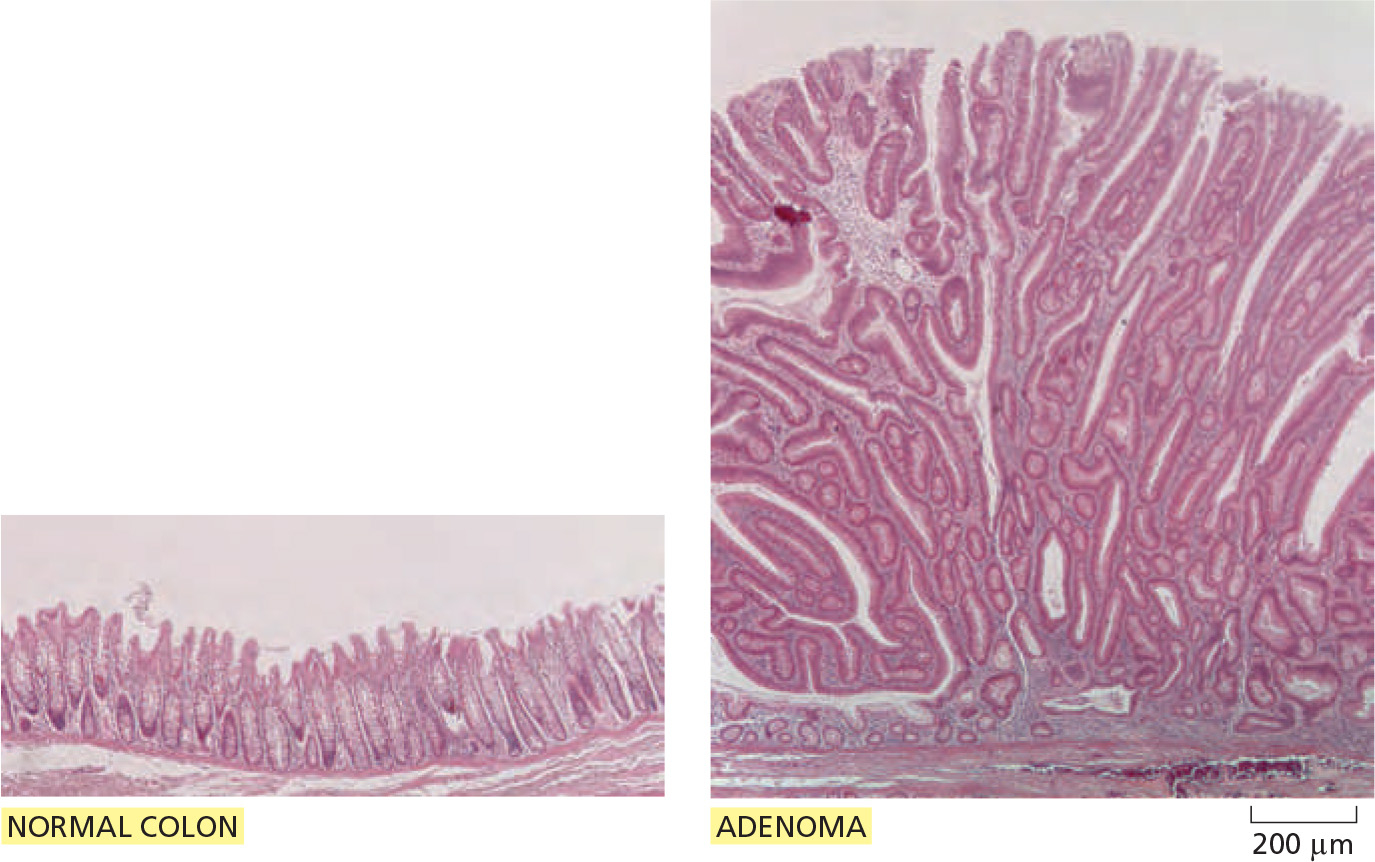
- Wnt signaling maintains cell proliferation in the crypts, and overactivation of the Wnt pathway gives rise to tumors.
- A mutation in the other Apc (Adenomatous polyposis coli) gene copy, occurring in a colon epithelial cell during adult life, has given rise to a clone of cells that behave as though the Wnt signaling pathway is permanently activated
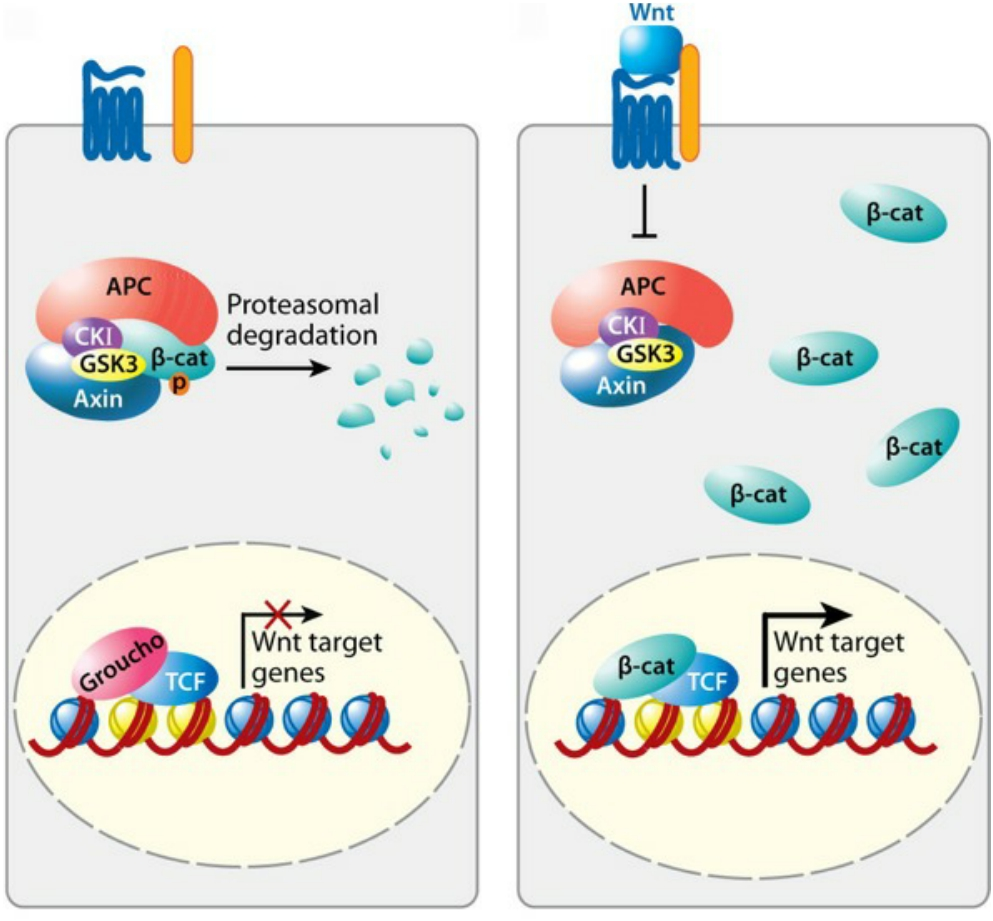
APC is tumor suppressor that mediates degradation of β-catenin
Lgr5-expressing cell in culture can generate an entire organized crypt-villus system
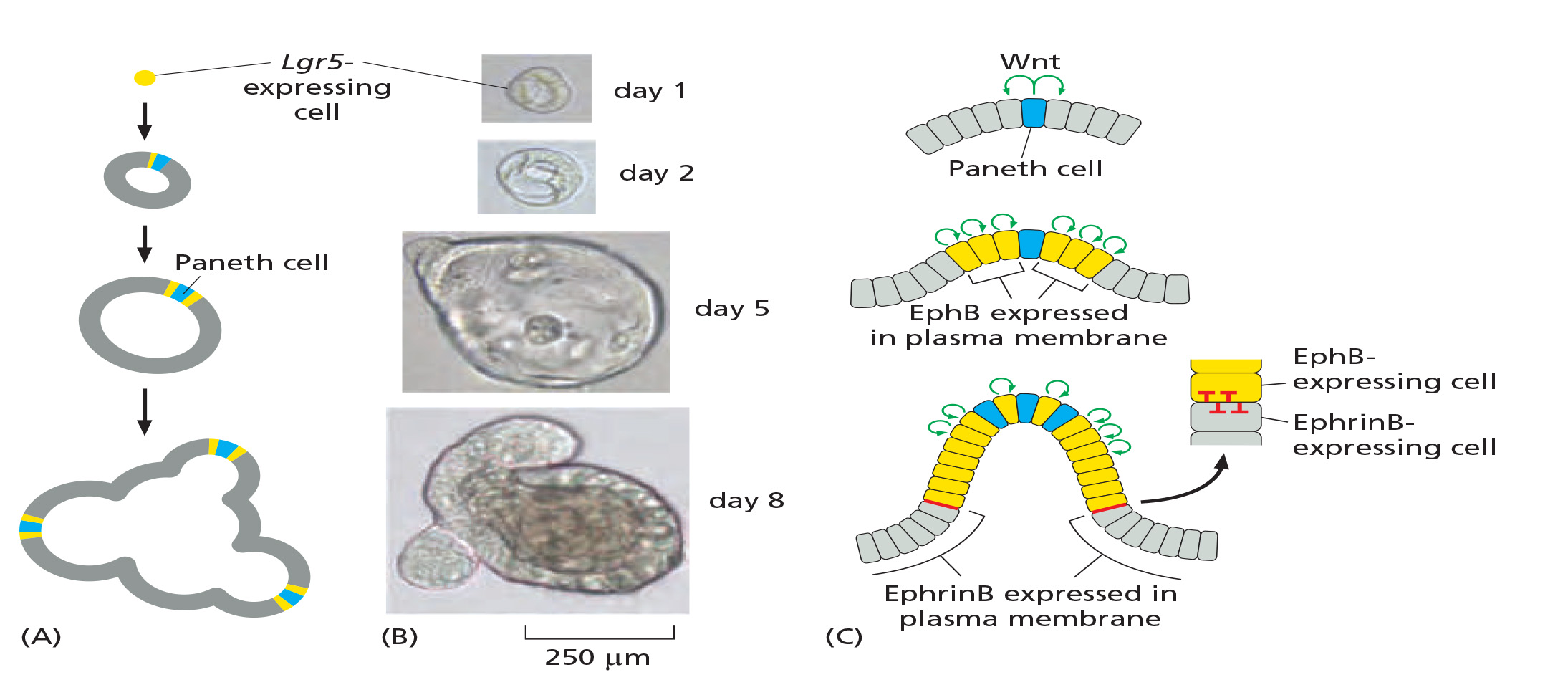
Lgr5 is one of Wnt-signaling responsive genes, which encodes a receptor for the signal molecules called R-spondin.
Genesis of mini-gut from single in vitro cultured Lrg5-expressing cell
(1) ephrin induces growth cone collapse through activating Rho GTPase
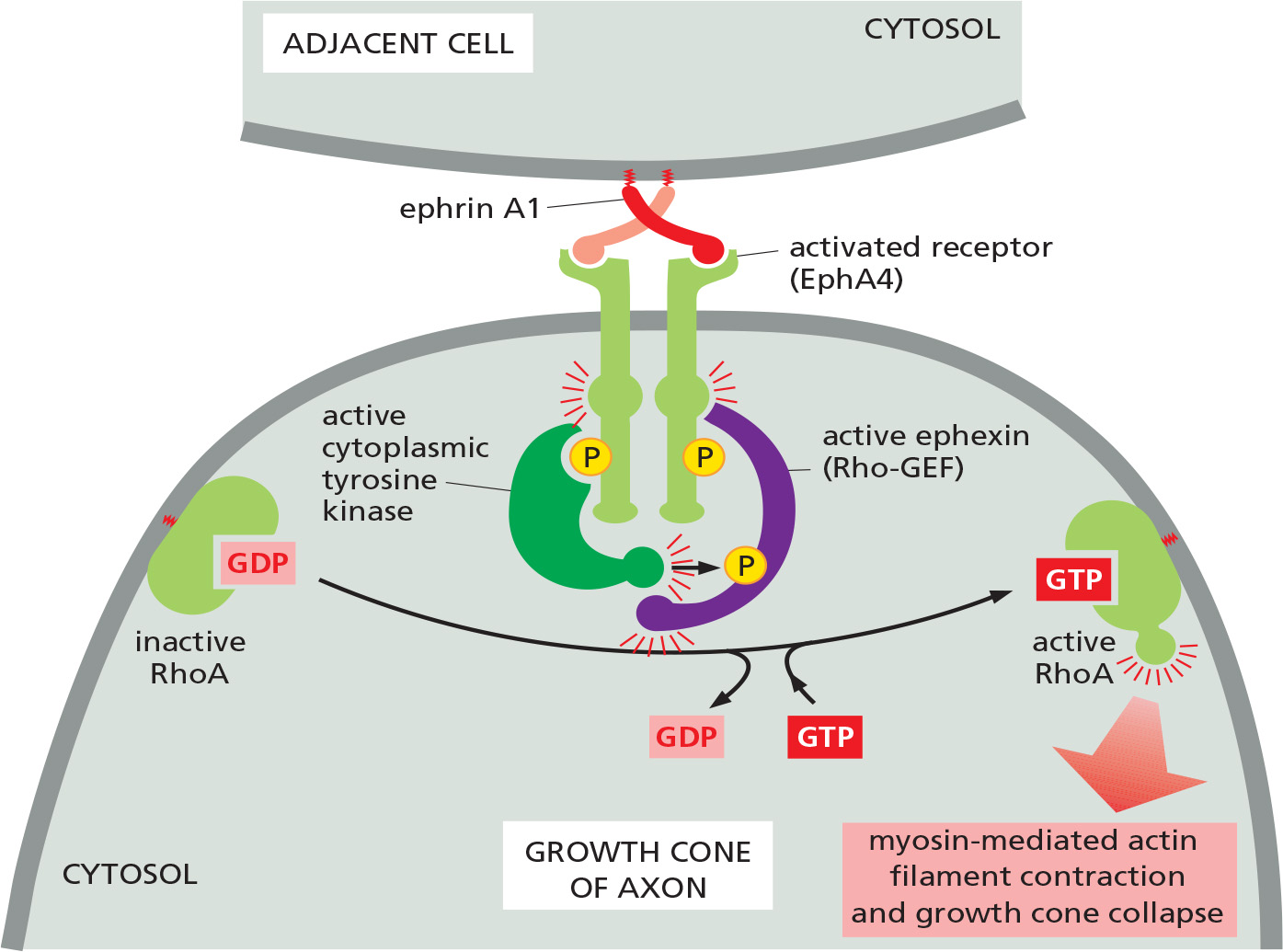
Ephrin (extracellular signaling proteins ) binds and activates the Eph family of RTKs (EphA4) on the surface of motor neurons
Helps guide the migrating tip of the axon (called a growth cone) to its muscle target
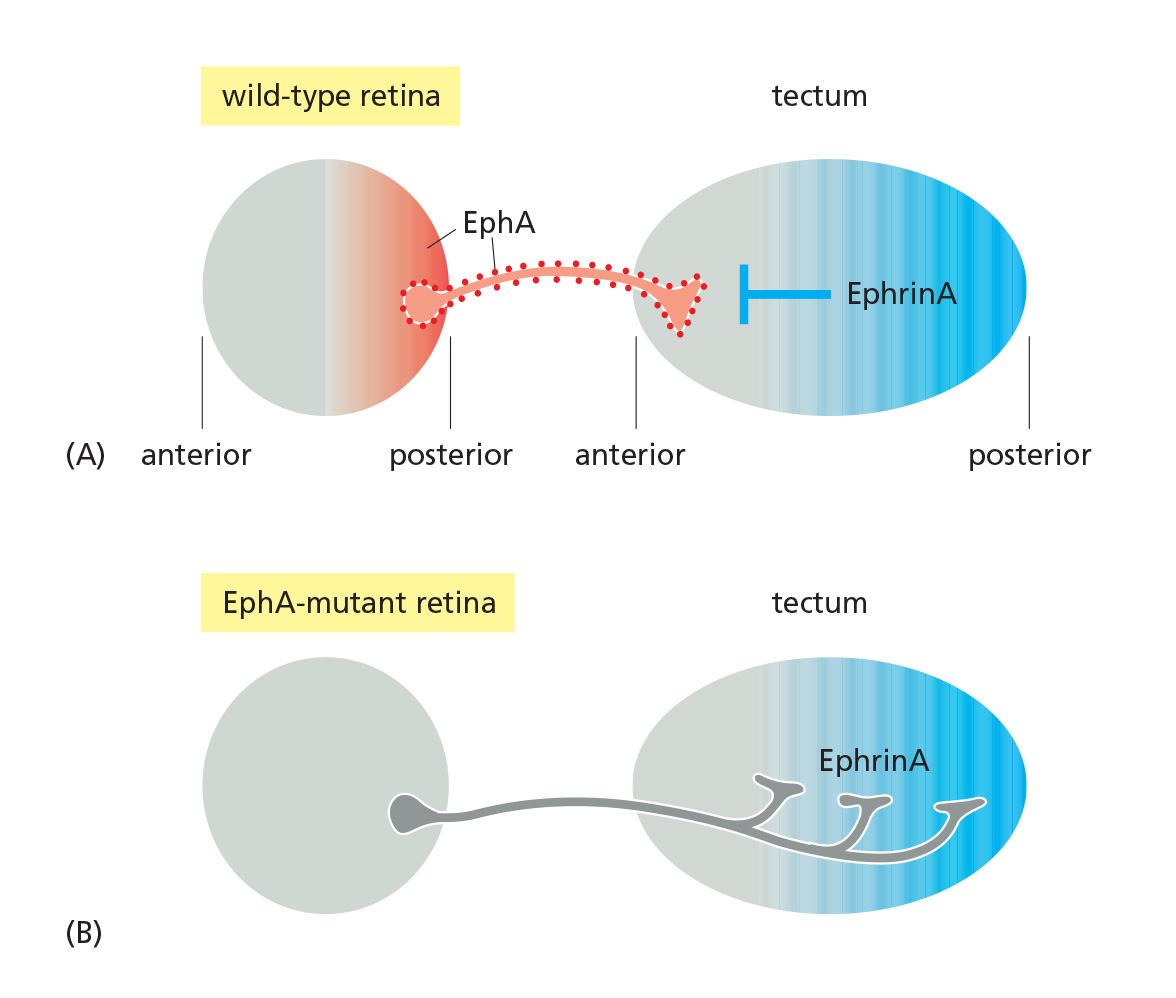
(2) A repulsive interaction based on ephrin-Eph interaction
2. Notch pathway and differentiation
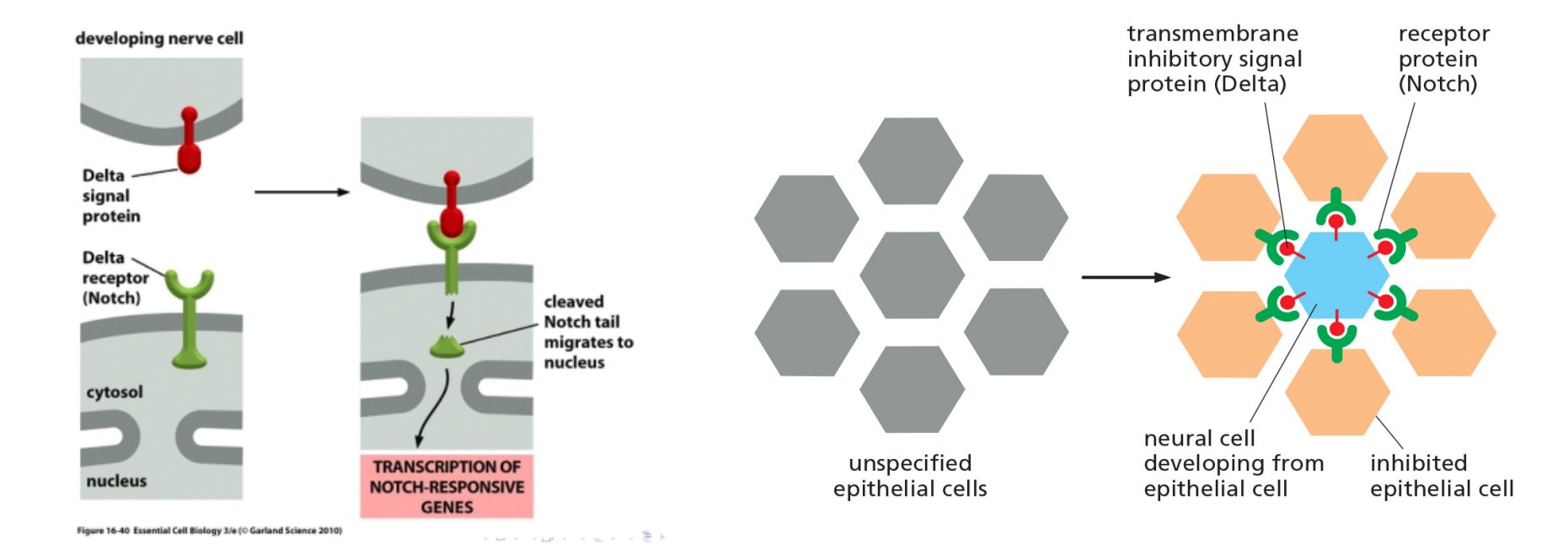
In fly neural cells, when a precursor cell commits to becoming a neural cell, it signals to its immediate neighbors not to do the same; the inhibited cells develop into epidermal cells instead. This process is called lateral inhibition.
This depends on a contact-dependent signaling mechanism that is activated by a single-pass transmembrane signal protein called Delta that activates Notch receptors.
By activation, Notch cleaved and its tail migrates to nucleus where activates responsible genes
The processing and activation of Notch
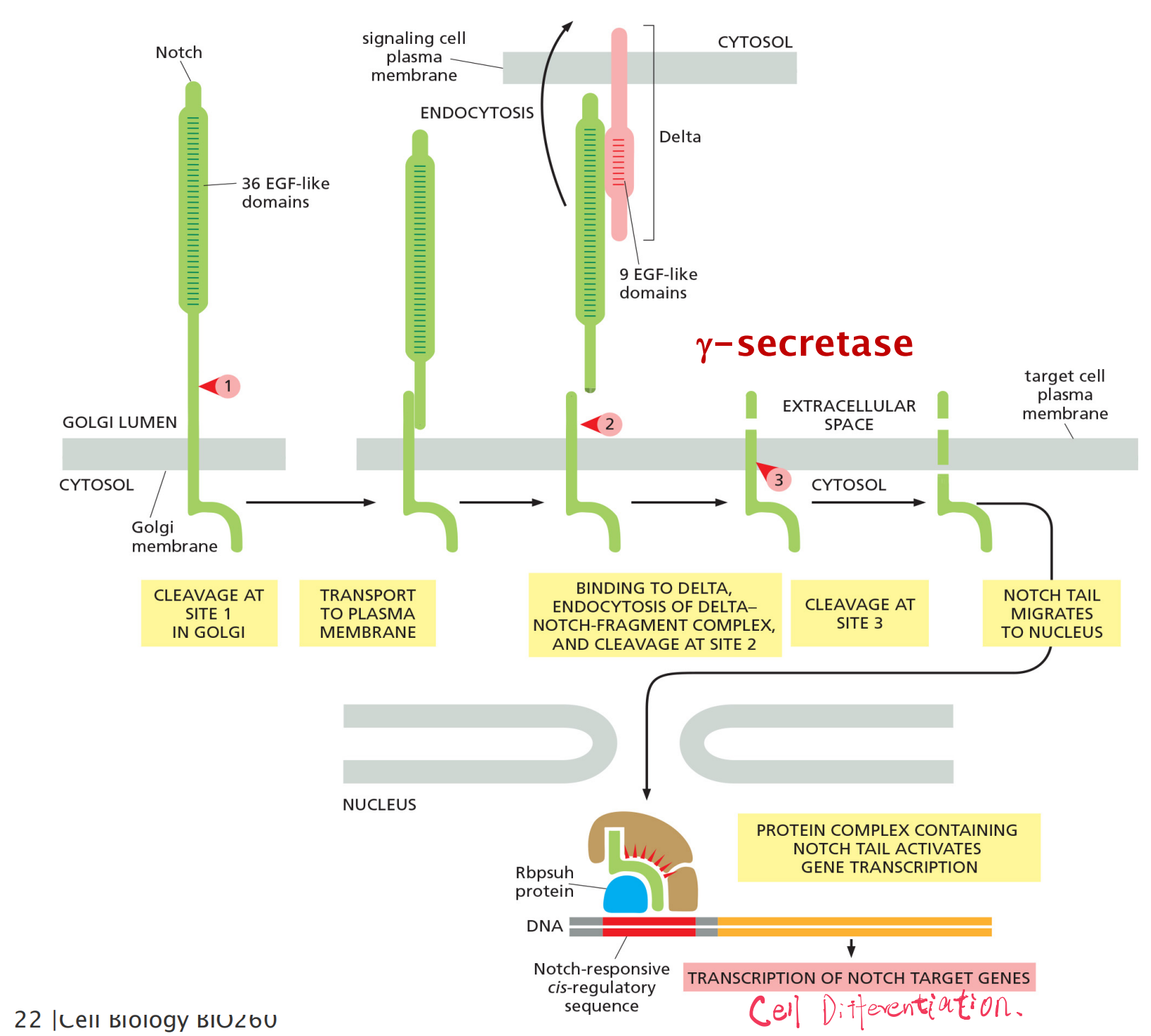
3-step proteolytic processing
- The first proteolytic processing step occurs within the trans Golgi network to generate the mature heterodimeric Notch receptor that is then displayed on the cell surface.
- The binding to Delta on a neighboring cell triggers the next two proteolytic steps:
- Delta-Notch complex is endocytosed by the Delta expressing cell, exposing the extracellular cleavage site in the transmembrane Notch subunit.
- The released Notch tail migrates into the nucleus, where it binds to the Rbpsuhprotein, which it converts from a transcriptional repressor to a transcriptional activator.
Notch signaling controls gut cell diversification and helps maintain the stem-cell state
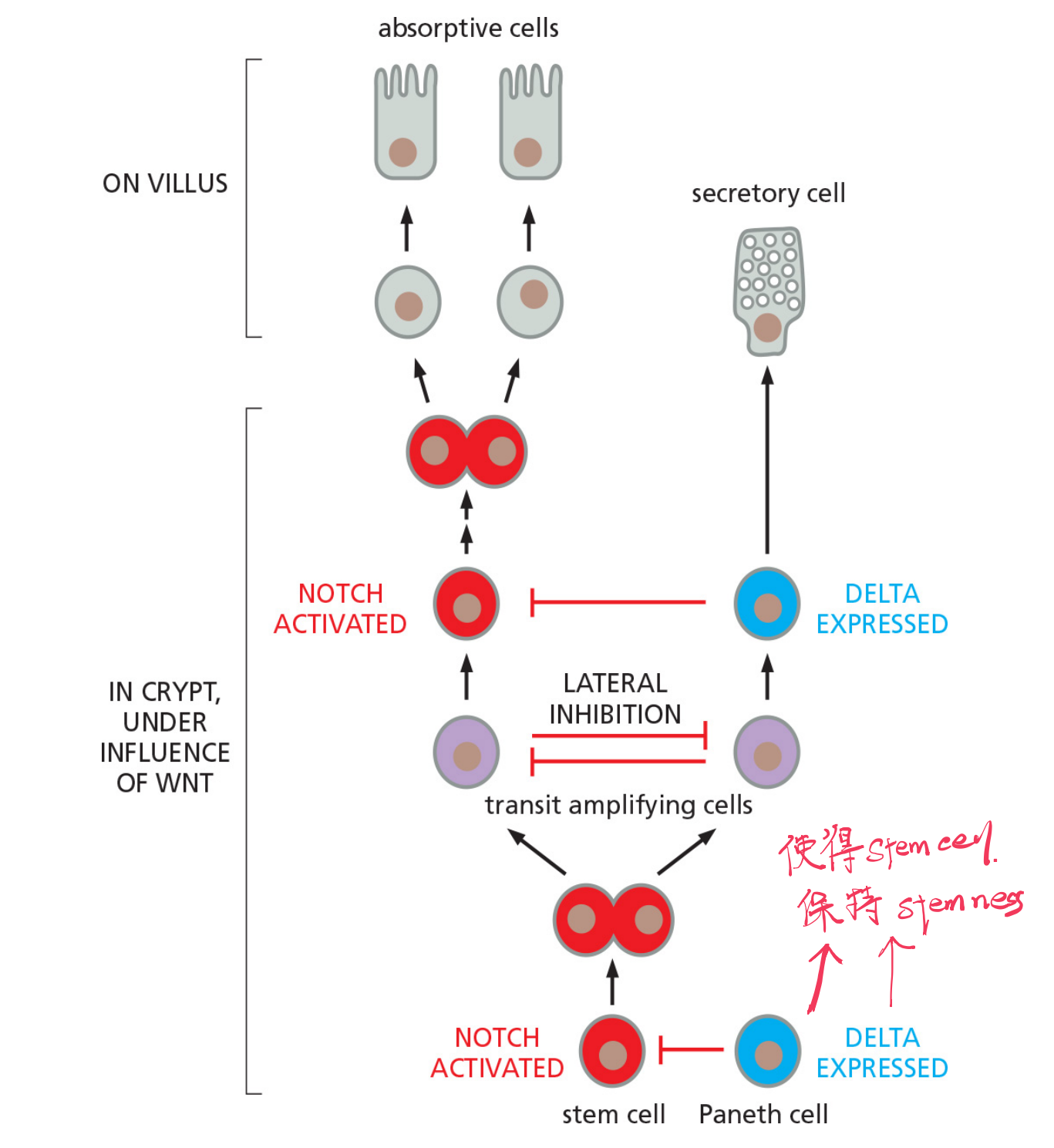
Paneth cells create stem cell niche
- The paneth cells generate signals, including a strong Wnt signal, that act over a short range to maintain the stem-cell state.
- Signal proteins from the connective tissue surrounding the crypt base help to reinforce the localizing signal from the Paneth cells
The cell lineage: tracing the origin of a cell
Pathway from stem cells to lineage-restricted progenitors to differentiated cells.
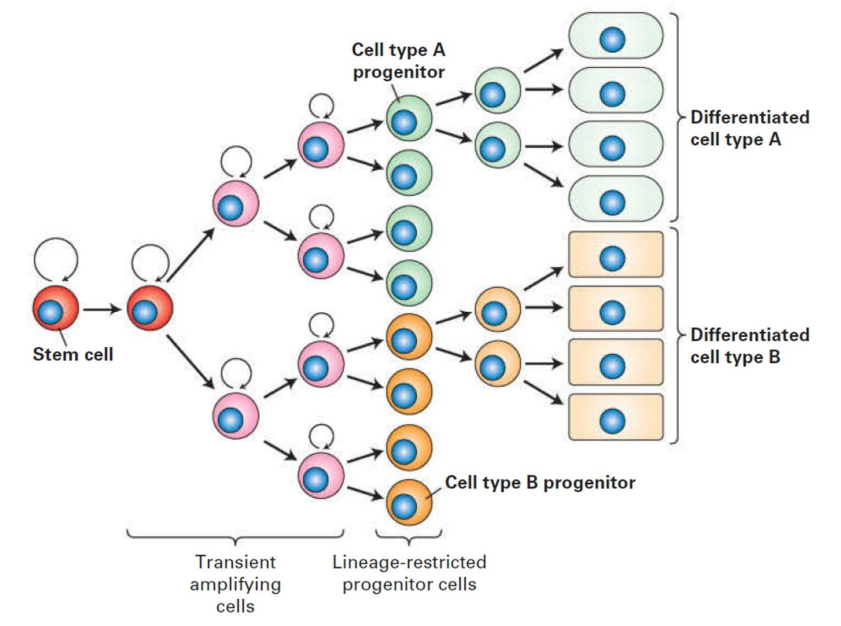
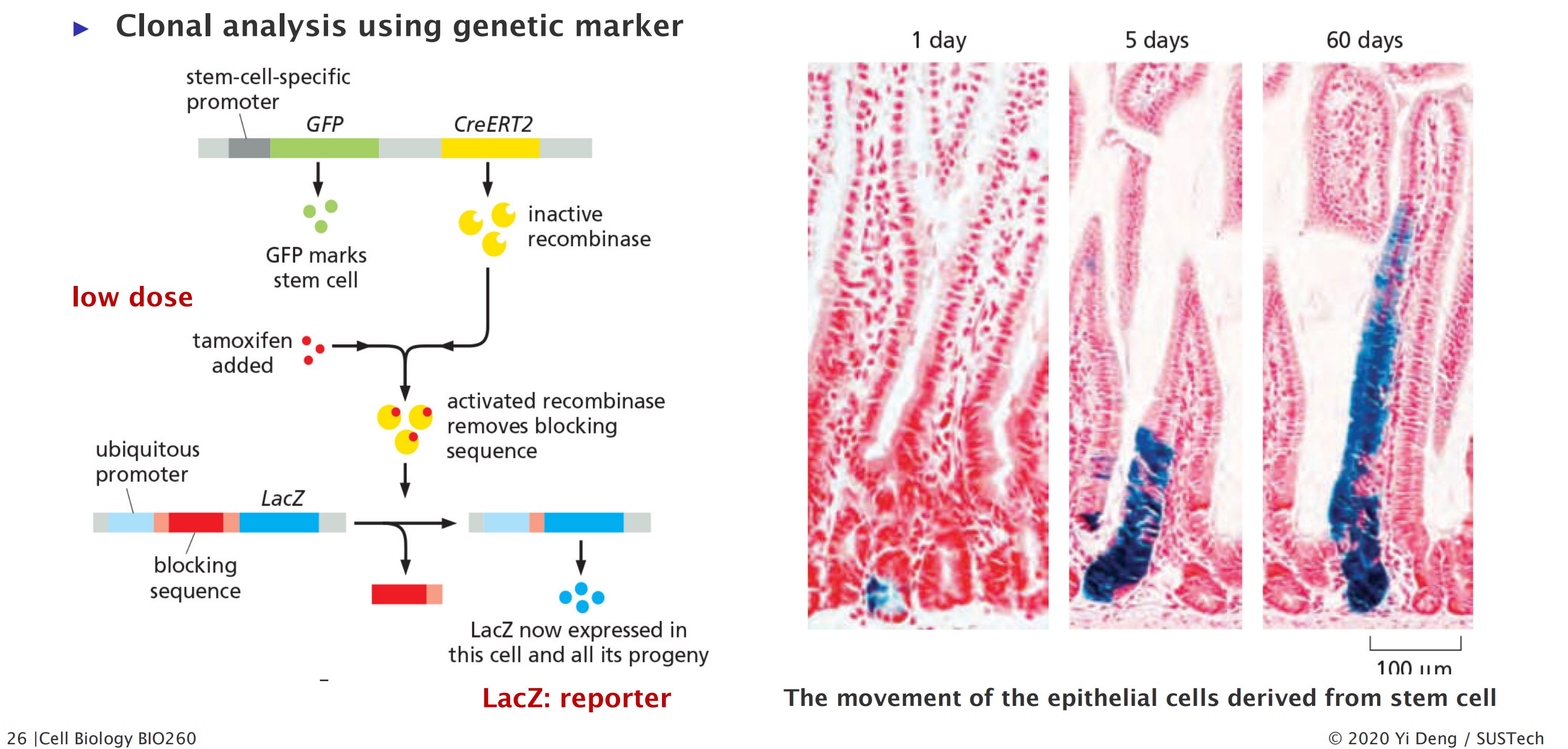
1. Stem cells at the crypt base are multipotent
Clonal Analysis
Cell Fate Determination
Two ways for a stem cell to produce daughters with different fates
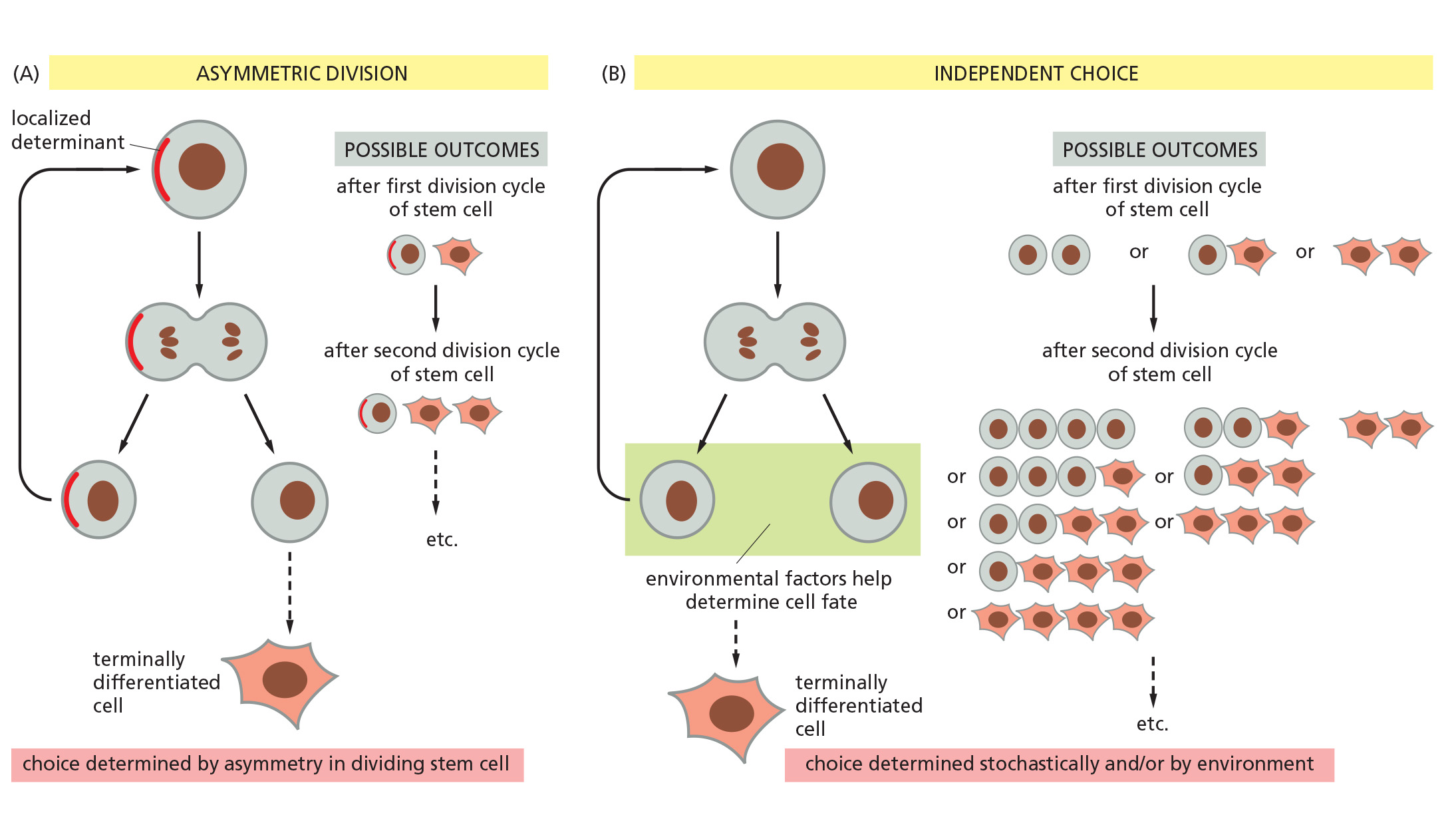
1. Example: The epidermal stem-cell system Renewal
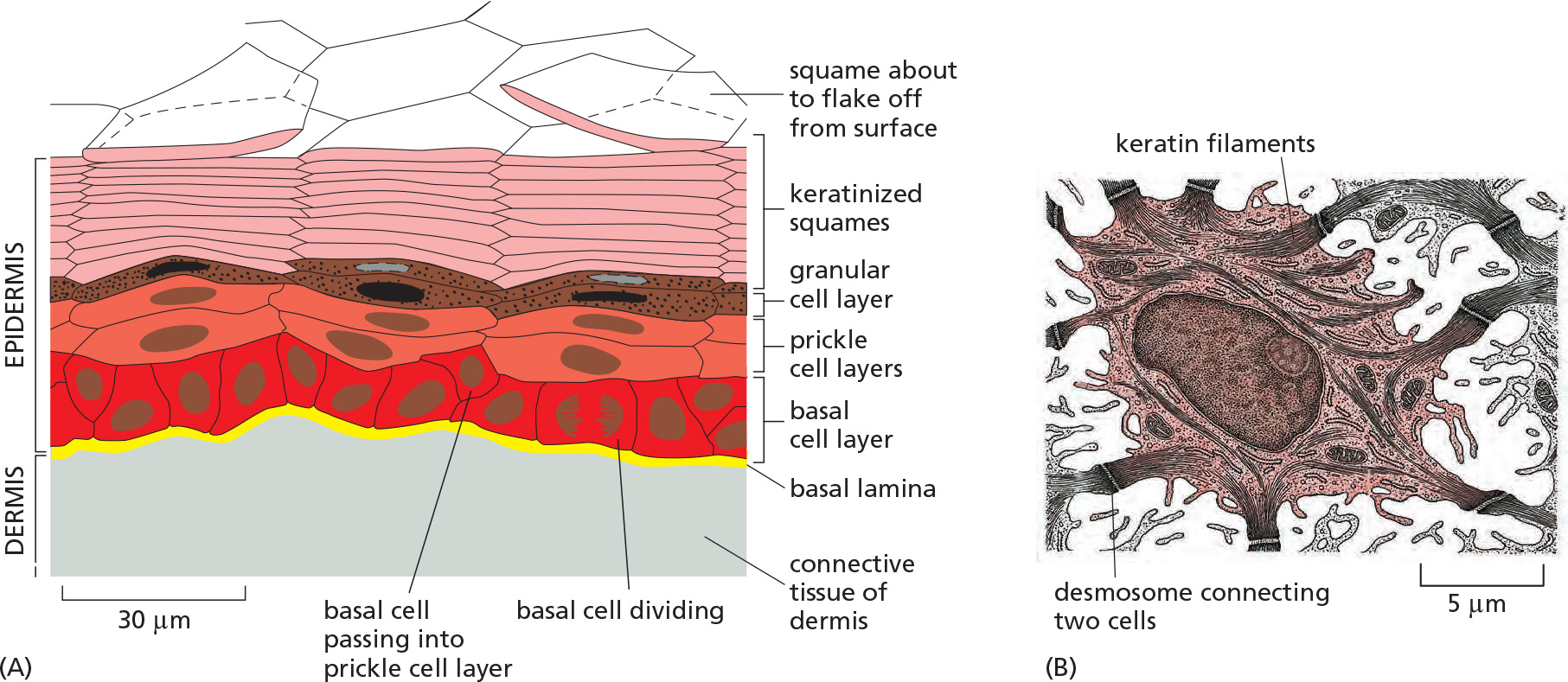
2. Other tissue renewal
Tissue renewal that do not depend on stem cells
- Insulin-secreting cell (β-cell)
- Hepatocytes
Some tissues lack stem cells and are not renewable
- Auditory epithelium
- retina
二、Cell reprogramming and pluripotent stem cells
Animal cloning: reverse differentiation
Cloned animal by the transfer of a nucleus of a differentiated somatic cell into an enucleated egg
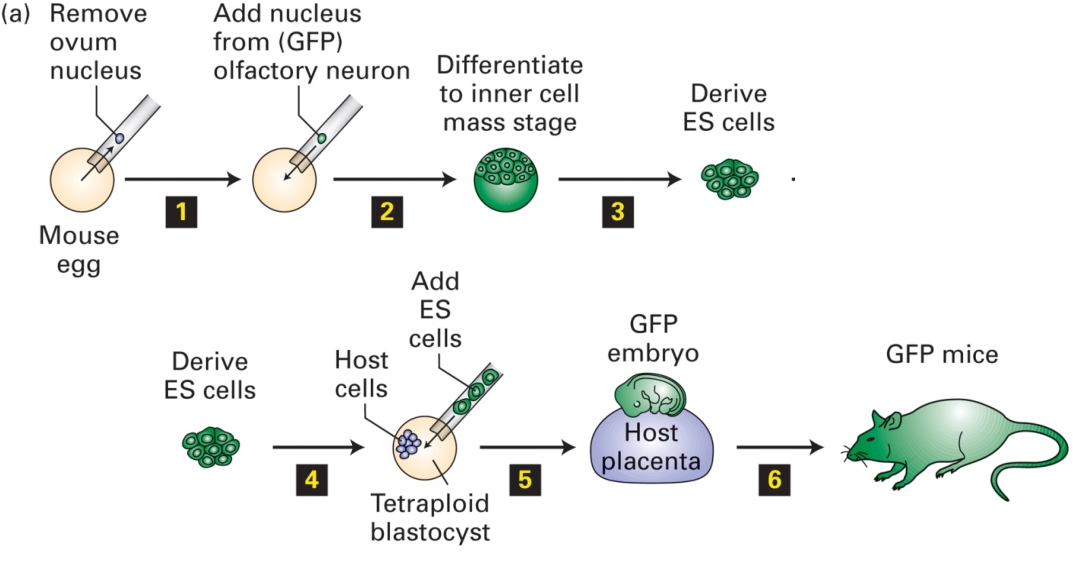
Different types of stem cells
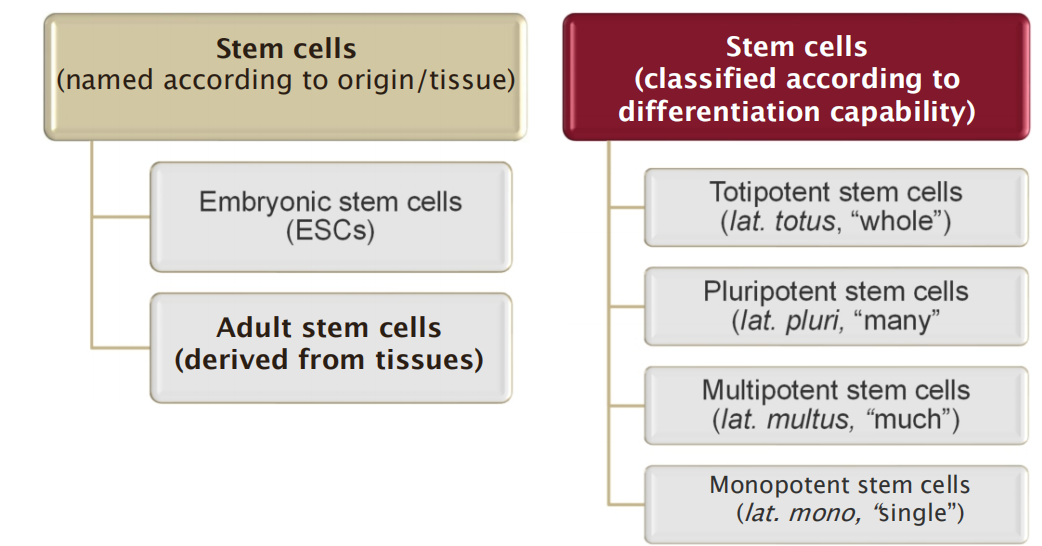
- Stem cells can be named after their location/fate or according to their differentiation capability
A fertilized egg or an equivalent cell produced by nuclear transplantation, is said to be totipotent
but in a strict sense it is not a stem cell because it is not self-renewing, and instead dedicated to a program of progressive differentiation.
1. Pluri-, multi- & mono-potent stem cells
Pluripotent cells can differentiate into a wide variety of cells, but can not differentiate to form the whole organism.
- Examples:
- embryonic stem cells
- bone marrow stromal stem cells
- nerve stem cells
- embryonic germ
Multi- and mono-potent stem cells can differentiate into a few types of cells or only in a special type of cells, respectively
- Examples:
- neuroglial cells
- nerve stem cells
- satellite cells
- epithelial stem cells
- hematopoietic stem cells
Inner cell mass of early embryos: embryonic stem (ES) cells
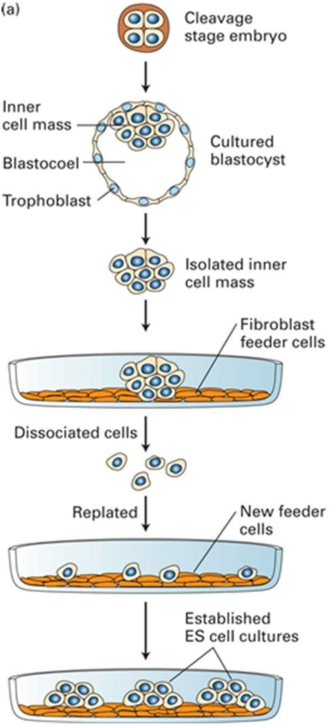
Cells taken from the inner cell mass of an early mammalian embryo are embryonic stem (ES) cells.
ES cells cultured in suspension develop into embryoid bodies upon stimulation with the transcription factor STAT3 (Signal transducer and activator of transcription 3)
1. ES cells and their pluripotency
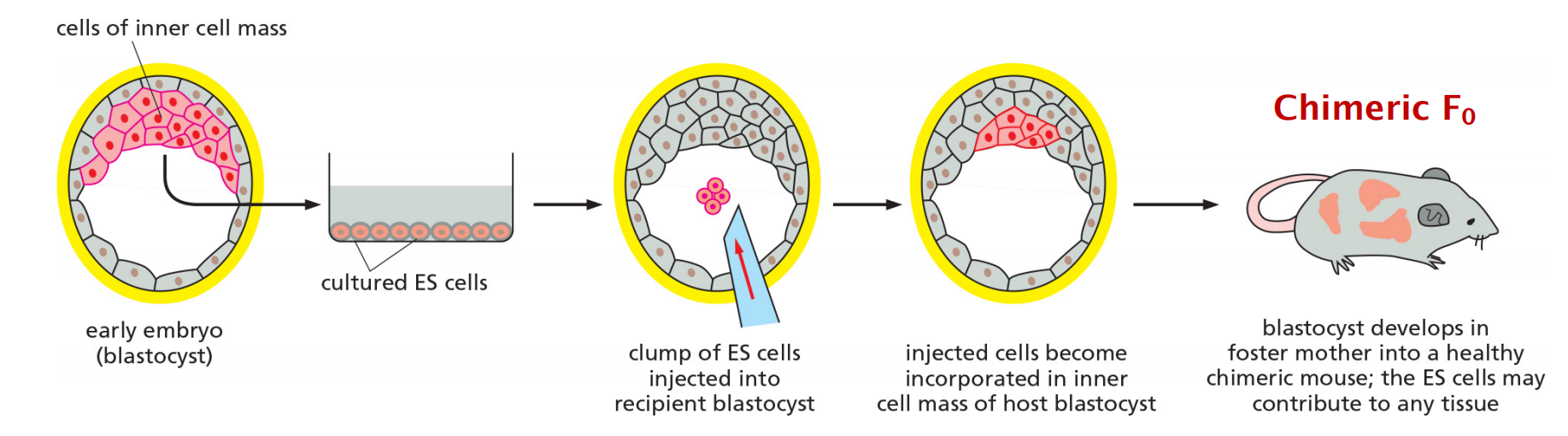
ES cells are derived from inner cell mass of early embryos
ES cells can be propagated in culture indefinitely in a pluripotent state. When transplanted back into a host early embryo, they can contribute cells to any tissue, including the germ line (germ cell).
How the pluripotency of ES cells Is maintained?
- What are mechanisms underlying stemness, cell differentiation, and the stability of a differentiated state?
2. Identification of factors control/maintain the pluripotency of ES cells in animals
ES cells express high levels of active telomerase (端粒酶), allowing them to escape senescence and continue dividing indefinitely.
How the whole complex pattern of gene expression in an ES cell is organized and maintained that ensure its stemness, cell differentiation potential?
Gene expression analysis
- → Identification of Oct4 (octamer binding transcription factor 4)
- Oct4 is exclusively expressed in ES cells, in the germ-cell lineage and in the inner cell mass and its precursors.
- Oct4 is a transcription regulator.
- Loss of Oct4 in ES cells leads to losing the ES cell character
- Loss of Oct4 in an embryo, the precursors of inner cell mass are diverted into an extraembryonic pathway of differentiation and their development is aborted.
Multiple transcription regulators: Oct4, Sox9, Nanog…
3. Transcription network of ES cells
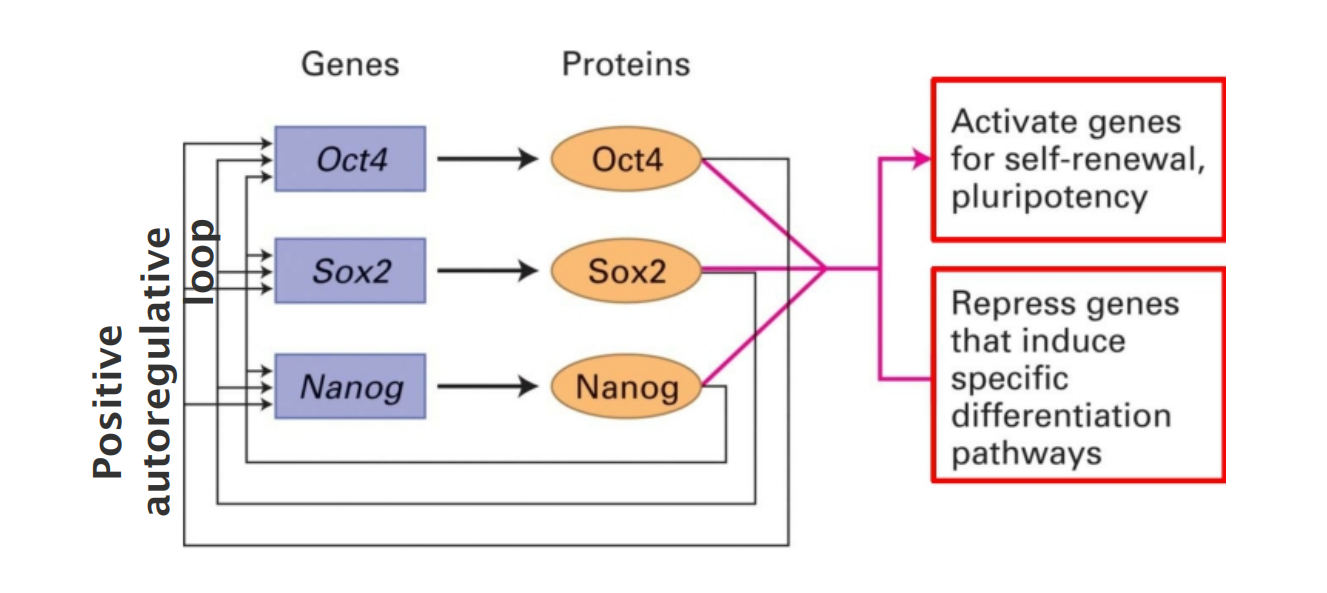
Each of these transcription factors has been found to regulate over 1000 different genes.
- OCT4 (octamer binding transcription factor 4)
- Sox2 (sex determining region Y- box 2)
- Nanog (Irish, Tír na nÓg , “land of eternal youth”)
The master regulators control their own expression.
Many target genes are bound by more than one regulators.
OSKM/Yamanaka transcription factors and induced pluripotent stem (iPS) cells
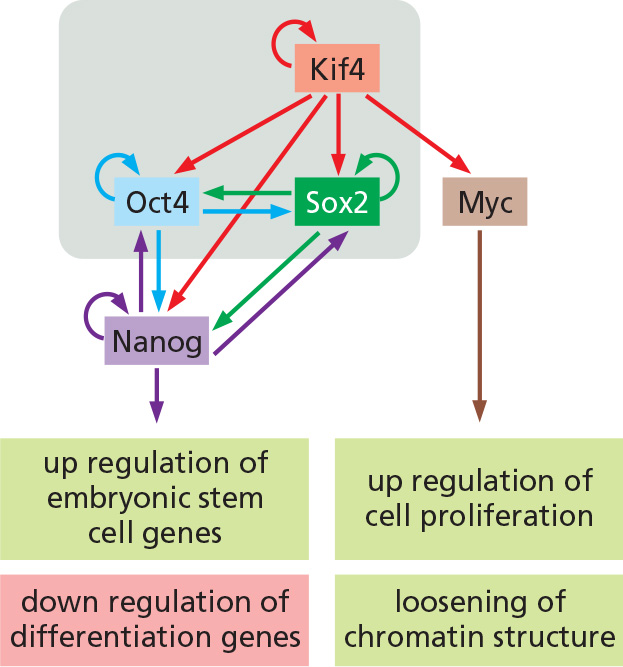
A total of 24 candidate ES-critical genes were tested to reprogram fibroblast to create Induced pluripotent stem cells (iPS)
By introducing four different transcription factors, Kif4, Sox2, Oct4, c-myc (they are also called OSKM/Yamanaka transcription factors).
- Core set of transcription regulators, which, when co-expressed, could reprogram mouse fibroblasts, permanently converting them into cells closely similar to ES cells, ie. induced pluripotent stem cells
Master transcription factors
- Self-induction
- Regulate other genes, leading to changing patterns of
- histone modification
- DNA methylation (at early stage of development, a wide-spread DNA demethylation occurs)
- Chromatin remodeling
- miRNAs
Major events accompanying the reprogramming of mouse fibroblasts to iPS cells
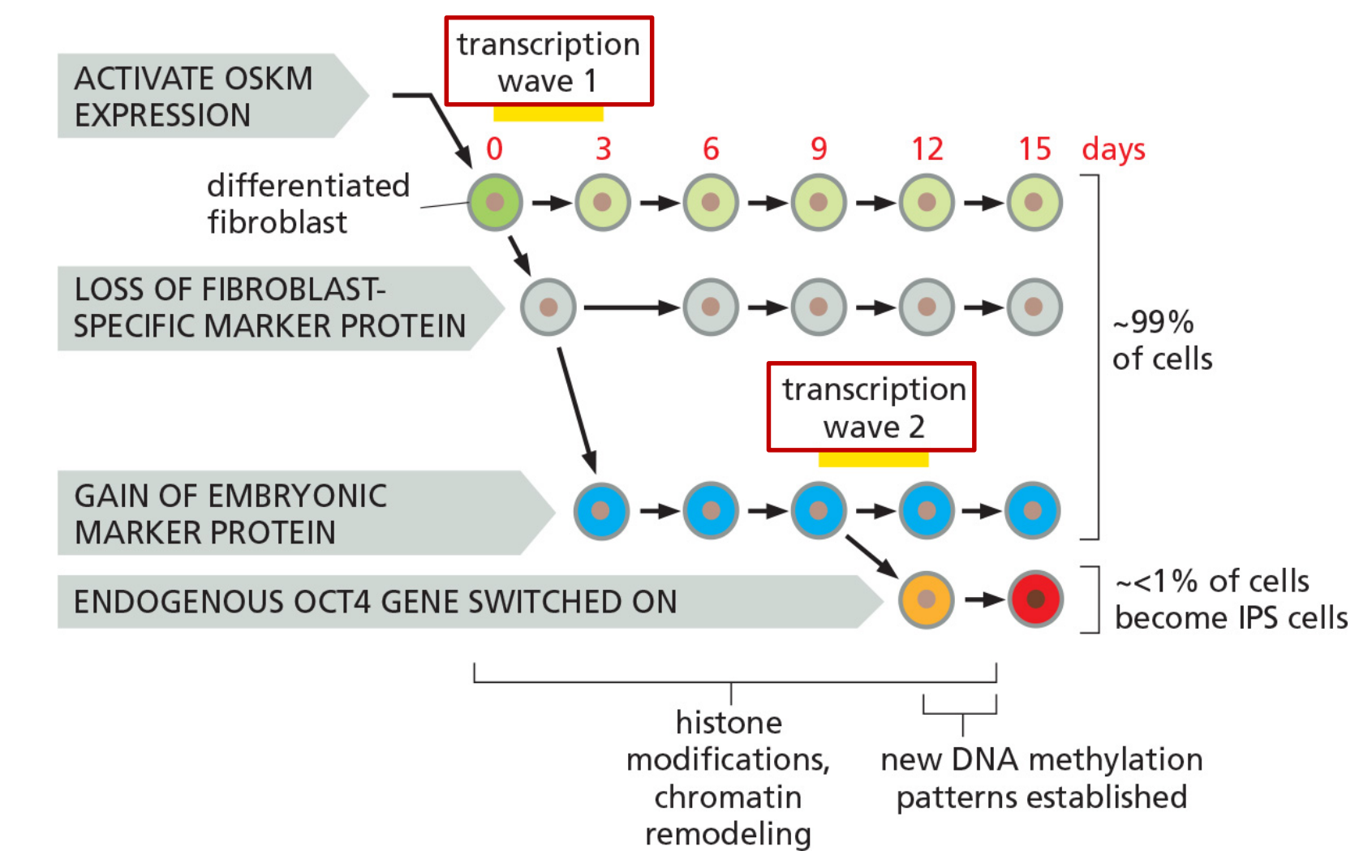
Biochemical analysis of iPS cells at different stages after transfection of OSKM/Yamanaka transcription factors.
Factors that have been observed to enhance reprogramming efficiency
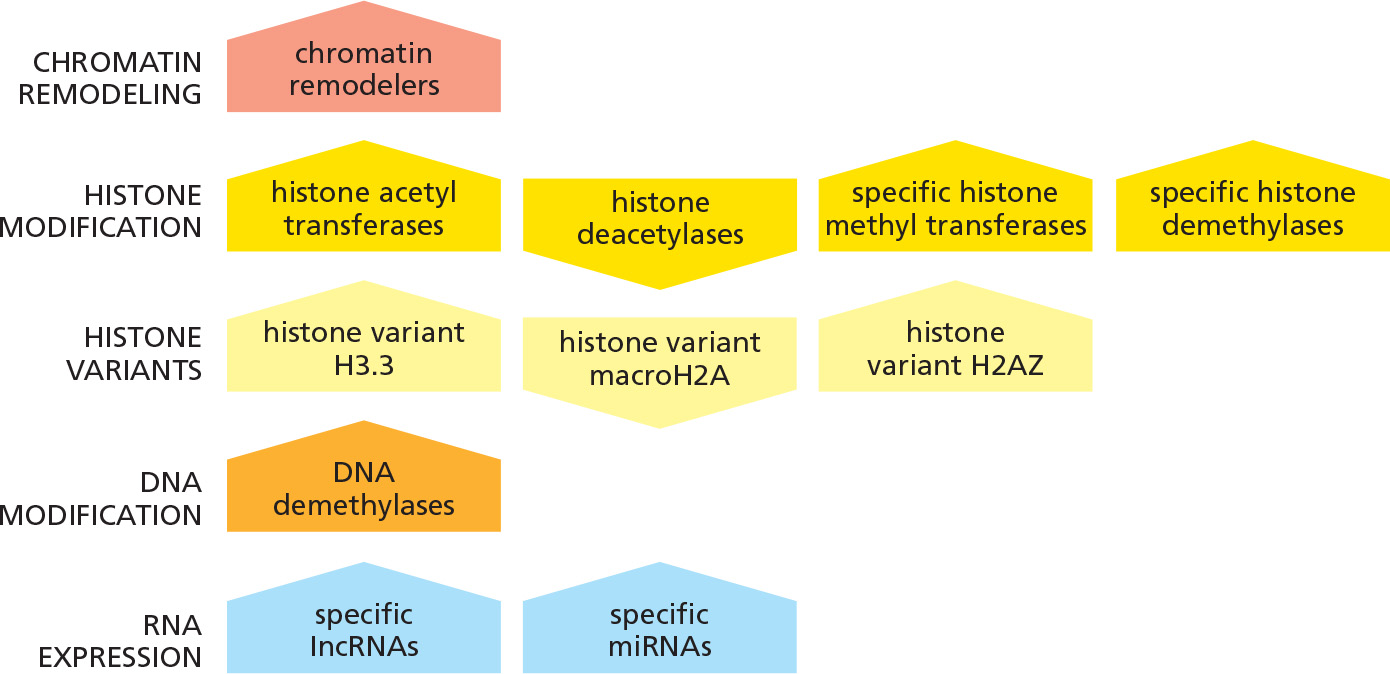
- 上图“尖角”朝上的表明enhance reprogramming, “尖角”朝下的表明inhibit
A strategy used to select cells that have converted to an iPS cell
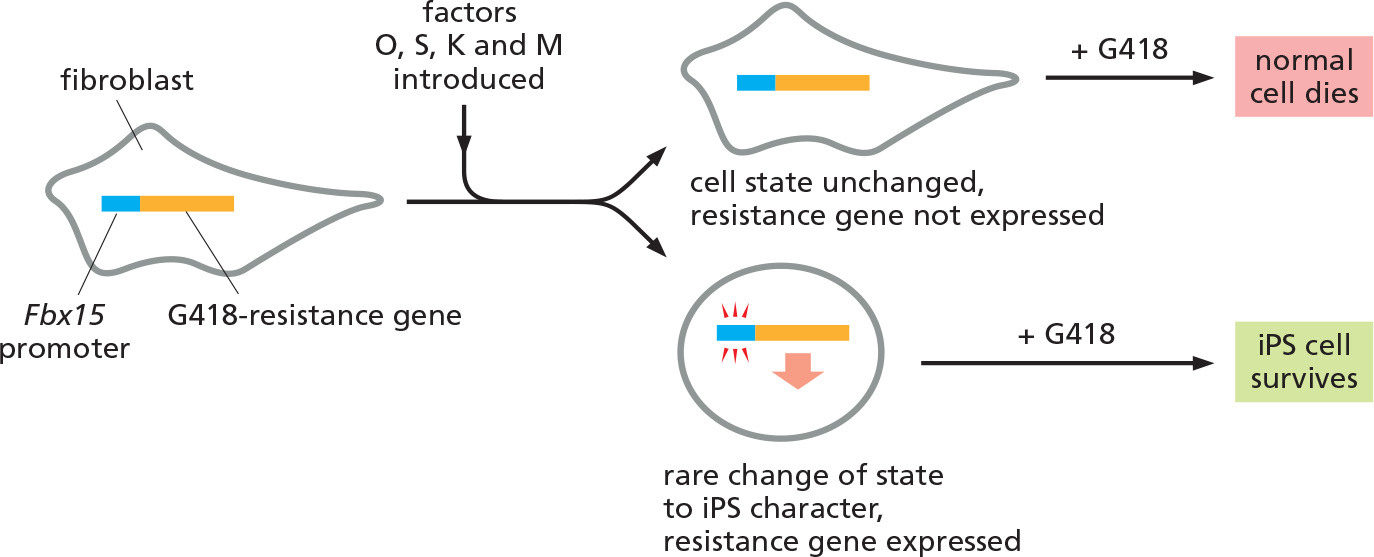
Fbx15 is present in all cells but is normally expressed only in ES and early embryonic cells
Using of stem cells
Cloning (via unfertilized egg): reproductive and therapeutic
The use of embryonic stem cells to cure diseases such as diabetes.
- Embryonic stem cells were induced to differentiate into pancreatic endoderm, which were then transplanted into mice.
- These mice will produce functional beta islet cells to produce insulin.
- Danger in introducing undifferentiated embryonic stem cells, because they will produce tumor like mass, containing large amounts of poorly differentiated cells
New tissue generation via iPS stem cells
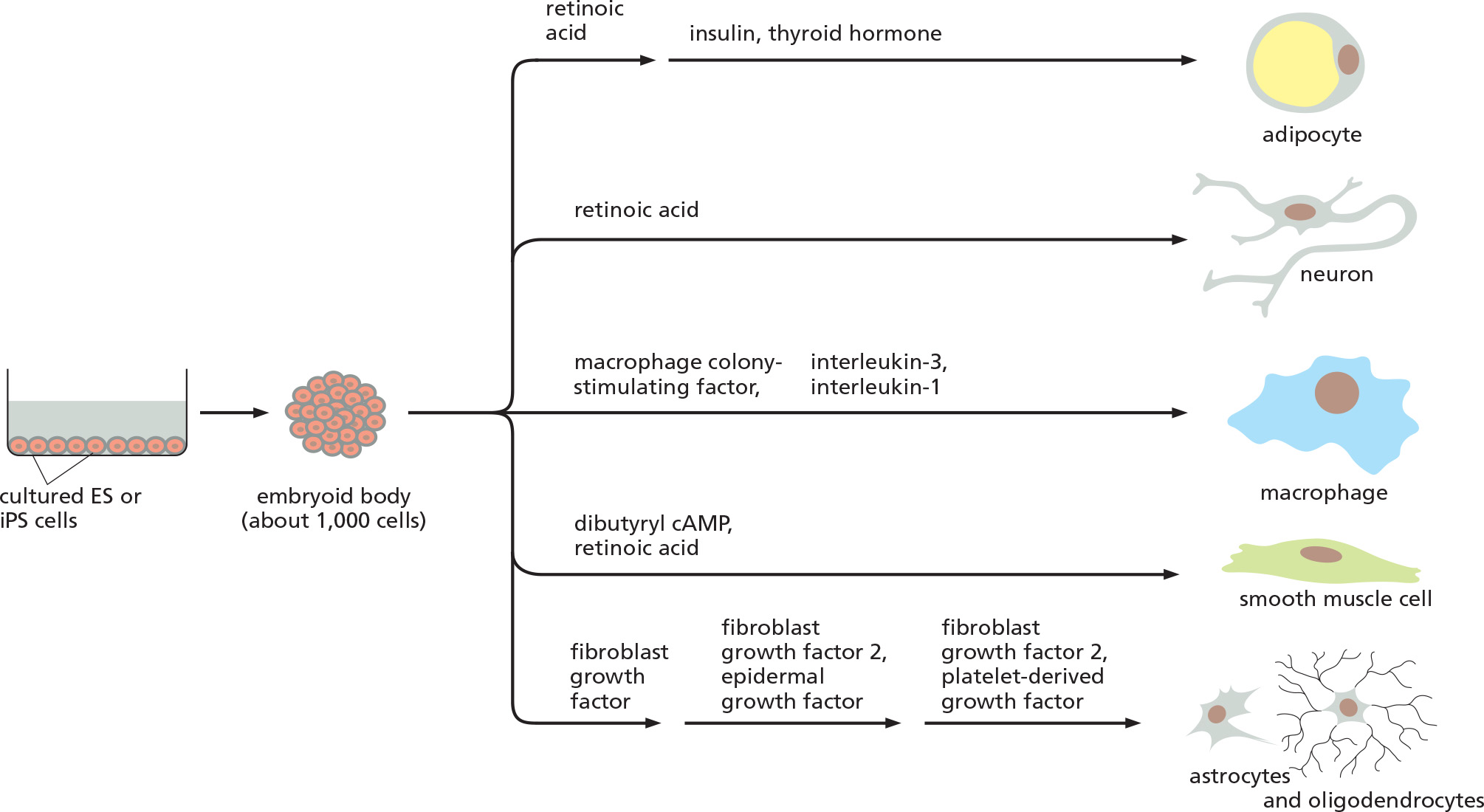
1. Cultured ES cells can give rise to a three-dimensional organ
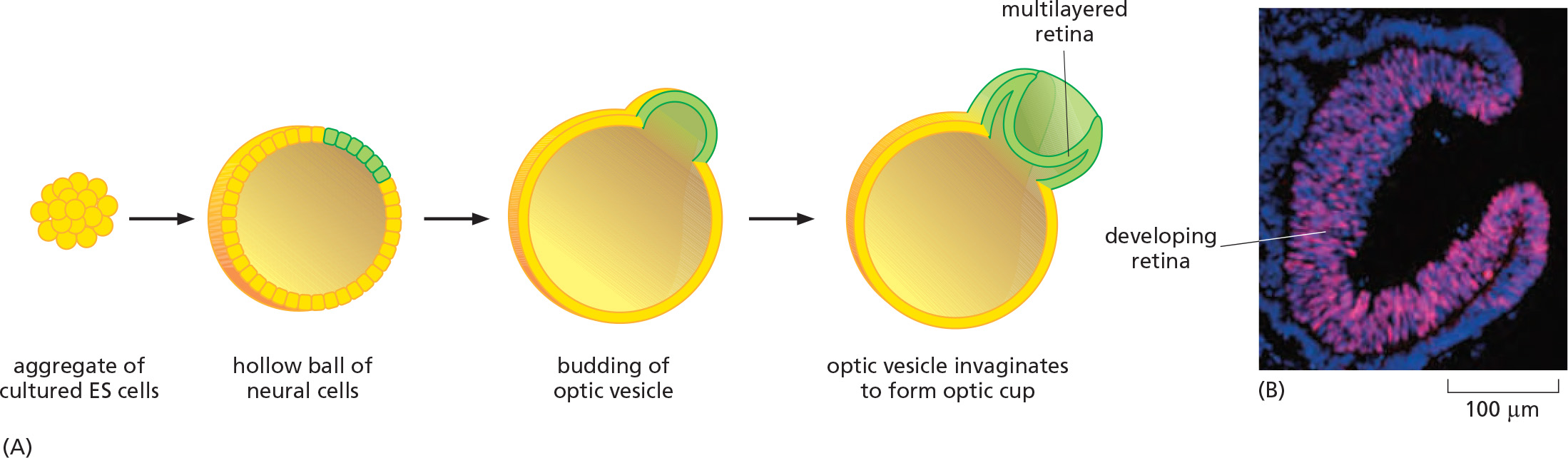
2. Medical applications for induced pluripotent stem cells (iPS)
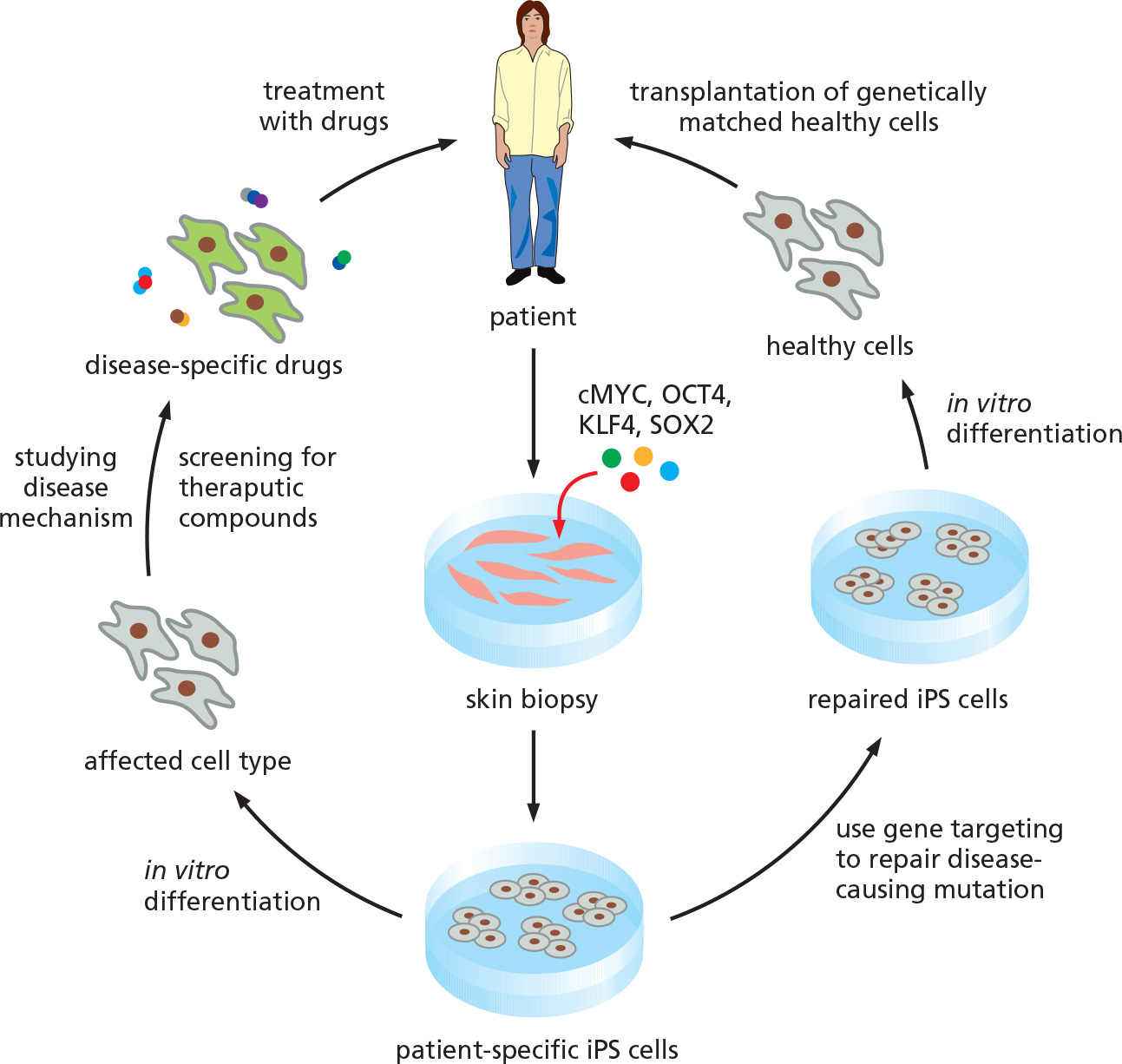
Two options to use iPS cells to cure the diseases.
- Option 1: Use of iPS cells to produce a patient-specific drug to cure the disease
- Option 2: Use of iPS cells to repair a genetic defect and to regenerate patient-specific cells for implantation