L9 Introduction to pharmacology of CSN drugs , sedatives and hypnotics
一、Introduction
Information from studies
Almost all drugs with CNS effects act on specific receptors that modulate synapse, exceptions?
Drugs are among the most important tools for studying all aspects of CNS physiology
Hypotheses regarding the mechanisms of disease from the actions of drugs with known clinical Efficacy: Dopamine, schizophrenia;
- GABA: anxiety and epilepsy
Ion channels and neurotransmitter receptors
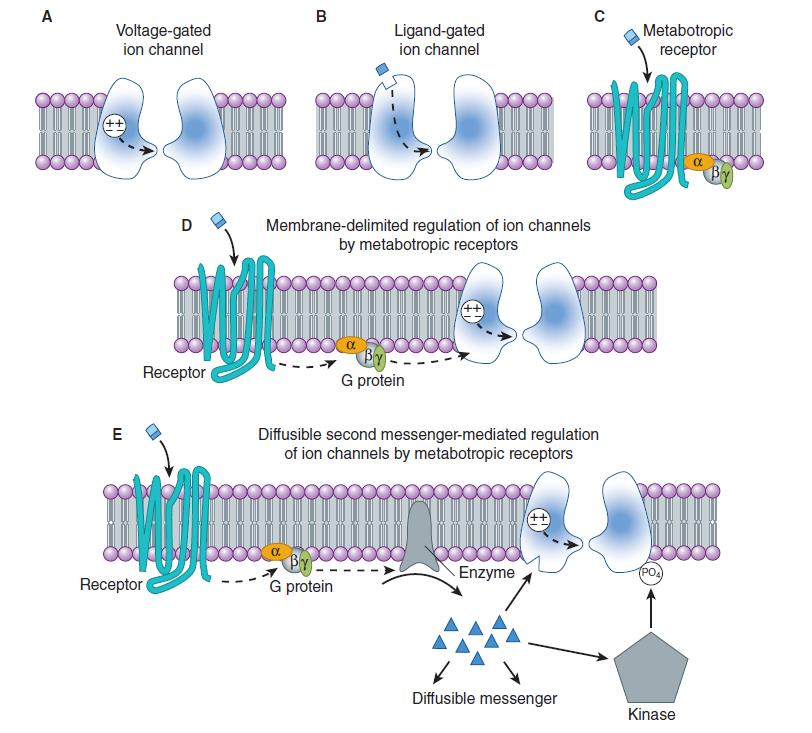
Ion Channels
1. Voltage-gated ion channels
Sodium (Na^+^) channels: have similar functional properties across many different cell types. While ten human genes encoding for sodium channels have been identified, their function is typically conserved between species and different cell types. (initiators)
Calcium (Ca^2+^) channels: With sixteen different identified genes for human calcium channels, this type of channel differs in function between cell types. Ca^2+^ channels give rise to action potentials similarly to Na^+^ channels in some neurons. They also play a role in neurotransmitter release in pre-synaptic nerve endings. (final executors)
Potassium (K^+^) channels: the largest and most diverse class of voltage-gated channels, with over 100 encoding human genes. some inactivating extremely slowly and others inactivating extremely quickly. This difference in activation time influences the duration and rate of action potential firing, which has a significant effect on electrical conduction along an axon as well as synaptic transmission. (modulators)
Chloride (Cl^−^) channels: are present in every type of neuron. With the chief responsibility of controlling excitability, chloride channels contribute to the maintenance of cell resting membrane potential and help to regulate cell volume.
Proton (H^+^) channels: carry currents mediated by hydrogen ions in the form of hydronium, and are activated by depolarization in a pH-dependent manner. They function to remove acid from cells.
Voltage-gated Sodium Channels
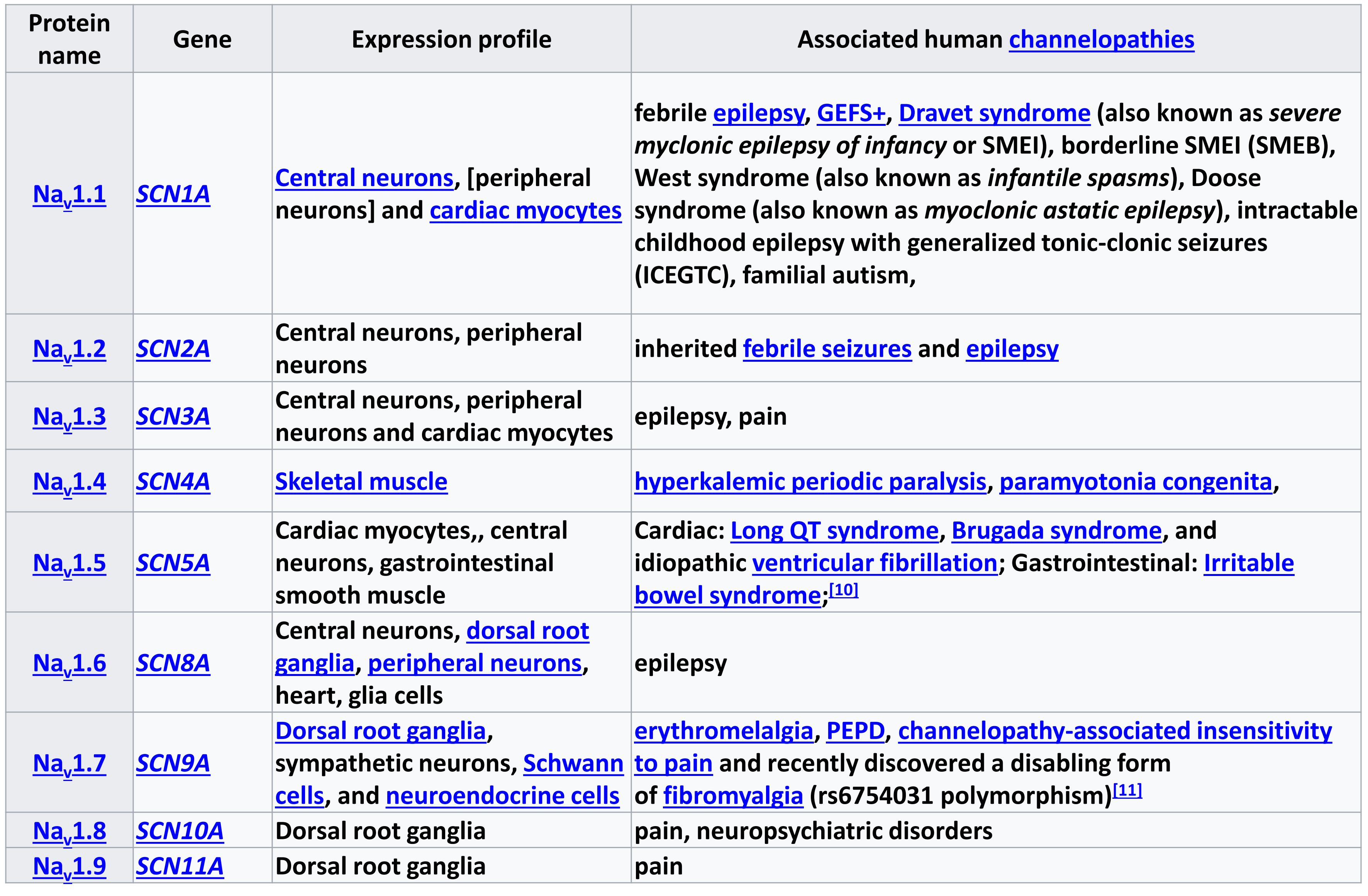
| Protein Name | Gene | Expression Profile | Associated Human Channelopathies |
|---|---|---|---|
| Na |
SCN1A | Central neurons, [peripheral neurons] and cardiac myocytes | febrile epilepsy, GEFS+, Dravet syndrome (also known as severe myclonic epilepsy of infancy or SMEI), borderline SMEI (SMEB), West syndrome (also known as infantile spasms), Doose syndrome (also known as myoclonic astatic epilepsy), intractable childhood epilepsy with generalized tonic-clonic seizures (ICEGTC), familial autism, |
| Na |
SCN2A | Central neurons, peripheral neurons | inherited febrile seizures and epilepsy |
| Na |
SCN3A | Central neurons, peripheral neurons and cardiac myocytes | epilepsy, pain |
| Na |
SCN4A | Skeletal Muscle | hyperkalemic periodic paralysis, paramyotonia congenita, |
| Na |
SCN5A | Cardiac myocytes,, central neurons, gastrointestinal smooth muscle | Cardiac: Long QT syndrome, Brugada syndrome, and idiopathic ventricular fibrillation; Gastrointestinal: Irritable bowel syndrome; |
| Na |
SCN6A | Central neurons, dorsal root ganglia, peripheral neurons, heart, glia cells | epilepsy |
| Na |
SCN7A | Dorsal root ganglia, sympathetic neurons, Schwann cells, and neuroendocrine cells | erythromelalgia, PEPD, channelopathy-associated insensitivity to pain and recently discovered a disabling form of fibromyalgia (rs6754031 polymorphism) |
| Na |
SCN8A | Dorsal root ganglia | pain, neuropsychiatric disorders |
| Na |
SCN9A | Dorsal root ganglia | pain |
Voltage-gated Calcium Channels
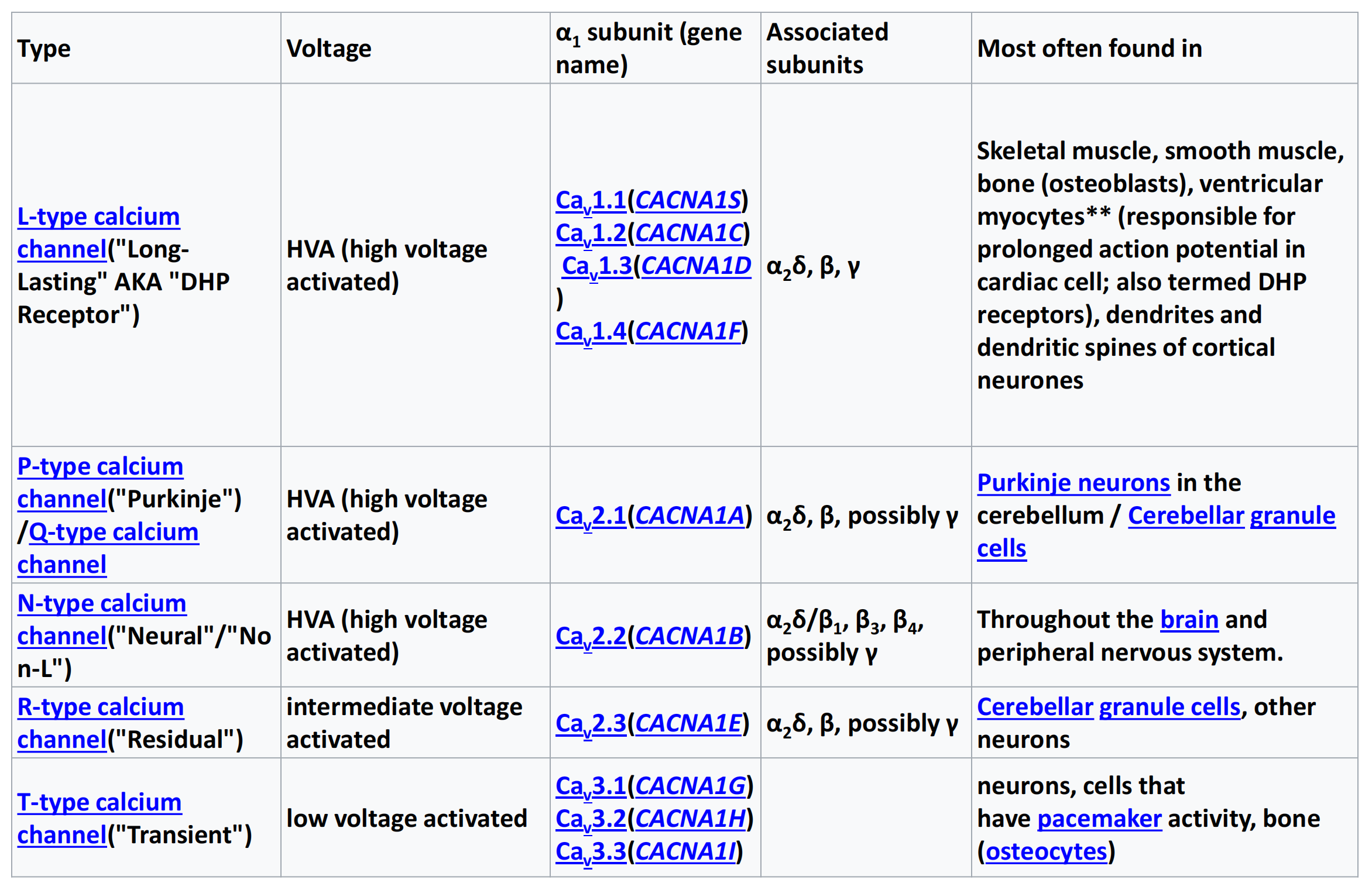
| Type | Voltage | α |
Associated subunits | Most often found in |
|---|---|---|---|---|
| L-type calcium channel(“Long-Lasting” AKA “DHP Receptor”) | HVA (high voltage activated) | Ca Ca Ca Ca |
α |
Skeletal muscle, smooth muscle, bone (osteoblasts), ventricular myocytes** (responsible for prolonged action potential in cardiac cell; also termed DHP receptors), dendrites and dendritic spines of cortical neurones |
| P-type calcium channel(“Purkinje”) /Q-type calcium channel | HVA (high voltage activated) | Ca |
α |
Purkinje neurons in the cerebellum / Cerebellar granule cells |
| N-type calcium channel(“Neural”/“No n-L”) | HVA (high voltage activated) | Ca |
α |
Throughout the brain and peripheral nervous system. |
| R-type calcium channel(“Residual”) | intermediate voltage activated | Ca |
α |
Cerebellar granule cells, other neurons |
| T-type calcium channel(“Transient”) | low voltage activated | Ca Ca Ca |
neurons, cells that have pacemaker activity, bone (osteocytes) |
2. Ligand-gated ion channels (post-synaptic initiators)
Cys-loop receptors
Named after a characteristic loop formed by a disulfide bond between two cysteine residues in the N terminal extracellular domain.
exhibit receptor specificity for
- acetylcholine (AcCh) (non-selective)
- serotonin (5-HT3 ,non-selective)
- glycine (Cl^-^)
- glutamate (Ca^2+^)
- γ-aminobutyric acid (GABA) (Cl^-^)
The prototypic ligand-gated ion channel is the nicotinic acetylcholine receptor
**Ionotropic glutamate receptors (iGluR):**The ionotropic glutamate receptors bind the neurotransmitter glutamate. Further divided into AMPA (GluA), Kainate (GluK),NMDA (GluN) and Orphan (GLuN) subtypes
ATP-gated channels: ATP-gated channels open in response to binding the nucleotide ATP.
- P2X1-7
PIP2-gated channels: Phosphatidylinositol 4,5-bisphosphate (PIP2) binds to and directly agonizes Inward rectifying potassium channels(Kir).
3. Some Toxins Used To Characterize Ion Channels
| Channel Types | Mode of Toxin Action | Source |
|---|---|---|
| Voltage-gated | ||
| Sodium Channels | ||
| Tetrodotoxin (TTX) | Blocks channel from outside | Puffer fish |
| Batrachotoxin (BTX) | Slows inactivation, shifts activation | Colombian frog |
| Potassium channels | ||
| Apamin | Blocks “small Ca-activated” K channel | Honeybee |
| Charybdotoxin | Blocks “big Ca-activated” K channel | Scorpion |
| Calcium channels | ||
| Omega conotoxin ($\omega$-CTX-GVIA) | Blocks N-type channel | Pacific cone snail |
| Agatoxin ($\omega$-AGAIVA) | Blocks P-type channel | Funnel web spider |
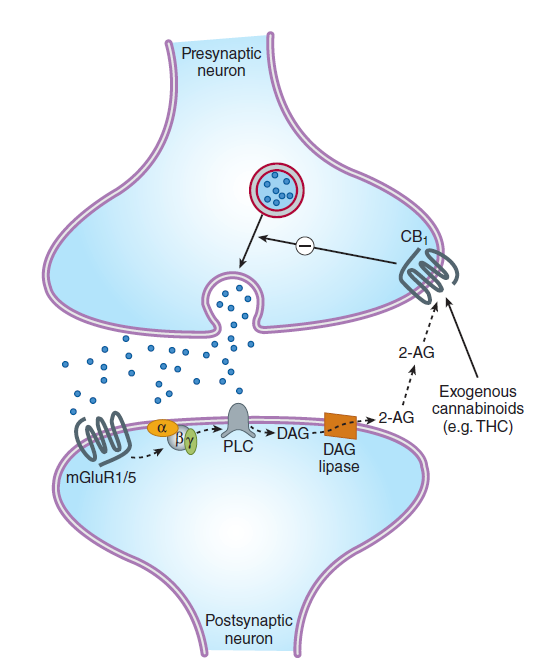
Metabotropic receptors
- This class of receptors includes the metabotropic glutamate receptors, muscarinic acetylcholine receptors, GABA
Breceptors, and most serotonin receptors, as well as receptors for norepinephrine, epinephrine, histamine, dopamine, neuropeptides and endocannabinoids. - Metabotropic receptors have neurotransmitters as ligands, which, when bound to the receptors, initiate cascades that can lead to channel-opening or other cellular effects.
- G protein-coupled receptors are inherently metabotropic. Other examples of metabotropic receptors include tyrosine kinases and guanylyl cyclase receptors.
Action Potential: the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory
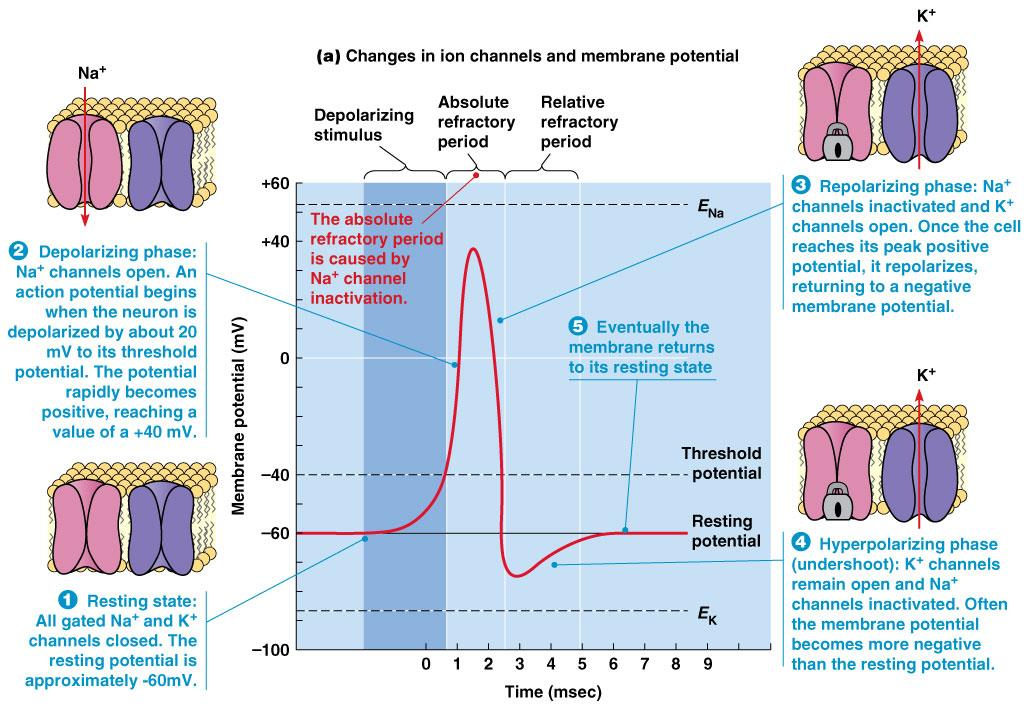
1. The synapse & synaptic potentials
EPSP: excitatory postsynaptic potential
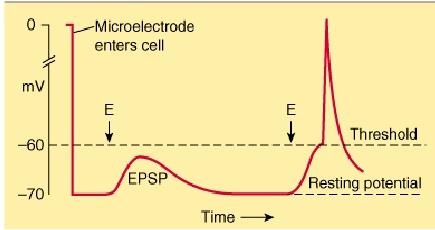
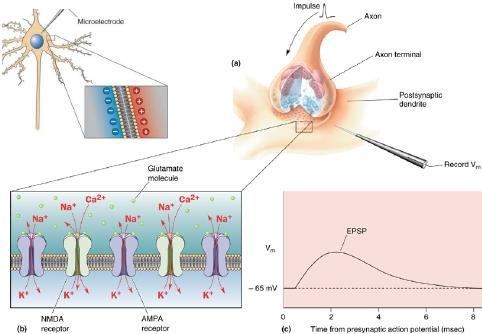
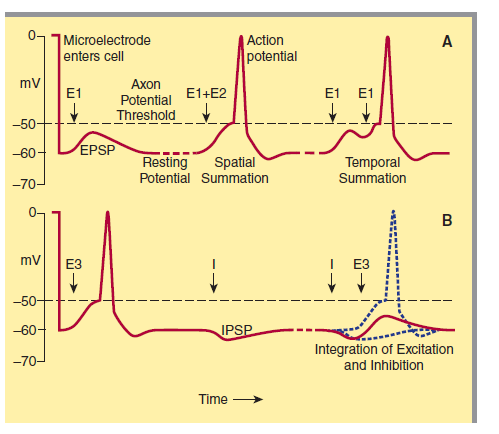
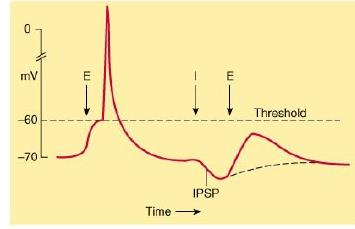
When an excitatory pathway is stimulated, a small depolarization or excitatory postsynaptic potential (EPSP) is recorded. This potential is due to the excitatory transmitter acting on an ionotropic receptor, causing an increase in cation permeability. Changing the stimulus intensity to the pathway, and therefore the number of presynaptic fibers activated, results in a graded change in the size of the depolarization. When a sufficient number of excitatory fibers are activated, the excitatory postsynaptic potential depolarizes the postsynaptic cell to threshold, and an all-or-none action potential is generated.
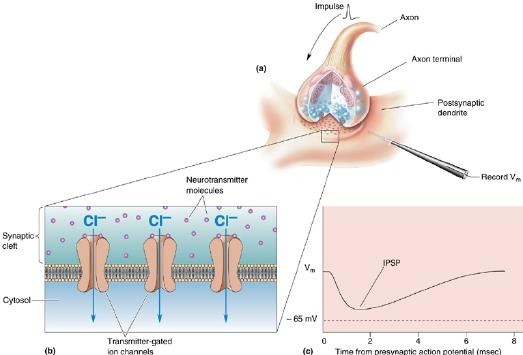
IPSP: inhibitory postsynaptic potential
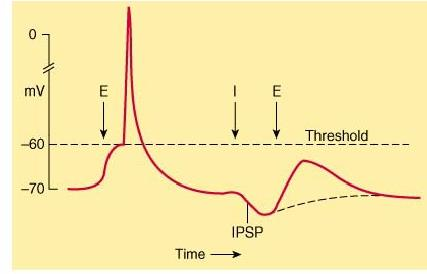
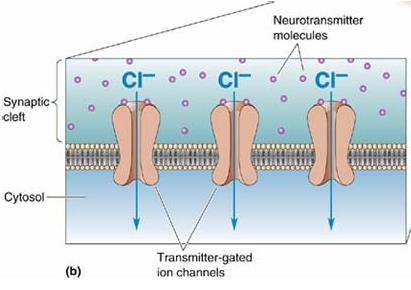
When an inhibitory pathway is stimulated, the postsynaptic membrane is hyperpolarized owing to the selective opening of Cl– channels, producing an inhibitory postsynaptic potential (IPSP)
However, because the equilibrium potential for Cl– is only slightly more negative than the resting potential (~ –65 mV), the hyperpolarization is small and contributes only modestly to the inhibitory action. The opening of the Cl– channel during the inhibitory postsynaptic potential makes the neuron “leaky” so that changes in membrane potential are more difficult to achieve. As a result, an excitatory postsynaptic potential that evoked an action potential under resting conditions fails to evoke an action potential during the inhibitory postsynaptic potential
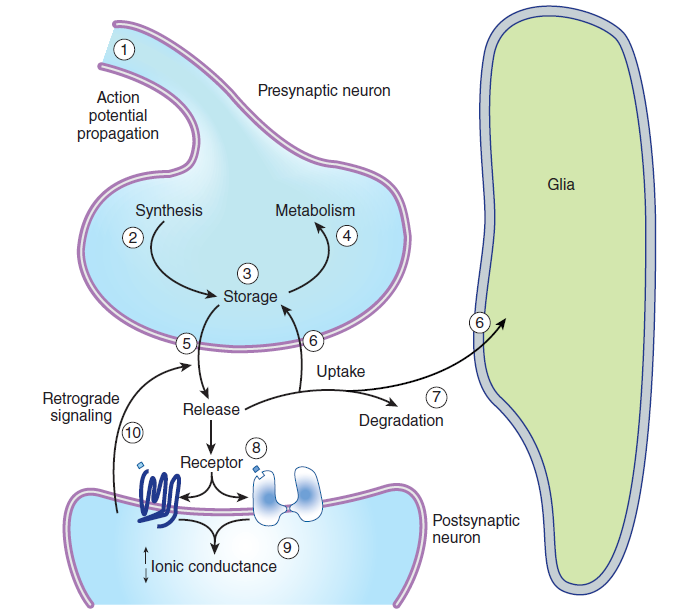
三、Sites of drug action
四、Cellular organization of the brain
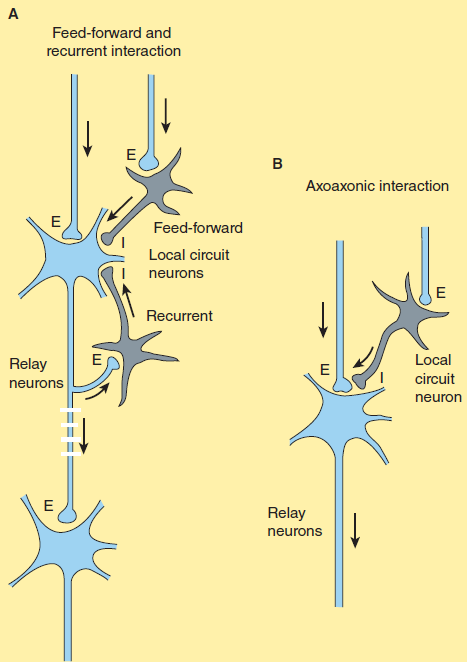
- E: Excitatory neuron, blue Neurotransmitters: Glutamate
- I: Inhibitory neuron, gray Neurotransmitters: Glycine; GABA
Hierarchical systems include all the pathways directly involved in sensory perception and motor control. The pathways are generally clearly delineated, being composed of large myelinated fibers that can often conduct action potentials at a rate of more than 50 m/s.
Central neurotransmitters
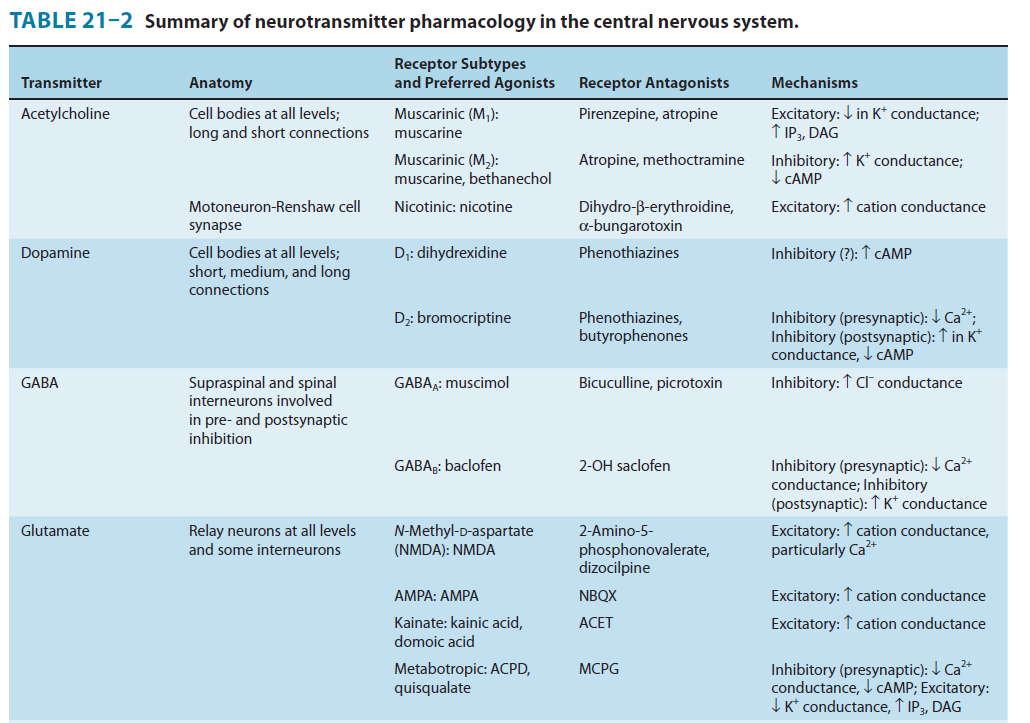
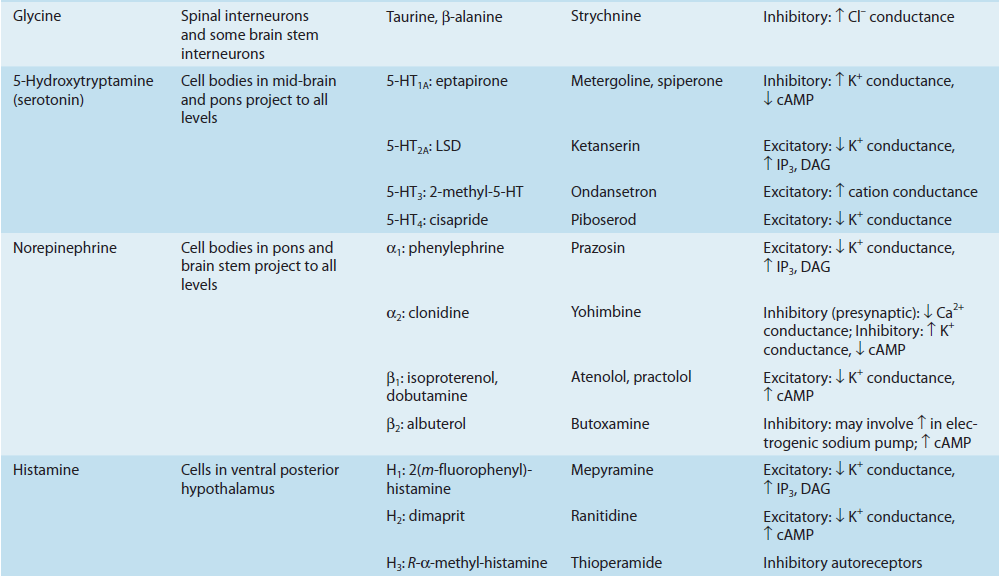
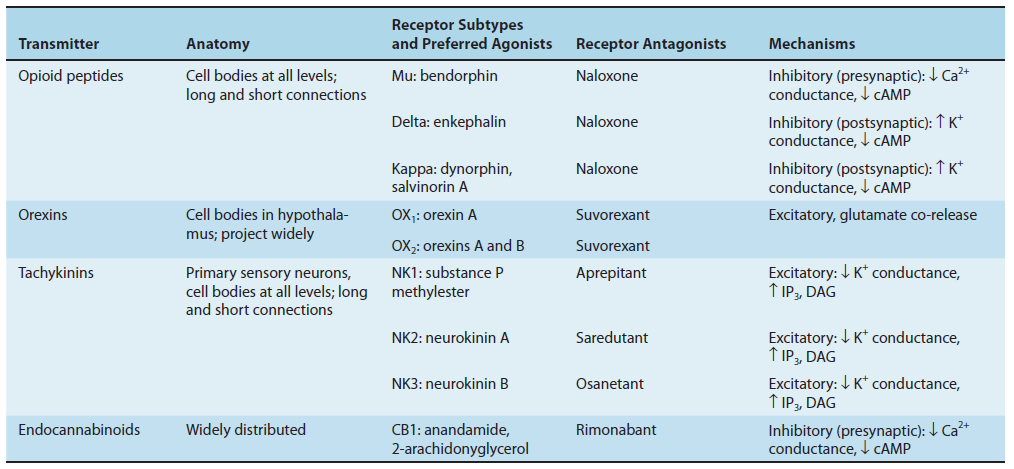
1. Amino acids: Glutamate - Excitatory Neurotransmitter
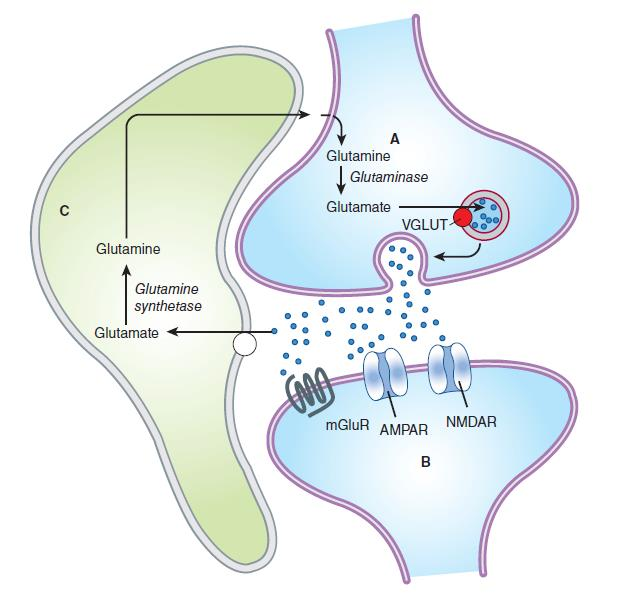
Glutamine is imported into the glutamatergic neuron (A) and converted into glutamate by glutaminase. The glutamate is then concentrated in vesicles by the vesicular glutamate transporter.
Upon release into the synapse, glutamate can interact with AMPA and NMDA ionotropic receptor channels (AMPAR, NMDAR) in the postsynaptic density (PSD) and with metabotropic receptors (MGluR) on the postsynaptic cell (B). Synaptic transmission is terminated by active transport of the glutamate into a neighboring glial cell (C) by a glutamate transporter. It is synthesized into glutamine by glutamine synthetase and exported into the glutamatergic axon.
(D) shows a model NMDA receptor channel complex consisting of a tetrameric protein that becomes permeable to Na^+^ and Ca^2+^ when it binds a glutamate molecule.
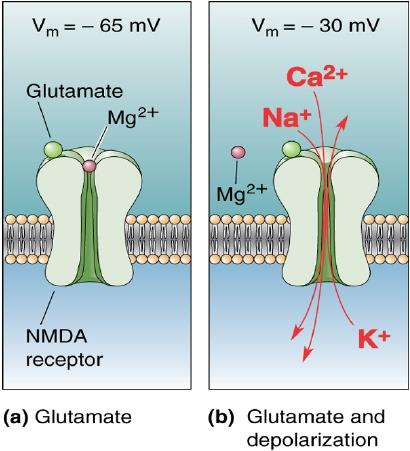
Unlike AMPA and kainate receptors, all NMDA receptors are highly permeable to Ca^2+^ as well as to Na^+^ and K^+^.
Another key difference between AMPA and kainate receptors on the one hand, and NMDA receptors on the other, is that AMPA and kainate receptor activation results in channel opening at resting membrane potential, whereas NMDA receptor activation does not. This is due to the voltage-dependent block of the NMDA pore by extracellular Mg2+. When the neuron is strongly depolarized, as occurs with intense activation of the synapse or by activation of neighboring synapses, Mg2+ is expelled and the channel opens.
- Thus, there are two requirements for NMDA receptor channel opening: Glutamate must bind the receptor and the membrane must be depolarized.
- The rise in intracellular Ca^2+^ that accompanies channel opening results in a long-lasting enhancement in synaptic strength that is referred to as long-term potentiation (LTP). The change can last for many hours or even days and is generally accepted as an important cellular mechanism underlying learning and memory.
2. Amino acids: GABA and GlycineInhibitory Neurotransmitter
Neurotransmitter crosstalk