L14 Cancer Pharmacology
一、Cancer Biology
What is Cancer
Cancer or Neoplasm is an abnormal mass of tissue as a result of abnormal proliferation of cells.
Neo-plasm: novel swelling mass in the body
 **Some neoplasms do not form a tumor**
**Some neoplasms do not form a tumor**
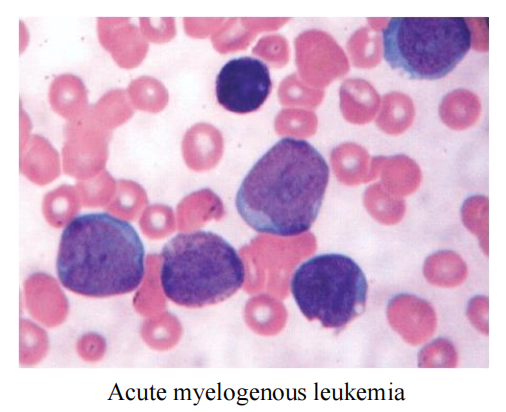
- Some blood cancers such as leukemia don’t form a tumor, it widely spread in the bloodstream and bone marrow.
What are the difference between a normal and cancer cells
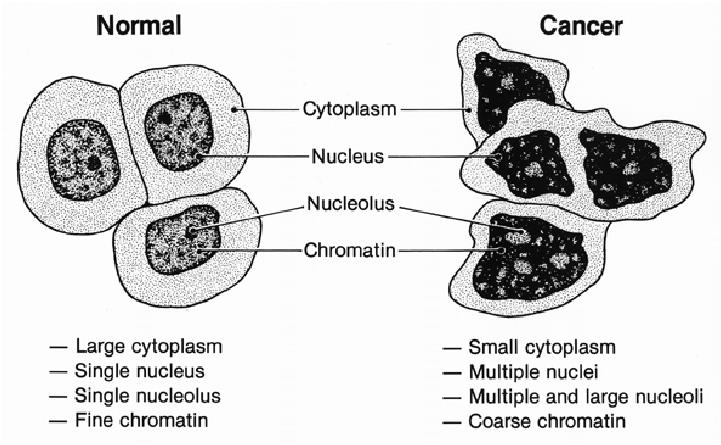
1. Cell shape
Normal cells:
- Committed Cells—divide only a defined number of times, and are limited in capacity for self-renewal
- Specialized Cells—carry out organ function and are incapable of cell division
- Restricted to its functional area
Normal and Cancer Cell Structure:
 ### 2. Genetic Aberrations
### 2. Genetic Aberrations
Genetic Aberrations
- Chromosomal Translocation
- Chromosomal Deletion
- Gene mutation
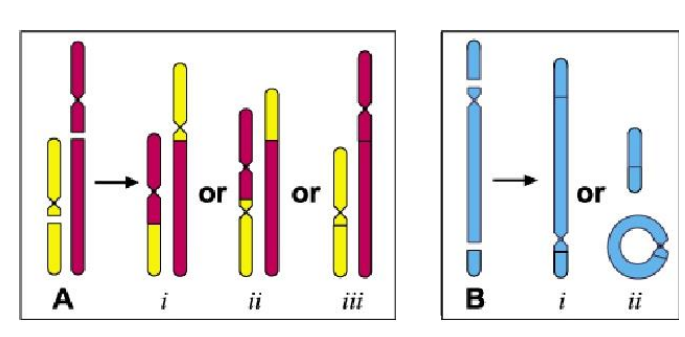
 #### Chromosomal Translocation
#### Chromosomal Translocation
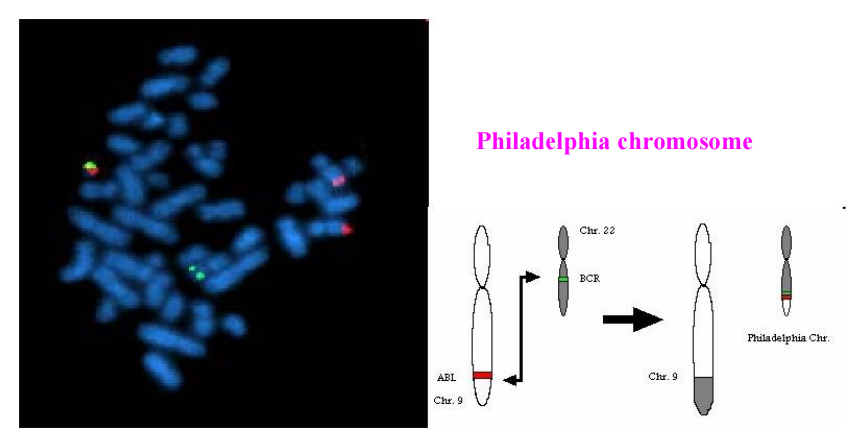 + Philadelphia chromosome
+ Philadelphia chromosome
 ## How does cancer take place (Causes of cancer)
## How does cancer take place (Causes of cancer)
- Sex: male>women
- Age: most cancers in people> 50 YO
- Race
- Genetic predisposition
- Environment - living and warking
- Chemicals
- Viruses
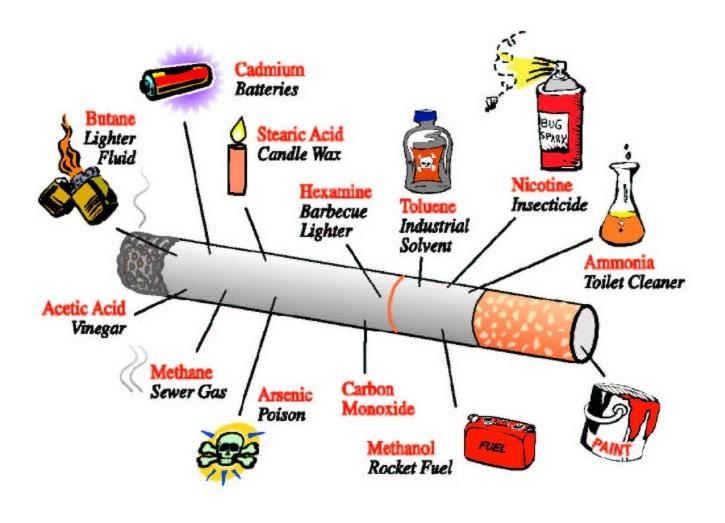
- Lifestyle—smoking, alcoholism, preserved food, BBQ…
1. Polluting Environment
Ionizing radiation
- Acute leukemia
- Thyroid cancer
- Breast cancer
- Lung cancer
- Basal cell skin cancers

Chemical exposure:
- azo dyes
- aflatoxins
- asbestos
- benzene
 #### Azo Dyes
#### Azo Dyes
synthetic colours that contain an azo group, - N=N-, as part of the structure. Azo groups do not occur naturally
Azo dyes account for approximately 60-70% of all dyes used in food and textile manufacture.
Most colored textile and leather articles are treated with azo dyes and pigments
Bladder cancer (dye factory workers, Azo dye workers still have a tremendous increase in urothelial cancer
 #### Oncovirus: that induce cancers
#### Oncovirus: that induce cancers
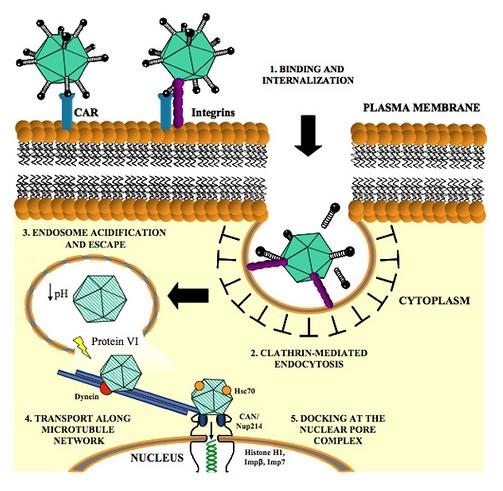
DNA Viruses
- Epstein-Barr virus → Burkitt’s lymphoma
- Hepatitis B virus → liver cancer
- Human papilloma viruses → cervical cancer
- Human herpes virus-8 → Kaposi sarcoma
RNA Viruses
- Human T lymphotrophic virus type 1 (HTLV-I), a retrovirus, → T-cell leukemia.
- Hepatitis C virus → liver cancer
Lifestyles
 + Smoking
+ Alcoholism
+ Preserved food
+ BBQ
+ Smoking
+ Alcoholism
+ Preserved food
+ BBQ
2. Pathogenesis of Neoplasia
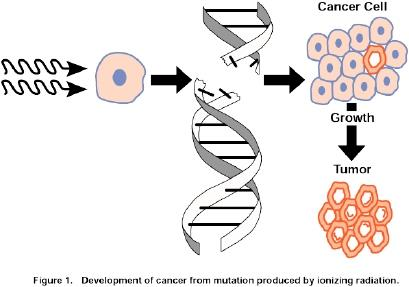
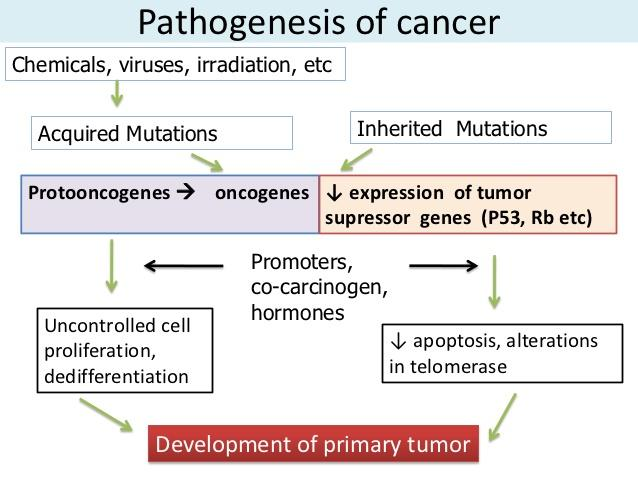
Cancer development can begin with a brief exposure (hours or days) to a chemical into an activated form and the chemical need not be present ever again
However, DNA is altered via mutagens including chemical carcinogens, viruses, and radiation. This mutations is inherted by at least one cell division (intiation).
This mutation mainly lead to activation of proto-oncogene into oncogenes (leading to uncontrolled cell proliferation) and/or inactivation of tumor suppressor genes (leading to resistance to apoptosis.)
Upon exposure to other epigenetic factors (hormones, cocarcinogens, immunosuppressant…which themselves are non carcinogenic) tumor growth is promoted (promotion)
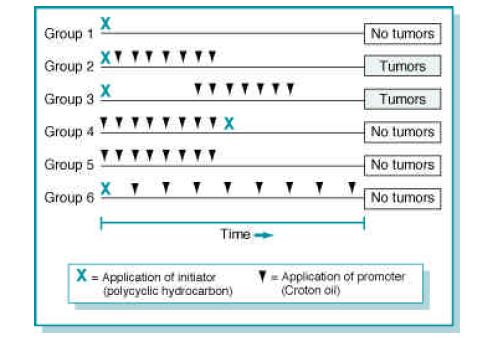
Initiation
Initiation point at which an irreversible alteration, usually genetic, is introduced into a target cell
Initiation:
- is essentially irreversible
- caused only by carcinogenic compounds
- occurs rapidly after carcinogen exposure
- alone does not result in tumor formation
Promotion
Promotion is the process whereby an initiated tissue or organ develop focal proliferations and it requires the presence of continuous stimulation.
Promotion:
- reversible
- acts only after exposure to an initiating agent
- requires repeated administration of a promoter
- is not carcinogenic in itself
Etiology and Pathogenesis of Neoplasia Initiation and Promotion
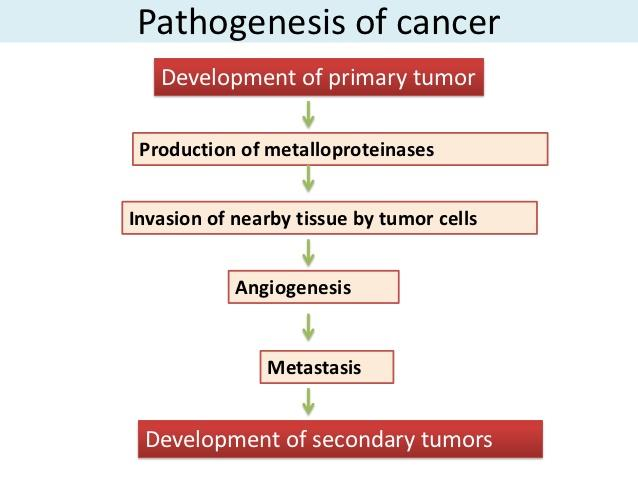 # 二、Principle and strategy for cancer therapy
# 二、Principle and strategy for cancer therapy
Cancer Therapeutic Modalities
| Name | Detail |
|---|---|
| Surgery | Isolated solid cancers |
| Chemotherapy | Almost all cases, in combined with other treatments. It alone cure 10-15% of all patients. |
| Radiation therapy | Used for half of all cases to either cure or improve the symptoms of cancer. |
| Palliative care | Symptom management |
The log-kill hypothesis
 + Infrequent scheduling of treatment courses with low (1 log kill) dosing and a late start prolongs survival but does not cure the patient (i.e., kill rate < growth rate)
+ More intensive and frequent treatment, with adequate (2 log kill) dosing and an earlier start is successful (i.e., kill rate > growth rate)
+ Early surgical removal of the primary tumour decreases the tumour burden. Chemotherapy will remove persistant secondary tumours, and the total duration of therapy does not have to be as long as when chemotherapy alone is used.
+ Infrequent scheduling of treatment courses with low (1 log kill) dosing and a late start prolongs survival but does not cure the patient (i.e., kill rate < growth rate)
+ More intensive and frequent treatment, with adequate (2 log kill) dosing and an earlier start is successful (i.e., kill rate > growth rate)
+ Early surgical removal of the primary tumour decreases the tumour burden. Chemotherapy will remove persistant secondary tumours, and the total duration of therapy does not have to be as long as when chemotherapy alone is used.
Treatment strategies
Surgery + Radiotherapy + Chemotherapy
Combined Chemotherapy: various classes of drugs in combination
- MP: Melphalan, prednisone
- VAD: Vincristine, Adriamycin, dexamethasone
- CHOP: Cyclophosphomide, doxorubicin, vincristine, prednisone
Correct selection of drugs in a regimen can result in decreased development of resistance, synergistic effects and decreased toxic effects.
Drug categories:
Cell Cycle Specifc (CCS)
| Category | Drugs |
|---|---|
| Antimetabolites | Capecitabine, Cytarabine, Fludarabine, 5-Fluorouracil, Gemcitabine |
| Antitumor antibiotic | Bleomycin |
| Epipodophyllotoxins | Etoposide, Teniposide |
| Taxanes | Docetexel, Paclitaxel |
| Vinca Alkaloids | Vinblastine, Vincristine |
Cell Cycle NonSpecifc (CCNS)
| Category | Drugs |
|---|---|
| Alkylating agents | Busulfan, Carmustine, Melphalan, Thiotepa |
| Anthracyclines | Daunorubucin, Doxorubicin, Idarubicin |
| Antitumor Antibiotics | Dactinomycin, Mtomycin |
| Camptothecins | Irinotecan |
| Platinum analogs | Cisplatin, Carboplatin |
Problems associated with chemotherapy
1. Resistance to chemotherapy
Resistance to chemotherapy may develop by several mechanisms:
- Decrease in the amount of drug uptake by cancer cells
- E.G. Methotrexate
- Increase in the amount of drug removed by cancer cells. (Transporters=P-glycoprotein).
- E.G. Vinblastine ,doxorubicin, bleomycin ,etapsoid….
- Decrease or alteration in target molecule sensitivity – this is caused by mutation in the molecule targeted by the drug
- E.G. Methotrexate,Mercaptopurine,doxorubicin
- Increase in DNA repair ability of the cell via an increased expression of DNA repairing enzymes.
- E.G. Alkylating agent
Resistance Mechanisms
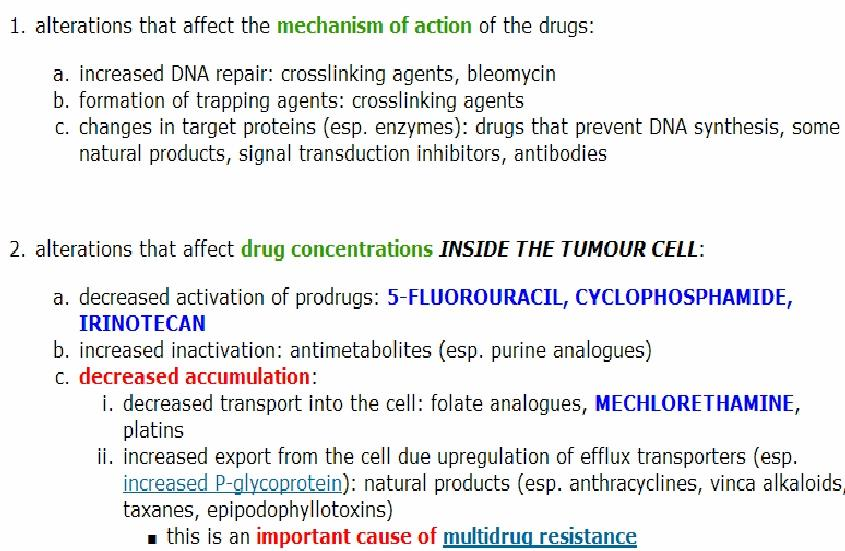
Alterations that affect the mechanism of action of the drugs:
- increased DNA repair: crosslinking agents, bleorsycin
- formation of trapping agents: crosslinking agents
- changes in target proteins (esp. enzymes): drugs that prevent DNA synthesis, some natural products, signal transduction inhibitors, antibodies
Alterations that affect drug concentrations inside the tumour cell:
- decreased activation of prodrugs: 5-fluorouracil, cyclophosphamide, trinotecan
- increased inactivation: antimetabolites (esp. purine analogues)
- decreased accumulation:
- decreased transport into the cell: folate analogues, mechlorethamine platins
- increased export from the cell due upregulation of efflux transporters(esp increased P-glycoprotein) natural products(esp. anthracyclines, vinca alkaloids taxanes, epipodophyllotoxins)
- this is an important cause of multidrug resistance
The P-Glycoprotein Structure
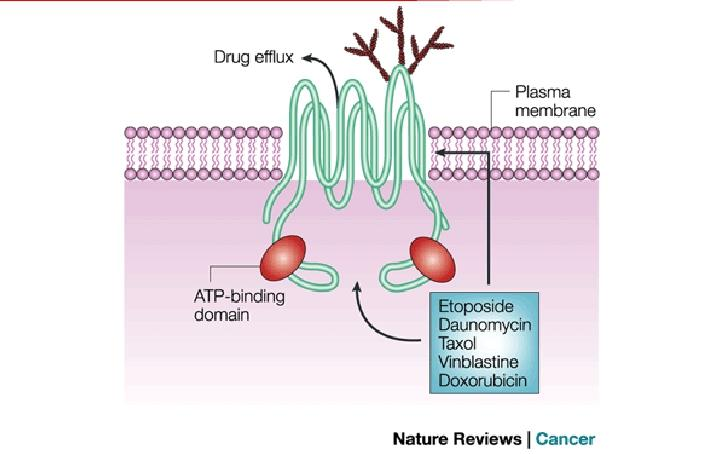 + Encoded by ABCB1 gene
+ Encoded by ABCB1 gene
2. Toxicity and side Effects of Antineoplastic Agents
Normal cells in the body that tend to be injured the most due to chemotherapy are those which have a high growth fraction. Those are bone marrow, GI Tract ,hair follicles, reproductive organs .Leading to the followings:
- Alopecia (脱发) - hair loss
- Myelosuppression (骨髓功能抑制) - bone marrow loss
- Emetic potential (呕吐): disruptive to cells in stomach which causes: Nausea/vomiting
- Low WBC count (低白细胞数目) - low immunity
3. Treatment-induced tumor
Many anticancer drugs are mutagens and can cause the rise of neoplasm ten or more years after the original cancer was cured
三、Anti-Cancer drugs and their Pharmacology
Why term chemtherapy
Like infective disease
- Some malignant cells can be cultured
- Some malignancies can be transmitted by innoculation
Cancer chemotherapy not as successful as antimicrobial chemotherapy
Metabolism in parasite differs qualitatively from host cells, while metabolism in cancer cells differ only quantitatively from normal host cells
- Hence target selectivity is more difficult in cancer
- cancer there is no substantial immune response
- Diagnostic complexity: delay in institution of treatment
Cancer cells differ from normal cells by
- Uncontrolled proliferation
- De-differentiation loss of function
- Invasiveness
- Metastasis
Cell Cycle and Cancer
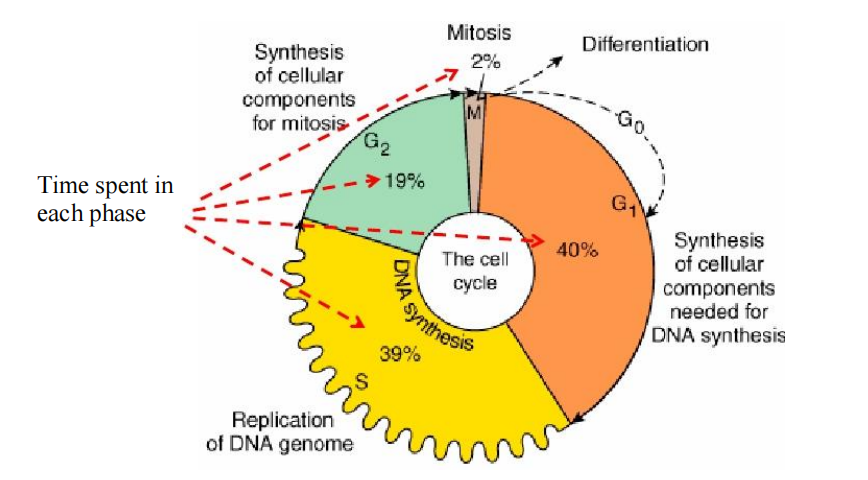 The cell cycle and Cancer: both Normal and Cancer cells must traverse before and after cell division.
The cell cycle and Cancer: both Normal and Cancer cells must traverse before and after cell division.
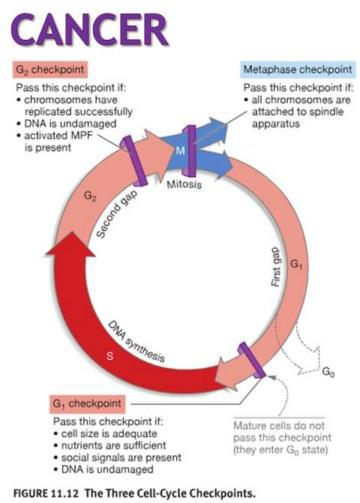
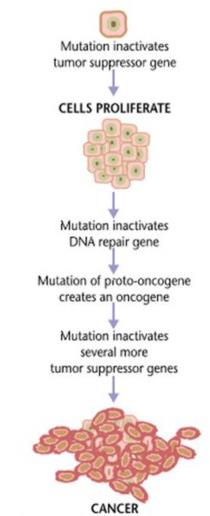
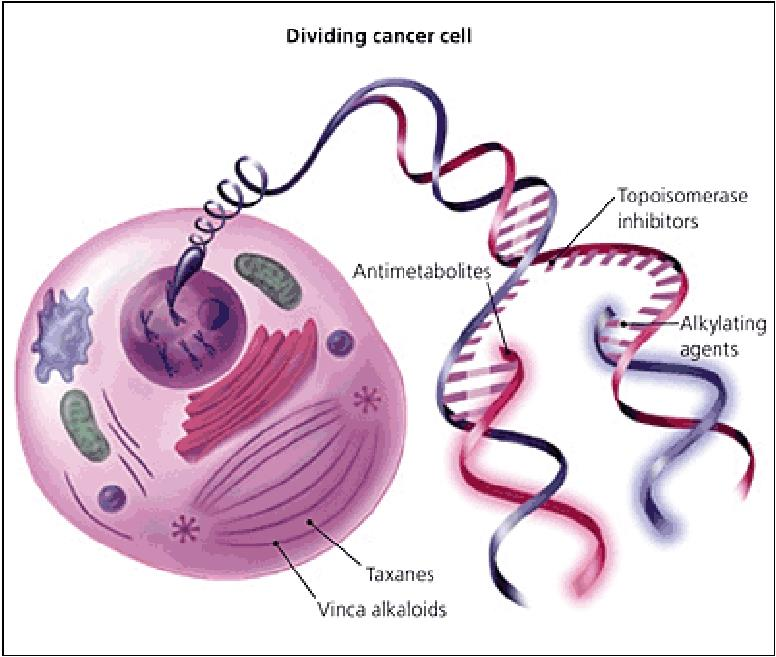
Classification of Anti-Cancer Drugs
1. Overall classifications
| Category | Drugs |
|---|---|
| Alkylating Agents | Cyclophosphamide, Nitrosourea, Platinum analogs: Cisplatin, Carboplatin |
| Antimetabolites | Cytarabine, Fludarabine, Methotrexate |
| Plant alkaloids | Camptothecins, Etoposide, Paclitaxel, Vinblastine |
| Antitumor antibiotics | Bleomycin, Doxorubicin, Mitomycin |
| Hormonal Agents | Flutamide, Leuprolide,Tamoxifen |
| Differential Inducer | Arsenic trioxide, Retinoic acid derivatives |
| Gene targeted drugs | Cetuximab, Dasatinib, Gefitinib, Imatinib |
| Immunomodulators | Including cytokines and growth factors |
| Antiangiogenesis drugs | sorafenib, sunitinib, pazopanib |
2. Polyfunctional Alkylating Agents
Introduction and classification
Alkylation is the transfer of an alkyl group from one molecule to another.
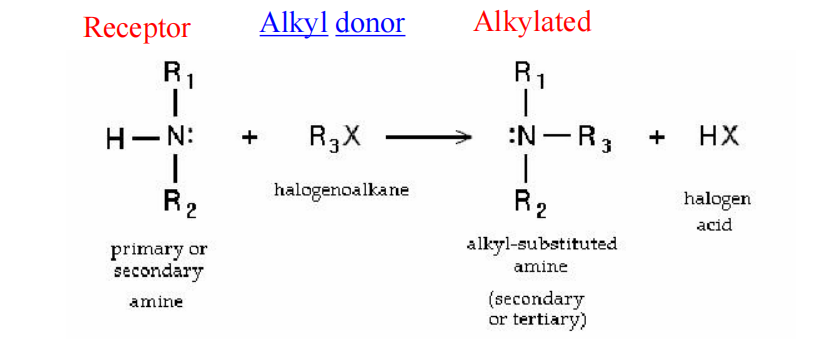 An **alkylating antineoplastic agent** is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA, especially on the guanine base of DNA, at the number 7 nitrogen atom of the purine ring
An **alkylating antineoplastic agent** is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA, especially on the guanine base of DNA, at the number 7 nitrogen atom of the purine ring
Alkylating agents classification:
 | Categories | Drugs |
| ----------------- | --------------------------------------------------------- |
| Nitrogen mustards | Mechlorethamine,Melphalan,Cyclophosphamide,Chlorambu cil |
| Ethyleneimines | Thiotepa |
| Nitrosoureas | Carmustine,Lomustine |
| Alkylsulfonates | Busulphan |
| Platinum | Coordination complexes: Cisplatin,Carboplatin,Oxaliplatin |
| Categories | Drugs |
| ----------------- | --------------------------------------------------------- |
| Nitrogen mustards | Mechlorethamine,Melphalan,Cyclophosphamide,Chlorambu cil |
| Ethyleneimines | Thiotepa |
| Nitrosoureas | Carmustine,Lomustine |
| Alkylsulfonates | Busulphan |
| Platinum | Coordination complexes: Cisplatin,Carboplatin,Oxaliplatin |
Structures of alkylating agents
 #### Alkylation: Mechanisms
#### Alkylation: Mechanisms
Attach an alkyl group to various cellular constituents:
- DNA—the major attacking subjects: Guanine (N7) , Adenine (N1, N3), Cytosine (N3) and Guanine (O6).
- Proteins in various groups, including:
- Sulfhydryl
- Hydrocyl
- Phosphate group
- Amino
- Carboxyl
- Carbomoylation of lysine residues by nitrosourea through formation of isocyanates.
- Non cell cycle specific, but preferentially attack cells at late G1 and S phases, thus blocking cells at G2
Mechanisms of Alkylation of DNA Guanine
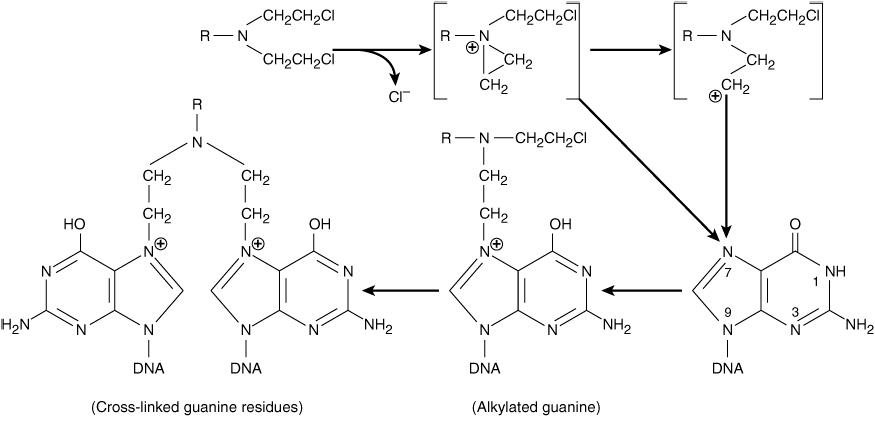 #### Mechanisms of Alkylating Action in DNA
#### Mechanisms of Alkylating Action in DNA
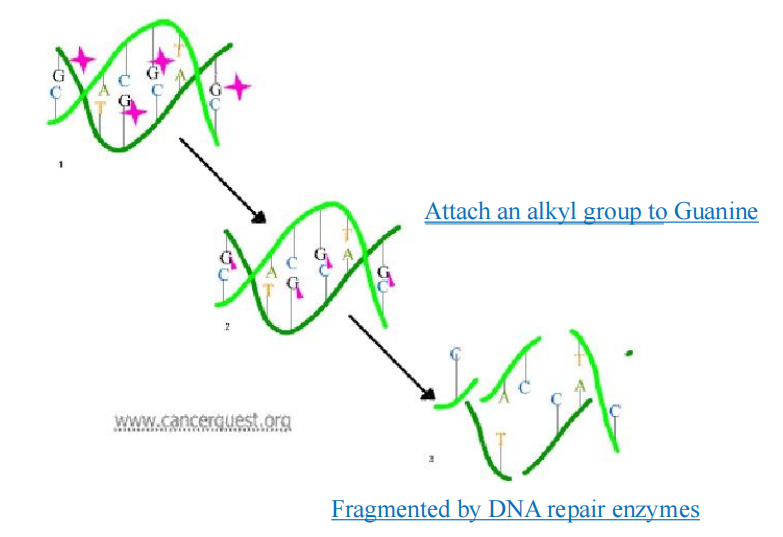
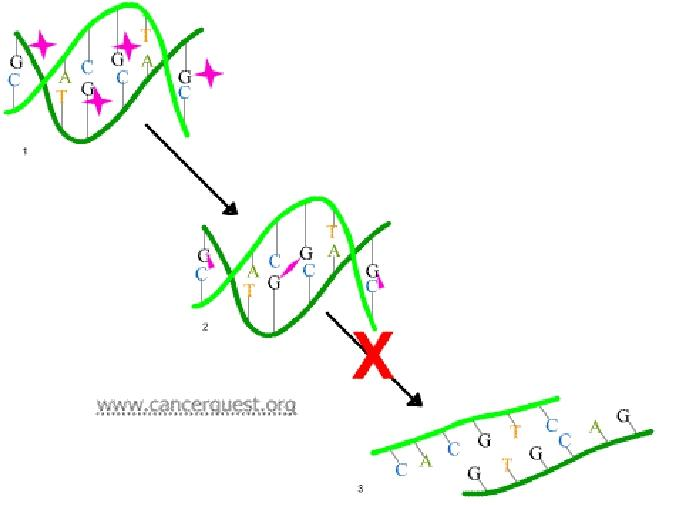
Resistance to Alkylating agents
Acquired resistance
- Increased capability to repair DNA lesions
- Decreased transport of the alkylating drug into the cell
- Increased production of glutathione and glutathione-associated proteins, which are needed to conjugate the alkylating agent, or increased glutathione S-transferase activity, which catalyzes the conjugation.
3. Platinum Analogs
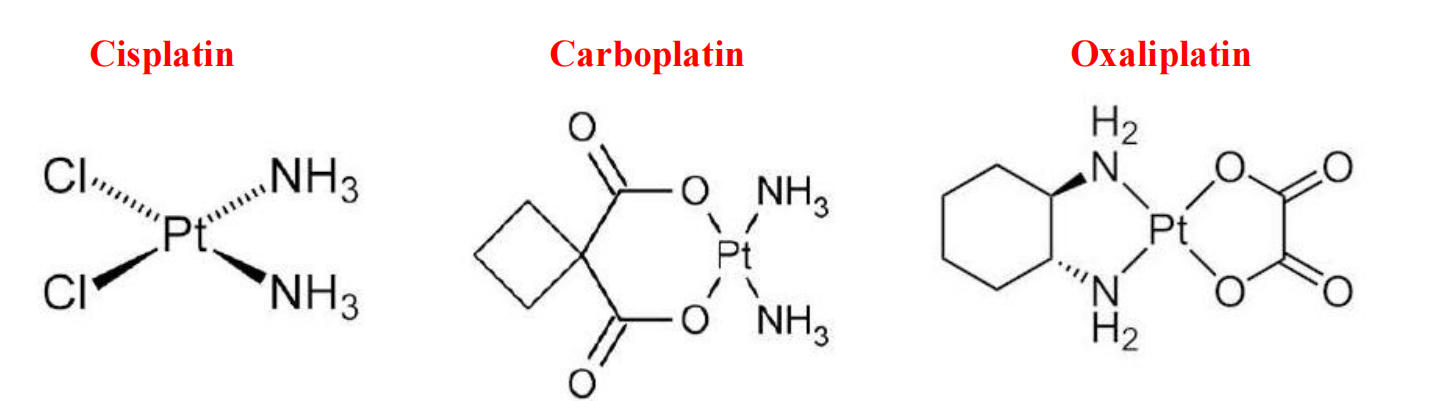 1. Inorganic metal complex
2. They kill tumor cells in all stages of the cell cycle
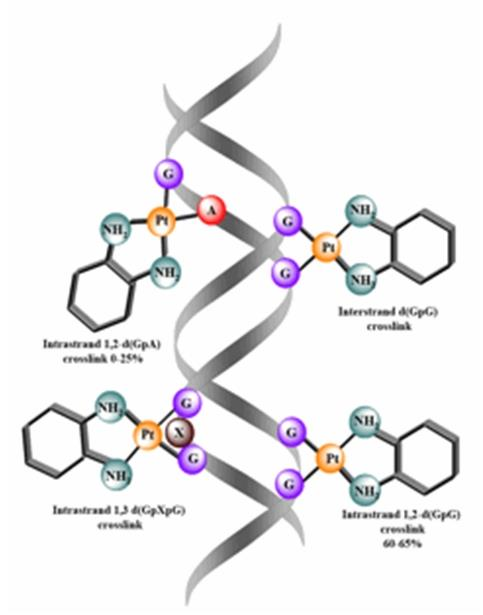
3. Bind DNA through the formation of intrastrand and interstrand cross-links
4. Leading to inhibition of DNA synthesis and function.
5. N7 Guanine, N3 Adenine,O6 cytosine can also occur.
6. Nephrotoxicity acould be attenuated by i.v. saline infusion.
7. Used for NSCLC, GU, gastric, head and neck, ovarian and bladder cancers
8. Synergistic with other anticancer agents :Alkylating agents, taxanes
1. Inorganic metal complex
2. They kill tumor cells in all stages of the cell cycle
3. Bind DNA through the formation of intrastrand and interstrand cross-links
4. Leading to inhibition of DNA synthesis and function.
5. N7 Guanine, N3 Adenine,O6 cytosine can also occur.
6. Nephrotoxicity acould be attenuated by i.v. saline infusion.
7. Used for NSCLC, GU, gastric, head and neck, ovarian and bladder cancers
8. Synergistic with other anticancer agents :Alkylating agents, taxanes
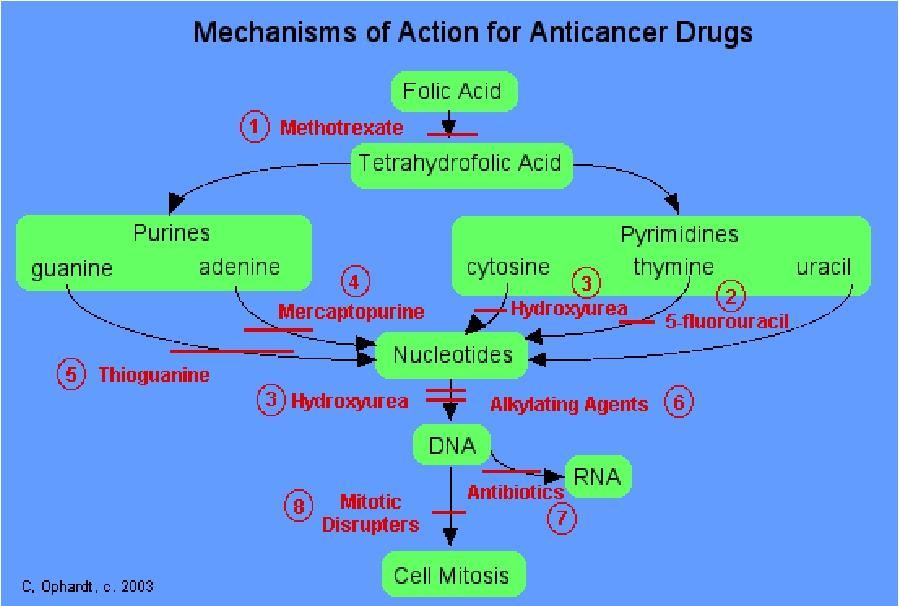
4. Antimetabolic Agents
- Structural analogues: Neoplastic cells’ metabolic differences – increased susceptibility to actions of antimetabolites.
- Most antimetabolites interfere with nucleic acid synthesis or nucleotide synthesis.
- Folic acid antagonist: methotrexate, pemetrexed
- Purine Antagonists: 6-Thiopurines, Fludarabine,
- Pyrimidine Antagonists: 5-Fluorouracil, Cytarabine, gemcitabine
- DNA synthesis (S phase–specific)
Methotrexate
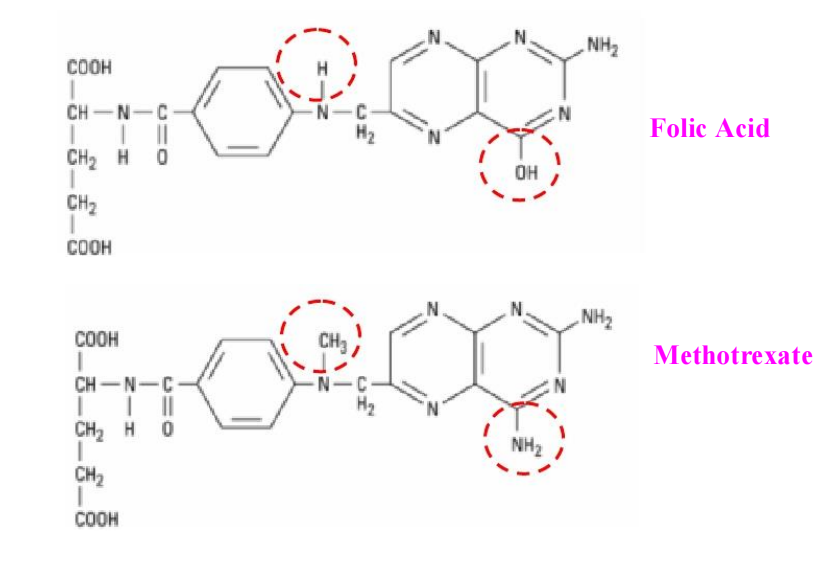 **Method of action:**
**Method of action:**
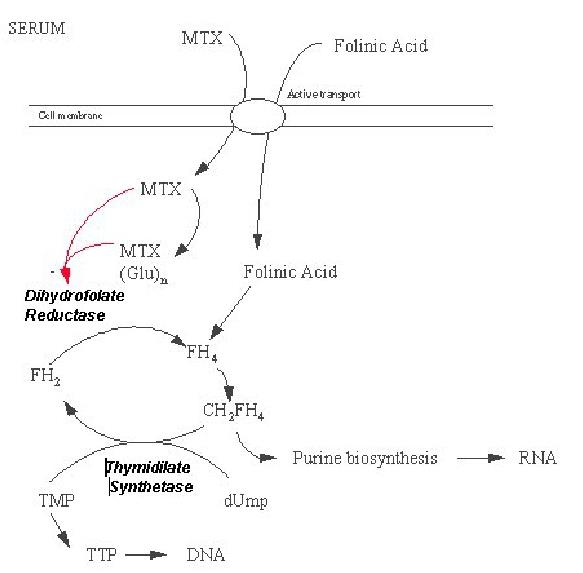 1. binds with high affinity to the active catalytic site of DHFR.
2. interfering with the synthesis of tetrahydrofolate (THF)
3. THF for de novo synthesis of thymidylate, purine nucleotides, and the amino acids serine and methionine.
4. DNA, RNA, and key cellular proteins
1. binds with high affinity to the active catalytic site of DHFR.
2. interfering with the synthesis of tetrahydrofolate (THF)
3. THF for de novo synthesis of thymidylate, purine nucleotides, and the amino acids serine and methionine.
4. DNA, RNA, and key cellular proteins
5-Fluorouracil
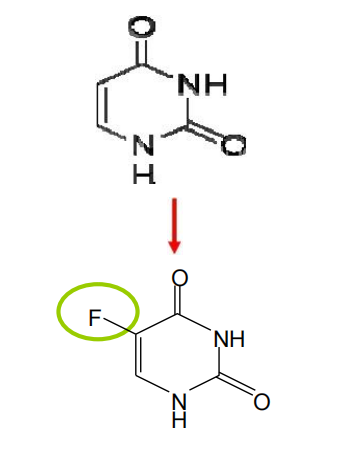
- Uridine derivatives
- One derivative, 5-fluoro-2’-deoxyuridine 5’-phosphate (FdUMP),inhibits thymidylate synthase and its cofactor,a tetrahydrofolate derivative,resulting in inhibition of thymidine nucleotide synthesis.
- Another derivative, 5-fluorouridine triphosphate is incorporated into RNA, interfering with RNA function.
- Cytotoxicity: effects on both RNA and DNA
Cytarabine (ara-C)
IV administration
S phase specificity: highly schedule-dependent
Clinical Use: almost exclusively for acute myelogenous leukemia
MOA
- S phase-specific antimetabolite
- Biotransformed to active forms: ara-CTP, competitive inhibitor of DNA polymerase.
- Blocks DNA synthesis; no effect on RNA or protein synthesis cytarabine incorporated into RNA and DNA – interfering with chain elongation
AE (side effect):
- nausea, alopecia stomatitis , severe myelosuppression
5. Natural Products for Cancer Chemotherapy: Plant Alkaloids
function can be totally different
Example: Vincristine, vinblastine, Etoptosides, Camptothecin, Taxanes: Paclitaxel, Doxetaxel
Alkaloid, any of a class of naturally occurring organic nitrogen-containing bases
vinblastine and vincristine
Resource: plant products, from periwinkle plant—vinca rosea

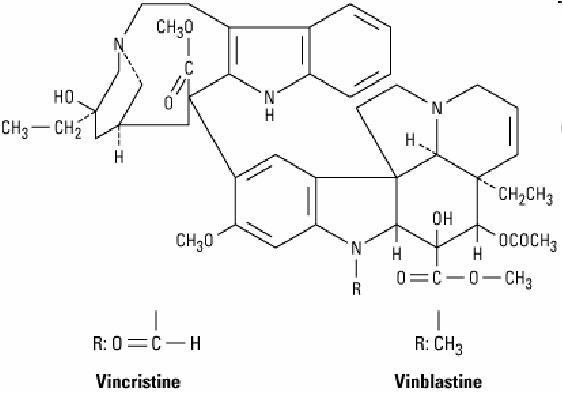
Function:
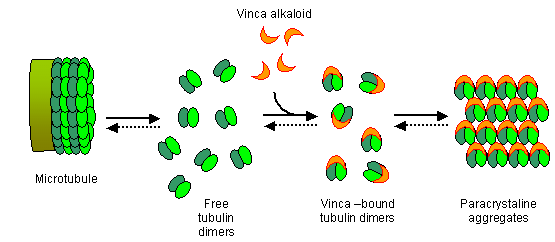 + **inhibition of tubulin polymerization**, which disrupts assembly of microtubules, an important part of the cytoskeleton and the mitotic spindle. This inhibitory effect results in mitotic arrest in metaphase, bringing cell division to a halt, which then leads to cell death.
+ **inhibition of tubulin polymerization**, which disrupts assembly of microtubules, an important part of the cytoskeleton and the mitotic spindle. This inhibitory effect results in mitotic arrest in metaphase, bringing cell division to a halt, which then leads to cell death.
Applications:
- Vinblastine
- Cure for:
- Hodgkin’s lymphoma
- Non-Hodgkin’s lymphoma
- Breast cancer
- Germ cell cancer
- AE:
- Nausea and vomitting
- bone marrow suppression
- alopecia
- Cure for:
Camptothecins: Topotecan, Irinotecan
Resource: camptotheca acuminata tree
MOA: inhibition of topoisomerase I, the key enzyme responsible for cutting and religating single DNA strands –DNA damage
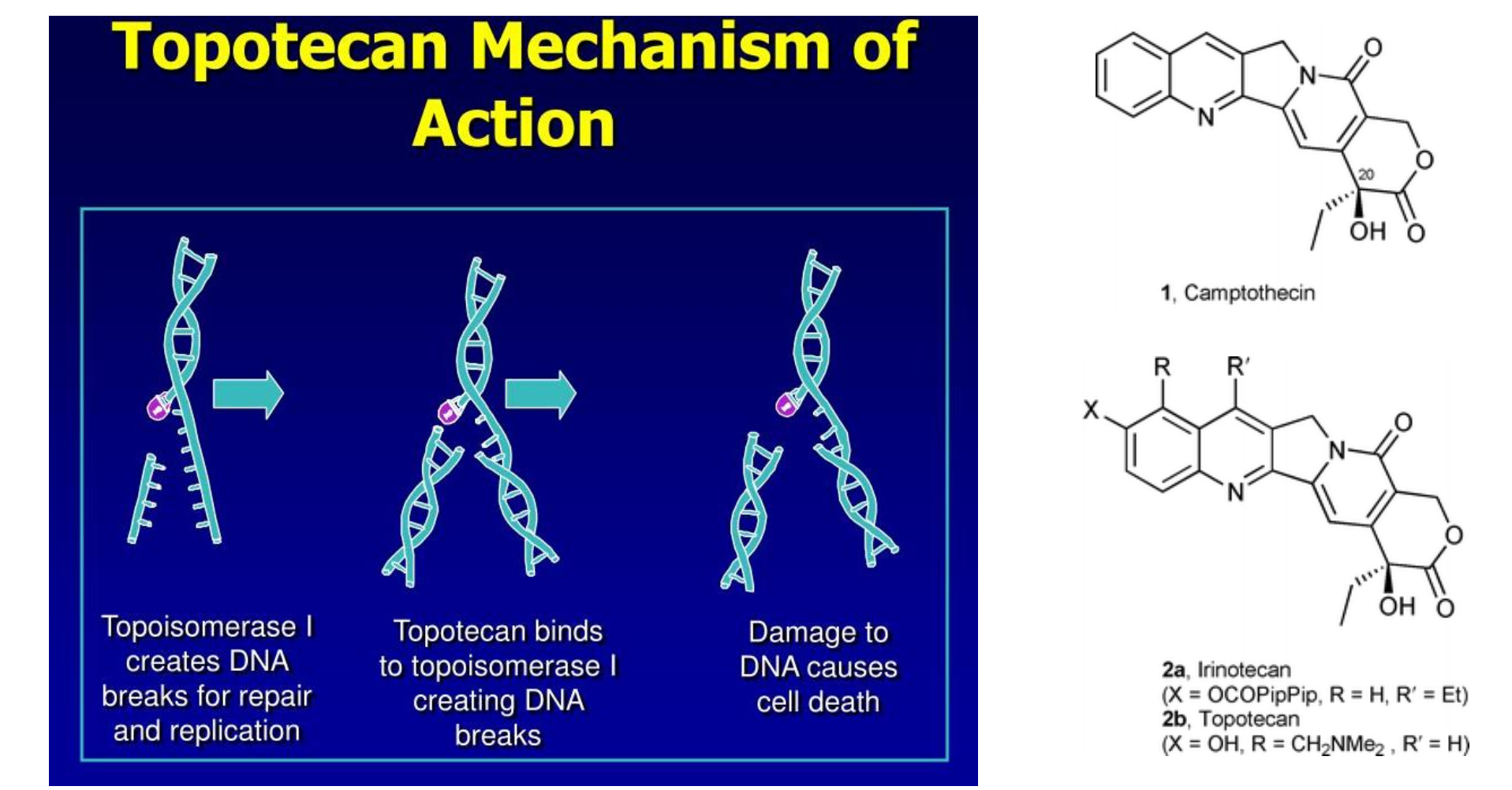
Indications(适应症):
- Topotecan (拓扑替康)–2nd line therapy for: Advanced ovarian cancer, small cell lung cancer
- Irinotecan (伊立替康;依立替康;抗癌妥)—metastatic colorectal cancer as a monotherapy who has failed to 5-FU. Or used as 1st line in combination with 5FU and leucovorin
AE: myelosuppression and diarrhea
Taxanes, Taxol
 + Paclitaxel
+ Paclitaxel
 #### The PK&PD of Taxol
#### The PK&PD of Taxol
Administration
- Intravenous(135~350 mg/m2, 3h or 24h)
- Intraperitoneal(60~65 mg/m2, every week)
- Lipid-soluble
- MW=853.91
Distribution
- Liver, lung, pancreas, salivary glands, stomach, heart, muscle, kidney, etc.
- Couldn’t pass the blood brain barrier
Elimination
- Cytochrome P450 occur(CYP2C&CYP3A)
- Form 6α-hydroxylated paclitaxel
Half-Life Elimination
Children: 4.6 to 17 hours (varies with dose and infusion duration)
Adults:
- 3-hour infusion: Mean (terminal): ~13 to 20 hours
- 24-hour infusion: Mean (terminal): ~16 to 53 hours
 #### Mechanism&Usage
#### Mechanism&Usage
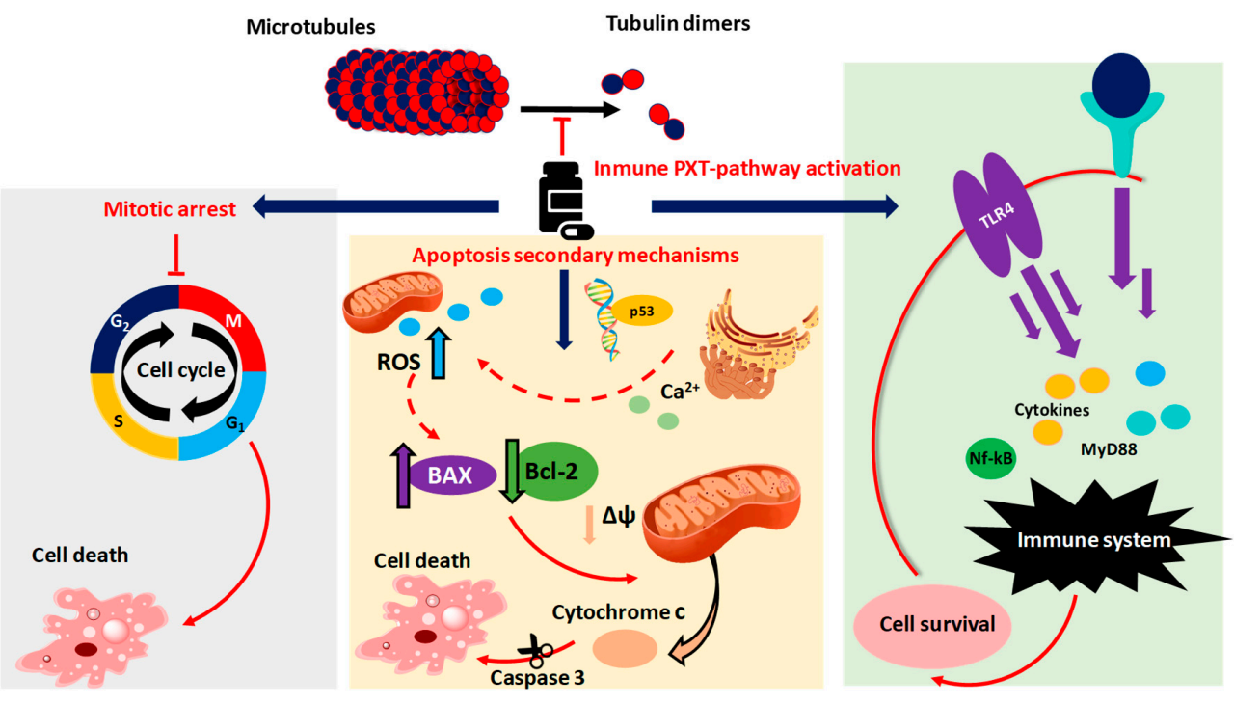
Paclitaxel is one of several cytoskeletal drugs that target tubulin. Paclitaxel-treated cells have defects in mitotic spindle assembly, chromosome segregation, and cell division.
Unlike other tubulin-targeting drugs, such as colchicine, that inhibit microtubule assembly, paclitaxel stabilizes the microtubule polymer and protects it from disassembly.
Usage
- Anal cancer, advanced
- Bladder cancer, advanced or metastatic
- Breast cancer, adjuvant treatment
- Breast cancer, metastatic or relapsed
- Cervical cancer, advanced
- Endometrial cancer, advanced or recurrent
- Esophageal cancer, metastatic or unresectable locally advanced
- Esophageal/esophagogastric cancer, preoperative chemoradiation
- Gastric cancer, metastatic or unresectable locally advanced
- Head and neck cancers, advanced
- Kaposi sarcoma, AIDS related
- Melanoma, advanced or metastatic
- Non-small cell lung cancer
- Ovarian cancer, advanced
- Penile cancer, metastatic
- Small cell lung cancer, relapsed/refractory
- Soft tissue sarcoma (angiosarcoma), advanced/unresectable
- Testicular germ cell tumors, relapsed/refractory
- Thymoma/thymic carcinoma, advanced
- Thyroid cancer, anaplastic
- Unknown primary adenocarcinoma
Side effects
Anaphylaxis
Alopecia, headache, edema, weakness, etc
9. Natural Products for Cancer Chemotherapy: Anti-tumor antibiotics
From microbes: Streptomyces (链霉菌属)
Example:
- Anthracyclines: doxorubicin, daunorubicin
- Mitoxantrone, Dactinomycin
- Mitomycin
- Bleomycin
MOA:
Bind to DNA through intercalation between specific bases and block the synthesis of RNA, DNA, or both
Cause DNA strand scission; and interfere with cell replication
Include the anthracyclines, bleomycin, and mitomycin
Anthracyclines
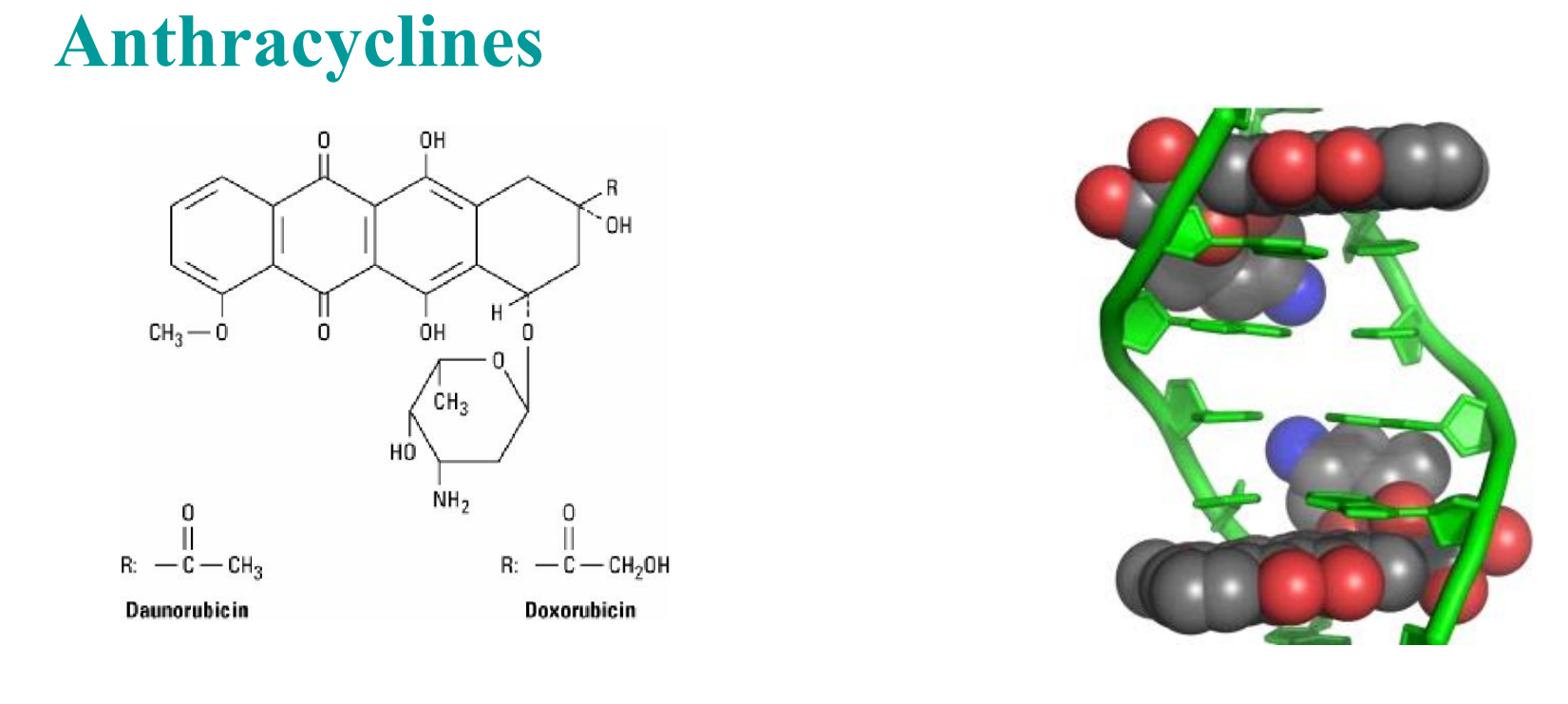
- Inhibition of topoisomerase II;
- high-affinity binding to DNA through intercalation, with consequent blockade of the synthesis of DNA and RNA, and DNA strand scission;
- generation of semiquinone free radicals and oxygen free radicals through an iron-dependent, enzyme-mediated reductive process;
- binding to cellular membranes to alter fluidity and ion transport
Indications
- Doxorubicin—Most used one
- Solid cancers : breast, endometrium, ovary, testicle, thyroid, stomach, bladder, liver, and lung
- Soft tissue sarcomas
- Childhood cancers, including neuroblastoma, Ewing’s sarcoma, osteosarcoma, and rhabdomyosarcoma
- Hematologic malignancies
- acute lymphoblastic leukemia
- multiple myeloma
- Hodgkin’s and non-Hodgkin’s lymphomas
- Daunorubicin—First identified one
- Acute myeloid leukemia.
- Limited for solid tumors
Mitomycin C
MOA
- Metabolic activation through an enzyme-mediated reduction;
- An alkylating agent that cross-links DNA
- Active for all cell cycles.
Used in combination with radiotherapy:
- Hypoxic tumor stem cells , the most sensitive one to MMC.
- Squamous cell cancer of the anus
- Squamous cell carcinoma of the cervix
- Breast, gastric, and pancreatic cancer.
- Superficial bladder cancer–intravesical treatment while no systemic toxicity
Bleomycin
Features:
- Glycopeptide antibiotics isolated from Streptomyces verticillus.
- Contains a DNA-binding region and an iron-binding domain
MOA:
- Binding to DNA, which results in single-stranded and double-stranded breaks following free radical formation
- Inhibition of DNA biosynthesis.
- DNA fragmentation is due to oxidation of a DNA-bleomycin-Fe(II) complex , which leads to chromosomal aberrations
Bleomycin is a cell cycle-specific drug that causes accumulation of cells in the G2 phase of the cell cycle
Indications:
- Hodgkin’s and non-Hodgkin’s lymphomas
- Germ cell tumor, head and neck cancer, and squamous cell cancer of the skin, cervix, and vulva.
Hormonal Agents
Estrogen inhibitors: Tamoxifen-antiestrogen, for breast cancer, endometrial cancer
Androgen inhibitor:
- Flutamide—binds to Androgen receptor; Prostate cancer
- Bicalutamide
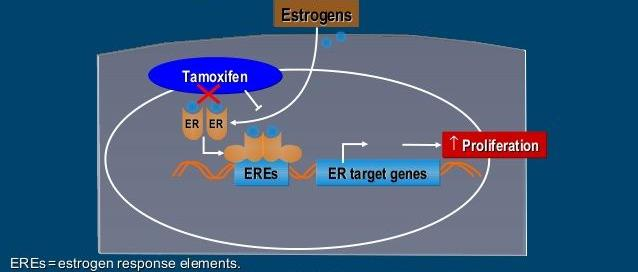
(1) Tamoxifen
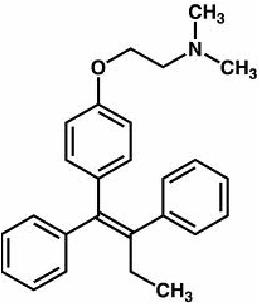
- Binds to the ER of the breast tumor
- Competitive inhibitor of the ER
- Suppresses IGF1 and TGF-β
- Therapies for: breast cancer, earlier or metastatic
- Prevention for: Breast cancer
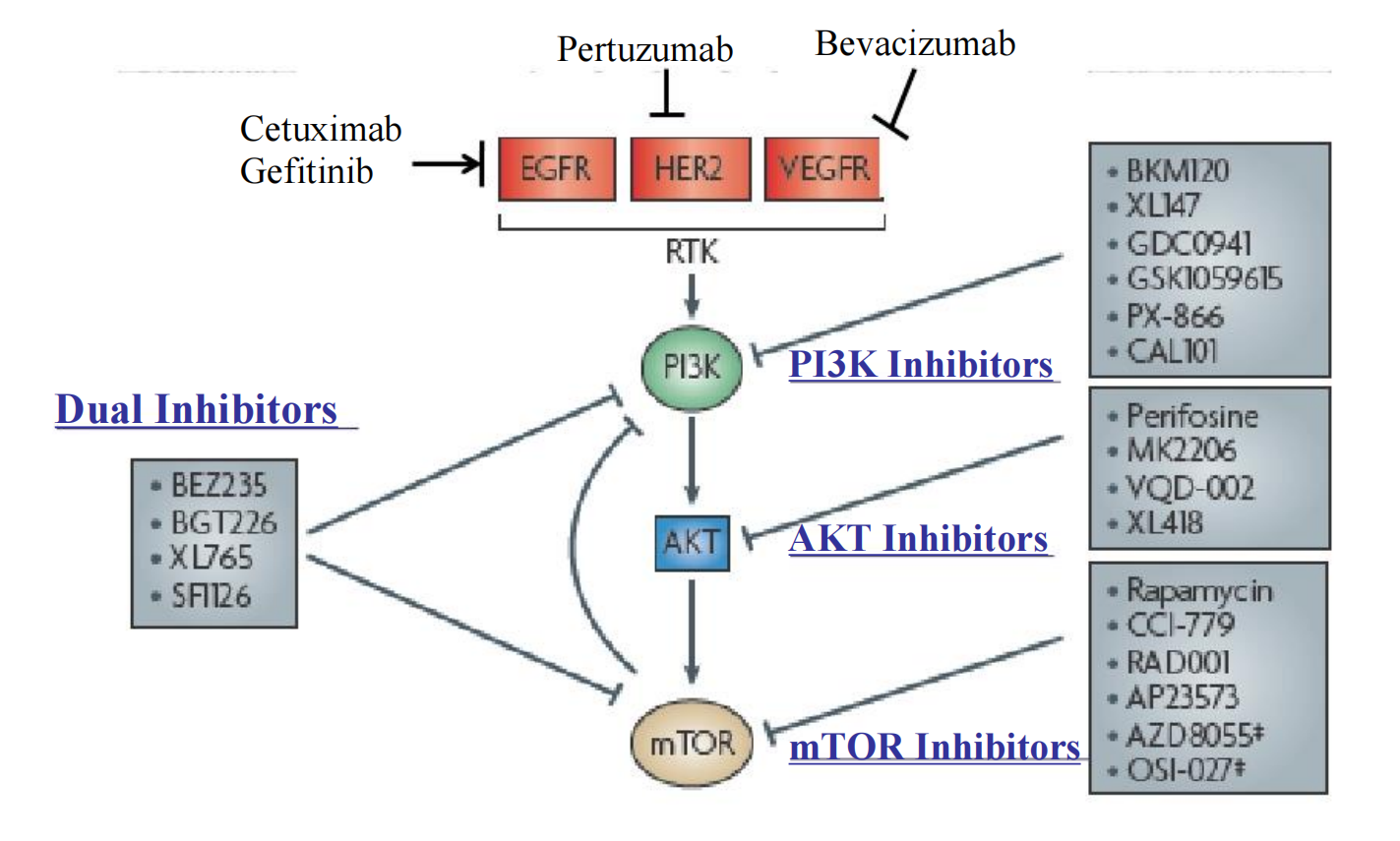
 ### 10. Molecularly Targeted Anti-cancer Drugs
### 10. Molecularly Targeted Anti-cancer Drugs
PI3K-AKT Signaling

PI3K Pathway Inhibitors:
Genetic Aberrations
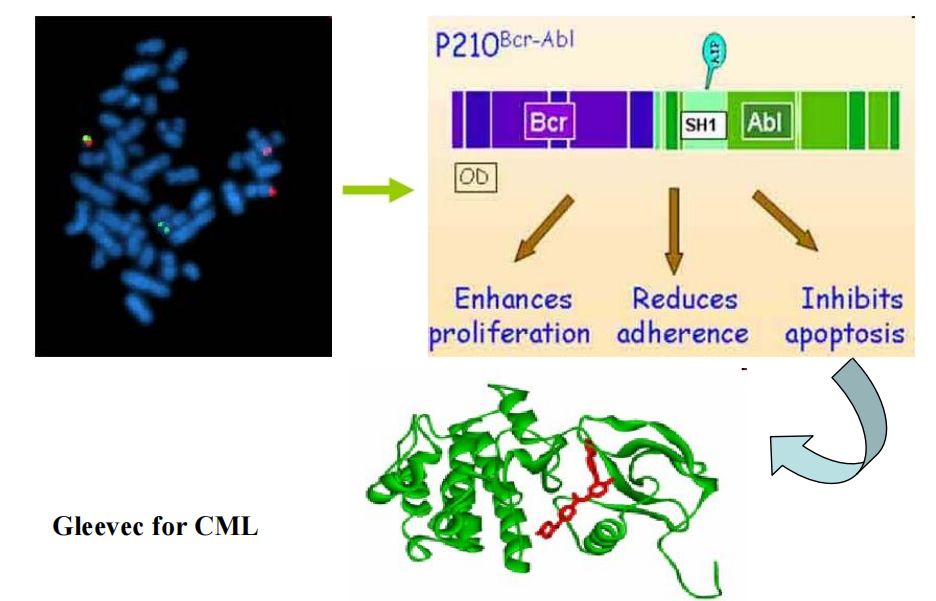
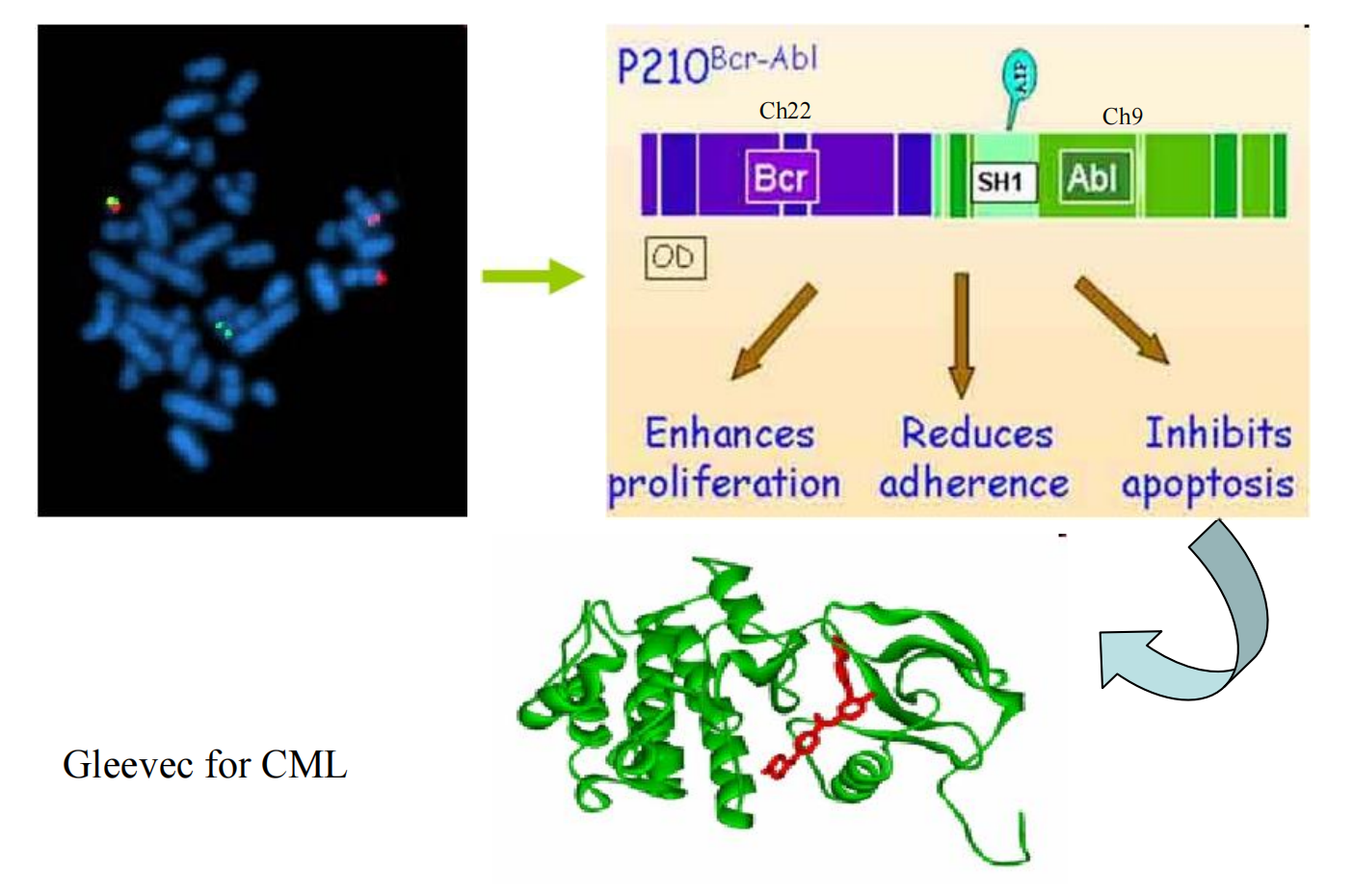 ##### (1) Imatinib (Gleevec)
##### (1) Imatinib (Gleevec)
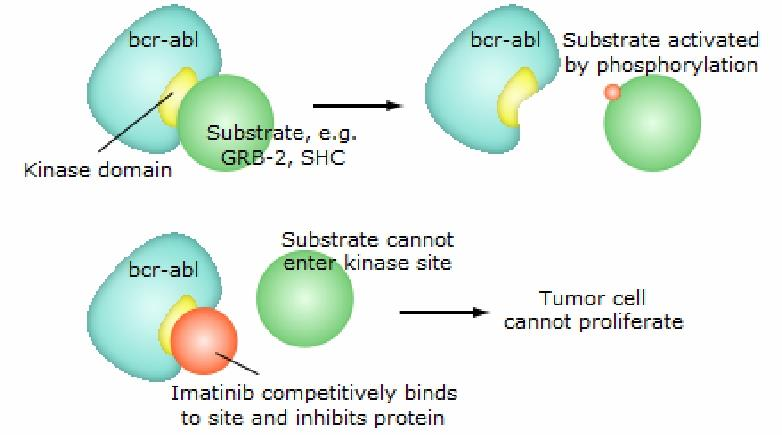 **MOA**: inhibition of the tyrosine kinase Bcl/ABL, prevents its phosphorylation by ATP.
**MOA**: inhibition of the tyrosine kinase Bcl/ABL, prevents its phosphorylation by ATP.
Indications:
- Approved for Ph+ chronic phase of CML (chronic myeloid leukemia),balst crisis that ahs progressed on prior interferon α
- Gastrointestinal stromal tumors expressing the c-kit tyrosine kinase
(2) Dasatinib (Sprycel)
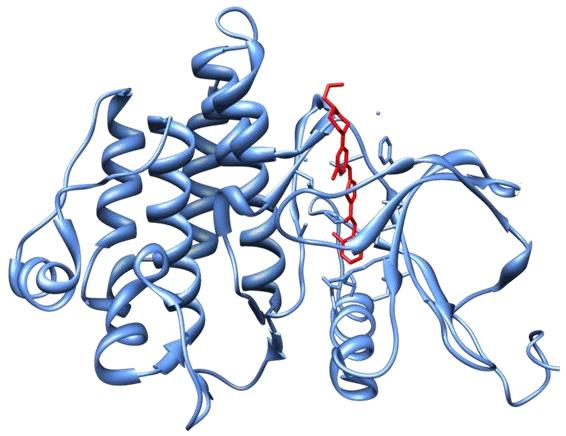
- Crystal structure of Abl kinase domain (blue) in complex with dasatinib (red).
The main targets: BCR/ABL, Src, c-Kit, ephrin receptors, and several other tyrosine kinases, but not erbB kinases such as EGFR or Her2
- Oral inhibitor
- Indications: CML , ALL with Ph+
- AE: Neutropenia, myelosuppression
(3) Gefitinib (Iresa) and Erlotinib (Tarcera)
- Gefitinib is an EGFR (epidermal growth factor receptor) inhibitor, like erlotinib, which interrupts signaling through the epidermal growth factor receptor (EGFR) in target cells. Therefore, it is only effective in cancers with mutated and overactive EGFR, such as breast and lung cancers.
- Is a drug used for certain breast, lung and other cancers
- It is not a chemotherapeutic in the traditional sense, since it does not cause the destruction of tumor cells. Instead, gefitnib interferes with the growth and spread of new cancer cells. Anti-tumor but not cytotoxic.
- Patients who don’t smoke with a bronchoaleolar subtype have a good resposiveness.
- Tarcera approved for advanced pancreatic cancer in combination with gemcitabine.
- AE: diarrhea, acneifrom skin rash
(4) Cetuximab
MOA:
- Epidermal growth factor receptor inhibitor.
- Chimeric monoclonal antibody directly against the extracellular domain of EGFR
Indications:
- For metastetic colorectal cancer in combination with irinotecan or oxaliplatin.
- For locally advanced head and neck cancer in combination with radiation therapy.
Proteasome and protein degradation
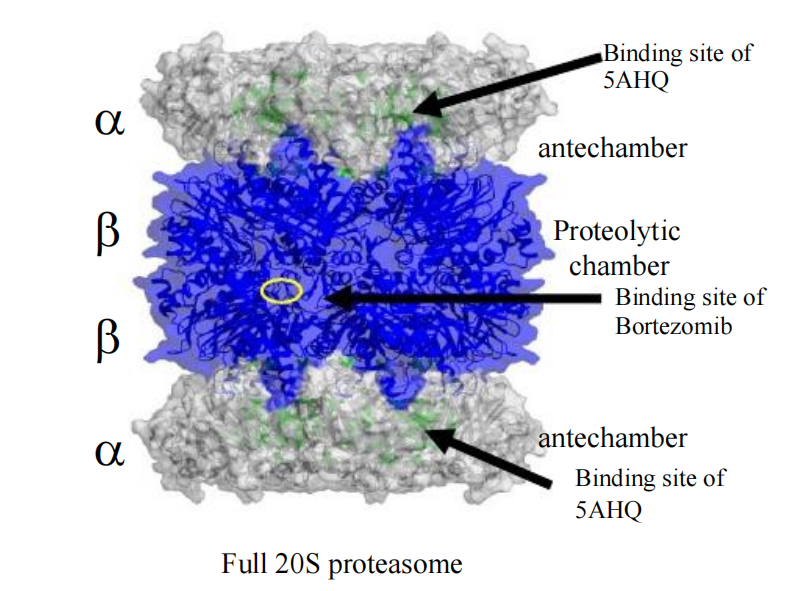
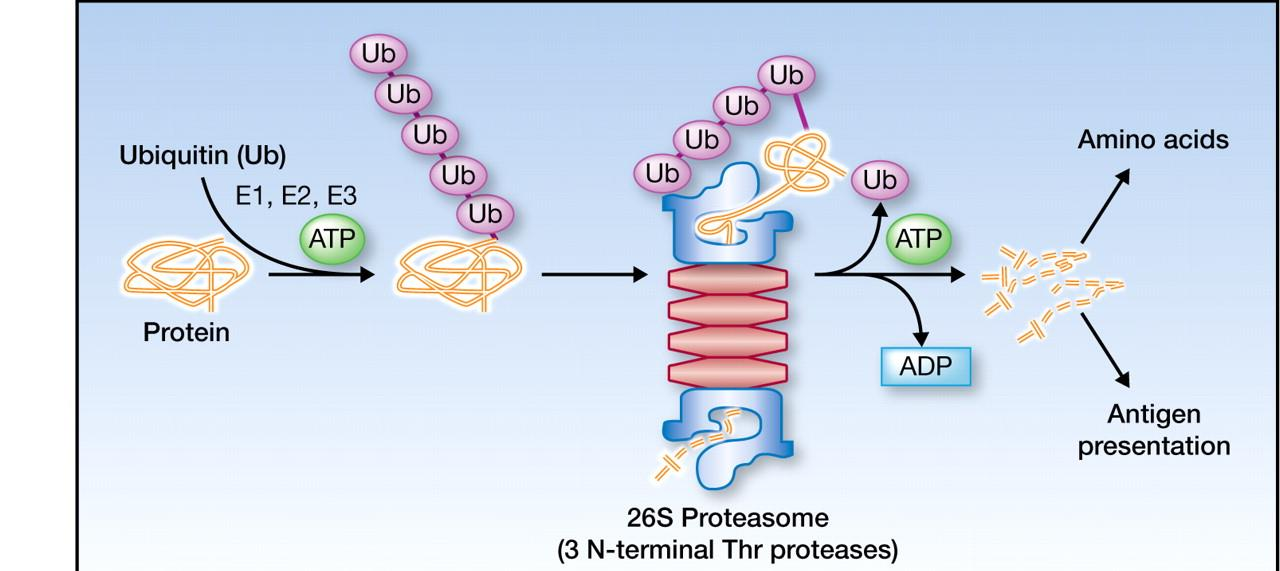 ##### (1) Bortezomib (Velcade)
##### (1) Bortezomib (Velcade)
- 1st line for mutliple myeloma
- 2nd line for mantle cell lymphoma
the first and only FDA approved anti-cancer agent among proteasome inhibitors
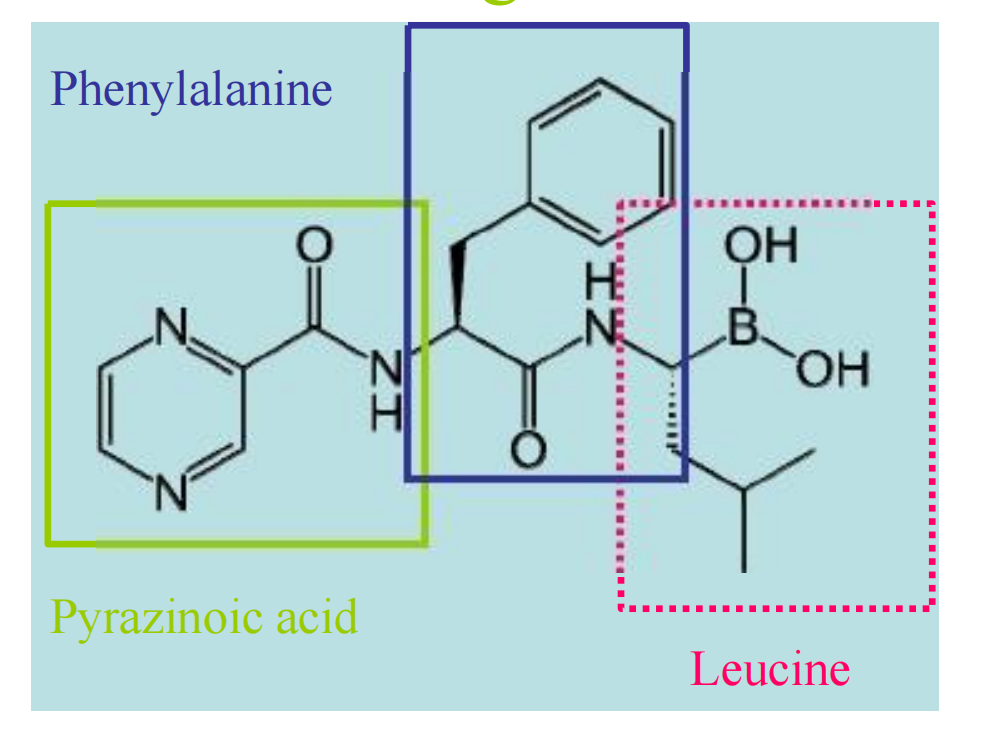
- highly selective
- Reversible
- 26S proteasome complex
- Most effective on Myeloma
- Effects the bone metabolism in MM
- Sensitizes them toward conventional chemotherapy
- Prolongs patients’ survival by overcoming resistance to conventional drugs
Differentiation Inducers
- Retinoic acid derivatives
- Arsenic trioxide
Specific for APL, acute promyelocytic leukemia by induction of terminal differentiation thus losing its ability to proliferate
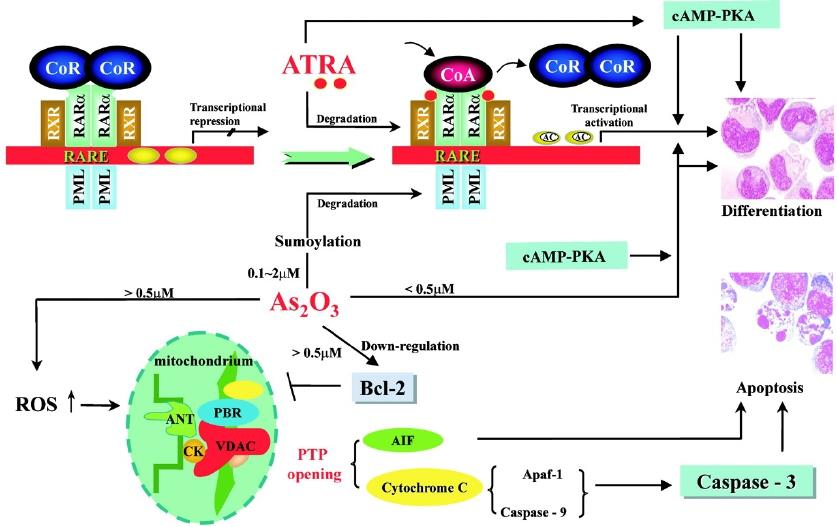
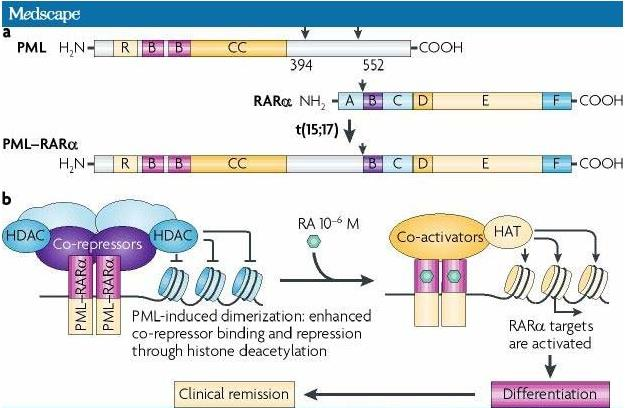 #### Arenic Trioxide (As2O3)
#### Arenic Trioxide (As2O3)
Indications: 2nd line for APL with t(15:17) translocation, refractory to ATRA or anthracyclines.
MOA:
- degradation of the chimeric protein PML/RARa
- mitochondria-dependent cell apoptosis
Administration: intravenous.
AE: fatigue, electrocardiographic changes (QT prolongation), arrhythmias.
Summary of Mechanisms of Action
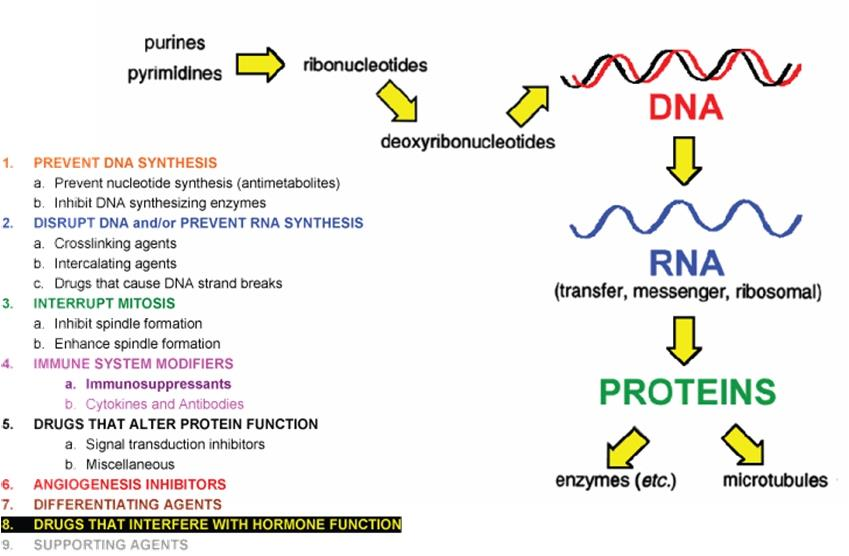
四、Chemotherapy: Pros and Cons
A broad spectrum : almost all kinds of cancers from all organs, including Brain cancer, blood cancers, breast cancer, colon cancer, gastric cancer, lung cancer cancer, prostate cancer….
Kill cancer cells that rapidly grow, or specific genetic presented cancers
More effective when used in combination with other drugs or other methods, such as surgery or radiotherapy.
No anticancer drugs are perfect that kill cancer cells only.
Most adverse effects are:
- Myelosuppression—anemia, hemorrhage, prone to infection
- Somatitis
- Alopecia
- Gastrointestinal effects.
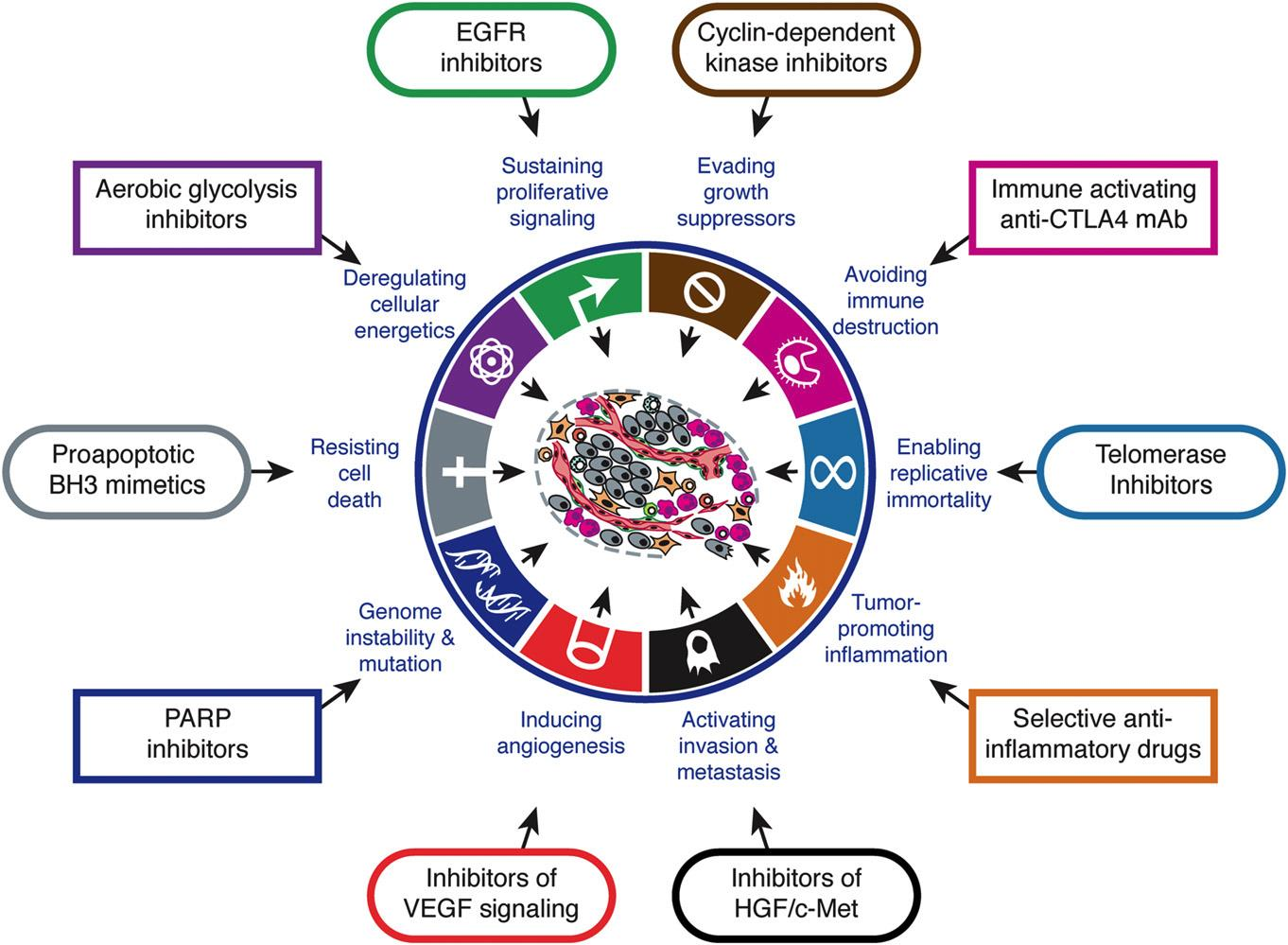 + Therapeutic targeting of the hallmarks of cancer.
+ Therapeutic targeting of the hallmarks of cancer.
五、Tumor-Immuno Therapy
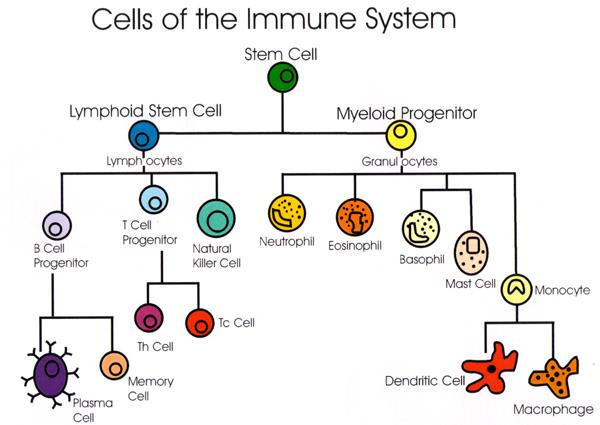 ## Immune system and cancer
## Immune system and cancer
The immune system serves as one of the primary defenses against cancer. When normal tissue becomes a tumor or cancerous tissue, new antigens develop on their surface.
These antigens send a signal to immune cells such as the T lymphocytes and macrophages, which in turn directly kill the tumor cells or release substances like cytokines that may bring about tumor cell death.
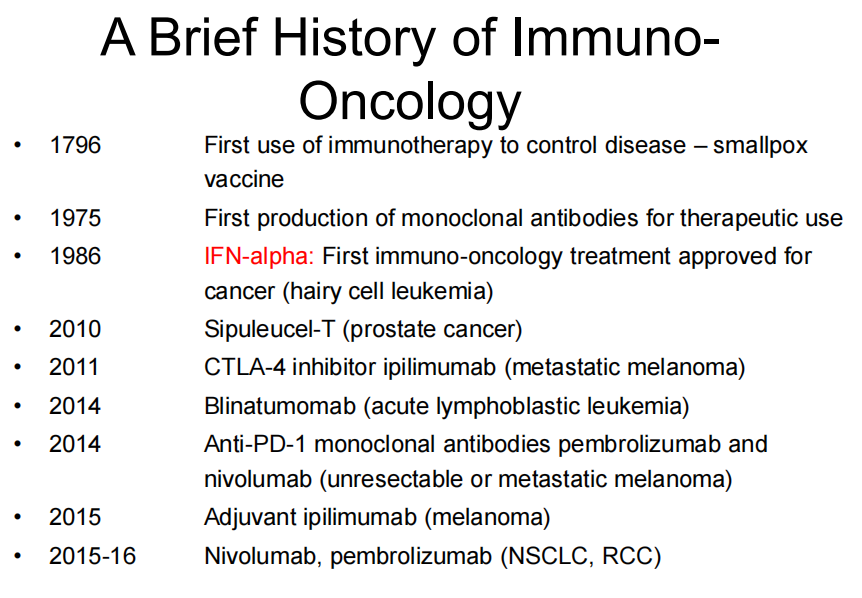 ## Abridged history of cancer immunotherapy: Disappointing until recently
## Abridged history of cancer immunotherapy: Disappointing until recently
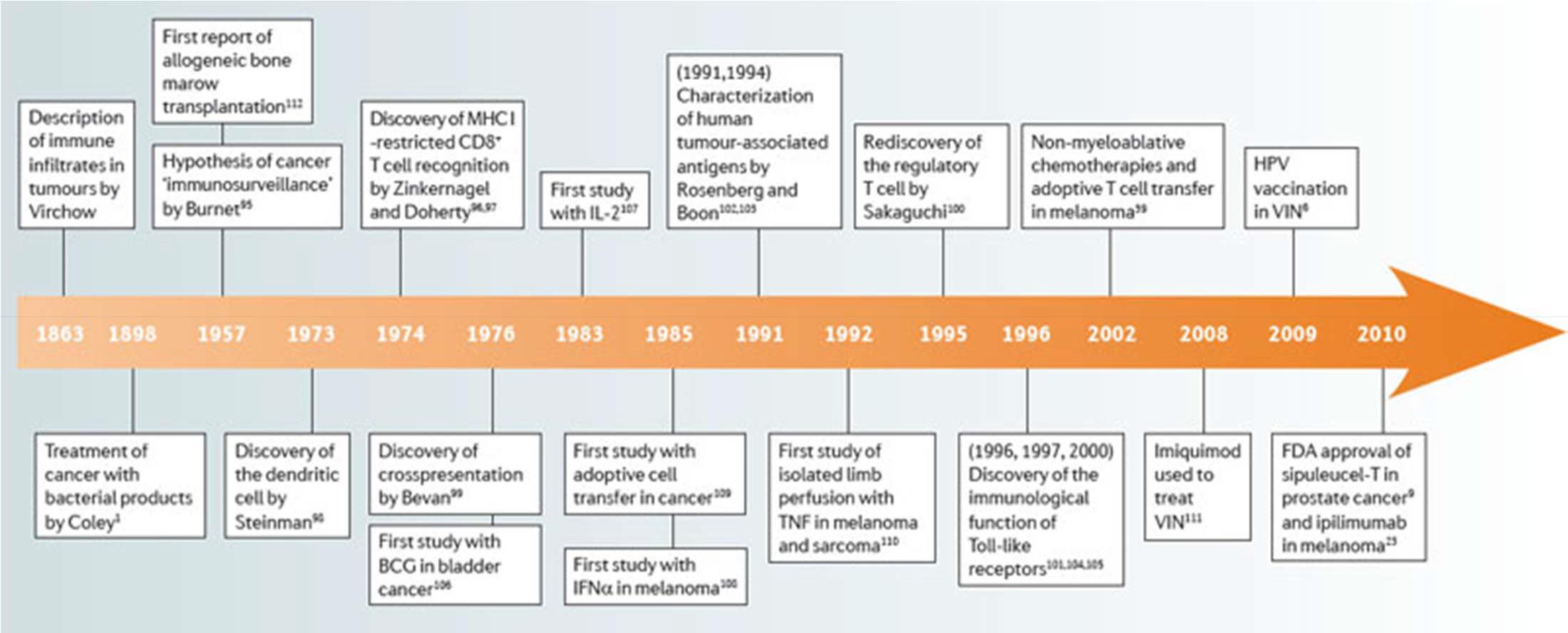 + 2011: Ipiliumumab shows overall survival benefit in melanoma
+ 2012-2014: PD1 and PD-L1 blockade has benefit in melanoma and lung
+ cancer 2011-2014: CAR-modified T cells show durable remissions in leukemia
+ 2011: Ipiliumumab shows overall survival benefit in melanoma
+ 2012-2014: PD1 and PD-L1 blockade has benefit in melanoma and lung
+ cancer 2011-2014: CAR-modified T cells show durable remissions in leukemia
Monoclonal Antibodies
Monoclonal antibodies are proteins produced in the laboratory from a single clone of a B−cell, the type of cells of the immune system that make antibodies. When used as a treatment for cancer, there are three general strategies with monoclonal antibodies:
- One uses the ability of the antibodies to bind to the cancer cells having the tumor antigens on their surface. The immune system will see the cancer cells marked with bound antibodies as foreign and destroy them
- A second strategy is to use the antibodies to block the binding of cytokines or other proteins that are needed by the cancerous cells to maintain their uncontrolled growth. Monoclonal antibodies designed to work like this bind to the cytokine receptors that are on the tumor cell surface.
- A final strategy involves special antibodies that are linked (conjugated) to a substance that is deadly to the cancer cells. E.G. radioactive isotopes, have been successfully conjugated to antibodies. The antibodies are then used to specifically destroy he tumor cells with the radioactivity or toxic substance
Trastuzumab (曲妥珠单抗)
- Trastuzumab is a humanized monoclonal antibody produced by recombinant DNA technology that binds specifically to the human epidermal growth factor receptor 2 protein (also known as HER2) that is found on the cell surface of some cancer tumors, most notably breast cancer(25−30% of breast malignancies) and also targets it for destruction by the natural killer cells of immune system.
Biological response modifiers
Researchers have been working on stimulating the immune cells during cancer with substances broadly classified as biological response modifiers. Cytokines are one such substance. These are proteins that are predominantly released by immune cells upon activation or stimulation.
Aldesleukin
- Aldesleukin is interleukin, that is used to treat metastasis renal cell carcinoma (a form of kidney cancer) and metastasis melanoma. Aldesleukin is also known as interleukin−2, IL−2
Interferons
- small, natural cytokines produced by leucocytes ,T−lymphocytes, and fibroblasts to activate tumor−specific cytotoxic T−lymphocytes. Thus, tumor cells would be destroyed based on immunotherapy
- used to treat cancers such as hairy cell leukemia, malignant melanoma, and Kaposi’s sarcoma (an AIDS−related cancer) as well as many other cancers
Immune therapy can overcome tolerance
Hallmark of cancer: induction of immune tolerance
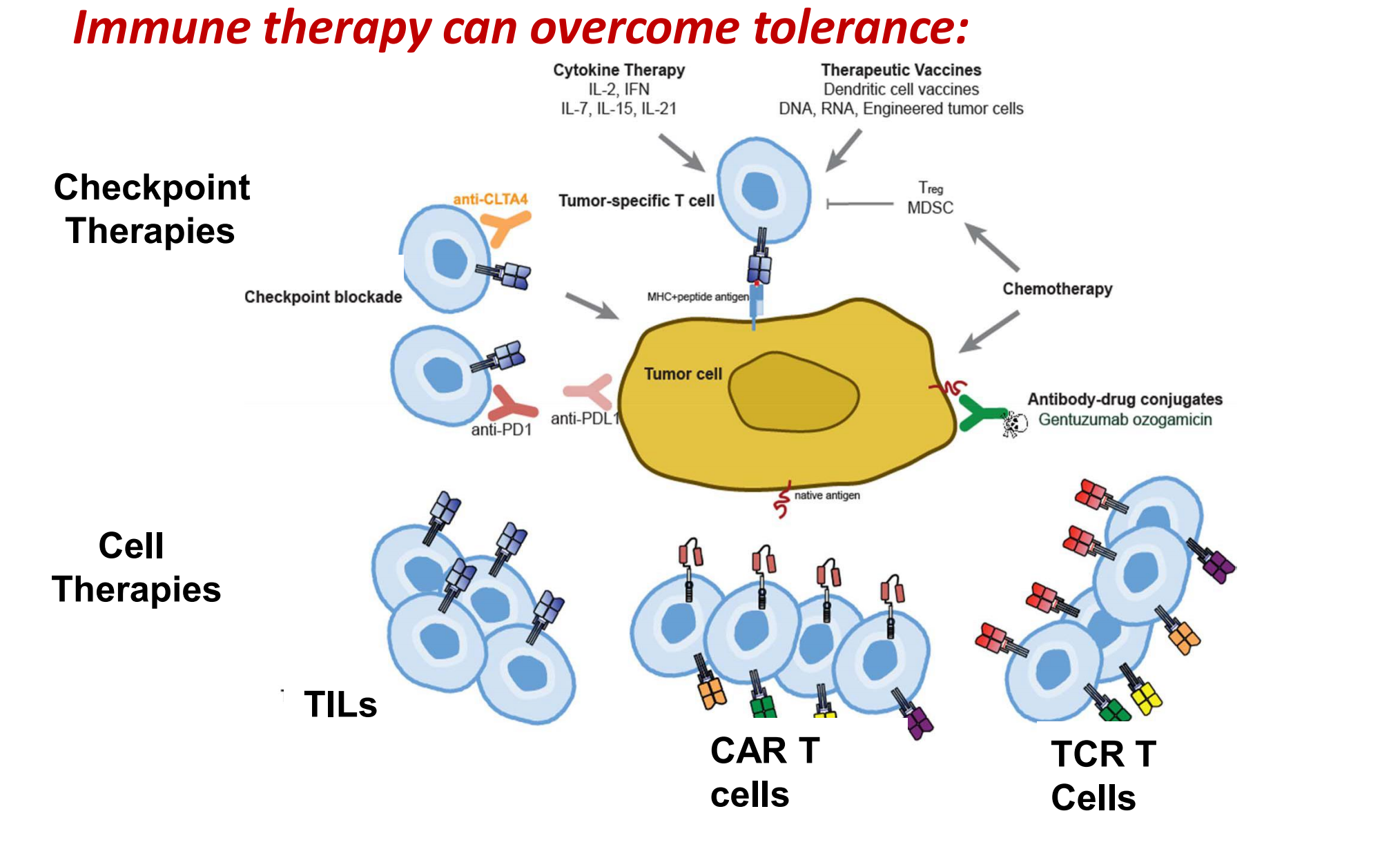 ### 1. CAR T cells: personalized “serial killer” cells
### 1. CAR T cells: personalized “serial killer” cells
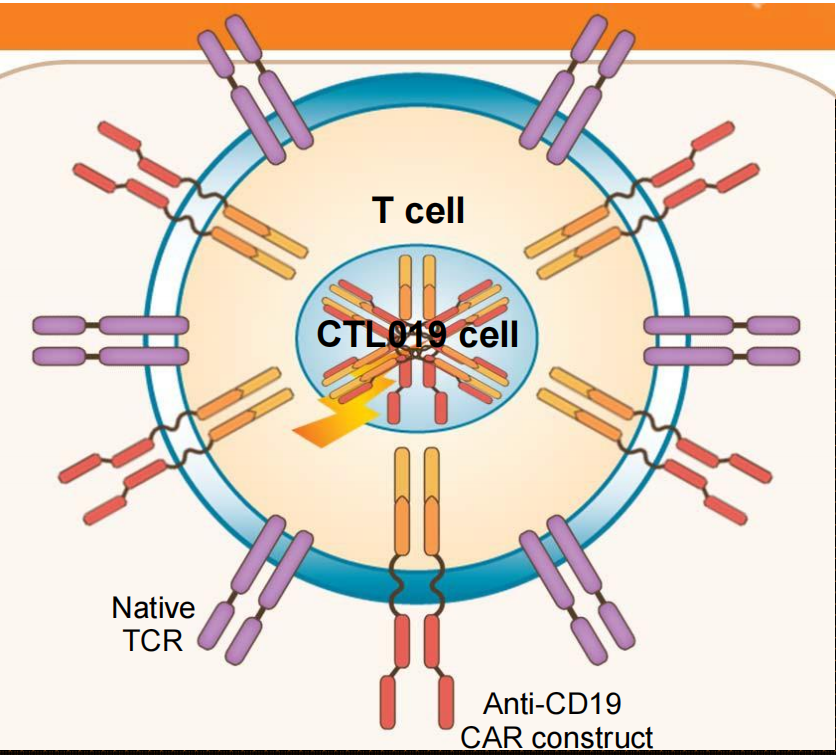
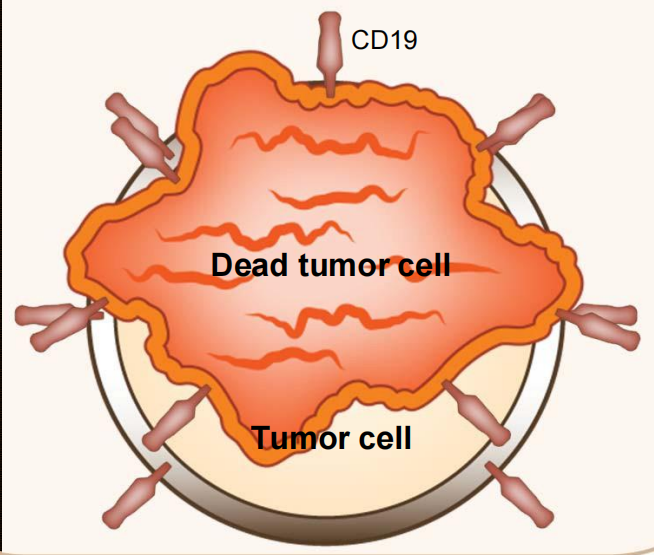
Gene transfer technology is used to stably express CARs on T cells, conferring novel antigen specificity
CART19 therapy takes advantage of the cytotoxic potential of T cells thereby killing tumor cells in an antigen-dependent manner
Persistent CART19 cells consist of both effector (cytotoxic) and central memory T cells
T cells are non-cross resistant to chemotherapy
Responses are cytolytic: no swelling!
2. Using Synthetic Biology to Overcome Tolerance Creation of Bi-specific CAR T cells
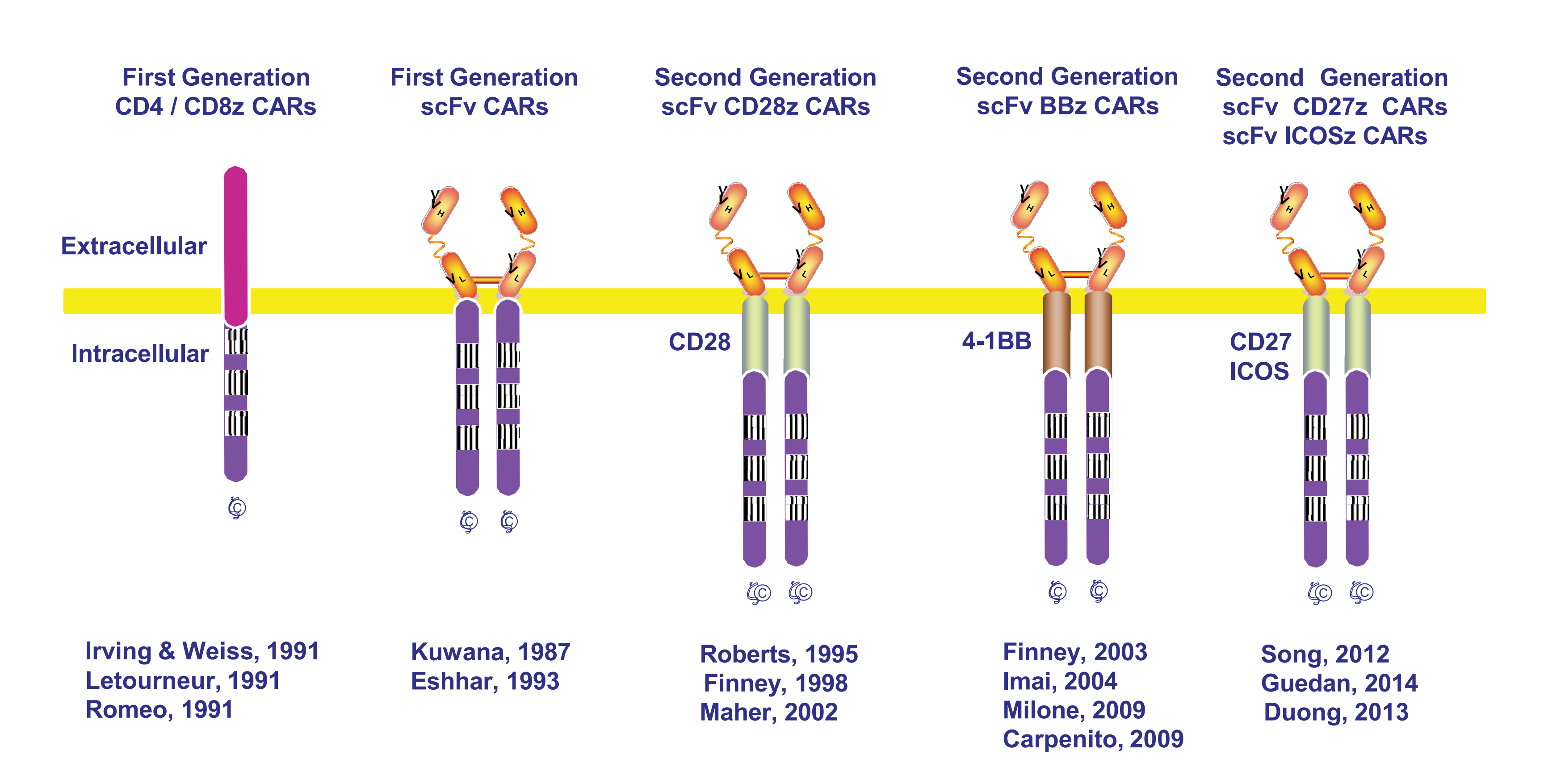 + Design of CAR T Cells
+ Design of CAR T Cells
3. Adult Chronic Leukemia Study Overview
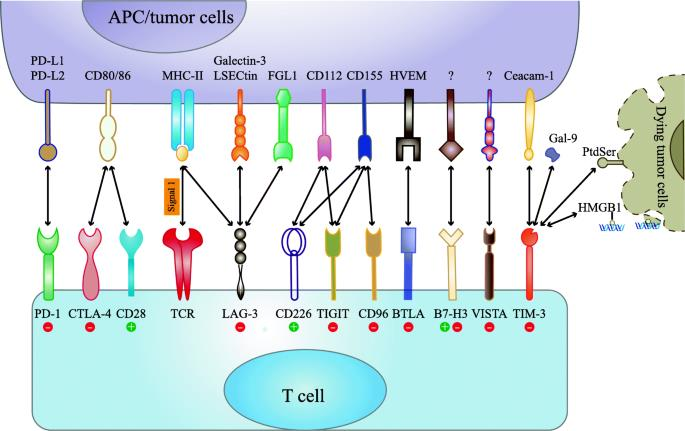
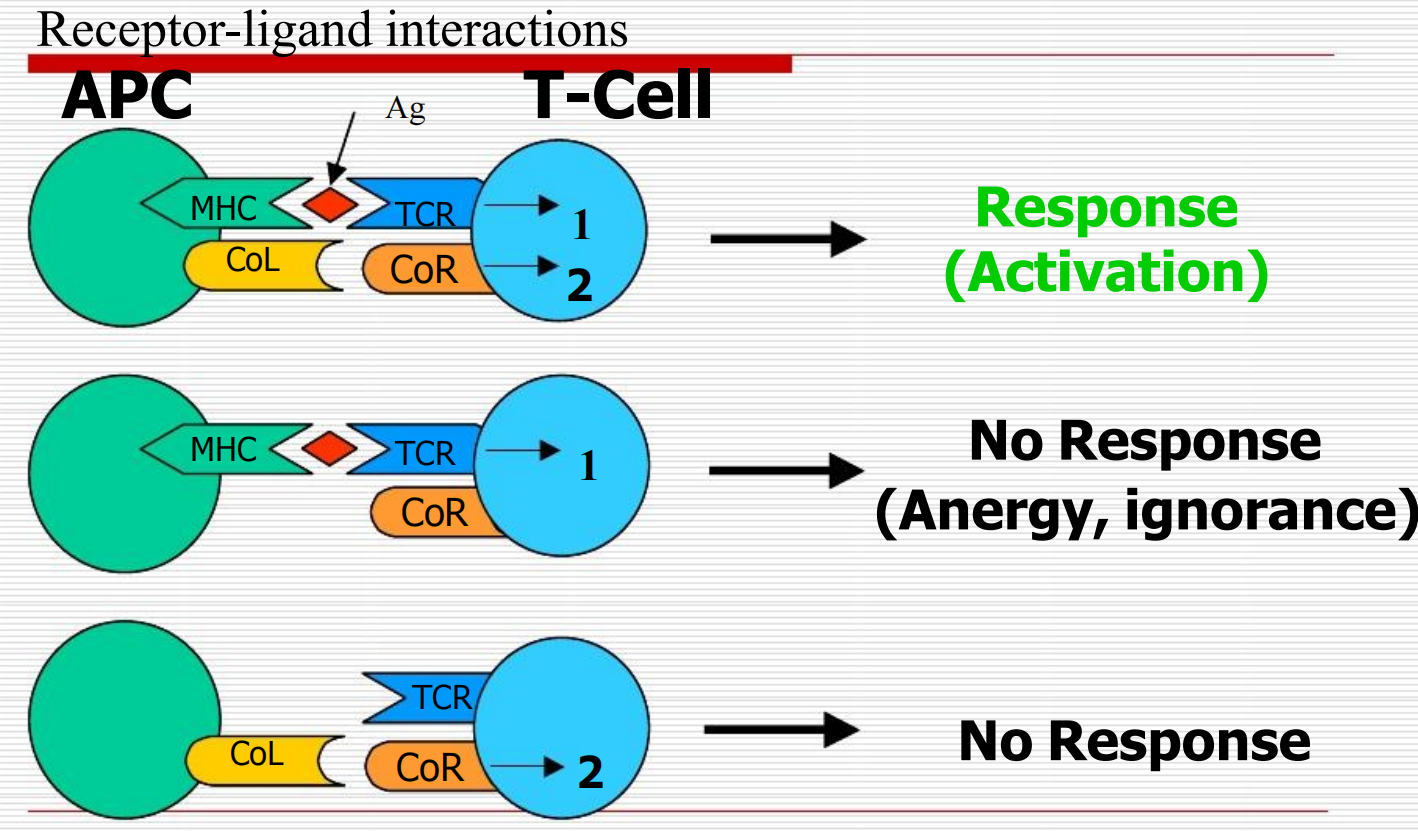
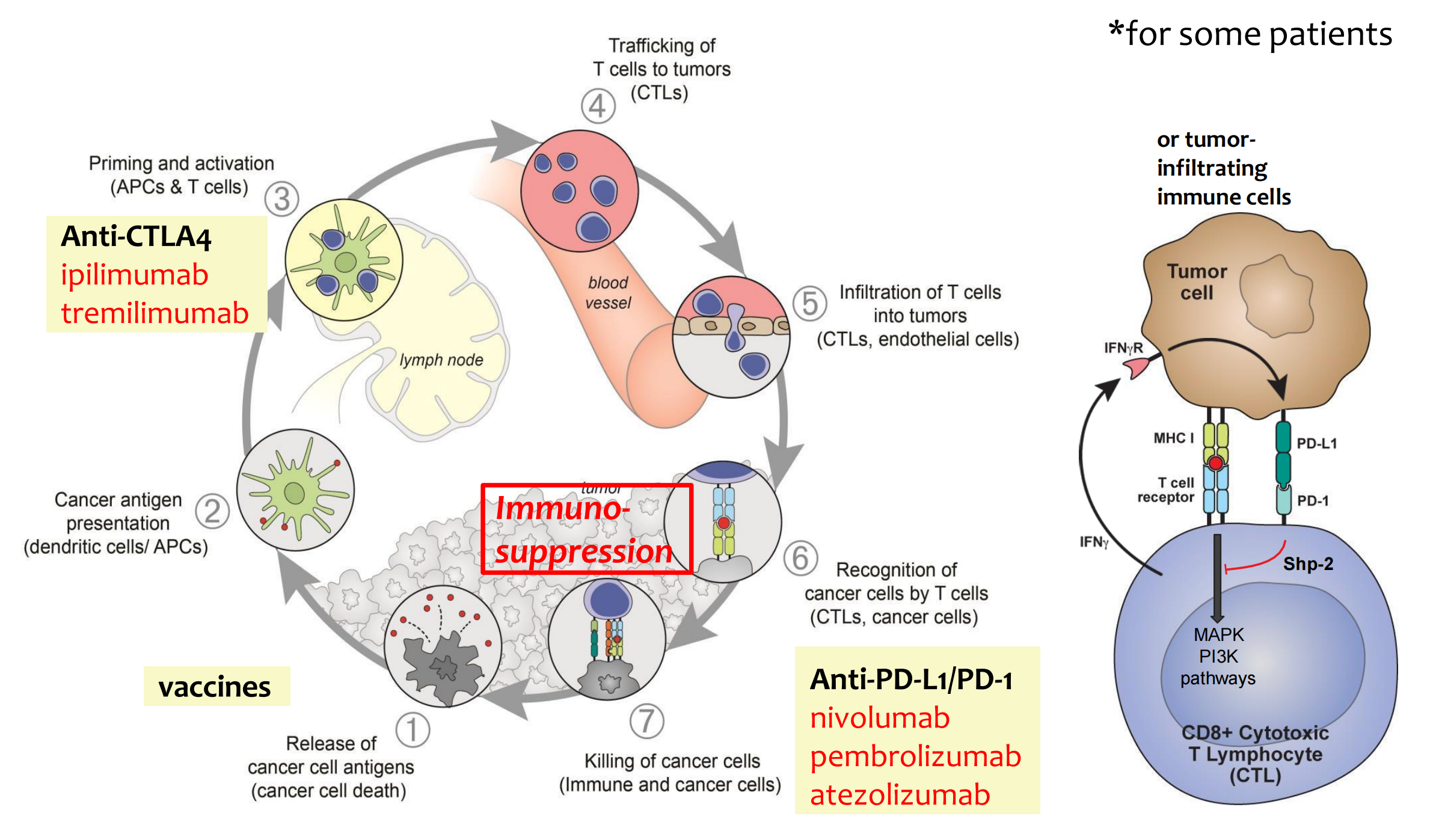
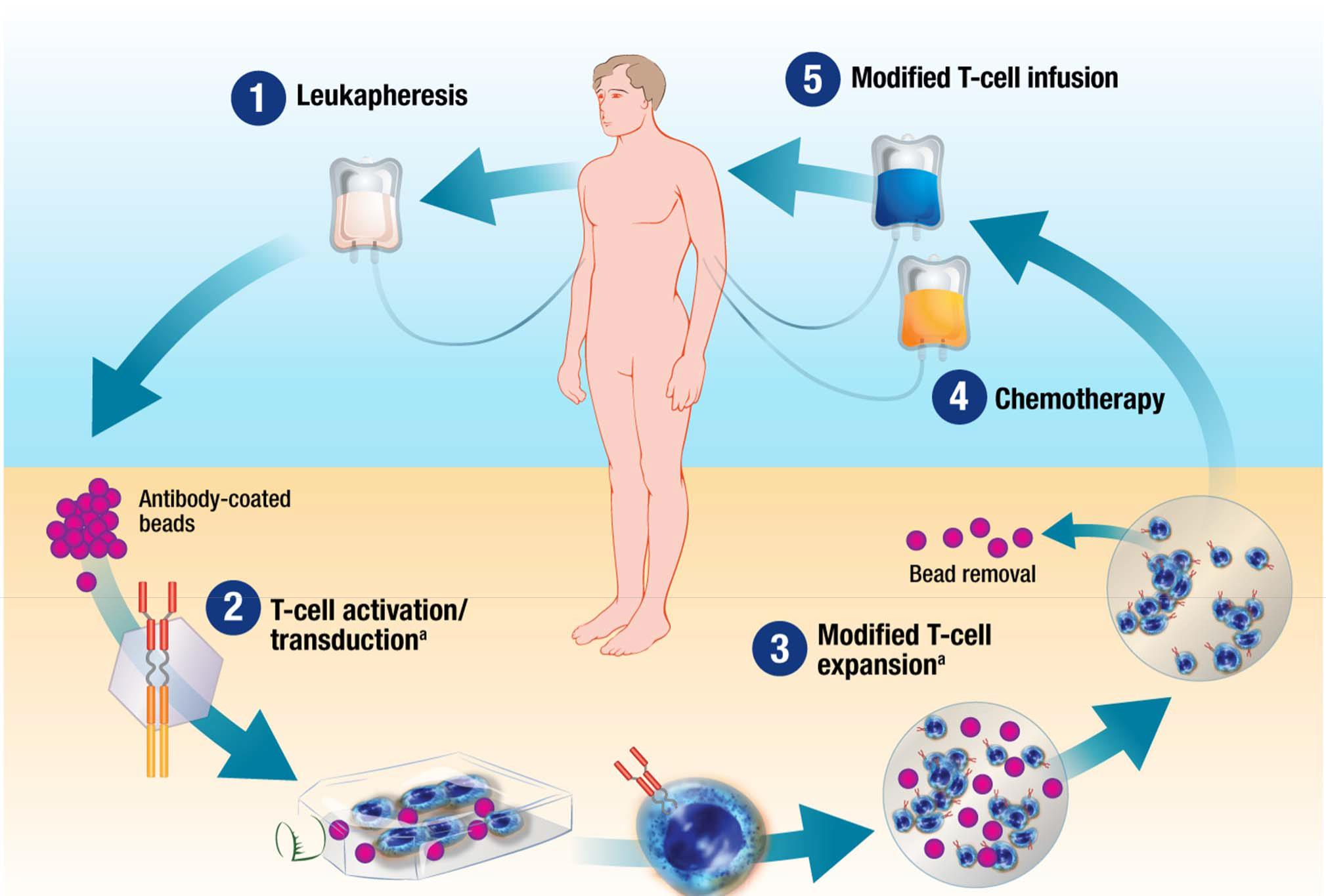 Immune Checkpoint Blockade: Mechanism of Action
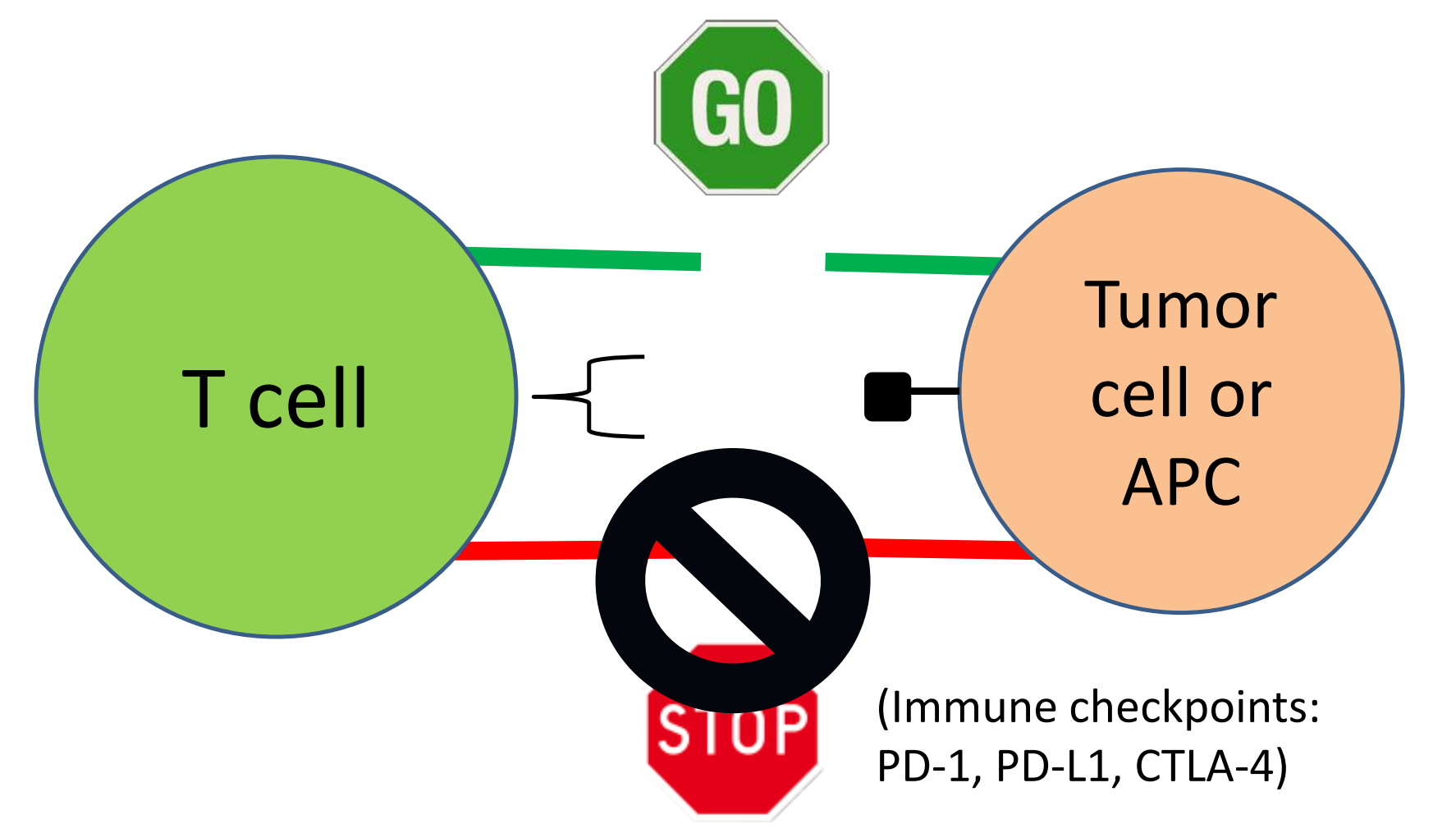
Immune Checkpoint Blockade: Mechanism of Action
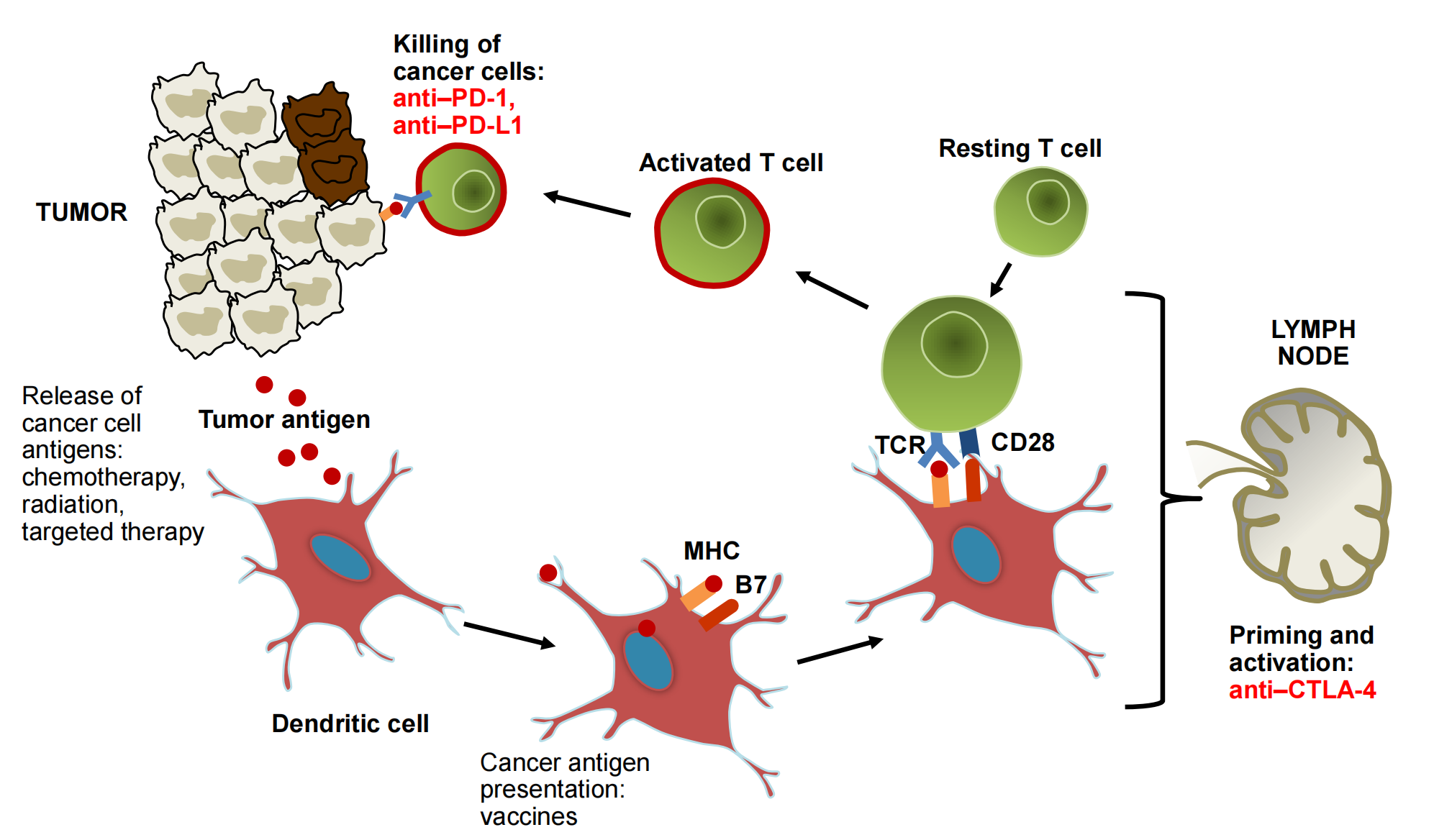
4. A Road Map of Immunotherapy Agents in the Cancer-Immune System Interaction
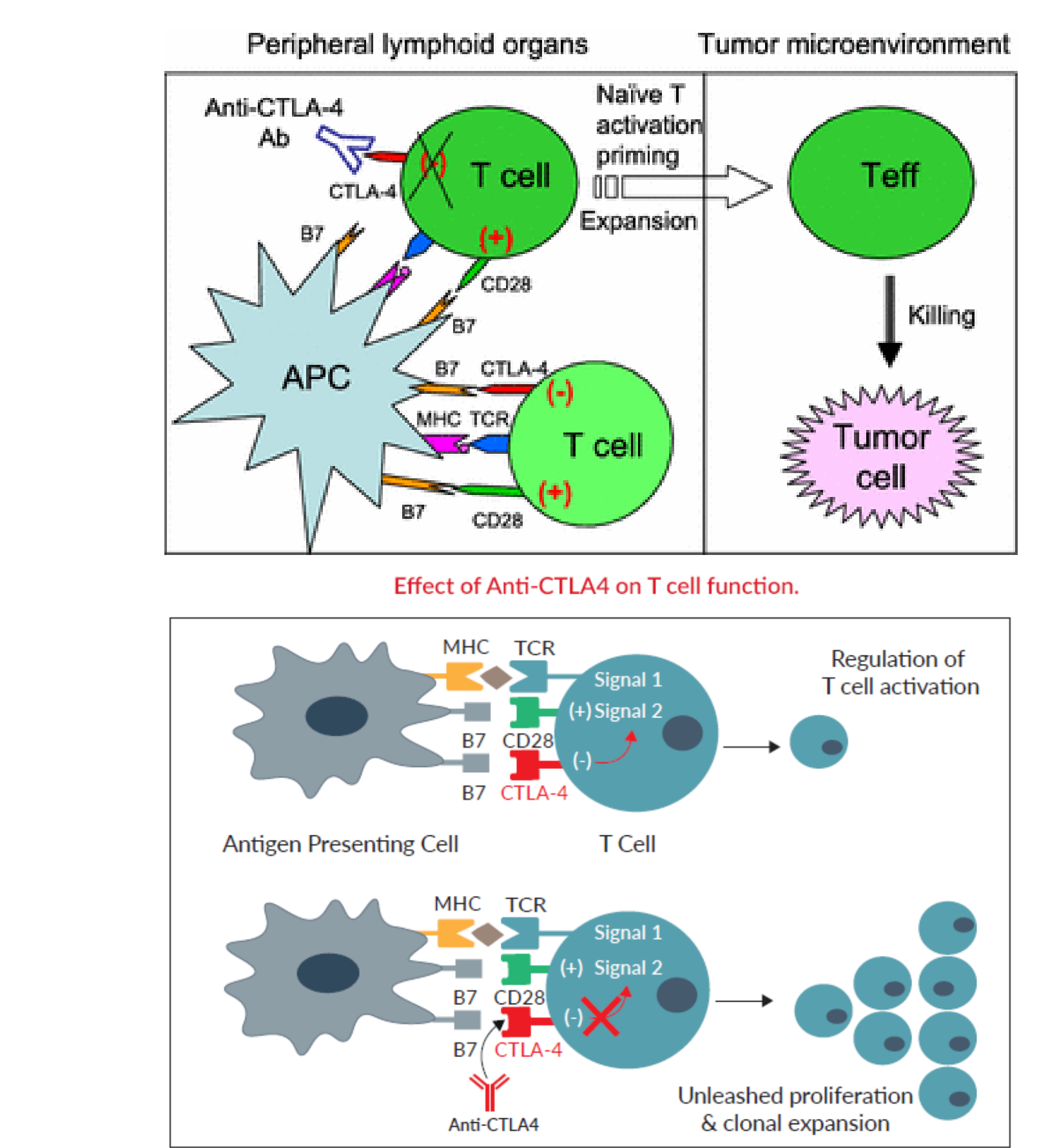 ### 5. Immunosuppression is a rate limiting step to effective antitumor immunity
### 5. Immunosuppression is a rate limiting step to effective antitumor immunity
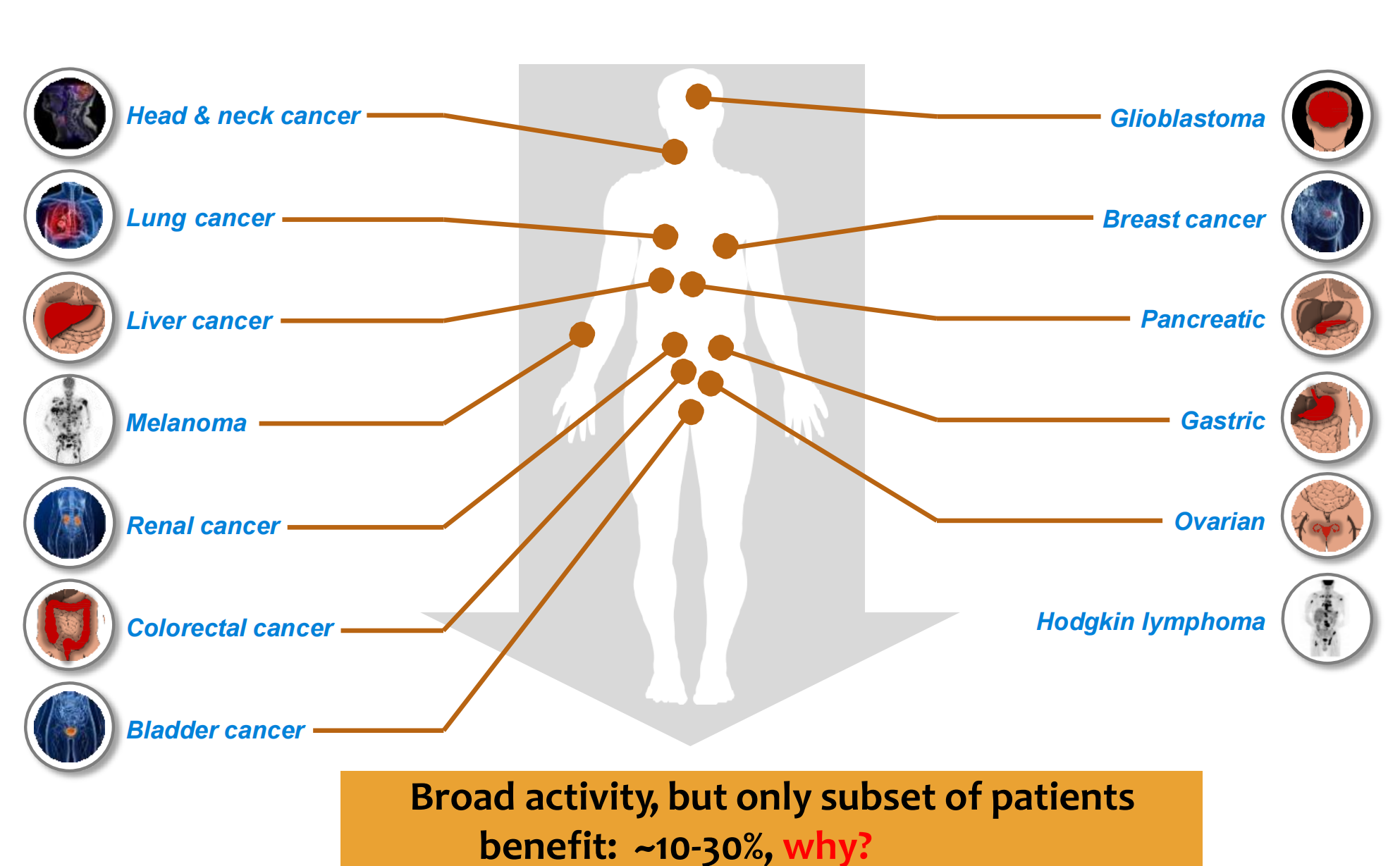
 ### 6. Broad activity for anti-PD-L1/PD-1 in human cancer
### 6. Broad activity for anti-PD-L1/PD-1 in human cancer
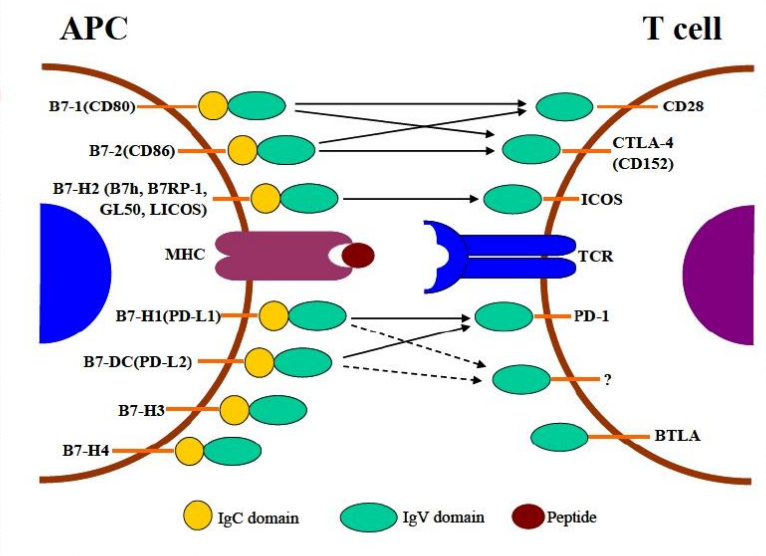
 ### 7. Immune checkpoints: more than PD-1 and CTLA-4
### 7. Immune checkpoints: more than PD-1 and CTLA-4