L1 Introdction
一、Introduction to Molecular Pharmacology
Objectives to pharmacology:
- Definition of the four basic terms (drug, pharmacology, clinical pharmacology, and therapeutics) for the study of pharmacology.
- Properties of an ideal drug
- Therapeutic objective of drug therapy.
- Factors that determine how an individual will respond to a specific drug and dosage
Four basic terms:
- Drug: any chemical that can affect living processes
- Pharmacology: the study of drugs and their interactions within living systems
- Physical and chemical properties
- Biochemical and physiological effects
- Knowledge of the history, source, and use of drugs
- Absorption, distribution, metabolism and excretion
- Clinical Pharmacology: study of drugs in humans
- Therapeutics: use of drugs to diagnose, prevent and treat illness( and/or pregnancy)=medical use of drugs
The Defination
1. Pharmacology
DEFINITIONS:
- Pharmacology is the study of how drugs exert their effects on living systems.
- Pharmacologists work to identify drug targets in order to learn how drugs work. Pharmacologists also study the ways in which drugs are modified within organisms.
- In most of the pharmacologic specialties, drugs are also used today as tools to gain insight into both normal and abnormal function.
- Pharmacologist
- Pharmacologists work to identify drug targets in order to learn how drugs work. Pharmacologists also study the ways in which drugs are modified within organisms
- In most of the pharmacologic specialties, drugs are also used today as tools to gain insight into both normal and abnormal function.
Pharmacy and pharmacist
- Pharmacy is a medical science concerned with the safe and effective use of medicines
- the study of techniques involved in the preparation compounding, dispensing, preservation and storage of the drugs for medical use
- Pharmacist: who is qualified and licensed
- Functions:
- to prepare and dispense drugs
- to responsible for the manufacture of the dosage form of drugs (e.g. tablets, capsules, etc)
- Functions:
2. Drugs
Drugs can be defined as chemical agents that uniquely interact with specific target molecules in the body, thereby producing a biological effect.
- Drugs can be stimulatory or inhibitory
- Drugs, as well as hormones, neurotransmitter, autocoids(自分泌物) and toxins can make possible the transfer of information to cells by interaction with specific receptive molecules called “receptors“
- Drugs interact with biological systems in ways that mimic, resemble or otherwise affect the natural chemicals of the body
- Drugs can produce effects by virtue of their acidic or basic properties (e.g. antacids(抗酸剂), protamine(鱼精蛋白)), surfactant properties (amphotericin(两性霉素)), ability to denature proteins (astringents(收敛剂)), osmotic properties (laxatives(泻药), diuretics(利尿剂)), or physicochemical interactions with membrane lipids (general and local anesthetics(麻醉剂))
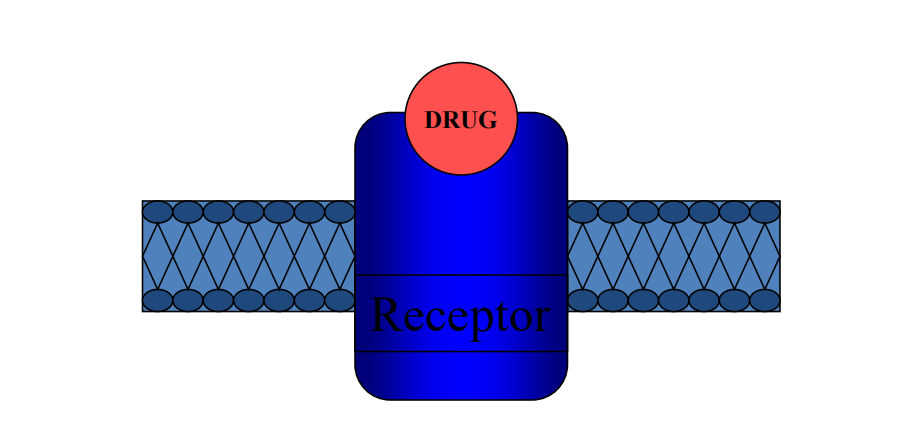
3. Receptors
Most drugs combine (bind) with specific receptors to produce a particular response. This association or binding takes place by precise physicochemical and steric interactions between specific groups of the drug and the receptor.
Classification:
- Proteins
- Carriers
- Receptors
- G protein coupled
- Ligand gated channers
- Intracellular
- Enzymes
- DNA
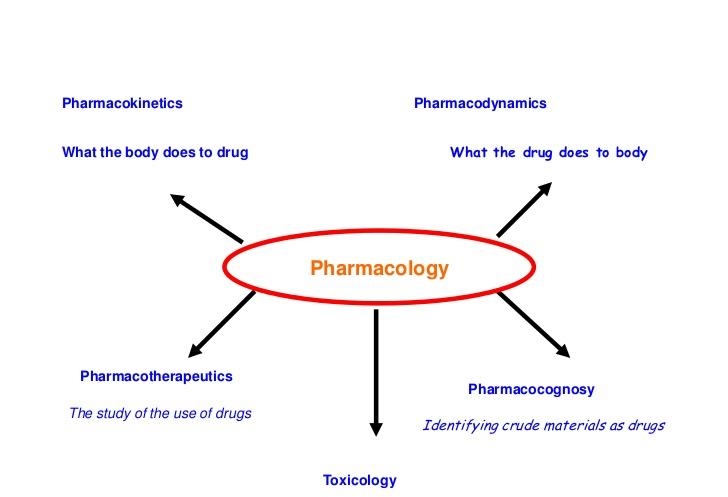
Divisions of Pharmacology
1. Pharmacognosy 生药学
What is Pharmacognosy?
- the branch of pharmacology dealing with the economic, biological and chemical aspects of natural drugs and their constituents
- study of the sources of drugs and the physical characteristics of crude or unrefined drugs
- study of drugs derived from herbal and other natural sources and how the body reacts to them
- Simply: the study of natural( plant and animal)drug sources
2. Pharmacokinetics 药物动力学
Pharmacokinetics Is what the body does to the drug
- The magnitude of the pharmacological effect of a drug depends on its concentration at the site of action.
It studies:
- Absorption
- Distribution
- Metabolism
- Elimination
3. Pharmacodynamics 药效学
Pharmacodynamics Is what the drug does to the body
- Interaction of drugs with cellular proteins, such as receptors or enzymes, to control changes in physiological function of particular organs
Drug-Receptor Interactions
- Binding
Dose-Response:
- Effect
Signal Transduction:
- Mechanism of action, pathways
4. Pharmatherapeutics 药物遗传学
Area of pharmacology concerned with unusual responses to drugs caused by genetic differences between individuals
Responses that are not found in the general population, such as general toxic effects, allergies, or side effects, but due to an inherited trait that produces a diminished or enhanced response to a drug.
Differences in Enzyme activity:
- Acetylation polymorphism 乙酰化多态性
- Butylcholinesterase alterations 丁基胆碱酯酶改变
- Cytochrome P450 aberration 细胞色素P450畸变
5. Pharmacogenomics 药物治疗学
What is Pharmacotherapeutics
- study of how drug may be used in the treatment of disease
- which among the drugs would be most effective or appropriate for a specific disorder or what dose would be required
- Use of drugs and clinical indications of drugs to prevent and treat disease
6. Toxicology 毒理学
Toxicology was defined as the basic science of poison, now defined as the quantitative and qualitative study of the adverse effects of toxicants on biological organisms
Toxicant is a chemical or physical agent that produces adverse effects on biological organisms.
How toxicants enter, effect, and being eliminated from the organism
All substances are toxic if taken in the wrong quantities
二、Drug Classifications
Molecular nature:
- Chemical agents
- Biologics 生物制剂
- Complementary therapies (补充疗法、辅助疗法)、
Methods of distribution:
- OTC drug
- Prescription drug
Classified by Molecular Nature
1. Traditional Drugs Are Chemical Agents
Synthesized in a laboratory
Produce biological responses in the body
- If desirable response—therapeutic
- If undesirable response—adverse
2. Examples of Biologics
- Hormones
- Monoclonal antibodies
- Natural blood products and components
- Interferon 干扰素
- Vaccines
3. Complementary and Alternative Therapies
Natural plant extracts, herbs, vitamins, minerals, dietary supplements
Acupuncture, hypnosis, biofeedback, massages
针灸,催眠,生物反馈,按摩
Classified by Methods of Distribution
1. Advantages and Disadvantages of Prescription Drugs
Advantages
- Health-care provider examines the client and orders proper drug
- Amount and frequency of drug is controlled
- Instructions on use and side effects of drug are discussed
Disadvantages
- Require a prescription to obtain
- Need for health-care-provider appointment
2. Advantages and Disadvantages of OTC Drugs
Advantages
- No health-care provider appointment required
- Often less expensive than prescription drugs
Disadvantages
- Client may choose wrong drug
- Client may not know reactions or interactions
- Ineffective treatment may
- result in progression of disease
Properties of Ideal Drug
1. Important properties
Effectiveness
- A drug that elicits the response it was meant to. It is the most important property.
- No effect=no justification of use (FDA approved with appropriate experiments)
Safety:
Pharmakon = poison in Greek
Safe even at high concentrations and for long periods of administration (no such thing as a safe drug
- Reduced by proper administration (iv, ip im, sc, etc,.)
- NO habit forming aspects
- No side effects
Selectivity
One that elicits only the response for which it is given
Selective for specific reaction with no side effects(there Is no such thing)
Drowsiness can be caused by antihistamines 抗组胺药
Morning sickness, cramps, and depression can be caused by oral contraceptives
口服避孕药可以引起孕吐、痉挛和抑郁症
Constipation, urinary hesitance, and respiratory depression can be caused by morphine
吗啡可引起便秘、尿潴留和呼吸抑制
2. Additional Properties of Ideal Drug
- Reversible action
- Effects be reversible, i. e, removal /subside w/i specific time (1/2 life is short but potent during that time)
- Example: General Anesthetic; Contraceptives
- Predictability
- Know how patient will respond Ease of Administration
- Ease of Administration
- Number of doses should be bw and easy to administer
- (1) increase compliance & (2) decrease errors
- Diabetic patient Multiple daily injection of insulin
- Intravenous infusion
- Freedom from drug interactions
- Should not augment or decrease action of other drugs or have adverse combined effects
- Respiratory depression caused by diazepam (valium) which is normally minimal, can greatly be intensified by alcohol
- Antibacterial effects of Tetracycline can be greatly reduced by taking ron or calcium supplements
- Should not augment or decrease action of other drugs or have adverse combined effects
- Low cost
- Easy to afford (especially with chronic illness)
- Growth hormone (somatrem)costs between $10, 000 ad 20000
- Lifelong medication: hyper tension, arthritis, diabetes
- Easy to afford (especially with chronic illness)
- Chemical Stability
- No lose of effectiveness with storage
- Possession of a simple generic name
- Easy to remember and pronounce
- Example: Viagra (sildenafil); Tyleno (acetaminophen)
- Easy to remember and pronounce
Ideal Drug should also have ideal Name
- Each drug has at least three names
- Chemical
- Description of the drug using chemical nomenclature
- Generic
- Non‐proprietary name
- Trade
- Proprietary or brand names
- Created by companies to sell drugs
- Chemical
三、Principles of Drug Action
Drugs do not produce new function
No drug has a single action
- have both therapeutic and adverse actions
- Or have multiple therapeutic effects
Drug vs poison: dose related
Therapeutic actions are interchangeable
- Actions can be considered therapeutic in one case and adverse in another
- e.g. acetaminophen (anti-fever, but causes liver toxicity)
Drug’s effects are variable?
- It depends
- Many sources of variability
- Disease state, age and sex
- route of drug administration, dose, drug interactions
- Deciding to use a drug
- weigh benefits vs. risks
- there are always risks
Therapeutic Objective
To provide maximum benefit with minimum harm
Factors that determine Intensity of Response:
- Administration- dosage size and route
- Because of errors in administration routes and dosage and at wrong time there are many discrepancies(差异) in what patient gets and could cause more harm than good
- Errors could be made by pharmacists, physicians, or nurses
- Should give patients complete instruction about their medication and how to take it
- Pharmacokinetic processes
- Determines how much of an administered dose gets to its sites of action
- drug absorption
- drug distribution
- drug metabolism
- drug excretion
- Determines how much of an administered dose gets to its sites of action
- Pharmacodynamics
- Once a drug has reached is site of action, pharmacodynamic processes determine the type of response and intensity
- Drug must first bind to its specific target site at (RECEPTOR) that may be a chemical, a protein on a cell or in blood or tissue spaces, or on a bacteria or virus (i.e., heparin, antibody, leukotriene receptor (new), penicillin, etc…)
- Followed by a sequence of events that result in response (inhibition of clotting, inhibition of peptidoglycan synthesis, inhibition of inflammation, blocking of virus, etc …).
- Functional state of the patient is also important Tolerance to morphine will cause less of a response & placebo effects may help determine response
- Once a drug has reached is site of action, pharmacodynamic processes determine the type of response and intensity
- Individual Variations
- Each patient is unique in ability to respond and to how they each respond, but formation of “IDEAL DRUG” will lessen this variation
- Age- very important factor
- Sex- due to hormonal differences
- Weight - less effective and longer lasting in obese individuals (storage in fat)
- Kidney & liver functions elimination of drug
- Genetic variables tolerance, allergy (though not always genetic)
- Each patient is unique in ability to respond and to how they each respond, but formation of “IDEAL DRUG” will lessen this variation
Factors that determine the intensity of drug response
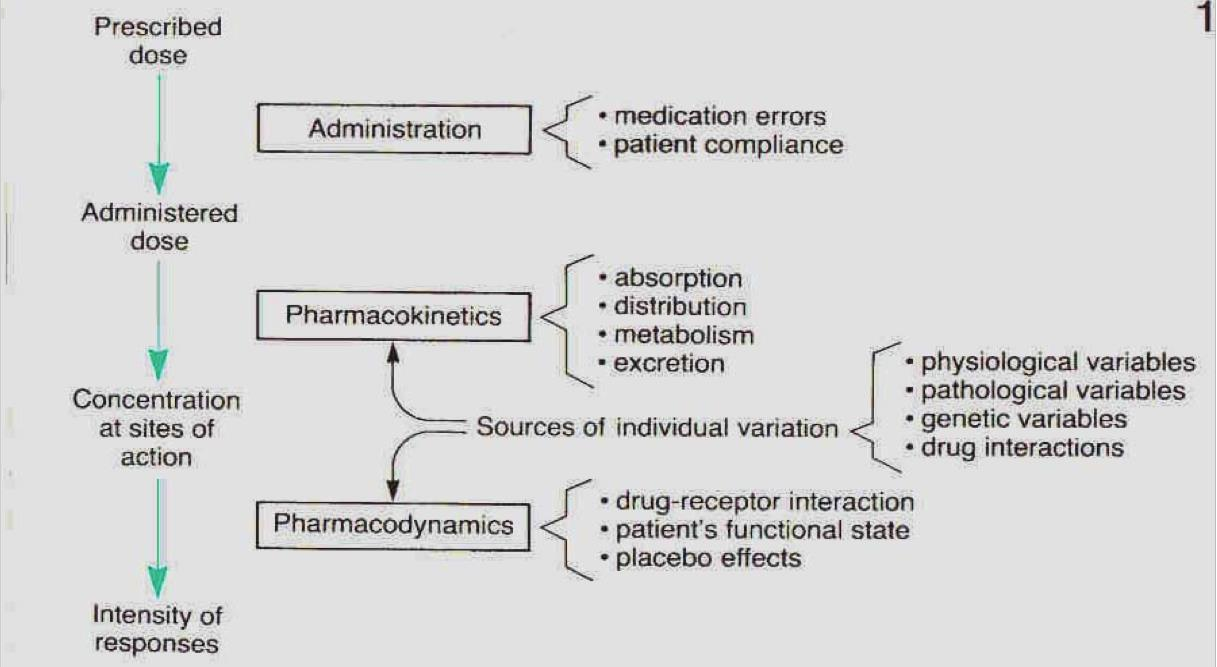
Prescribed dose → Administered dose: Administration
- Medication errors
- Patient compliance(病人的配合度)
Administered dose → Concentration at sites of action: Pharmacokinetics & Sources of Individual Variation
- Pharmacokinetics
- Absorption
- Distribution
- Metabolism
- Excretion
- Source of individual variations
- physiological variables
- pathological variables
- genetic variables
- drug interactions
Concentration at sites of action → Intensity of Responses: Pharmacodynamics
drug-receptor interaction
patient’s functional state
placebo effects
四、Modern Pharmacology
Began in Early 1800s
Chemists isolated pharmacological substances from natural substances
Effects on animals studied
Early researchers used themselves as test subjects
- Pharmacology Recognized as Distinct Discipline
First department of pharmacology established at Estonia in 1847
John Jacob Abe
- Father of American pharmacology
- Founded first pharmacology department in United States at University of Michigan in 1890
United States Pharmacopoeia (USP), 1820
- First comprehensive publication of drug standards
- Summarized standards of drug
- Purity
- Strength
- Directions for synthesis
Food and Drug Administration (FDA)
Officially established in 1988
Agency of US Department of Health and Human Services in 1988
1. Center for Drug Evaluation and Research
- Branch of FDA
- Determines safety and efficacy of drugs
- Pharmaceutical laboratories must solicit approval from FDA before marketing a drug
2. Center for Biologics Evaluation and Research (CBER)
- Branch of FDA
- Regulates use of biologics (serums, vaccines, and blood products)
- 1986 Childhood Vaccine Act result of CBER work
3. Four Stages of Approval for Therapeutic and Biologic Drugs
Step1: Preclinical investigation 临床前研究
Involves laboratory research
Tests done on cells and animals
Determines drug-dose range
Examines adverse effects
Results are considered inconclusive
Step2: Clinical Investigation 临床研究
Longest part of approval process
Termed ―clinical-trials phase
Evaluates human benefits
Step3: Review of New Drug Application (NDA)
Average review time 17–24 months
Drug approved: process continues
Drug rejected: process suspended
Step4: Post Marketing Surveillance 上市后监管
New drug placed on market
Surveyed for harmful effects in larger population
FDA holds annual public meetings
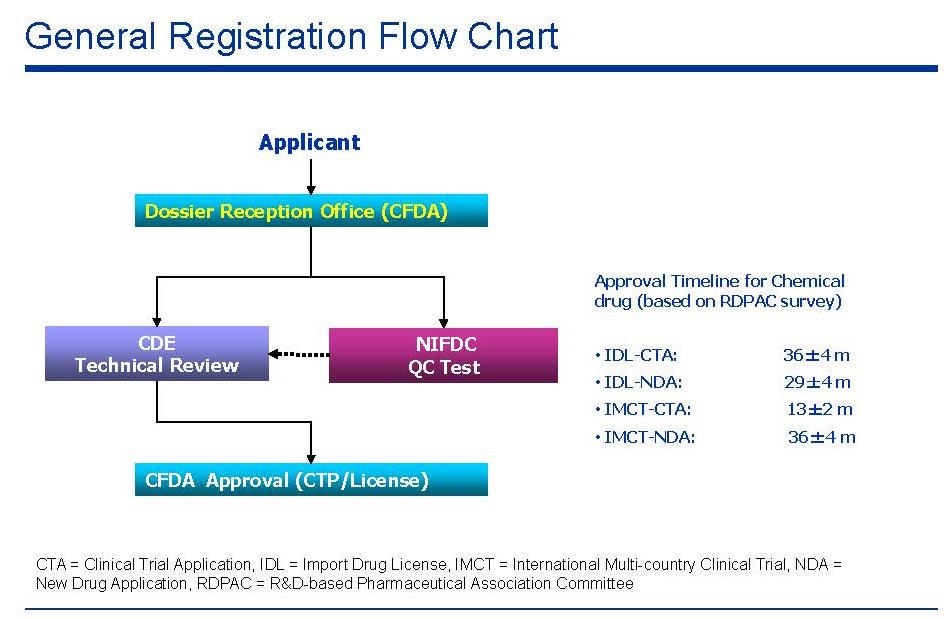
Introduction to the CTA & NDA process in China
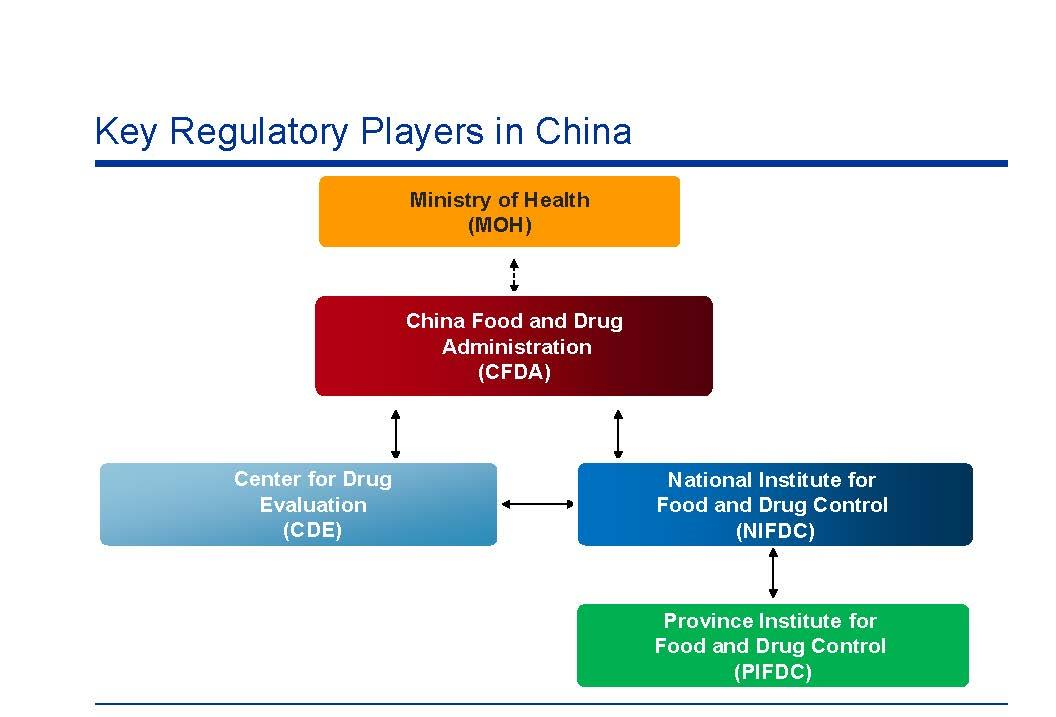
1. China Food and Drug Administration(CFDA)
Main responsibilities
- The implementation and enforcement of pharmaceutical rules and laws
- Regulations for all drug, food, supplement, medical device, and cosmetics in China
- The inspection, sales, research, and advertisement of drug, food, supplement, medical device, and cosmetics products
- Drug approvals