L2 Molecules and Cells in Animal Physiology
一、The organization of epithelial cells
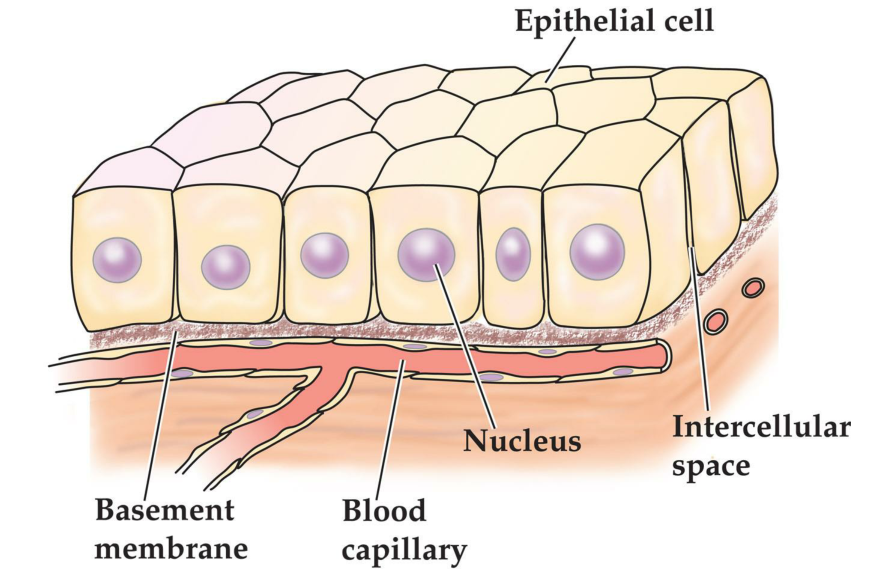
Generalized epithelium
1. Cellular Organization
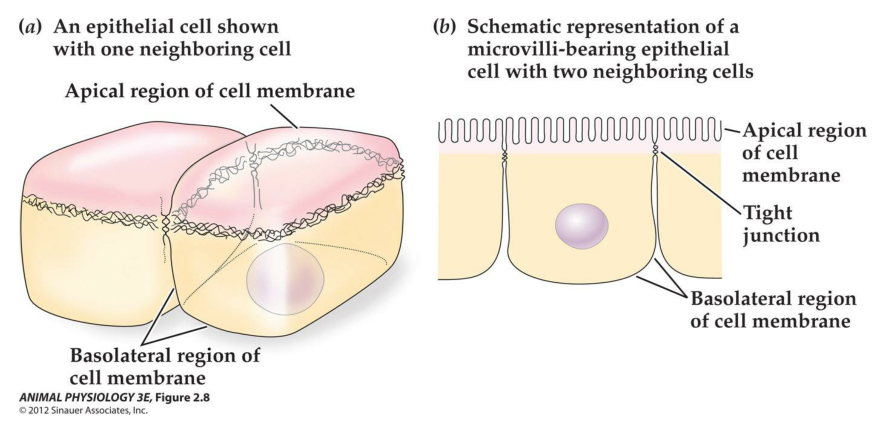
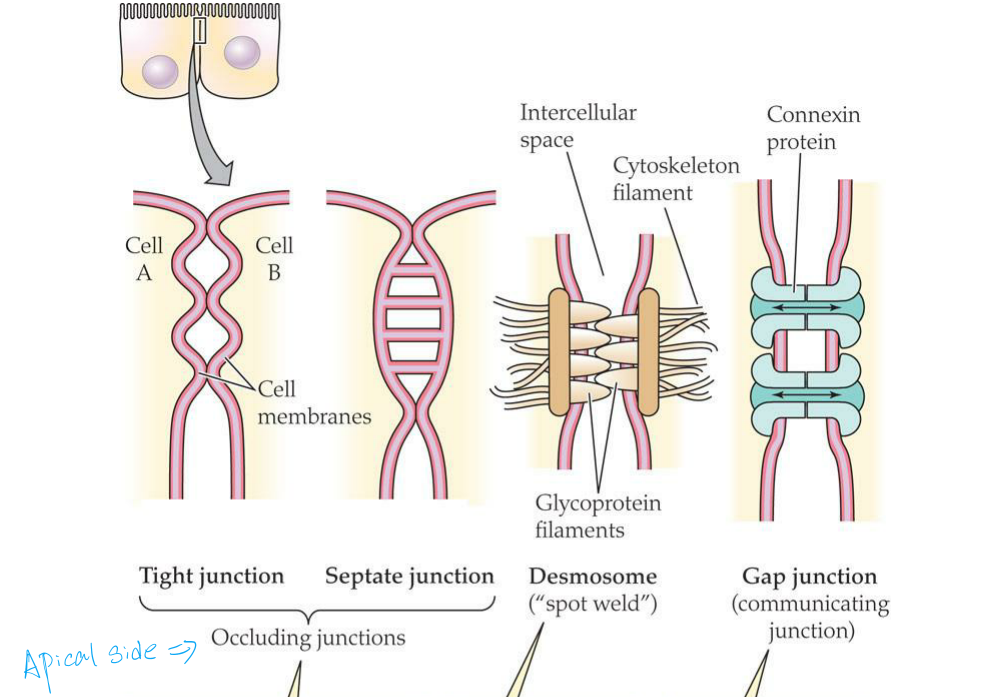
2. Cell Junctions
Tight junctions and septate junctions occlude the intercellular space between two cells because not only do the cell membranes meet or fuse at such iunctions, but also the junctions form continuous bands around cells
- In tight junctions, the cell membranes of the two cells make contact at ridges.
- (Occluding junctions: tight junction and septate junction)
A desmosome is a localized spot where the contact between cells is strengthened
A gap junction is a localized spot where the cytoplasms of two cells communicate through tiny pores, as symbolized by the double-headed arrows
3. Transcellular and paracellular paths across an epithelium
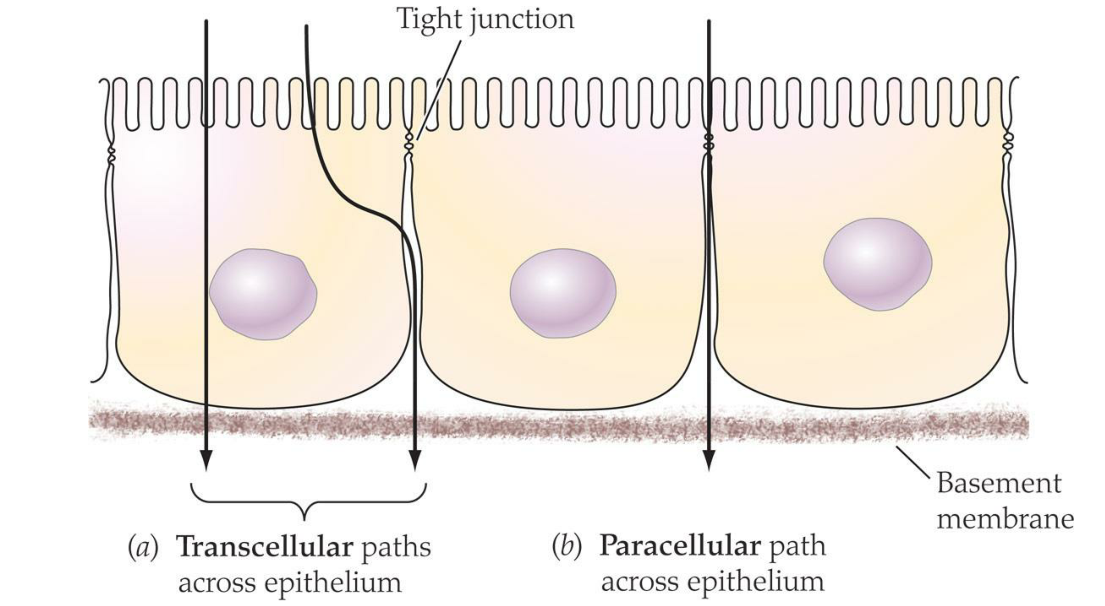
Materials following a transcellular path must cross both apical and basolateral cell membranes
Materials following a paracellular path must be able to move through the band of tight(or septate)junctions; in many epithelia, only very small molecules are able to do this, restricting the paracellular path to such molecules
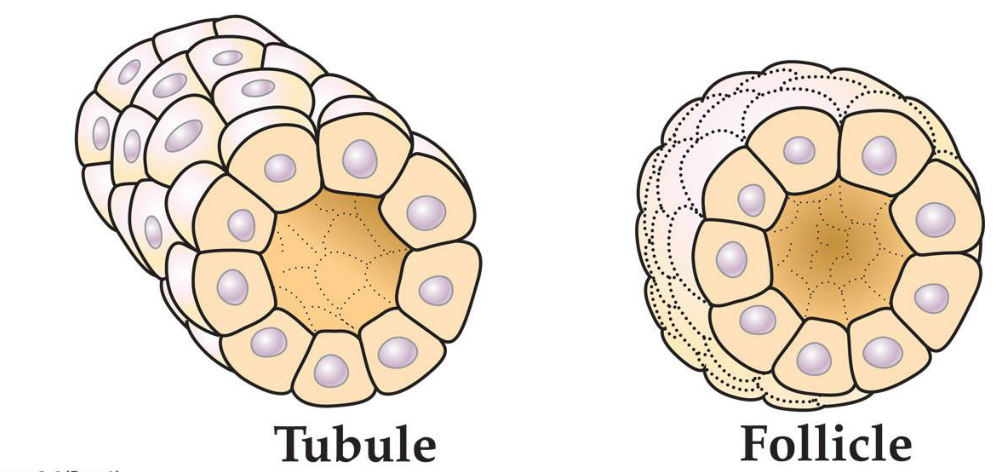
4. Epithelial cells can form tubules and follicles
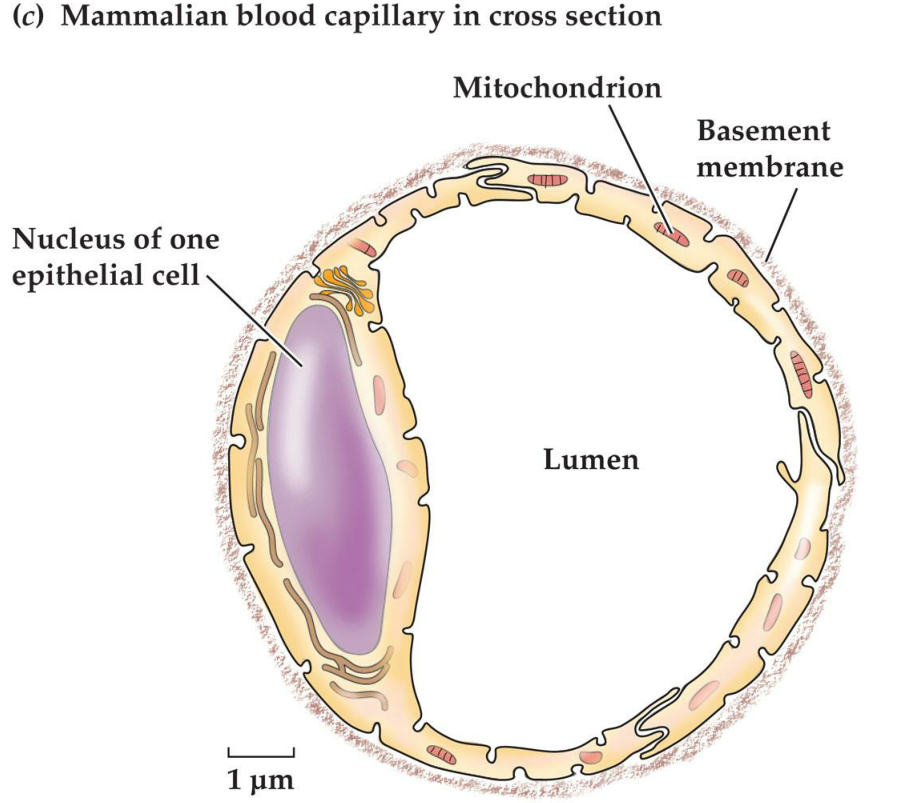
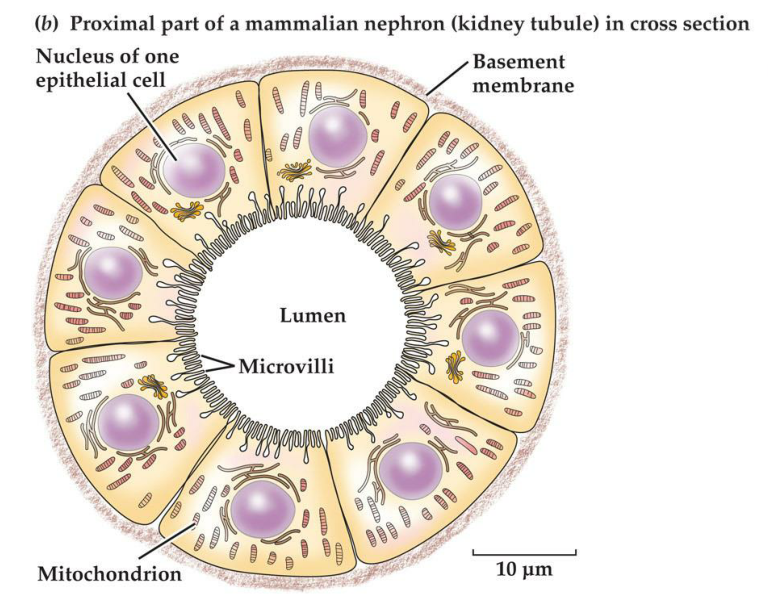
二、Cell membranes and Intracellular Membranes
The structure of a cell membrane:
- Outer cell membrane
- Intracellular membrane
- Endoplasmic reticulum
- Mitochondrion (mitochondria)
Membranes are very thin at 6-8 nm
1. Membrane Proteins
Integral membrane proteins (part of the membrane and can not be removed from the membrane without taking the membrane apart)
Peripheral membrane proteins (associated with the membrane and can be removed without affecting the membrane)
| Functional Type | Function performed (defining property) |
|---|---|
| Channel | Permits simple or quasi-simple diffusion of solutes in aqueous solution(see page 104)-or osmosis of water (see page 121)-through a membrane. A simplified view of a channel is that it creates a direct water path from one side to the other of a membrane(i.e. an aqueous pore)through which solutes in aqueous solution may diffuse or water may undergo osmosis |
| Transporter (Carrier) | Binds noncovalently and reversibly with specific molecules or ions to move them across a membrane intact. The transport through the membrane is active transport (see page 108) if it employs metabolic energy; it is facilitated diffusion(see page 108)if metabolic energy is not |
| Enzyme | Catalyzes a chemical reaction in which covalent bonds are made or broken (see page 41) |
| Receptor | Binds noncovalently with specific molecules and, as a consequence of this binding, initiates a change in membrane permeability or cell metabolism. Receptor proteins mediate the responses of a cell to chemical messages(signals )arriving at the outside face of the cell membrane(see page 58) |
| Structural Protein | Attaches to other molecules(e.g, other proteins)to anchor intracellular elements(e.g cytoskeleton filaments )to the cell membrane, creates Junctions between adjacent cells(see Figure 2.7), or establishes other structural relations. |
2. Membrane Phospholipids
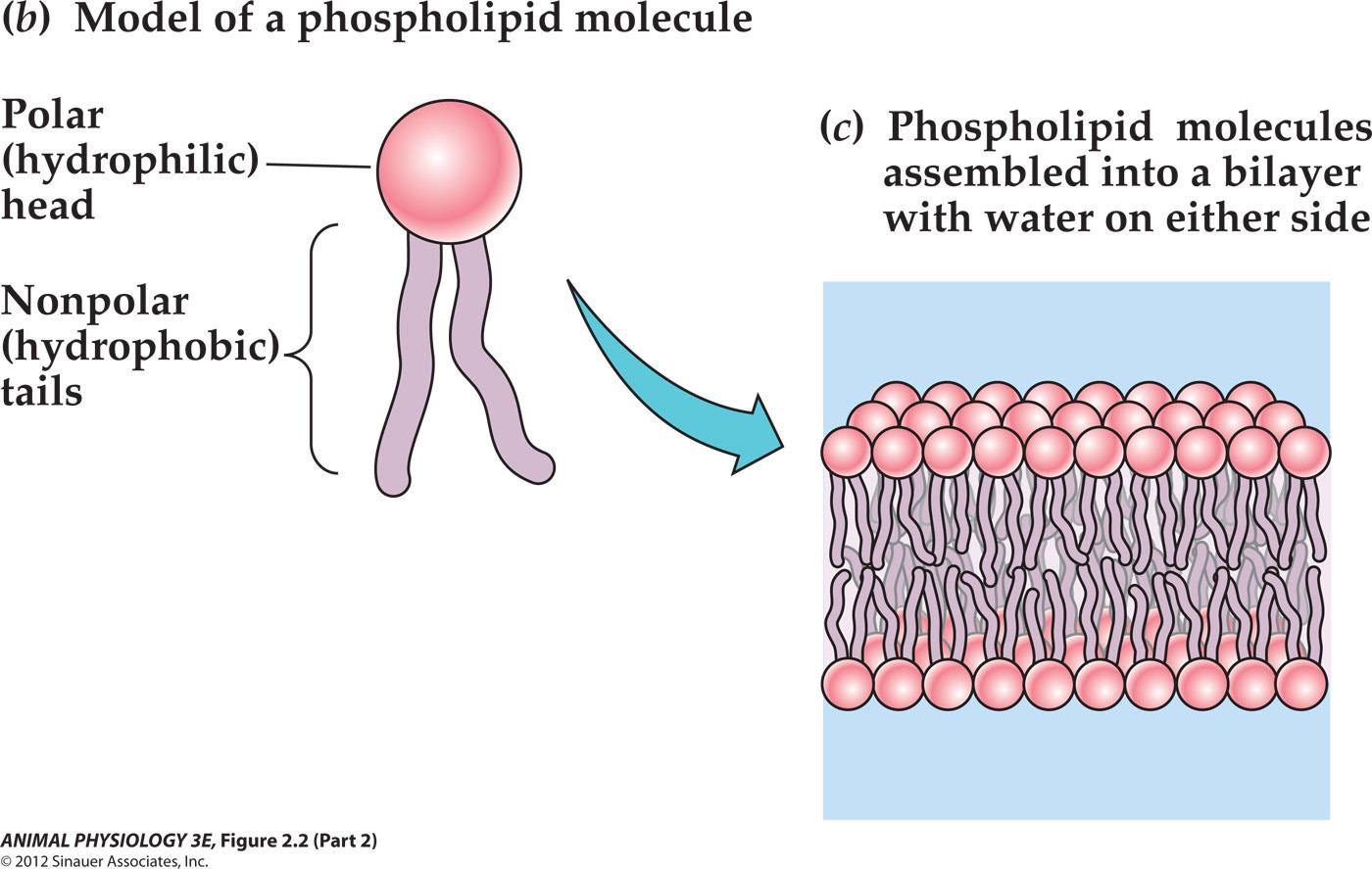
Phospholipid molecules assembled into a bilayer with water on either side
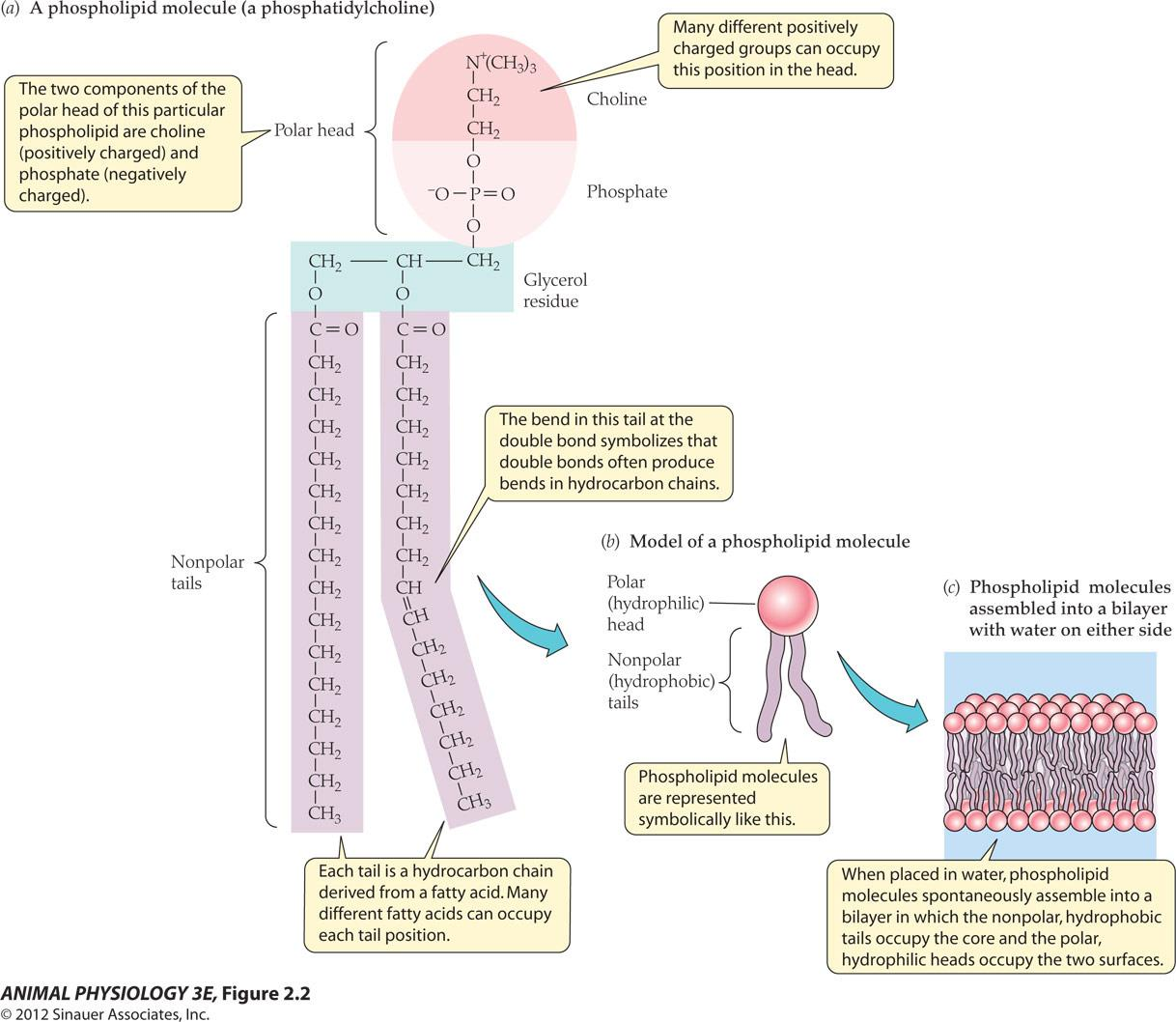
- Many different positively charged groups can occupy this position in the head.
- The two components of the polar head of this particular phospholipid are choline Positively charged)and phosphate(negatively charged).
- The bend in this tail at the double bond symbolizes that double bonds often produce bends in hydrocarbon chains.
- Each tail is a hydrocarbon chain derived from a fatty acid. Many different fatty acids can occupy each tail position.
- When placed in water, phospholipid molecules spontaneously assemble into a bilayer in which the nonpolar, hydrophobic tails occupy the core and the polar hydrophilic heads occupy the two surfaces.
The lipids of membrane are structured, diverse, fluidic and responsive to some environmental factors
Amphipathic(两亲) = each molecule consists of a polar part and a non-polar part (同时具有亲水和厌水部分).
- Fluidity: individual phospholipid moves within the leaflet of the membrane by diffusion
- Chemical saturation (fluidity) of the hydrocarbons of the phospholipid tail:
- Saturated: if the hydrocarbon contains no double bonds
- Unsaturated: if contains one or more double bonds
Nutrition – lipids – required for cell membrane and are the principal storage compounds of animals
Organic molecules contain carbon and hydrogen, nonpolar and hydrophobic
- Common lipids are fatty acids: hydrocarbon chains
- Triacylglycerols (fats and oils): three fatty acids molecules combined
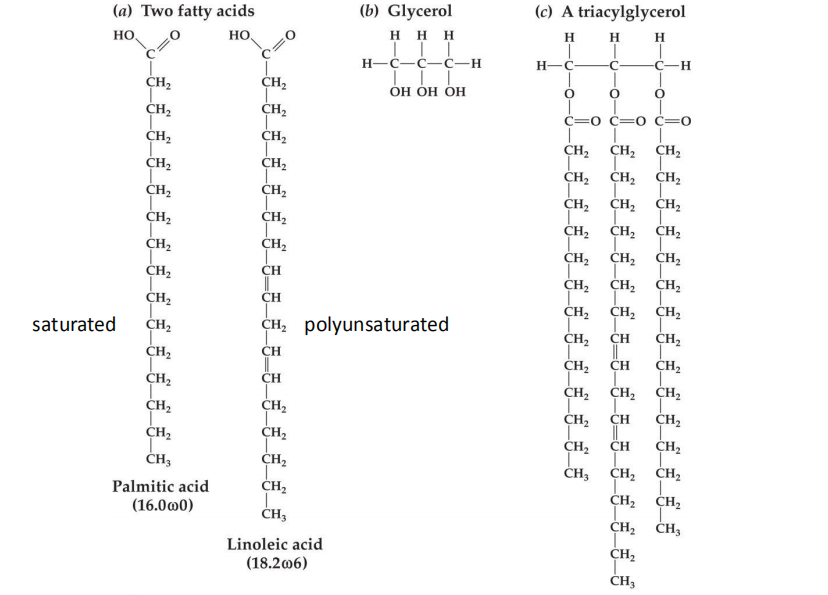
Fatty acids symbols
One example: 18.2 ω6
Fatty acids begin with carboxylic acid –COOH (alpha); ends with methyl group –CH3 (omega)
- 18: number of carbons in the fatty acids
- 2: number of double bounds
- Omega:6: position of the first double bond From its methyl end (-CH3)
Omega 3 and Omega 6 fatty acids Nutritional value
Functions of lipids
Principal components of the cell membrane
Storage compounds as energy stores, because they far exceed proteins and carbohydrates in their energy value per unit of weight.
lipids for terrestrial animals such as mammals and insects, to reduce the permeability of the integument to water.
- lipids in excessive of needs are stored in their body for future use.
Essential lipids: mammals lack of enzymes to create double bonds at the omega 3 and 6 positions. Therefore, omega 3, 6 must be obtained from outside sources
三、Receptions and Use of Signals by Cells
Extracellular signals initiate their effects by binding to receptor proteins
- Extracellular Signals are: mechanical stress, light, electrical current, eletro-magnetic wave, temperature, and other animals
Receptors for Extracellular Signals
- Ligand-gated channels
- G-protein-coupled receptors
- Enzyme/enzyme-linked receptors
- Intracellular receptors
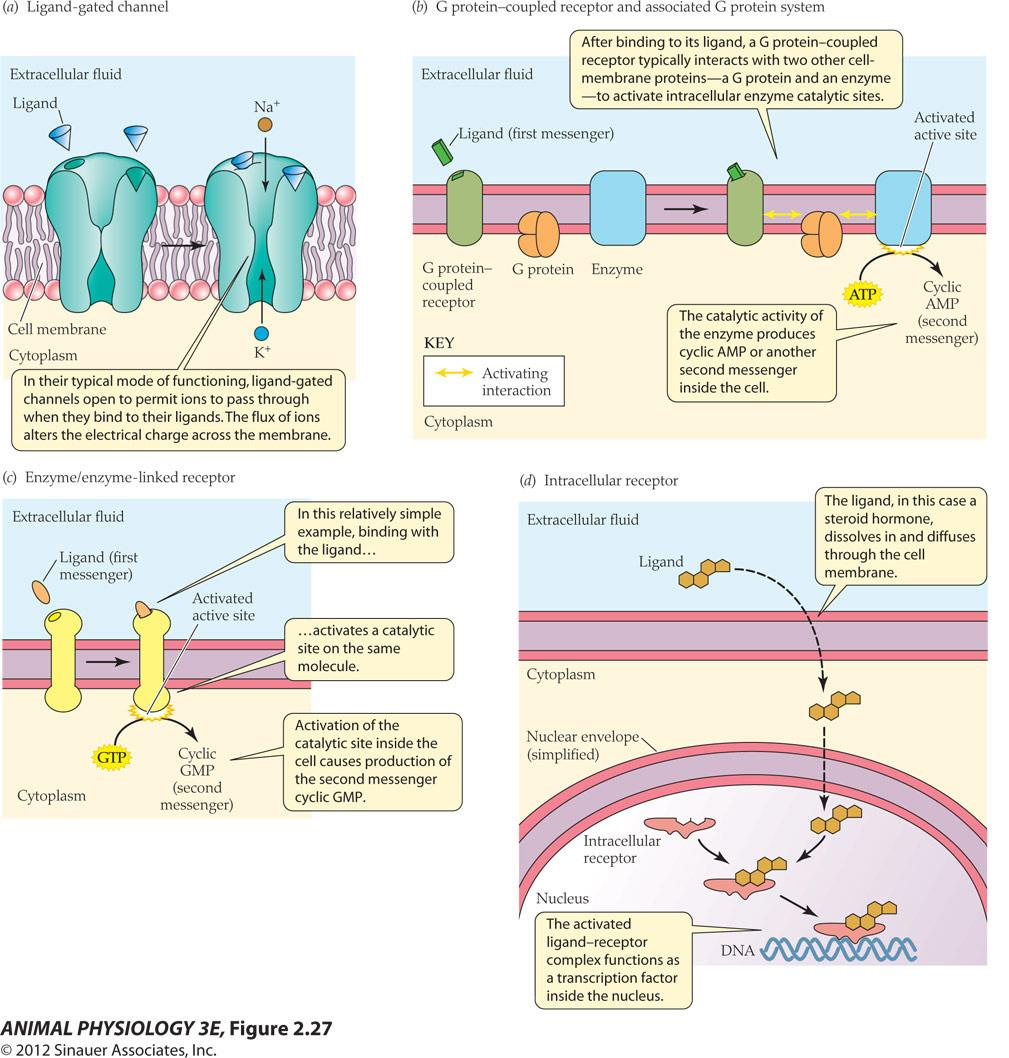
Ligand-gated channels
In their typical mode of functioning, ligand-gated channels open to permit ions to pass through when they bind to their ligands. The flux of ions alters the electrical charge across the membrane
Types:
Chemically gated channels (化学门控通道)
Ionotropic receptor (促离子型受体)
- Fast, directly cause permeability to ions
- Metabotropic receptor (代谢型受体 )
- Slow, long-lasting modulatory effects, through 2^nd^ messenger and G-proteins. ( GPCR are inherently metabotropic receptors)
- Voltage gated channels (mostly located on postsynaptic membrane and muscles cells)for example, Na+ , K+ , Ca++ channels
- Mechanically gated channels (stress caused change of membrane which activates mechanically gated channels such as non-selective Na, K, Ca channels.
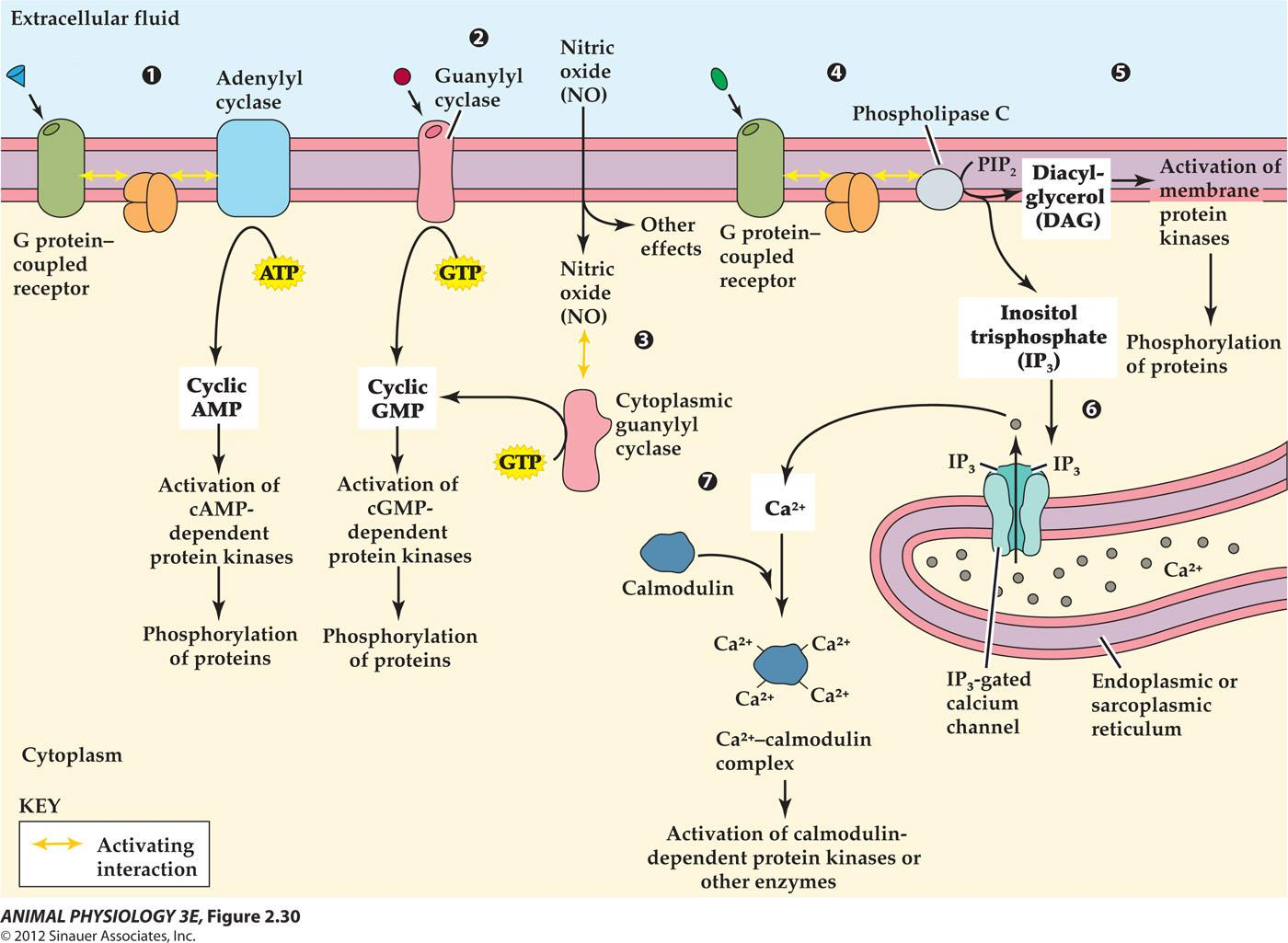
G-protein-coupled receptors
After binding to its ligand, a G protein-coupled receptor typically interacts with two other cell- membrane proteins-a G protein and an enzyme -to activate intracellular enzyme catalytic sites
The catalytic activity of the enzyme produces cyclic AMP or another second messenger inside the cell
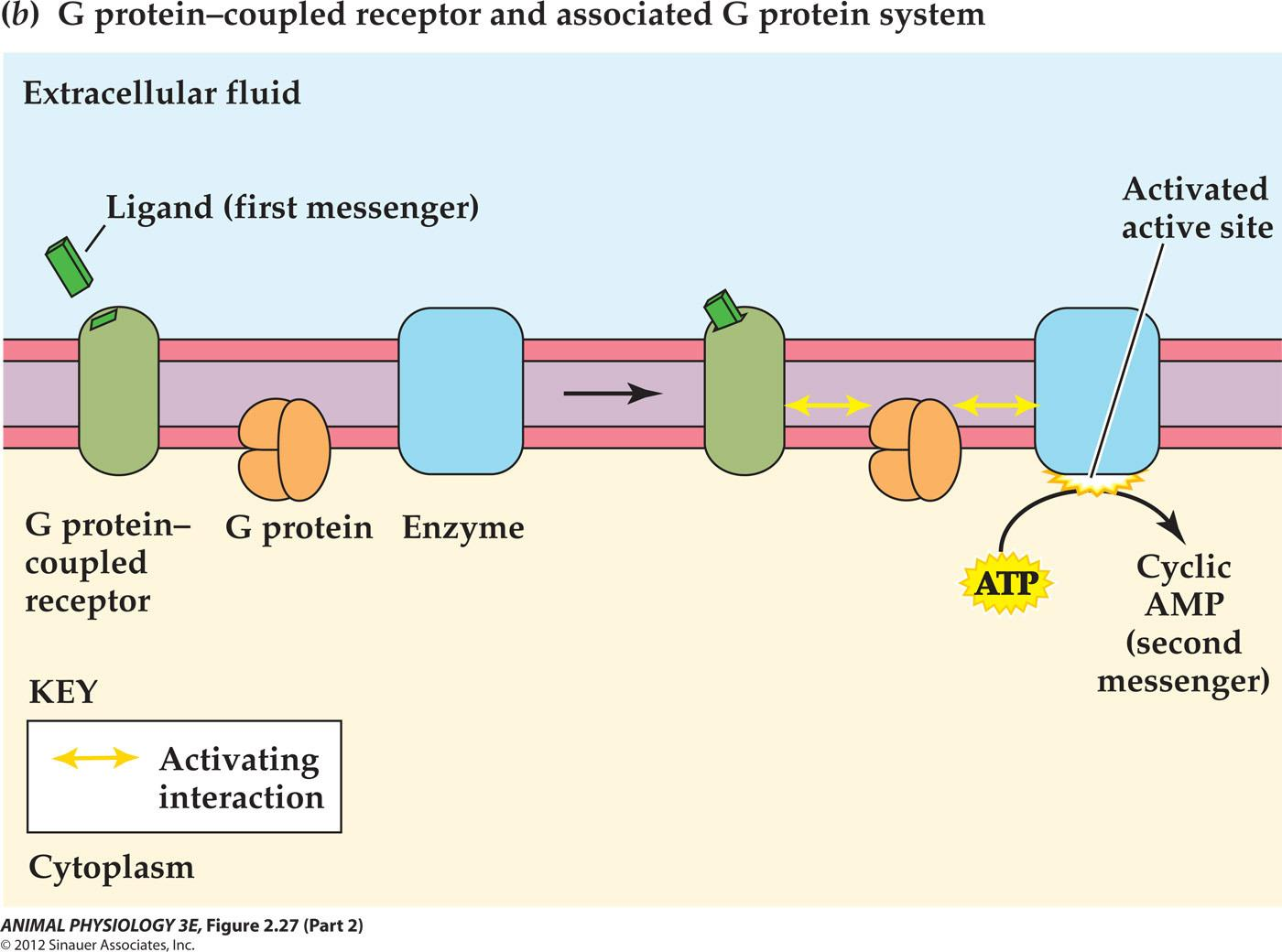
Four partners are involved in this reaction:membrane receptor, G protein, G protein effector, 2nd messenger
- G protein-linked receptor: 300 kinds, mAch receptor, 5-HT, small peptide hormones, etc. all have common features: long extracellular N-terminus, 7 $\alpha$-helical trans-membrane domains,intracellular C-terminus.
- G protein: Guanine nucleotide-binding protein
- G proteins were discovered when Alfred G. Gilman and Martin Rodbell investigated stimulation of cells by adrenaline. They found that, when adrenaline binds to a receptor, the receptor does not stimulate enzymes directly. Instead, the receptor stimulates a G protein, which stimulates an enzyme. An example is adenylate cyclase, which produces the second messenger cyclic AMP. For this discovery, they won the 1994 Nobel Prize in Physiology or Medicine.
- G protein effector: it catalyzes the production of2 nd messengers and activation of ion channels. Such as
- adenylyl cyclase (AC);
- phospholipase C(PLC),
- phosphodiesterase (PDE)
- phopholipase A2.
- 2^nd^ messenger: cAMP, IP3, DAG, cGMP, Calcium
Enzyme/enzyme-linked receptors
Activation of the catalytic site inside the cell causes production of the second messenger cyclic GMP.
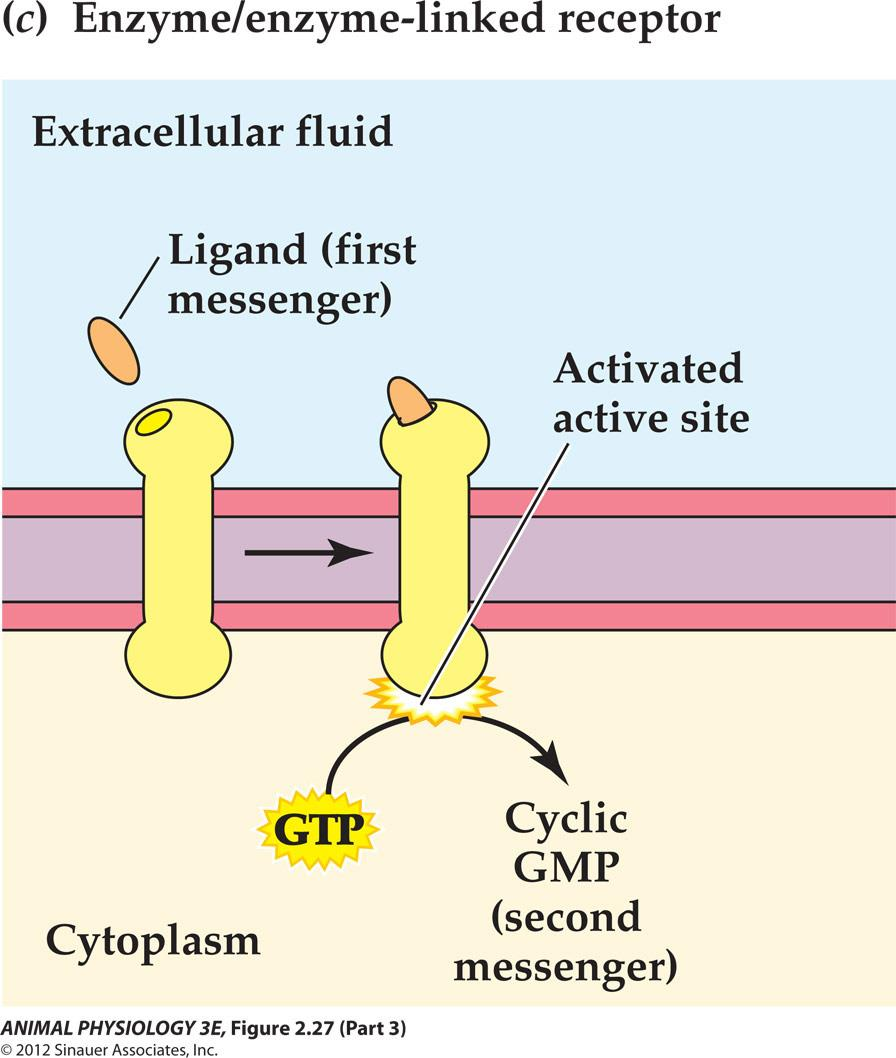
The hormone called atrial natriuretic peptide (ANP) acts on target cells in the kidney to increase Na+ excretion by way of this sort of receptors. [ANP 心钠素]
Receptor tyrosine kinases (insulin, growth factors receptors)
Intracellular receptors
The ligand, for example, a steroid hormone dissolves in and diffuses through the cell membrane
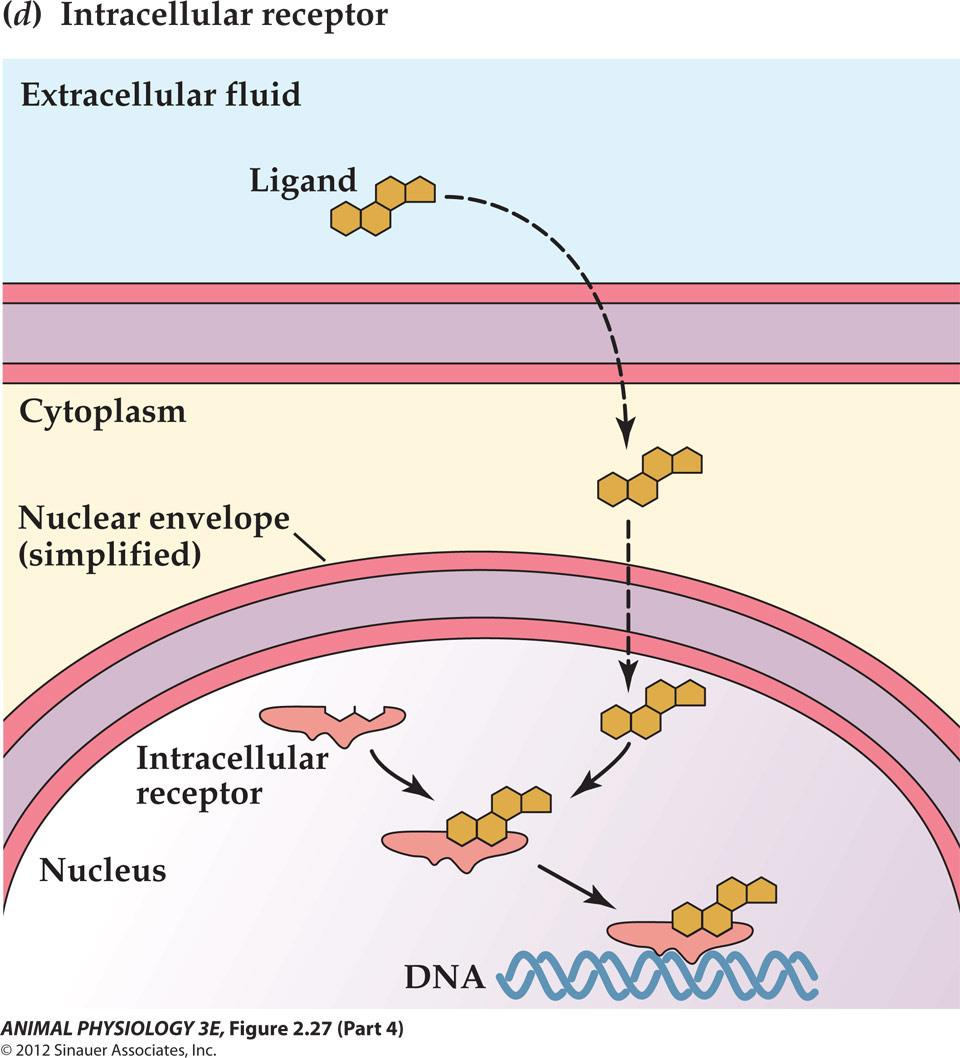
Steroid hormones (estrogen), thyroid hormones,retinoic acid, vitamin D and the gas nitric oxide (NO)
The receptors are often localized on cytoplasm or nucleus
After activation of these receptors, they interact with DNA to activate specific primary-response genes.[hormone-receptor complex is a transcription factor to bind to the specific promoter-enhance region of the DNA to alter gene expression.]
1. Cell signal transduction often entails sequences of amplifying effects
肾上腺素 [Epinephrine or adrenaline]
- Epinephrine activation of glycogen breakdown to produce glucose in the liver
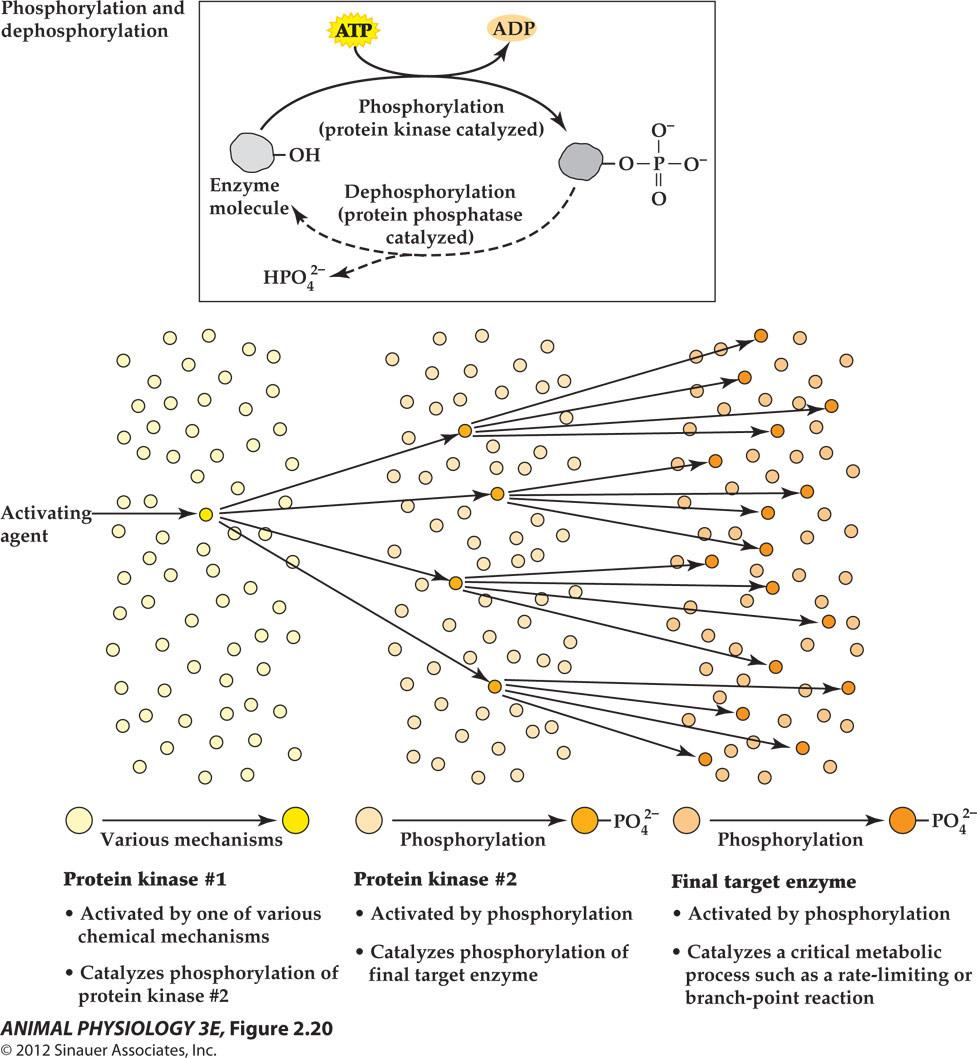
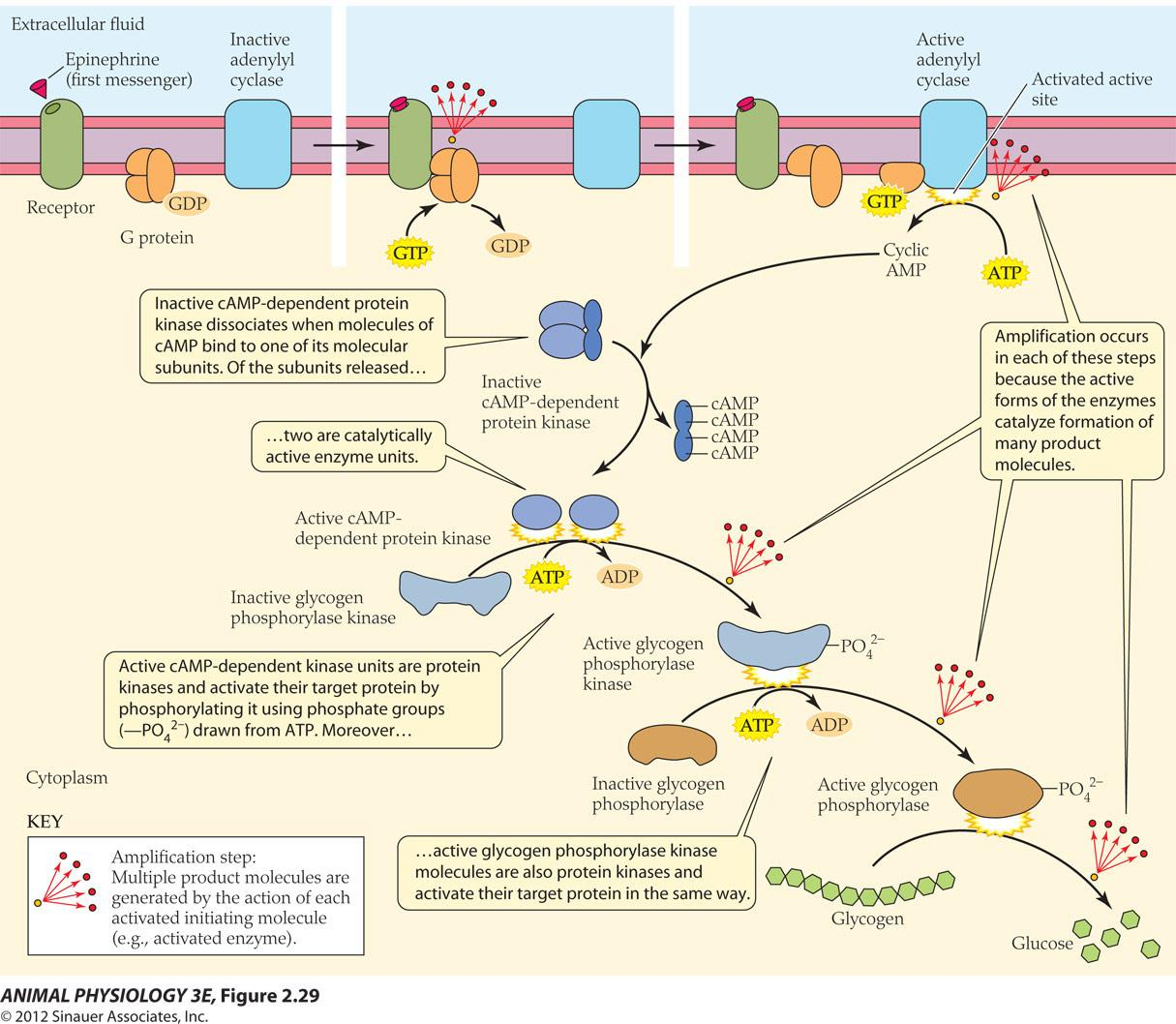
The cumulative effect of all the amplifications in this cell signal transduction pathway resulted in the effect of minute quantity of epinephrine to cause a flood of blood glucose(10,000 fold).
四、The Ionic Basis of Membrane Potentials
The Structure of Neuro Cells
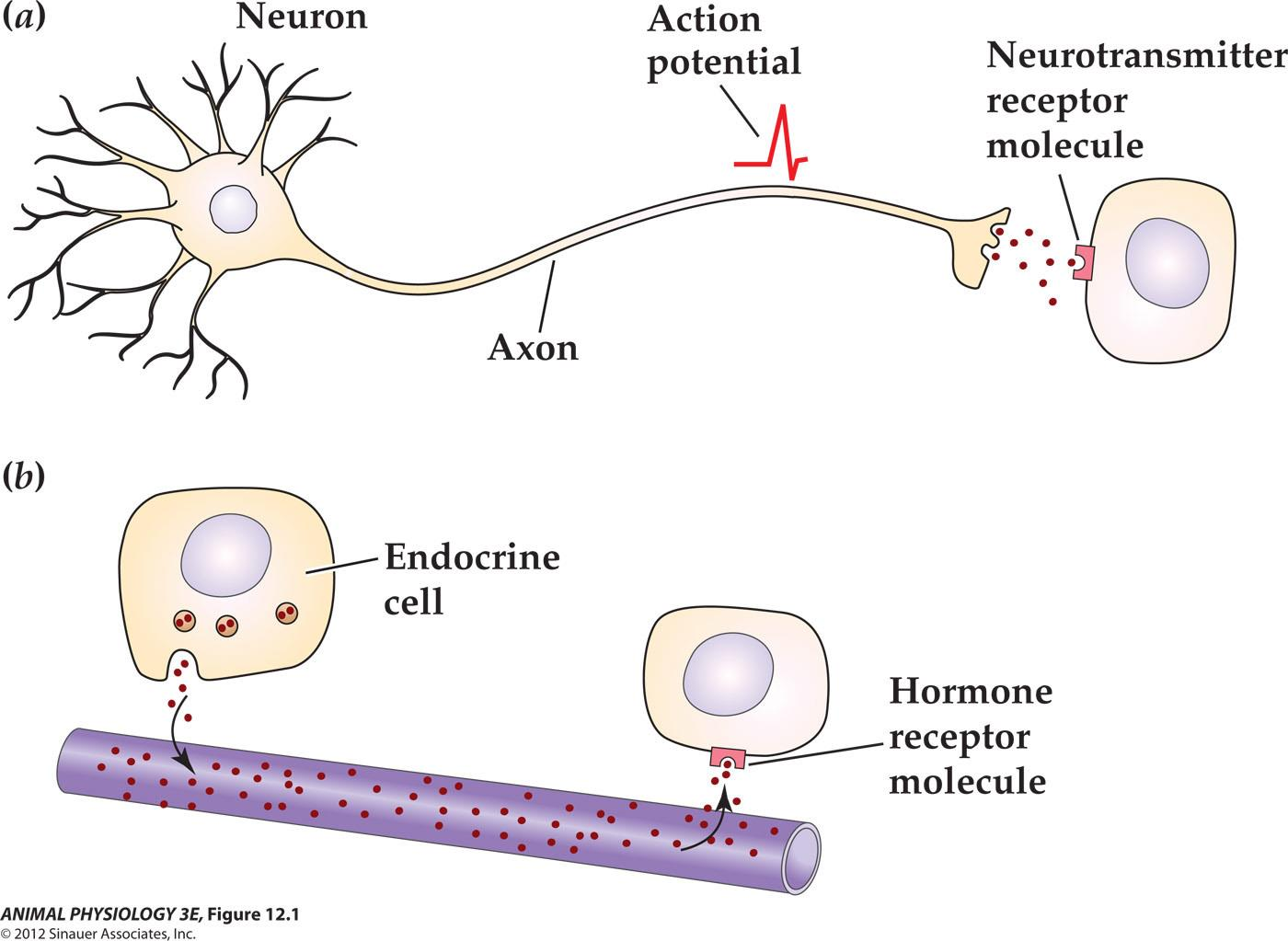
Cell membrane has passive electrical properties
Cell membrane exhibits:
- Resistance (R, in ohms, 电阻)[cell membrane is impermeable to ions and they have to go through restrictive ion channels]
- Capacitance (C, in farads, F, 电容) [Capacitance is the ability of a body to store an electrical charge; the lipid bilayer acts as an insulating layer between the conducting fluids on either side of the membrane]
Trans-membrane potential
- Resting membrane potential (静息电位)
- Action potential (动作电位)
All known cells, the polarity of the resting membrane potential is inside-negative.
By convention, the membrane potential is given as the inside value with the outside considered ZERO
What will happen to the resting membrane potential is we insert a second microelectrode and generated a pulse of electric current?
- Depolarization [a decrease in the absolute value of the membrane potential towards Zero]
- e.g. -65mV $\rarr$ -25mV
- Hyperpolarization [an increase in the absolute value of the membrane potential away from zero].
- e.g. -65mV $\rarr$ -85mV
Apply a current flows Outward across the membrane To cause a depolarization of the Membrane potential
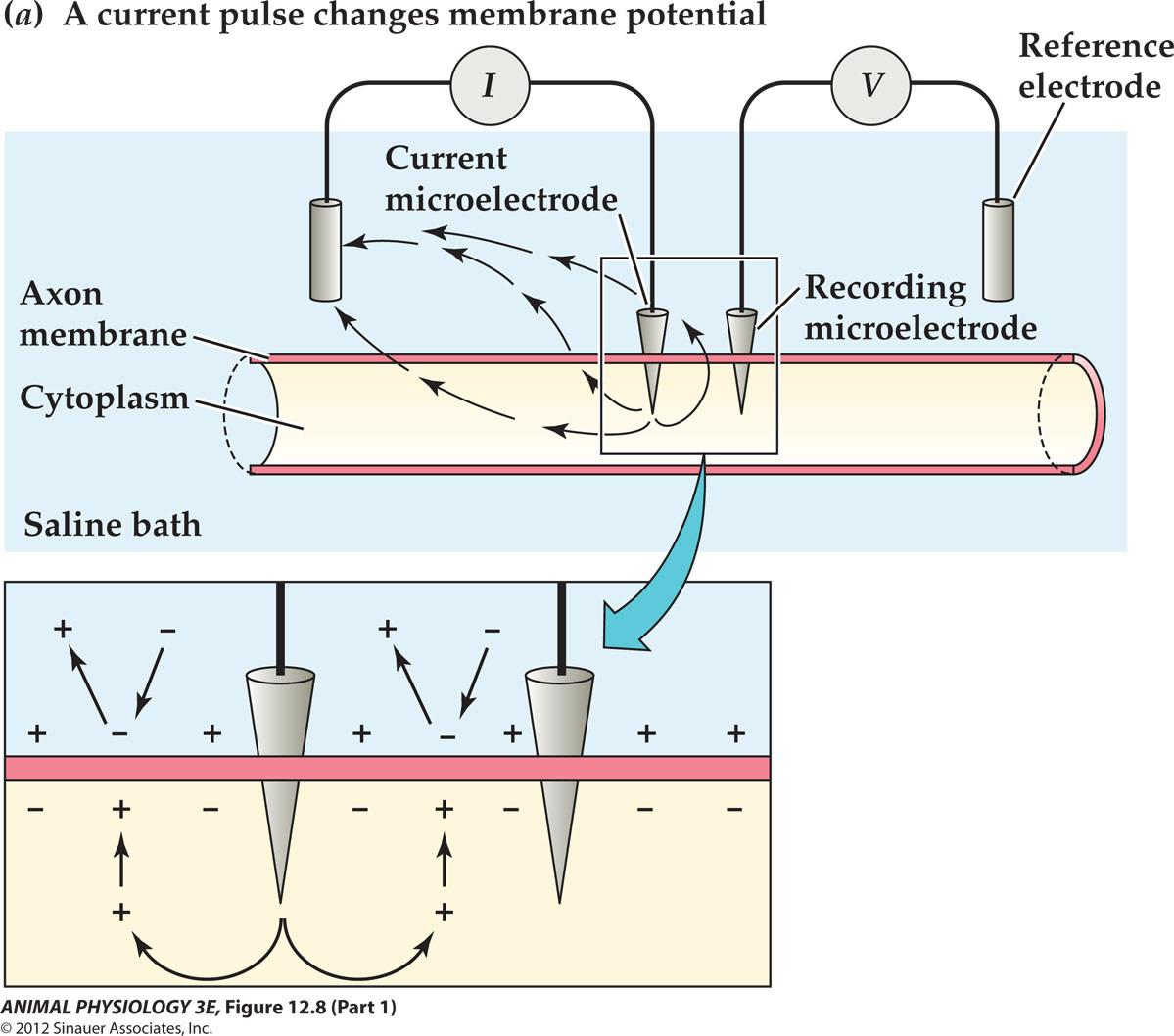
1. Some Important Constants
Time Constant: $\tau$
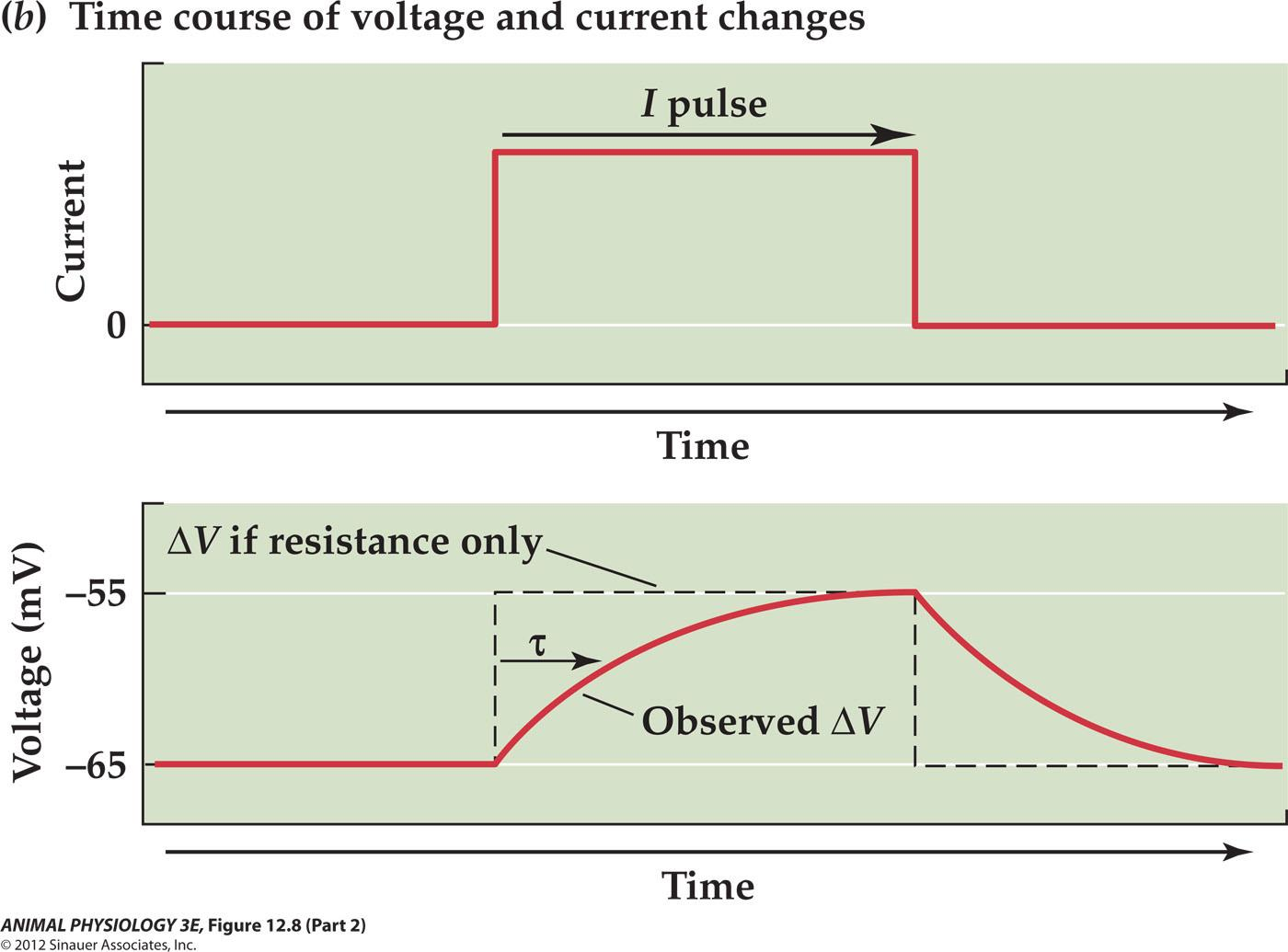
$\tau$ is the time constant tau is the time required for the observed ∆V to reach63% of the maximum
Ohm’s law:
- Graded potential ∆V=IR
- time constant tau = RC
- (for many cells, the time constant is in the range of 2-20 ms)
$\tau$ is corresponding to time, resistance and capacitance
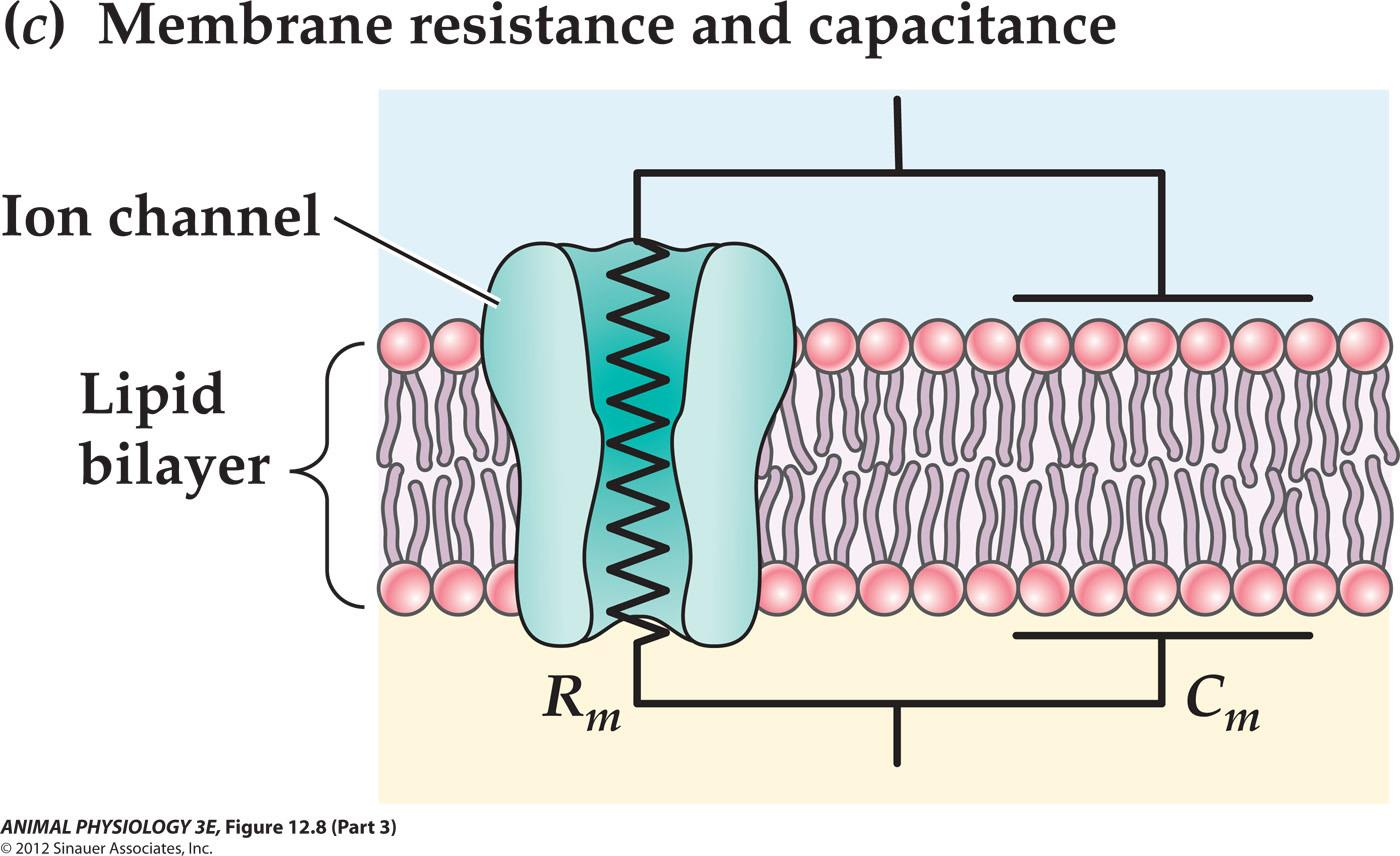
The resistance corresponds to ion channels through which ions can flow
The lipid bilayer corresponds to the dielectric layer of a capacitor that separates charges on its surface
Membrane Length Constant: $\lambda$
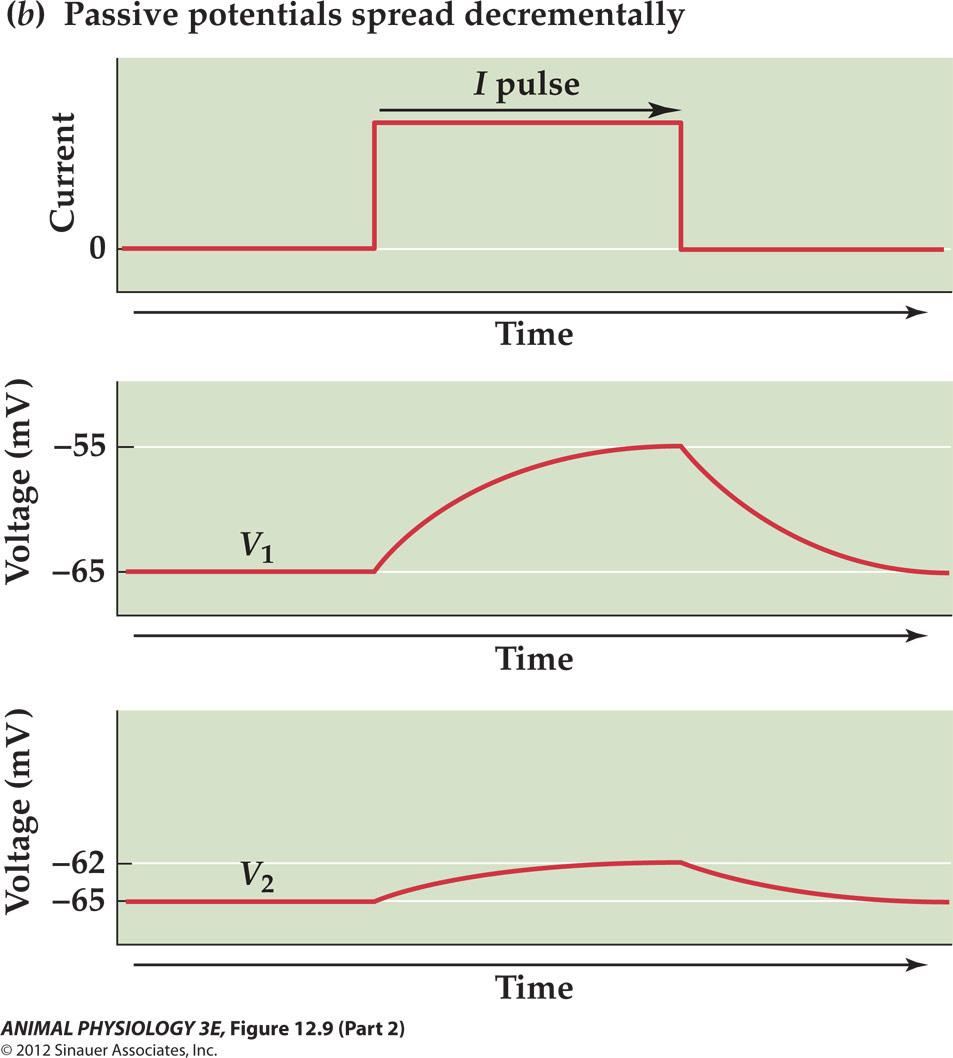
Passive spread (decremental spread) or electronic conduction will cause the signal decay
(1) The Signal Decay
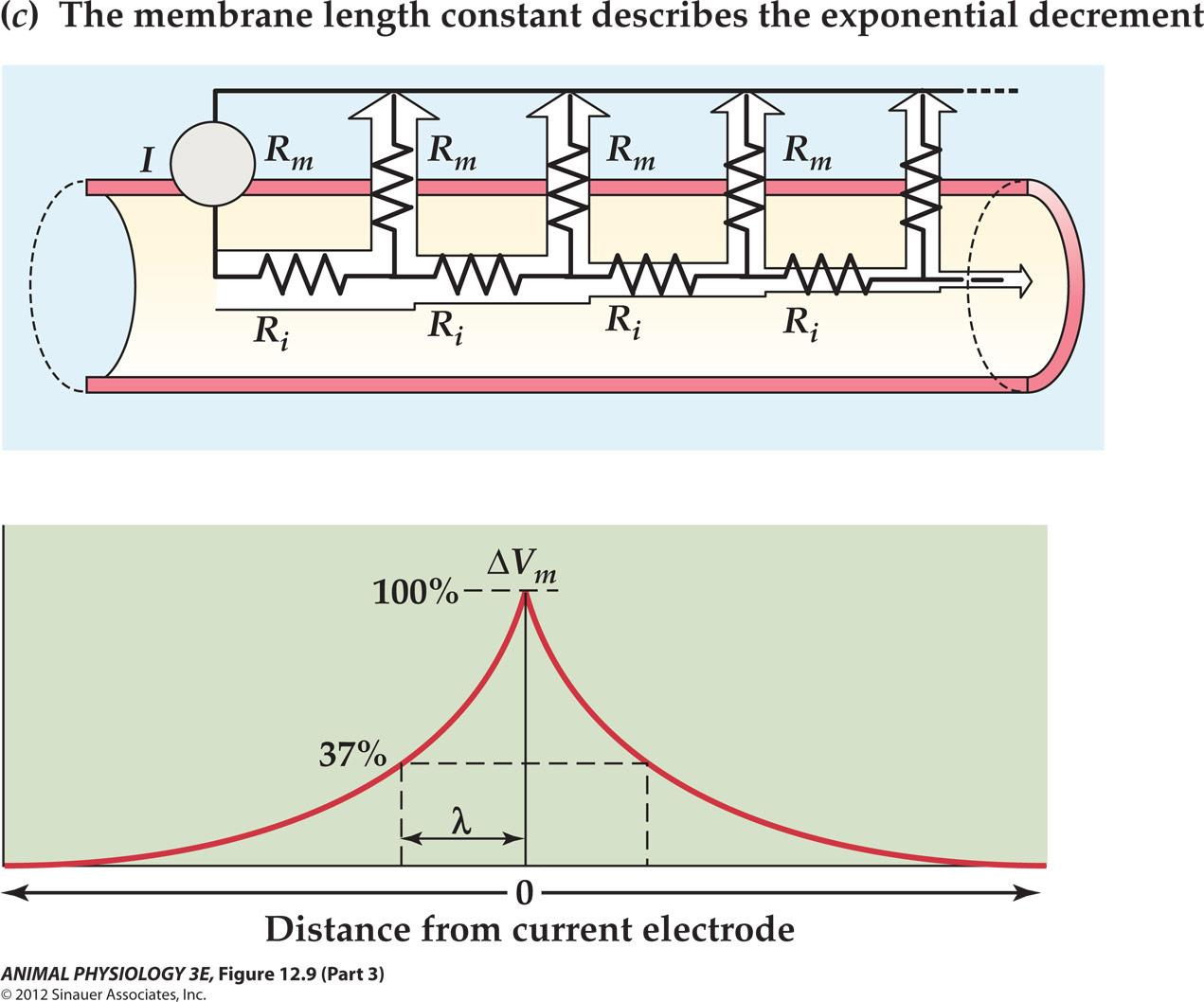
Because most of the current flows through shorter paths of lower resistance, less and less current is available to change the membrane potential at greater distance from the source
(2) The $\lambda$
The descremental spread of graded potential is referred to as electronic conduction
The steepness of this decrease with distance is described by the membrane length constant (λ: lambda) which represents the distance which the decaying voltage change is 37% of its value at the origin.
Passive electrical properties of a neuron or any other type of cells are similar to underwater cable, so these are often called cable properties.
- The equations are called cable equations
- Membrane resistance (R
m), membrane capacitance (Cm), resting membrane potential (Vm) and the related time constant (τ) and length constant (λ)
2. Electrochemical equilibrium
Selective permeability of a membrane gives rise to a membrane potential
Electrochemical equilibrium(there is no net movement of ions)
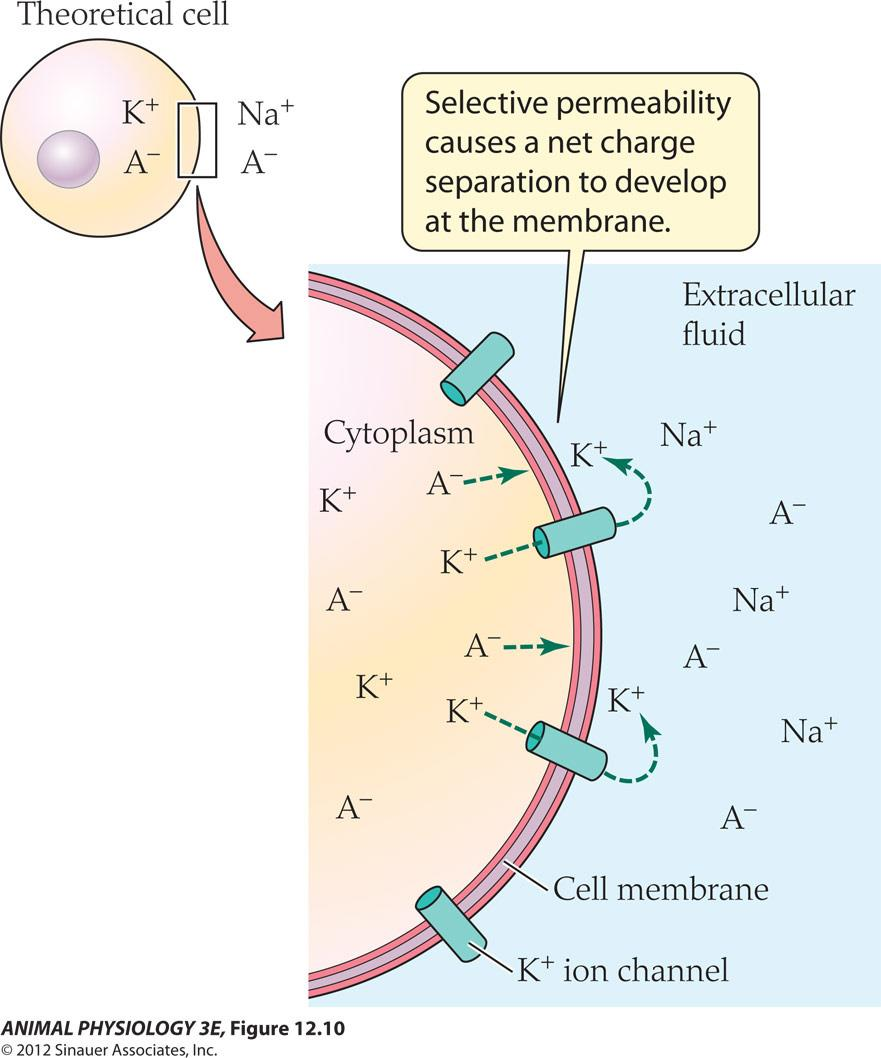
Cell membrane is impermeable to ions and ions must go through membrane Channels.(we stipulate that cell membrane is only permeable to K+)
All cells maintain low intracellular concentrations of Na+ and Cl- and high concentrations of K+ and nonpermeating anions (A-) within intracellular fluids, relative to extracellular fluid
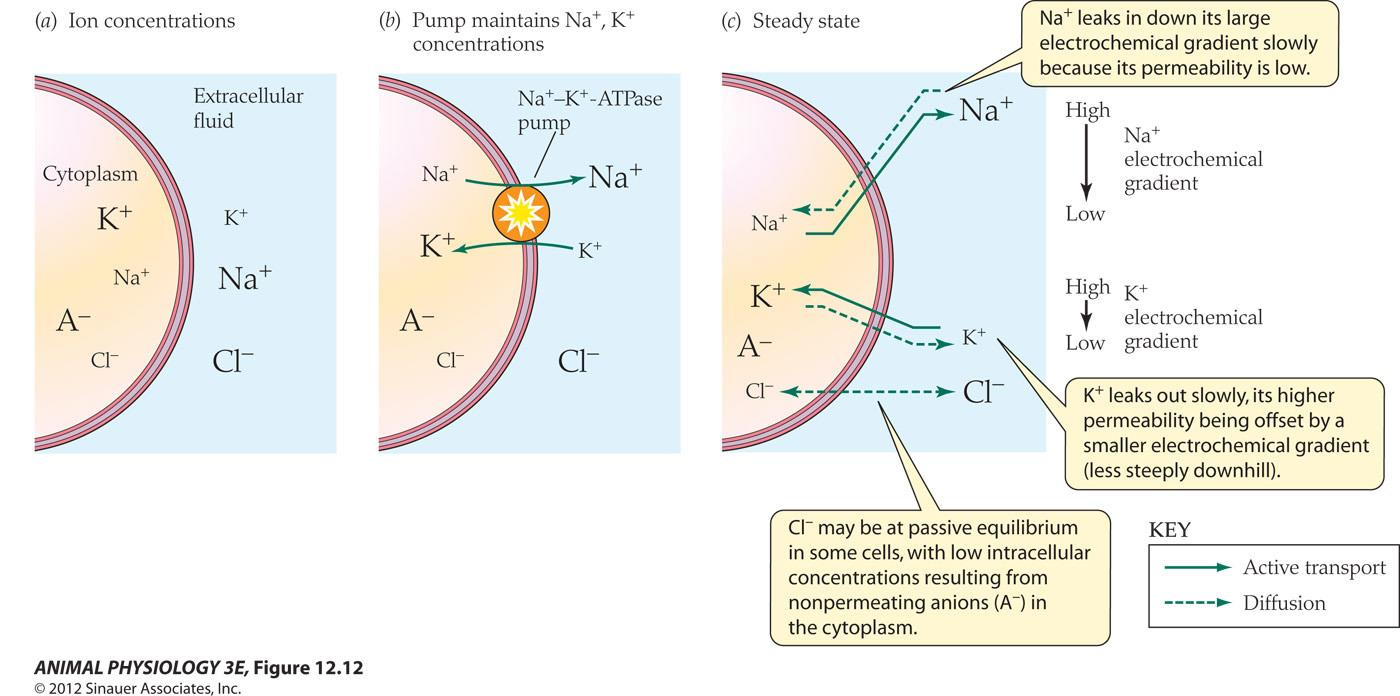
- The Na+-K+-ATPase pump to counteract leakage channels
- Na leaks in down its large electrochemical gradient slowly because its permeability is low
- K+ leaks out slowly, its higher permeability being offset by a smaller electrochemical gradient (less steeply downhill)
- Cl may be at passive equilibrium in some cells, with low intracellular concentrations resulting from nonpermeating anions (A-) in the cytoplasm
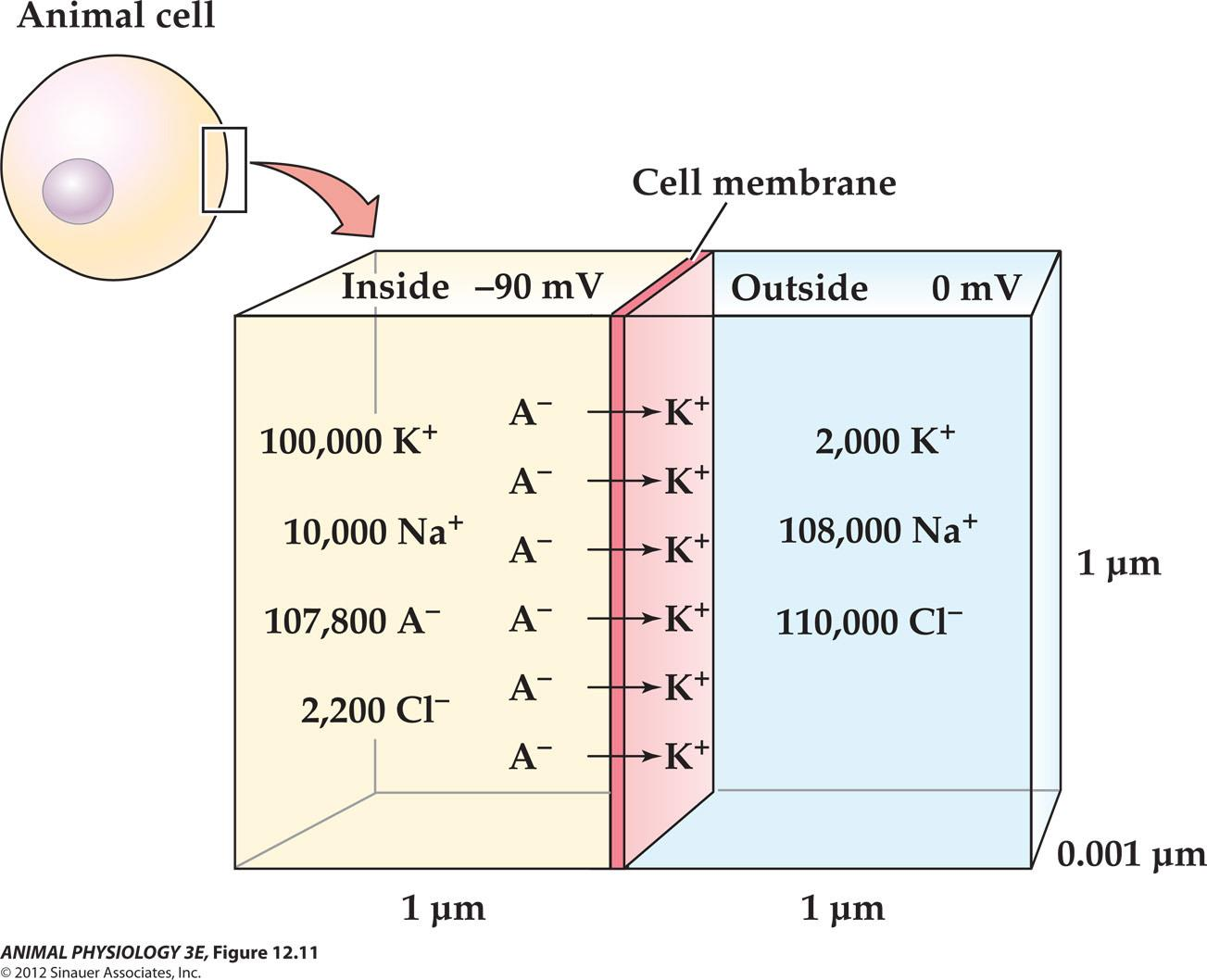
Remember: the movement of only a few Ions in in a region can establish or change A membrane potential without disrupting The overall charge neutrality of the intracellular And extracellular fluids
Here only six pairs of ions need to sit on the membrane and charge its capacitance to produce a membrane potential of -90 mV.
Nernst Equation
Electrochemical equilibrium can be calculated to reflect the resting membrane potential
Consider an ion at a given valence(化合价) at a given temperature (K+, a monovalent cation,at 18 °C; or at 37 °C)
- E (in mV) = 58log
10Cout/Cinor (18°C) - E (in mV) = 61log
10Cout/Cin(37°C) - (C
out= 10mM, Cin= 100mM at 18°C, E = 58log10(0.1) = -58 mV)
Action potentials
Voltage-dependent, ALL-or-None electric signals
A momentary reversal of membrane potential from -65 mV (inside-negative) to about +40mV (inside-positive), a change of about 100mV and lasting about 1 ms.
Action potential is triggered by depolarization of the membrane that reaches a critical value of depolarization, the voltage threshold
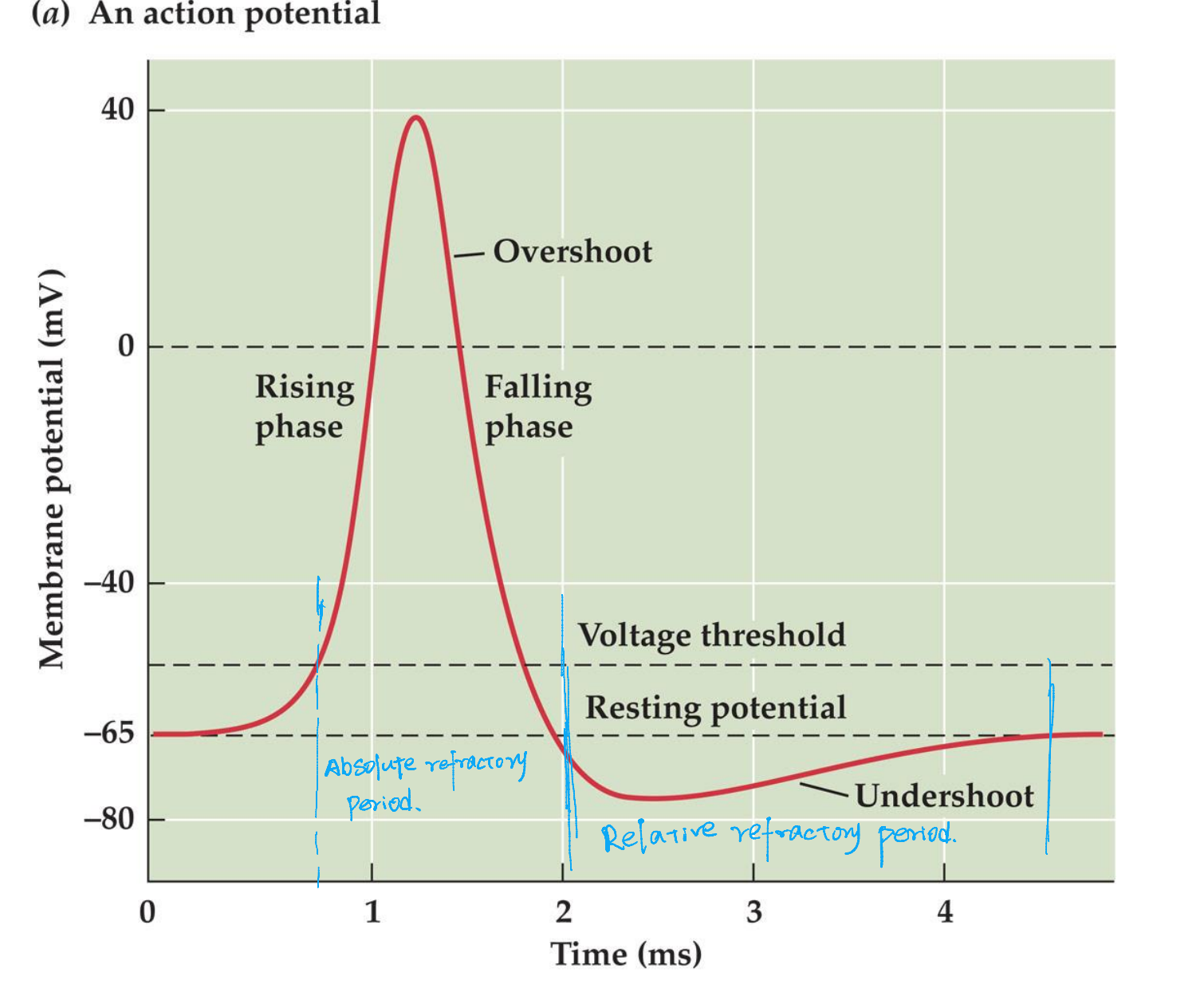
Important features:
- Hyperpolarization can not induce action potential
- Action potentials are All-or-None
- Action potential can not be generated immediately after another one (I ms is required, absolute refractory period)
- Because of the all-or-none property and the succeeding refractory period, impulses can not summate, instead, super-threshold depolarizing current elicit a train of discrete action potentials
- Action potential, once initiated, propagates along the axon without a decrease in amplitude and at a constant velocity
- Action potential results from intense, localized increase in permeabilities to specific ions –increase that are both voltage and time dependent!
- The permeability increase are selective for specific ions, first sodium and then potassium
Summary of action potential resulting from three overlapping permeability changes:
- Increase in permeability to Na+, caused by rapid opening of voltage-gated Na+ channels
- Decrease in permeability to Na+, caused by inactivation of Na+ channels
- Increased in permeability to K+, caused by the slower opening of voltage-gated K+ channels
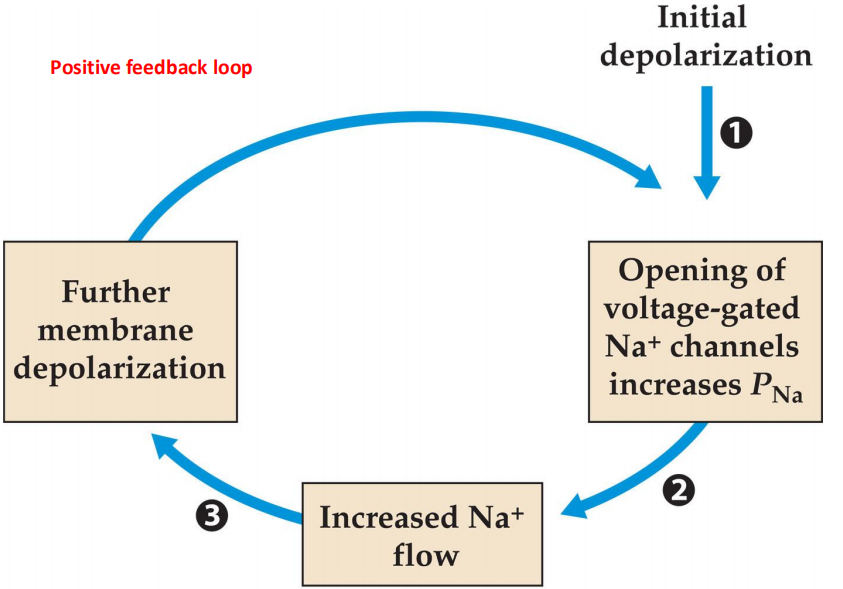
1. The Hodgkin cycle