L10 Contractile proteins and molecular motors
一、Contractile proteins and molecular motors
They are:
• Large complex structures
• Related to motion
• Cytoskeleton and motor proteins
Cytoskeletons
二、Actin and Myosin
The major proteins in muscle tissue are actin and myosin; however, actin and myosin are also found in many other types of cells and are involved in several kinds of cellular and intracellular motions.
1. Motor Proteins
The mechanical work of motion is carried out by motor proteins.
This motion may involve the whole organism, parts thereof, cells, or subcellular constituents.
Protein conformational change mediated by the binding, hydrolysis, and release of ATP is a key feature of motor protein function.
2. Actin-Myosin Contractile System And Some Other System
Actin-Myosin Contractile System
Almost all muscles, as well as some other contractile systems, are based on the inter-action of two major proteins, actin and myosin. We often refer to these systems as actin–myosin contractile systems.
Others
Certain kinds of directed motions exist—motions of individual cells and parts of cells—that do not depend on the
actin–myosin system at all but use other protein mechanisms
Example:
The beating of cilia and flagella and the movement of chromosomes and organelles within cells are accomplished by the interactions of a number of proteins with microtubules, filamentous structures made of a protein called tubulin.
(1) molecular motors
These include ATP synthase , which uses a proton gradient to drive ATP synthesis in mitochondria, and RNA polymerase , which uses nucleoside triphosphate hydrolysis to move along and untwist a DNA template.
The common future of movement system
The biological systems that produce movement share one common feature—they hydrolyze ATP.
Thus, proteins can be energy transducers.
Actin 肌动蛋白
Under physiological conditions, actin exists as a long, helical polymer (filamentous actin, or F-actin) of a globular protein monomer (G-actin).
The actin is ATPase, catalyze ATP hydrolysis.
Actin filament is in equilibrium.
1. G-actin
The binding of ATP by a G-actin monomer leads to polymerization; the ATP is subsequently hydrolyzed, but the ADP is held in the actin filament.
2. F-actin
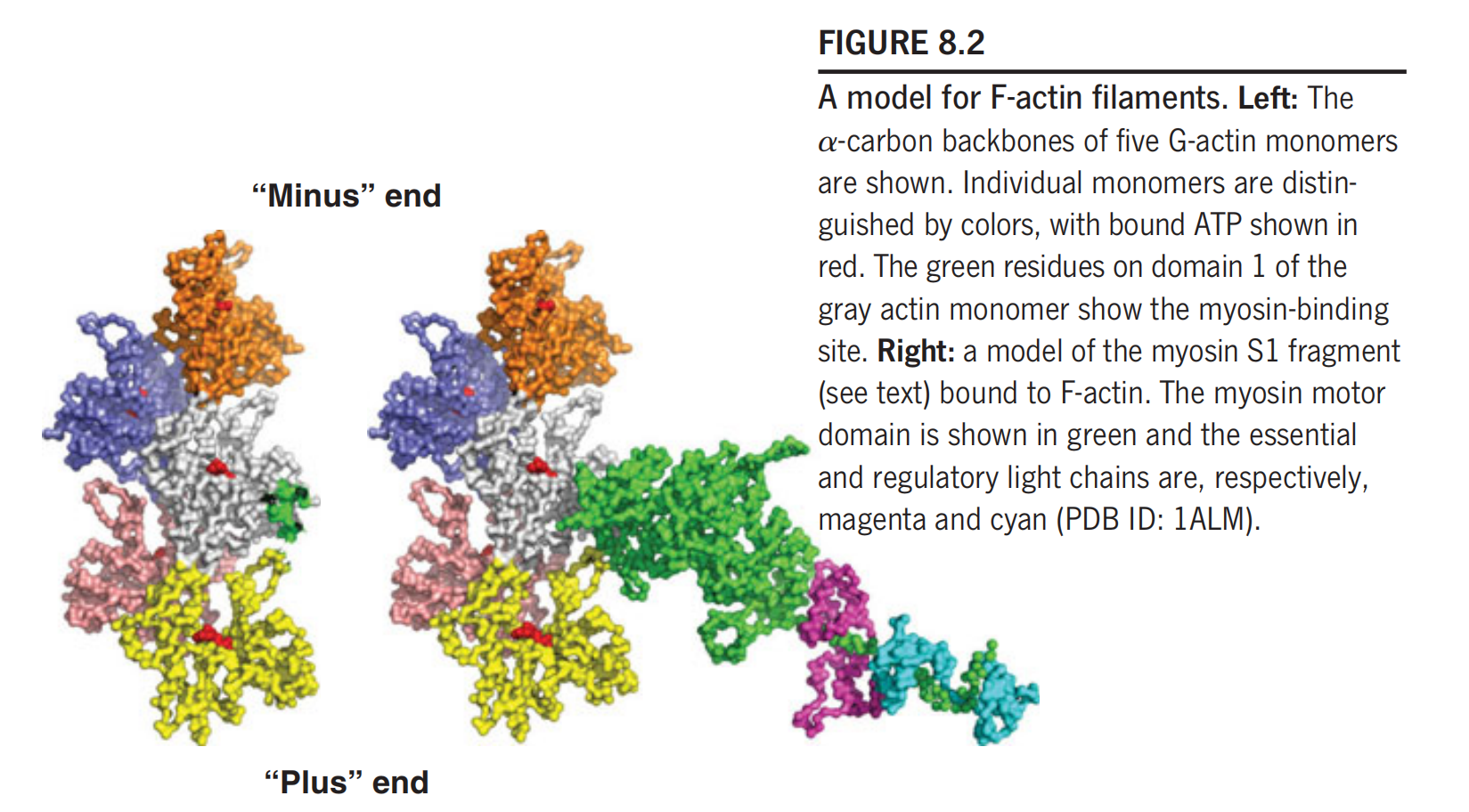
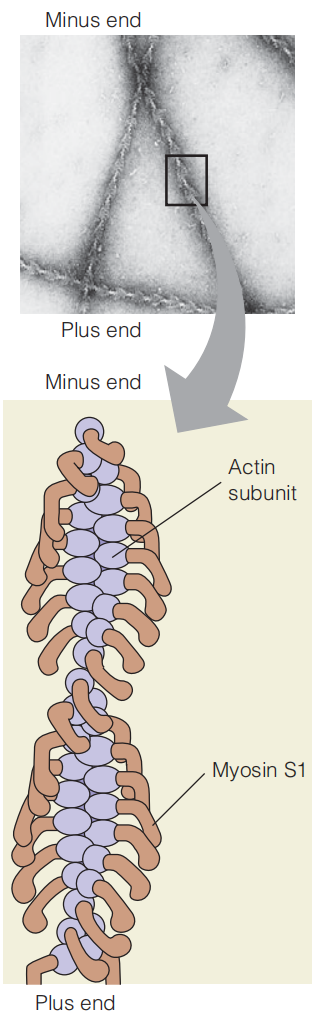
In F-actin filaments, the G-actin monomers are arranged in a two-strand helix (Figure 8.2)
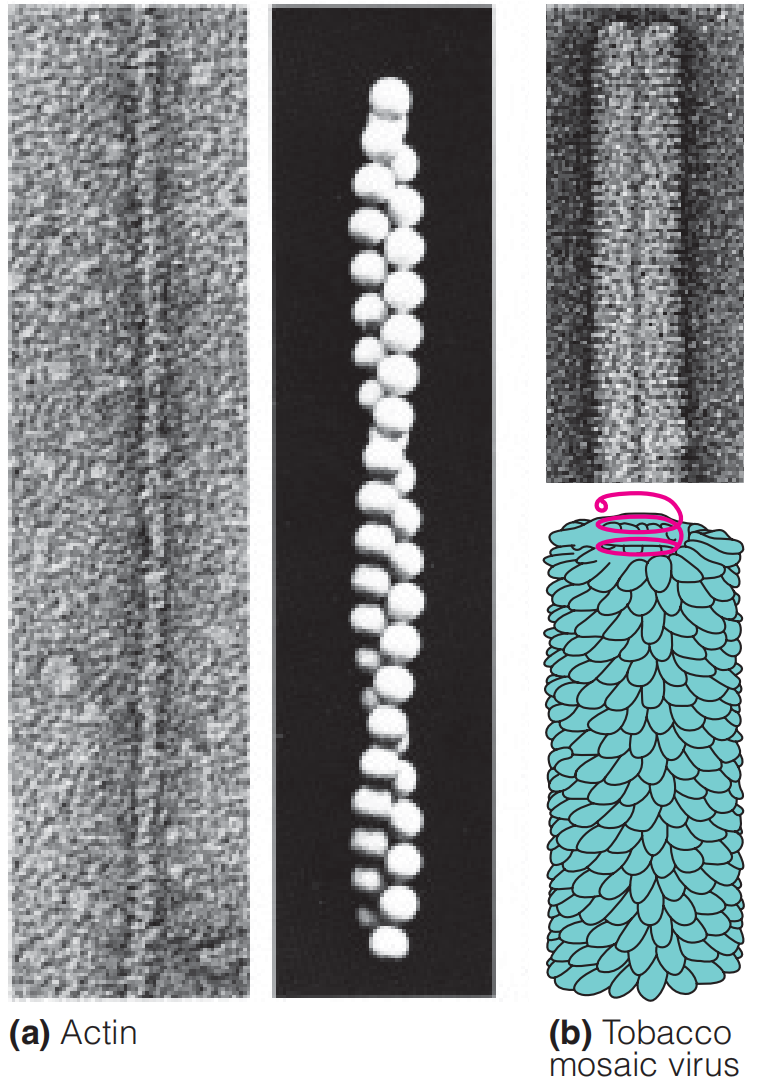
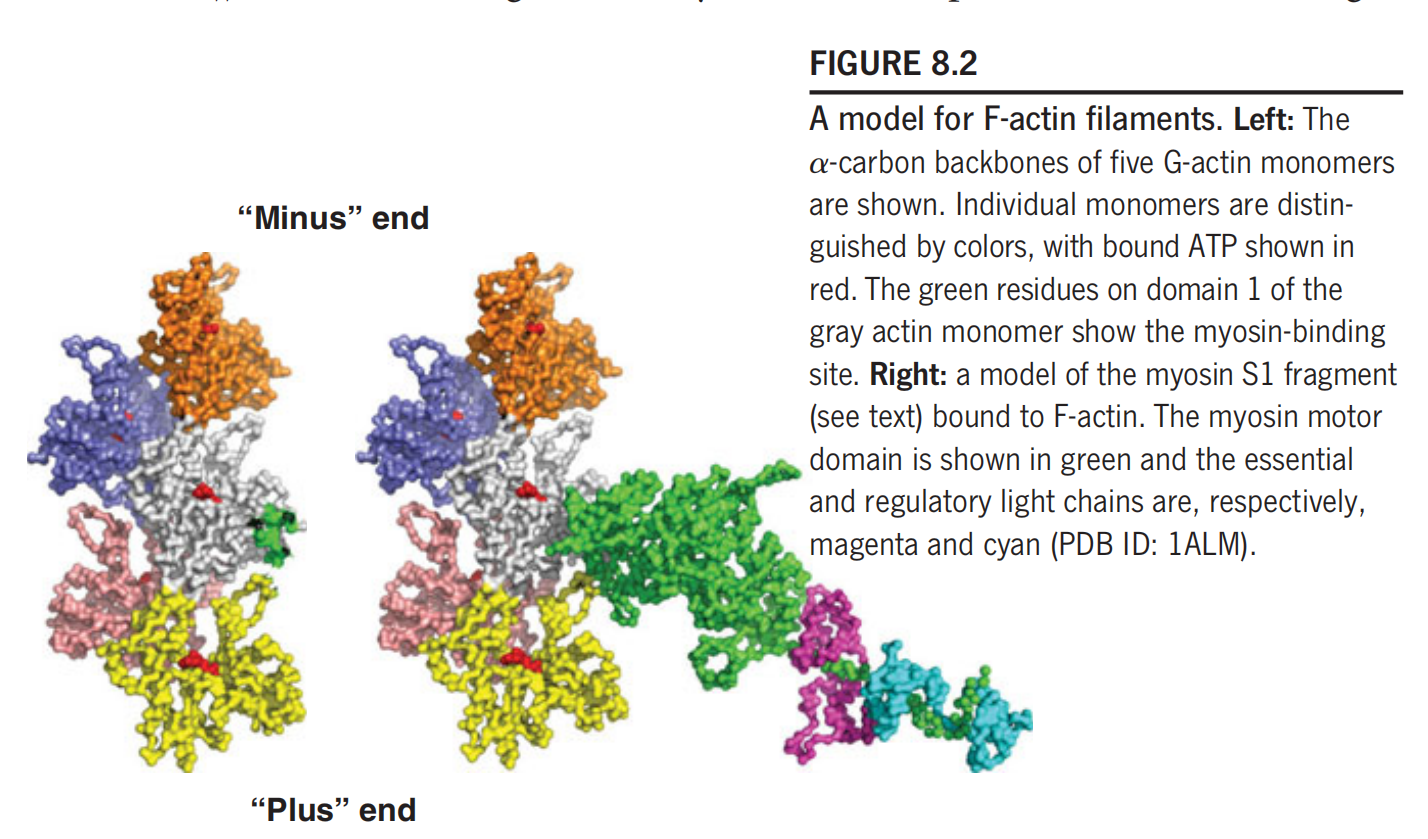
Because of the asymmetry of the subunits, the F-actin filament has a defined directionality, and the two ends have been called the “barbed” or “plus” end, and the “pointed” or “minus” end.
The polymerization reaction exhibits a preferred direction, and the plus end is defined as that end that grows more rapidly under physiological conditions. The sites on the F-actin filament that bind to myosin are located on domain 1 of each actin subunit.
Myosin 肌球蛋白
1. Different Types of Myosin
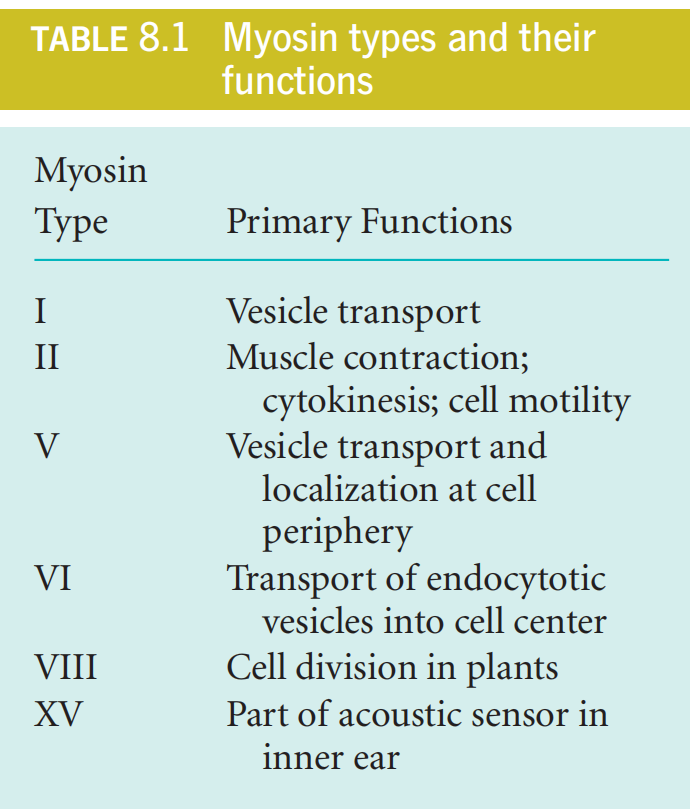
2. Myosin Ⅱ
The myosin Ⅱ is well studied and the following “myosin” refer to “myosin Ⅱ“.
We call Myosin Ⅱ “Ⅱ type” because this type of myosin form dimer.
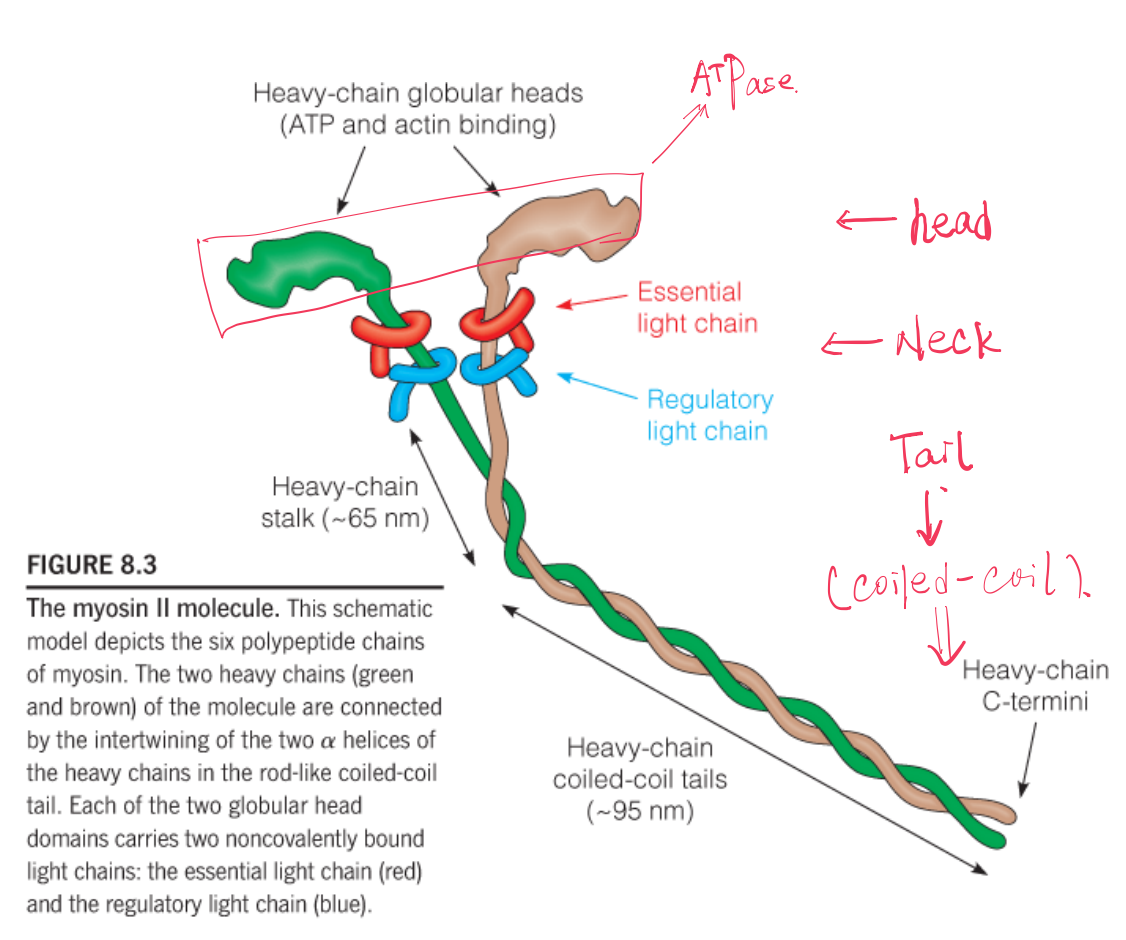
Structure
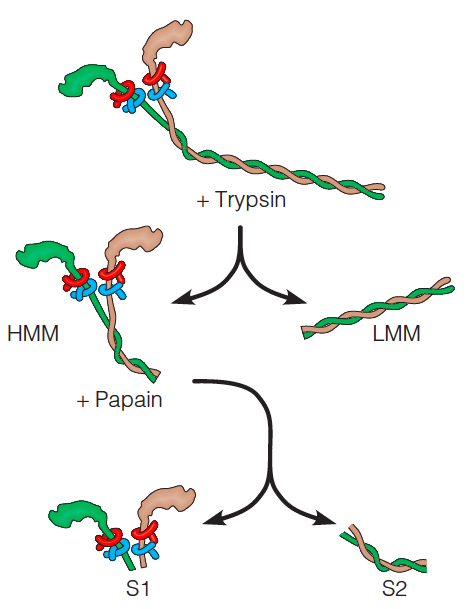
整体结构分为S1和S2 domain.
(1) Overall Structure
The functional myosin molecule (Figure 8.3) is composed of six polypeptide chains: two identical heavy chains (Mw = 230 kDa) and two each of two kinds of light chains (Mw = 20 kDa). Together, they form a complex of molecular weight 540 kDa.
Myosin exhibits aspects of both fibrous and globular proteins, and its functional domains play quite different roles.
- The tail domains have a pronounced tendency to aggregate, causing myosin molecules to form the kind of thick bipolar filaments.
- The S1 fragment, or headpiece, includes the globular motor domain, which binds ATP and actin.
- Two light chains—the essential and the regulatory light chains.
S1
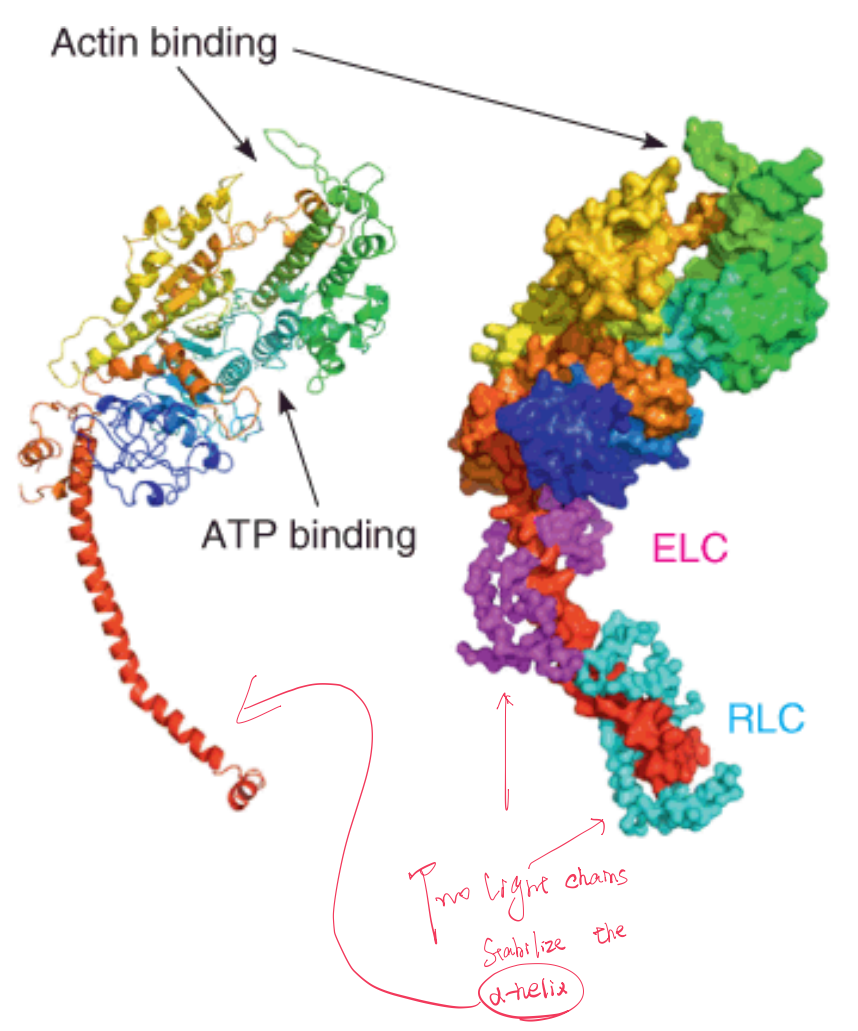
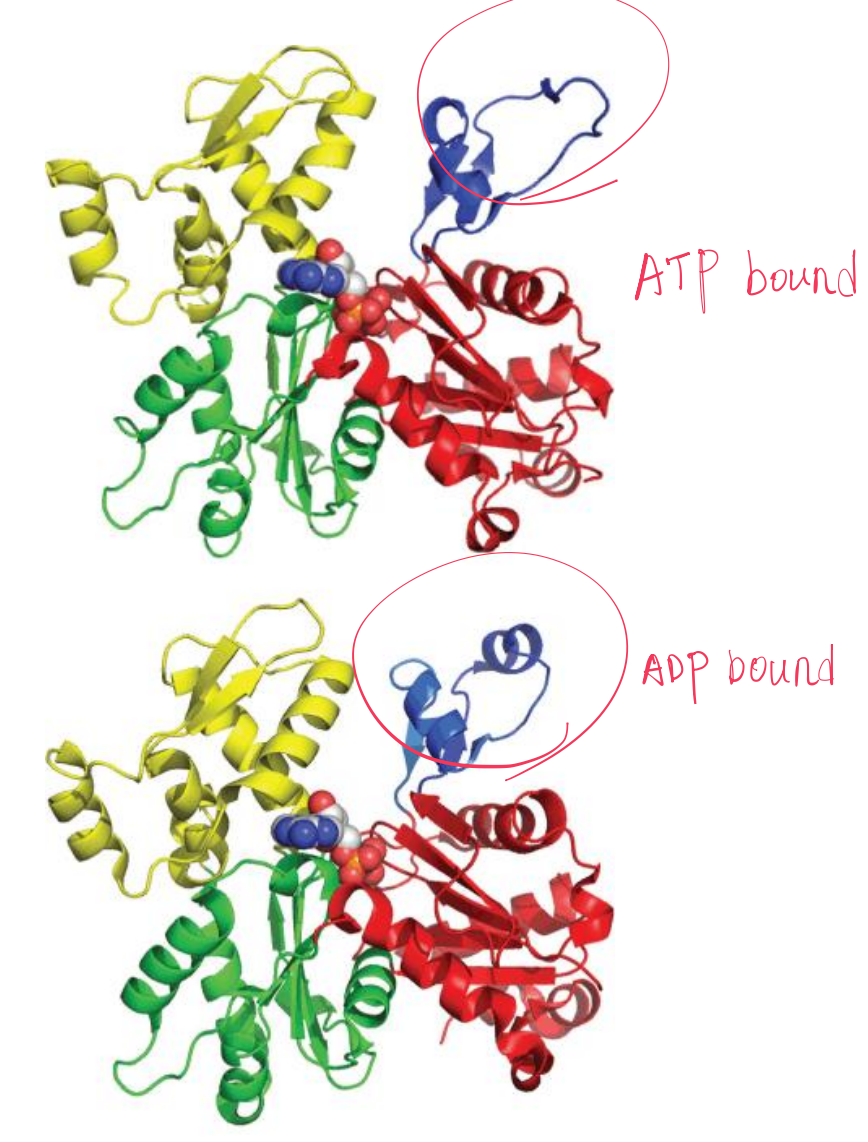
Explanation to the figure :
Left: a cartoon rendering of the heavy chain in rainbow coloring. The “neck” of the S1 fragment is shown by the extended red helix.
Right: the heavy chain in a surface representation with the a–carbon backbone of the essential (ELC, magenta,品红(色)) and regulatory (RLC, cyan,蓝绿色) light chains.
The position of ATP binding is shown, as well as the point of contact with actin.
The protease act as flexible region(蛋白酶可以在myosin的flexible region将其裂解).
(2) Heavy and Light Chain
The heavy chains have long α-helical tails, which form a two-stranded coiled coil, and globular head domains to which the light chains are bound.
Between each head domain and tail domain the heavy chain acts as a flexible stalk(茎). The coiled-coil structure of the tails is reminiscent of the structure of α-keratin.
Reaction of Myosin And Actin
If an actin filament is allowed to react with isolated S1 fragments, the filament will become decorated with these myosin headpieces, producing an asymmetric arrowhead pattern that demonstrates the polarity of the actin filament.
三、Structure of Muscle
In muscle tissue, the actin and myosin filaments interact to produce the contractile structure.
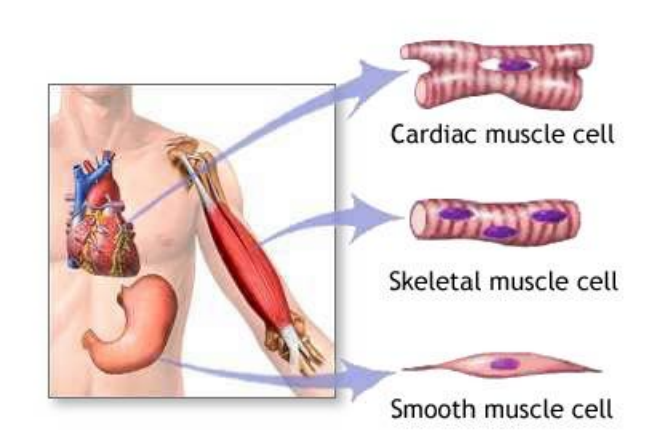
Vertebrates have three morphologically distinct kinds of muscle.
1. Striated Muscle 横纹肌
Striated muscle is the kind most often associated with the term muscle
- Muscles in arms, legs, eyelids(眼睑), etc. that make voluntary motions possible.
2. Smooth Muscle 平滑肌
Smooth muscle surrounds internal organs
- Muscles in blood vessels, intestines(肠), and gallbladder(胆囊), which are capable of slow, sustained contractions that are not under voluntary control.
3. Cardiac muscle 心肌
Cardiac muscle can be considered a specialized form of striated muscle,
- adapted for the repetitive(重复的), involuntary beating of the heart.
Striated Muscle
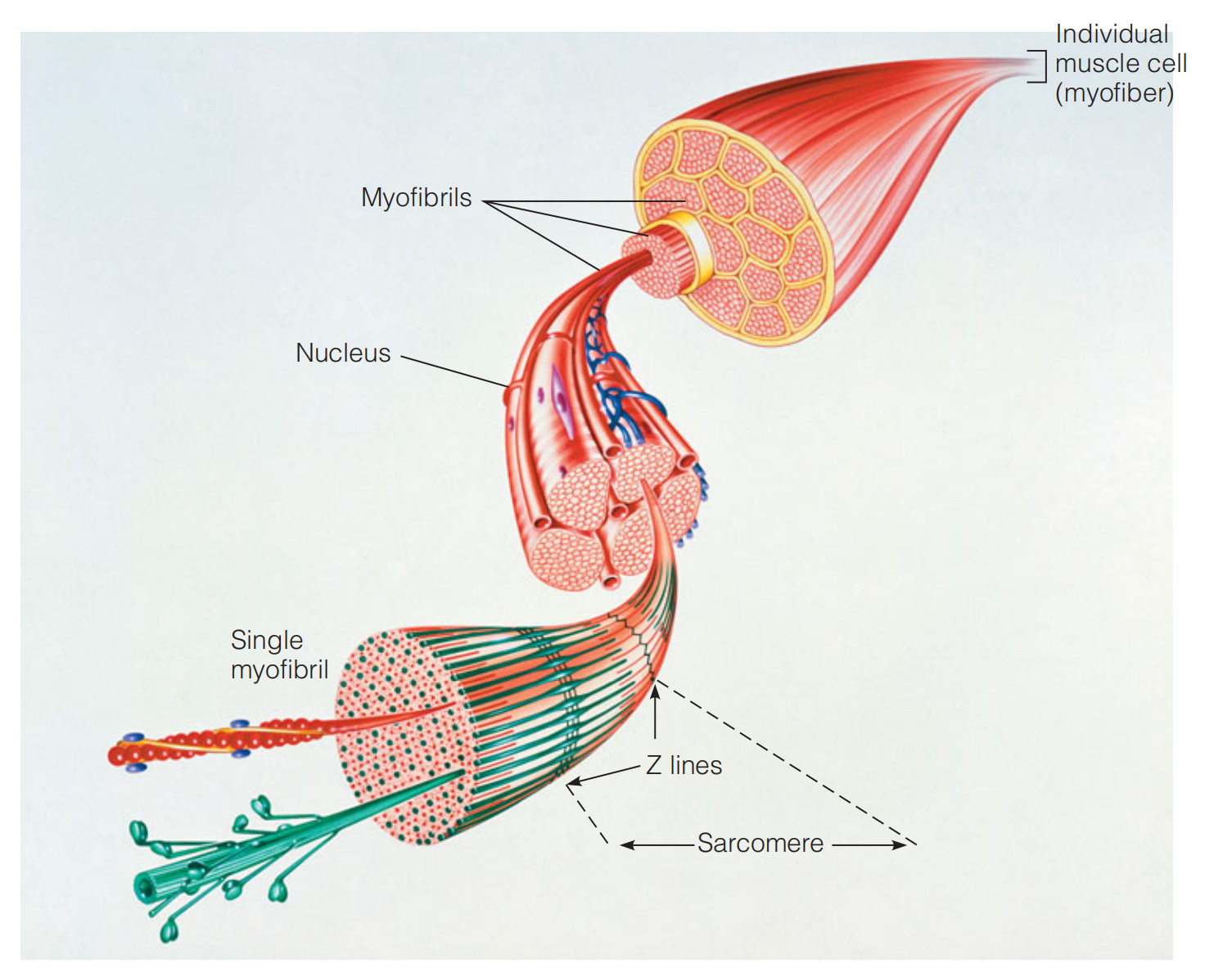
Myofibers(肌纤维)And Myofibrils(肌原纤维)
The individual muscle fibers, or myofibers, are very long (1–40 mm) multinucleate(多核的) cells, formed by the fusion of muscle precursor cells(前体细胞).
Each myofiber contains a bundle of protein structures called myofibrils.
A myofibril exhibits a periodic structure when examined under the light microscope.
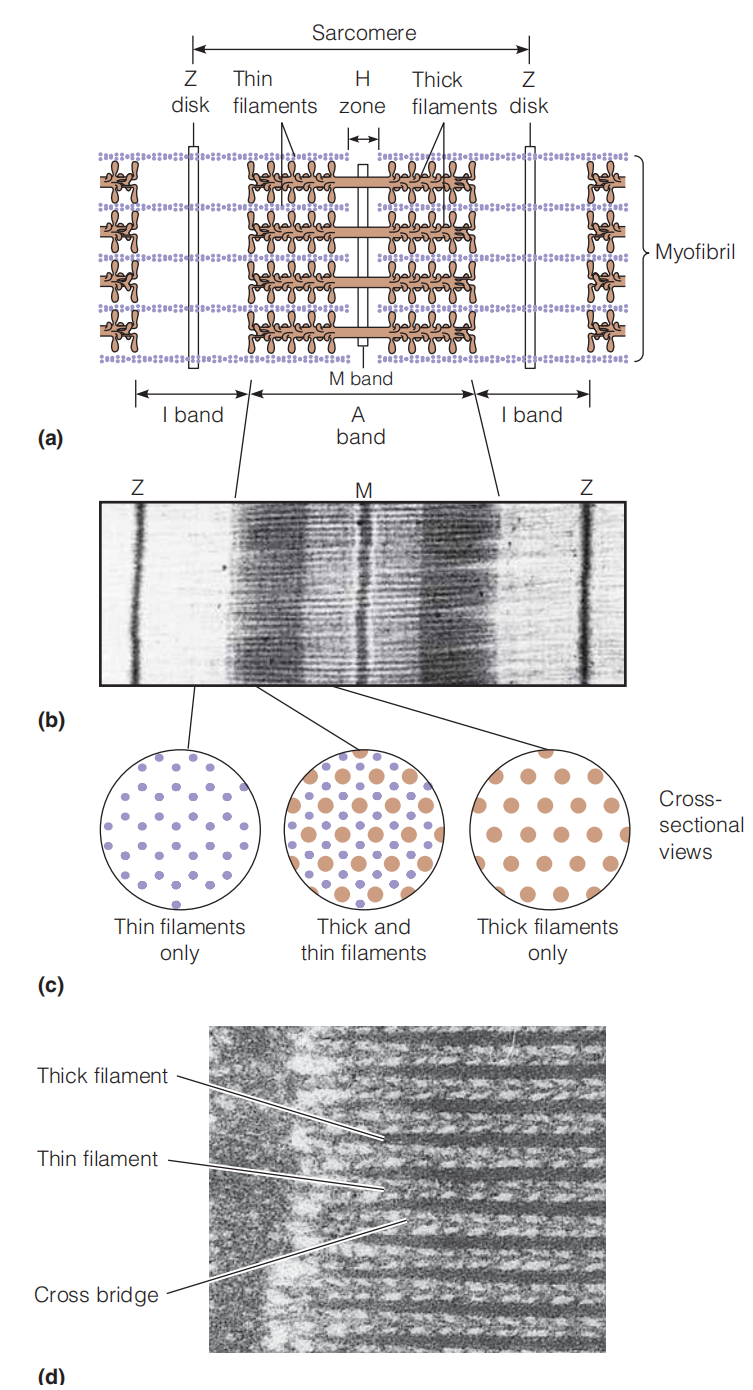
Dark A bands alternate with lighter I bands. The I bands are divided by thin lines called Z disks (or sometimes Z lines). At the center of the A band is found a lighter region called the H zone. The repeating unit of muscle structure can be taken as extending from one Z disk to the next. It is called the sarcomere(肌(原纤维)节,肌小节) and is about 2.3 μm long in relaxed muscle.
The dark part in the figure is because that is the protein-rich domain, 打过去的电子被吸收.
Thin filaments of actin extend in both directions from the Z disks, interdigitating(相互交叉) with myosin thick filaments.
The regions in which the thick and thin filaments overlap form the dark areas of the A band
The I bands contain only thin filaments, which extend to the edges of the H zones. Within the H zones, only thick filaments are found.
The dark line at the center of the H zone (sometimes called the M band) is believed to indicate positions where thick filaments associate with one another.
(1) The Thick Filament
- The composition of the thick and thin filaments has been demonstrated by extracting myofibrils with appropriate salt or detergent solutions to remove virtually all of the myosin. Because the A bands are abolished in this process, the thick A-band filaments must be composed of myosin.
The myosin thick filaments are bipolar(有两极的) structures of the kind shown in Figure 8.5, in which the helical tails of the myosin molecules join together, with the headpieces projecting with a regular 14.3-nm spacing at either end.
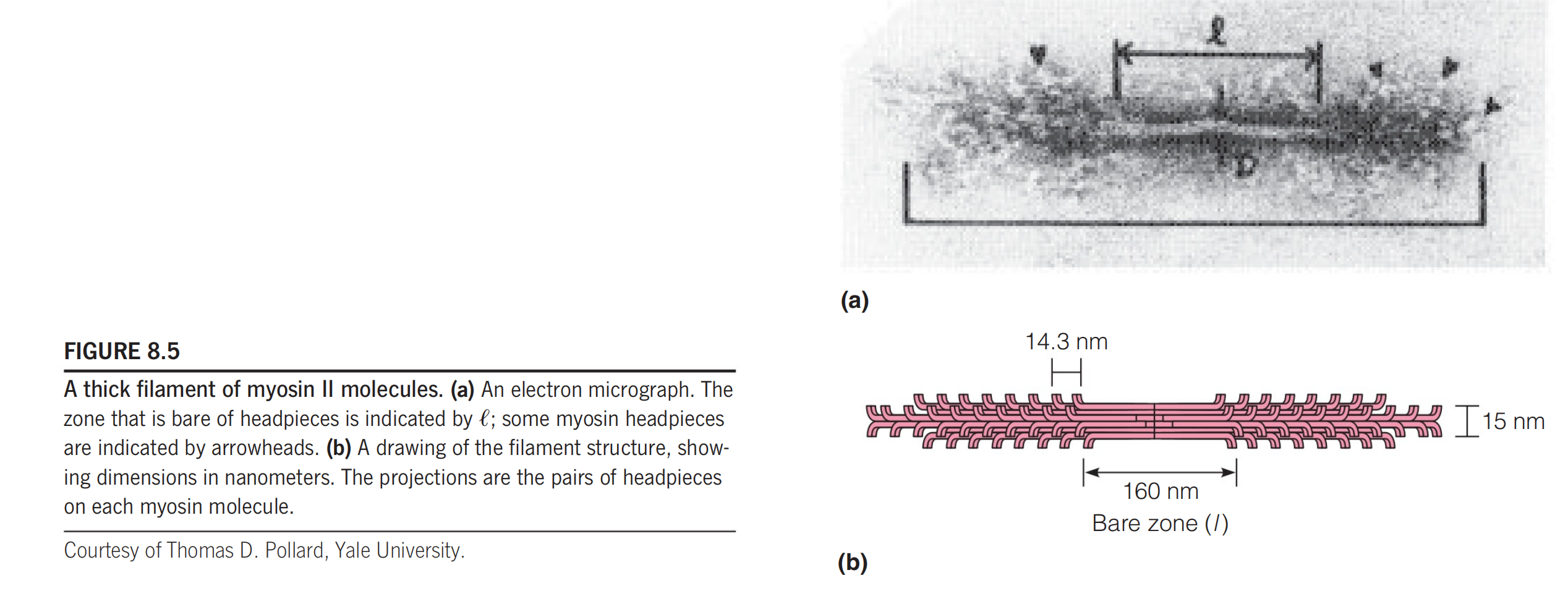
This spacing appears to be generated by a 14.3-nm periodicity in the amino acid sequence in the myosin tails.
(2) Thin Filament
- The thin filaments contain actin, however the thin filament is not composed of actin entirely.
The arrowhead patterns always point outward from the Z disks, demonstrating the polarity of these thin filaments (i.e., the “plus” end of each F-actin filament is anchored(锚) to the Z disk).
(3) Cross Bridge
The cross bridge is the motor domain. These cross-bridges between myosin and actin filaments are
the key to muscle contraction.
四、The Mechanism of Contraction: The Sliding Filament Model
In a fully contracted muscle, each sarcomere shortens from a length of about 2.3 μm to 2.0 μm
During this process, the I bands and H zones disappear, and the Z disks move right up against the A bands
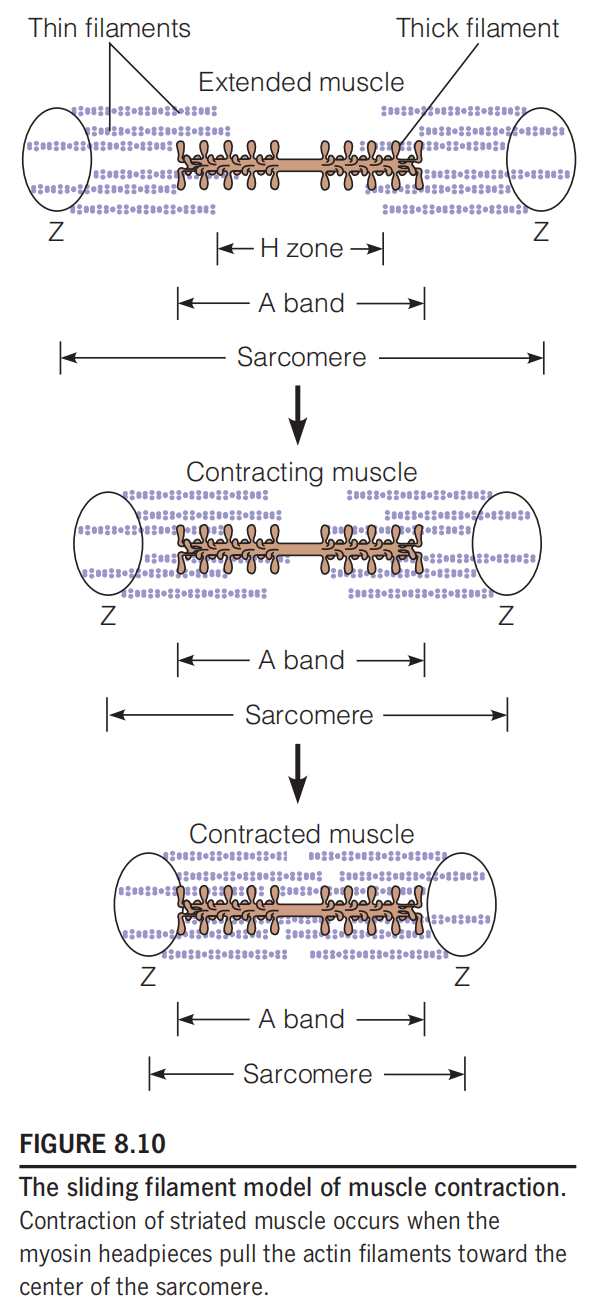
**The Sliding Filament Model: **Contraction of striated muscle occurs when the myosin headpieces pull the actin filaments toward the center of the sarcomere.
Motion Cycle And Energy (ATP) Use Cycle
According to a refinement of the sliding filament model called the swinging cross-bridge model, each myosin headpiece takes part in a repetitive cycle of making and breaking cross-bridges to an adjacent thin filament.
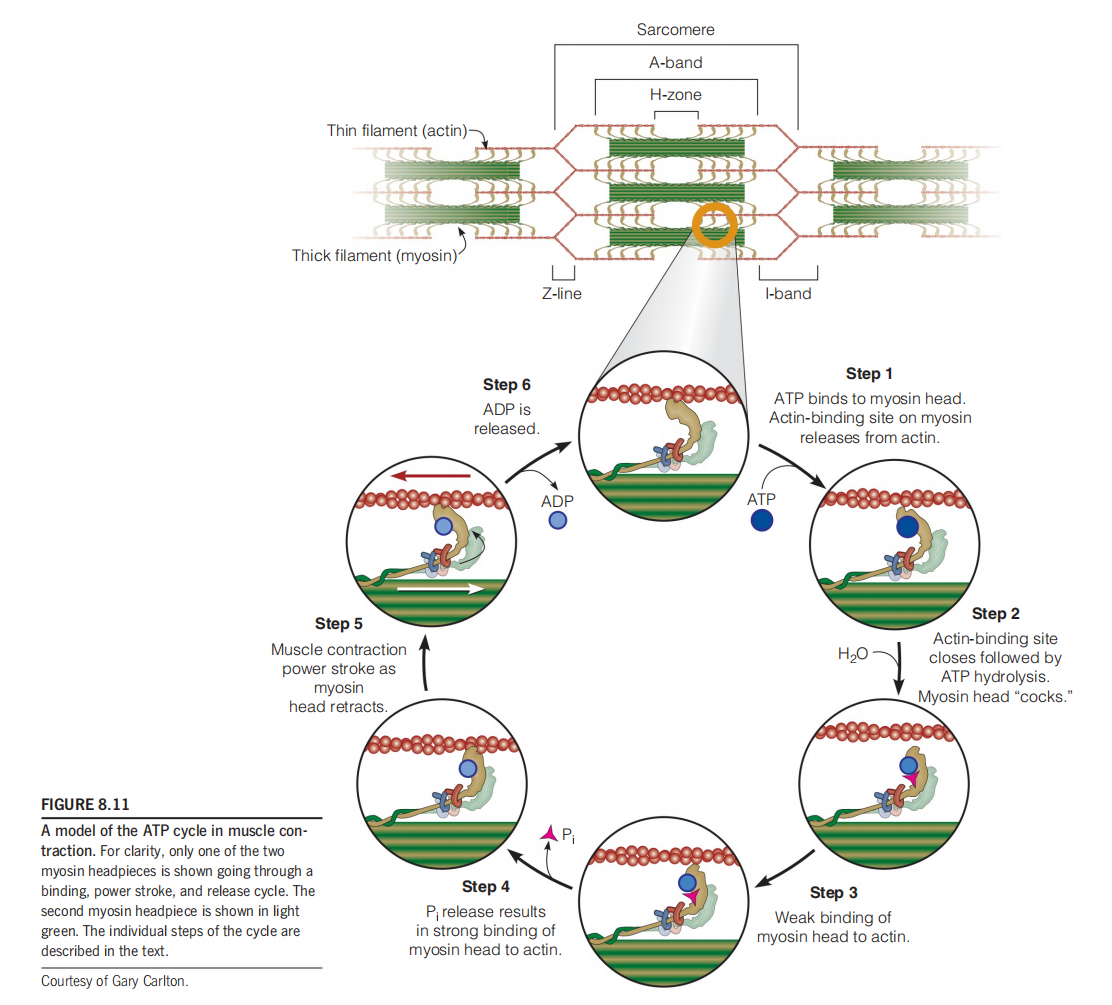
We imagine the cycle starting with the myosin attached to actin, as shown at the top of Figure 8.11.
- Binding of ATP leads to release of the myosin cross-bridge (step 1).
- Hydrolysis of ATP then causes a conformational change, “cocking” the headpiece (step 2).
- Cocked (竖起的) myosin binds to the thin filament at a site that is closer to the Z disk—where the end of the actin filament is anchored (step 3).This initial binding is weak
- Phosphate release (step 4). Phosphate release results in strong binding to the thin filament prior to the power stroke
- The power stroke (step 5), which pulls the thin filament toward the center of the sarcomere.
- Release of ADP (step 6) and binding a new ATP will then restart the cycle.
It is the “neck region” of the S1 fragment , where the light chains are bound, that undergoes the greatest conformational change during the power stroke.
Based on comparisons of X-ray crystal structures of myosin in the ADP-bound and nucleotide-free states, it is thought that the C-terminal part of the myosin neck moves about 10 nm during the power stroke
1. The distance moved by each use of ATP
The force is found to depend upon the load placed on the myosin–actin complex, but averages about 5 pN (piconewtons) at high load.
This corresponds to an energy expenditure of about $10^{-20}$J per power stroke—approximately one-fifth of the energy released when a single ATP molecule is hydrolyzed.
At lower loadings, there is evidence that several power strokes can be accomplished per ATP cycle. The distance the actin filament is moved per power stroke is about 10–20 nm, and this is what can be accomplished per ATP cycle at high loading. When working against weaker resistance, the multiple power strokes can propel the actin for as much as 100 nm per ATP.
五、Regulation of Contraction
ATP control?:
- NO, for ATP is used by many other proteins and molecules. The removal of ATP can cause these other proteins lost their functions.
Muscle contraction is stimulated by an influx of $Ca^{2+}$ into the sarcomere.
$Ca^{2+}$ binding by troponin C (肌钙蛋白C) causes a rearrangement of the troponin–tropomyosin–actin complex (肌钙蛋白-原肌球蛋白-肌动蛋白复合物), allowing actin–myosin cross-bridges to form.
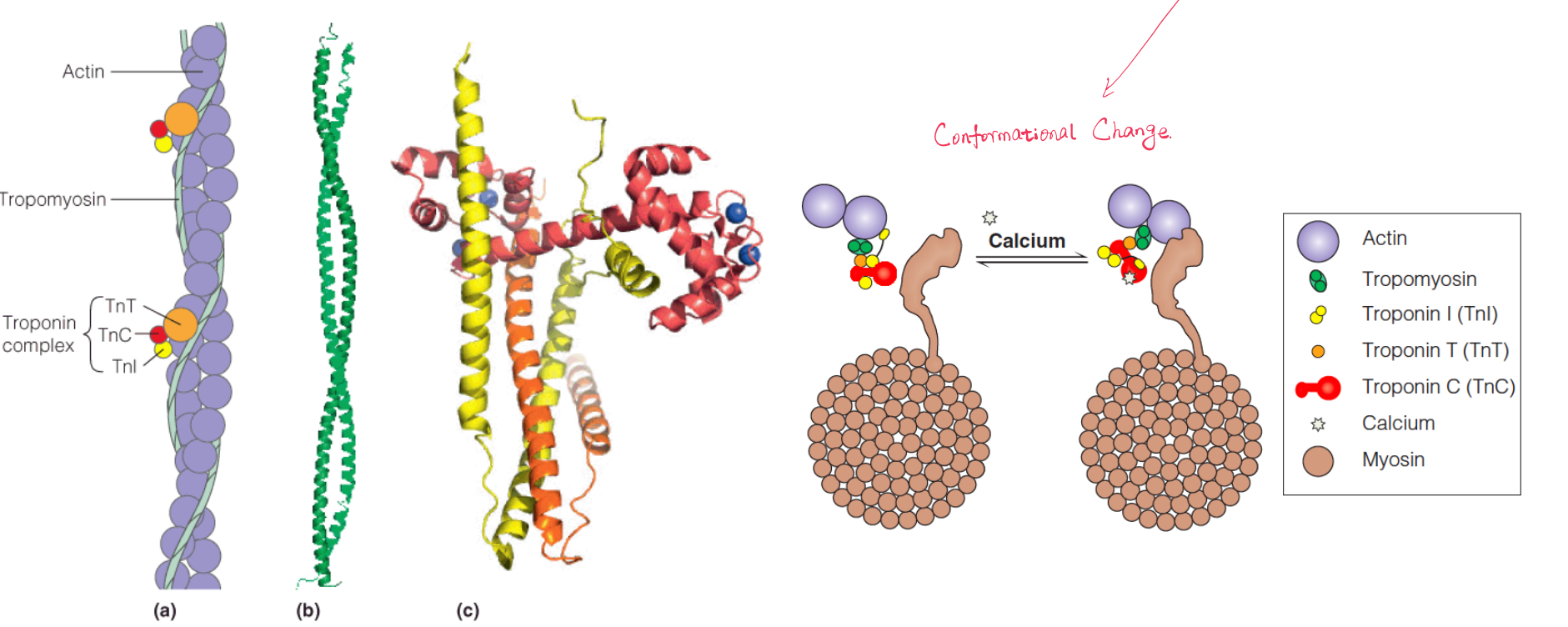
1. Tropomyosin 原肌球蛋白
Tropomyosin, a fibrous coiled-coil protein that overlaps the myosin-binding site in the F-actin helix. Bound to each tropomyosin molecule are three small proteins called troponins I, C, and T.
2. Troponins 肌钙蛋白
Troponin C (TnC) is homologous to the $Ca^{2+}$-binding protein calmodulin (钙调蛋白,钙调素), and like calmodulin, TnC undergoes a conformational change upon binding $Ca^{2+}$.
The presence of tropomyosin and the troponins inhibits the binding of myosin heads to actin unless calcium is present at a concentration of about $10^{-5}$M.
In resting muscle, concentrations are approximately $10^{-7}$M, so new cross-bridges cannot be formed.
How the Ca2+ Influences The Motion Cycle
An influx of $Ca^{2+}$ stimulates contraction because the ion is bound by troponin C, causing a rearrangement of the troponin–tropomyosin complex, which moves the tropomyosin ~1.0 nm closer to the central groove of the F-actin helix.
This shift reveals the sites on actin to which the myosin headpieces bind. The postulated mechanism, shown in Figure 8.14, permits step 3 and the subsequent steps in the cycle.
How Does The Ca2+ Influx Occur
Within the cell, each myofibril is surrounded by a structure called the sarcoplasmic reticulum (肌质网), formed of membranous tubules.
The sarcoplasmic reticulum (SR) is a network of specialized endoplasmic reticulum tubules that surrounds th额myofibrils within the myofiber. In resting muscle, the SR accumulates $Ca^{2+}$, which it discharges into the myofibrils when a neural signal reaches the plasma membrane.
In resting muscle, concentrations are approximately $10^{-7}$M, the concentration in the SR can be ~10000-fold higher.
Impulses from motor nerves depolarize the membrane of the sarcoplasmic reticulum, opening gated $Ca^{2+}$ channels (see Chapter 10), and $Ca^{2+}$ pours out of the lumen (内腔) into the myofibrils, stimulating contraction.
The signal is rapidly transmitted to the entire sarcoplasmic reticulum of a myofiber via transverse tubules, invaginations of the plasma membrane that connect at periodic intervals with the reticulum.
在收缩之后,特殊的钙离子转运蛋白会将钙离子泵出肌小节,使得肌小节中的钙离子浓度回归正常。
六、Nonmuscle Actin and Myosin
The functions of actin and myosin are not limited to muscle contraction, they also exists in many other cells.
Actin and myosin play significant roles in cell motility (Cell motion) and changes of cell shape.
Actin is the major component of the microfilaments of the cytoskeleton—the fibrous array that pervades (遍及,弥漫) almost every kind of cell and gives it a specific shape
The nonmuscle myosin II (NM II) in such intracellular networks is different in sequence from muscle myosin II, and its assembly into filaments is regulated by phosphorylation of the associated regulatory light chains.
Several cellular proteins are involved in anchoring microfilaments to the cell membrane. Organized contraction and relaxation of such anchored microfilament and NM II networks (Figure 8.16c) can lead to a wide variety of cell distortions and movements, including amoeboid crawling.
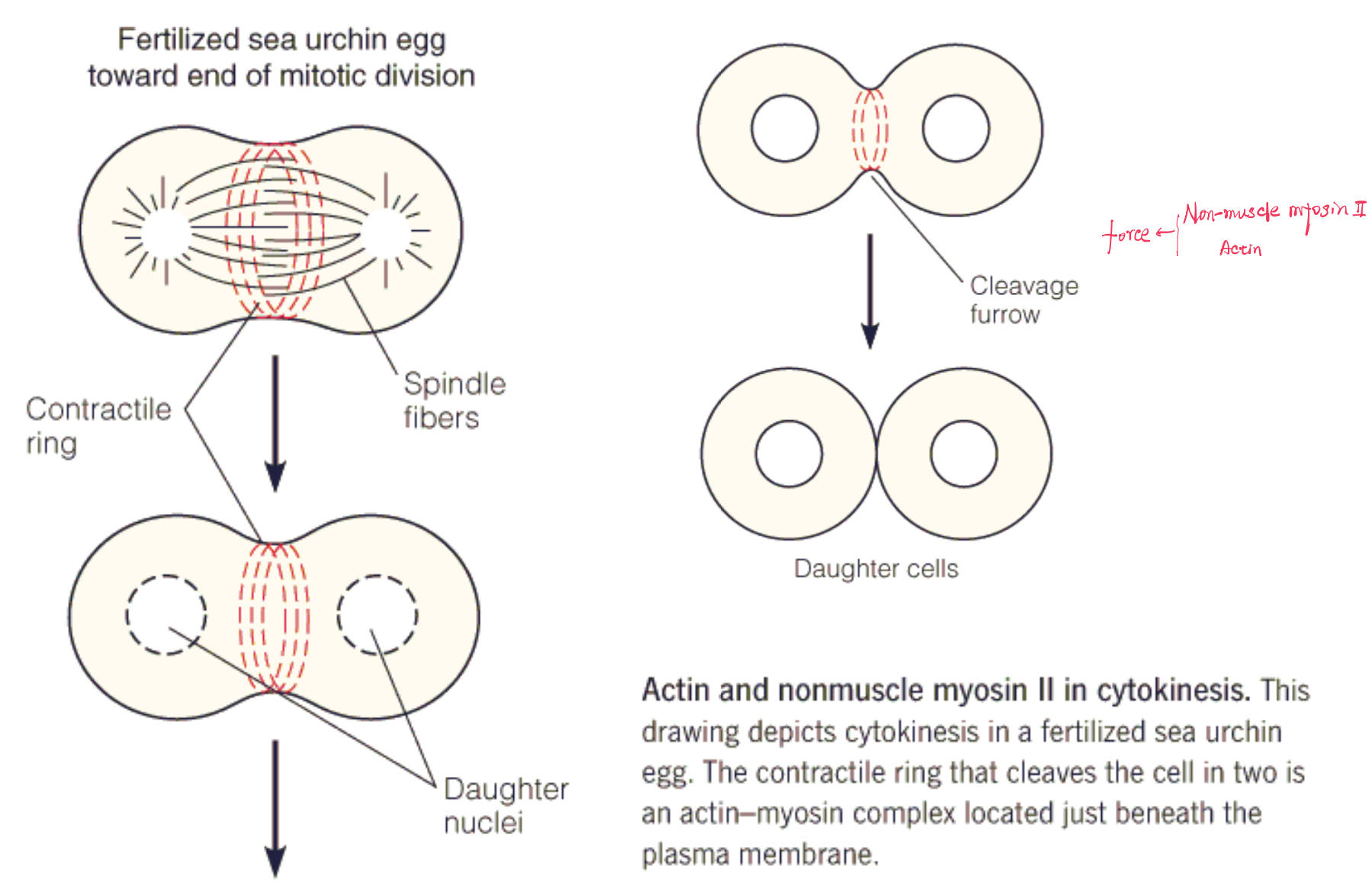
Cytokinesis
Actin–myosin contractile complex involves cytokinesis, the division of cells in the last stages of mitosis
Toward the end of mitosis, when the daughter nuclei are clearly separated at the two poles of the cell, a ring of indentation is observed in the cell surface, defining a plane perpendicular to the mitotic spindle. This ring then contracts, as proposed in Figure 8.16c, forming the cleavage furrow (卵裂沟) and eventually cutting the cell in two.
Electron microscopy shows that the ring consists of fibers, and staining with fluorescent antibodies shows that the fibers contain both actin and myosin.
七、Microtubule Systems for Motility
Introduction
A class of motile systems completely different from, and unrelated to, the actin–myosin contractile systems is used in places as diverse as the mitotic spindle, protozoan(原生动物) and sperm flagella, and nerve axons, to name only a few.
These systems are constructed from microtubules(微管), very long, tubular structures built from a helical arrangement of the protein tubulin(微管蛋白).
Tubulin
There are two homologous tubulin subunits, α and β, each of molecular weight 55 kDa. They are present in equimolar quantities in the microtubule, which can be considered a helical array of αβ dimers.
Alternatively, we can view the microtubule as consisting of 13 rows, or protofilaments (原型丝), of alternating and subunits.
由于 αβ subunits 是不对称的蛋白质,所以它们具有方向
A helical array of and tubulin forms a microtubule
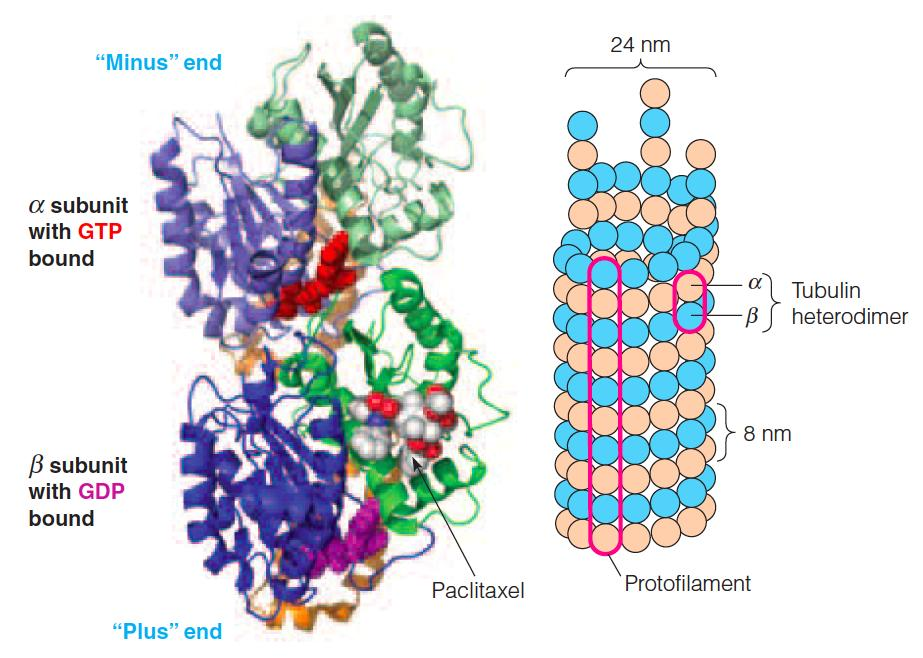
Explanation of the figure:
Left: the crystal structure of an αβ dimer is shown.
- The α subunit is on top and has GTP bound. The β subunit has GDP and the anticancer drug paclitaxel bound.
Right: A schematic model of a microtubule.
- The microtubule can be thought of either as a helical array of αβ dimers or as 13 parallel rows of αβ dimers. These rows are referred to as protofilaments (初纤维;原丝).
Assembly of microtubules
The assembly of microtubules bears resemblance to that of actin, but GTP are used instead of ATP.
During an initial lag period, αβ dimers form oligomers large enough to nucleate fiber formation. The microtubules then grow until most of the free dimers are used up and equilibrium between growth and dissociation is reached.
Both microfilaments and microtubules的存在都是一个动态的过程(equilibrium),组装与拆解形成了一个平衡
The dynamic microtubule network has important roles in the cell, and cancer cells are rapidly dividing, the disruption of microtubule assembly during mitosis (Block cell division) is the target of several anti-cancer treatments. Colchicine(秋水仙碱), paclitaxel(Taxol紫杉醇). Nocodazole is often used in cell biology experiments.
The α and β subunits each bind GTP and then associate to form dimers and oligomers. These oligomers form nucleation sites for the growth of microtubules
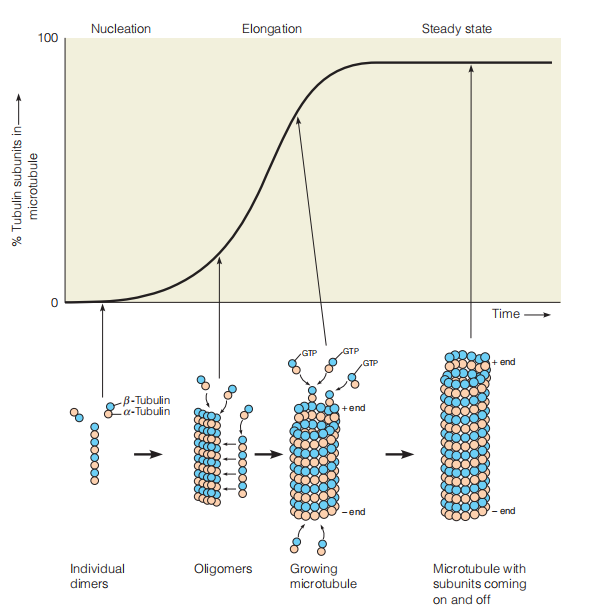
One end, called the plus end, grows more rapidly than the other, minus end. The β subunit has GTPase activity, and as in actin polymerization, the nucleotide is hydrolyzed but is held in the filament.
The Comparision
Microtubules are larger and appear to be more mechanically stable than actin microfilaments; thus, microtubules are better suited to high-stress functions.
microtubule-associated proteins (MAPs)
The final assembly of a functional microtubule usually involves the binding of other proteins to its surface.
Other MAPs stabilize microtubule structure and/or promote the association of microtubules into bundles (捆,束).
1. tau(τ) MAP
Tau MAP is found in neuronal tissue
- Phosphorylation of tau results in its dissociation from microtubules, leading to destabilization of the microtubule structure.
- Hyperphosphorylation has a much more dramatic effect, resulting in the formation of tangles of -filaments in neural axons, one of the major cellular symptoms of Alzheimer’s disease.
八、Cilia and flagella
Many kinds of prokaryotic and eukaryotic cells are propelled (推进) by the motions of cilia or flagella. Cilia are shorter than flagella and exert a coordinated rowing motion to move a microorganism through solution.
Eukaryotic flagella, such as sperm tails, are longer and propel the cell by an undulatory (波动的) motion
人体内的细胞也可能具有cilia,for information sensing。
Cilium
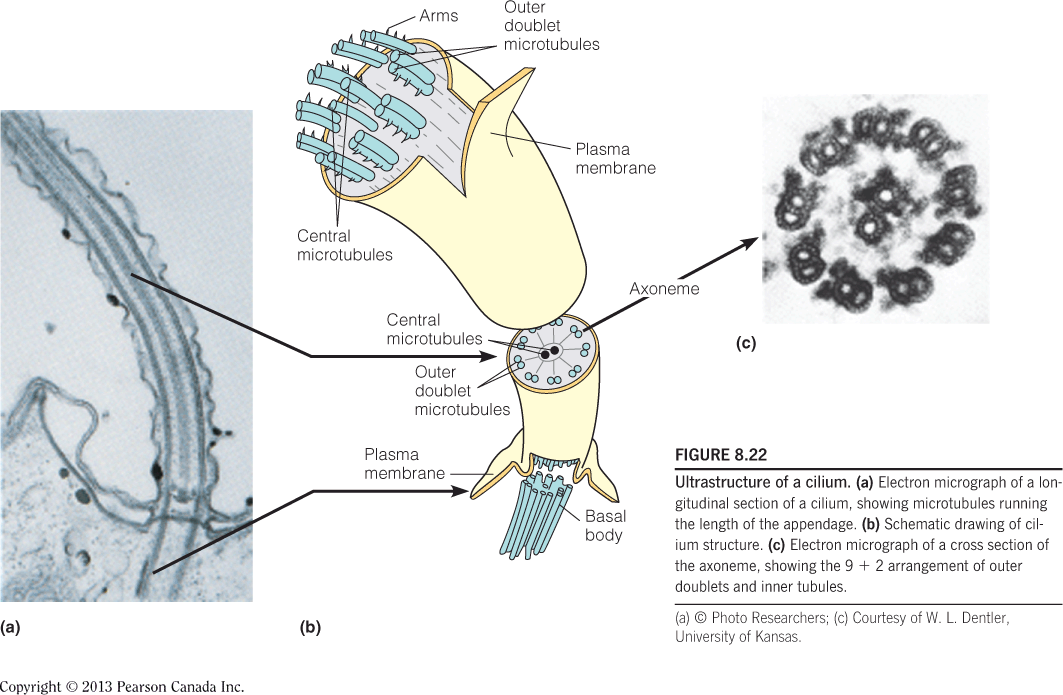
Ultrastructure of a cilium:
- Electron micrograph of a longitudinal section of a cilium, showing microtubules running the length of the appendage.
- Schematic drawing of cilium structure.
- Electron micrograph of a cross section of the axoneme, showing the 9 + 2 arrangement of outer doublets and inner tubules
Axoneme 基因丝;鞭毛轴丝
The Axoneme is connected to a basal body (基体;基部小体), an anchoring (锚泊) structure within the cell.
Diagram of the cross section of an axoneme:
- The dynein (动力蛋白) arms have ATPase activity and can cause adjacent doublets (成对物;一对中的一个) to slide with respect to each other.
- The nexin (微管连接蛋白) connections between doublets and the radial spoke (放射状辐条) system give stability to the whole assembly.
1. Structure Inside Axoneme
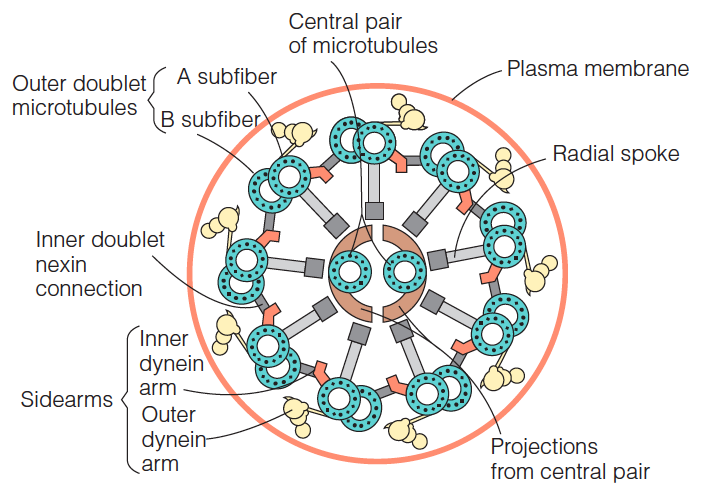
- The most obvious feature is the arrangement of microtubules known as a $9+2$ array
- Two central microtubules ringed by nine microtubule doublets. The single microtubules in the center are complete, each having 13 protofilaments of αβ tubulin dimers.
- By contrast, each of the nine surrounding doublets is composed of one complete microtubule (the A fiber) to which is fused an incomplete microtubule, carrying only 10 or 11 protofilaments (the B fiber).
- The outer doublets are periodically interconnected by a protein called nexin(微管连接蛋白) and carry at regular intervals sidearms composed of the motor protein dynein(动力蛋白).
- Radial spokes, each consisting of a head and an arm, project from the outer doublets to connect with the central pair. 、
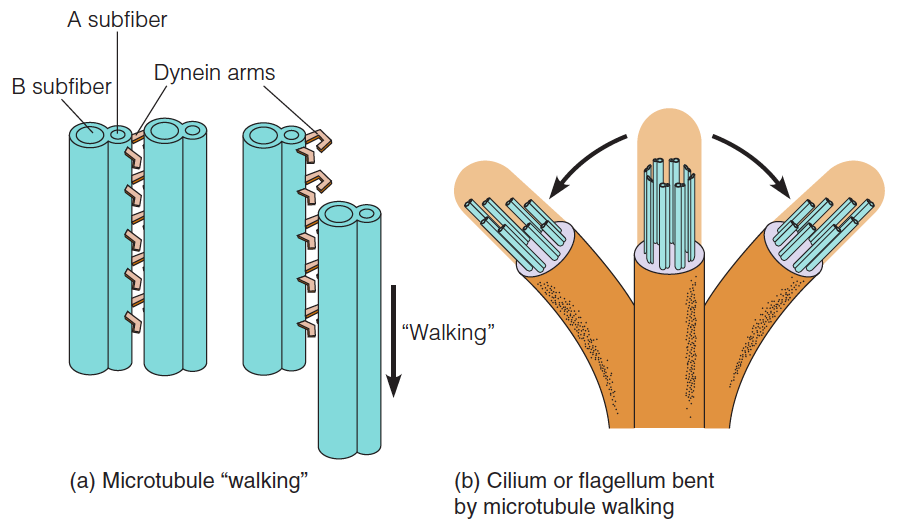
It has been demonstrated that dynein has ATPase activity, with binding of ATP associated with the breaking of dynein cross-bridges. Thus, an obvious similarity exists between the mechanisms of beating of cilia and flagella and the ATP-driven walking of myosin heads along the actin fiber.
Model for the bending of cilia and flagella
- Isolated microtubule doublets can “walk” past one another by making and breaking dynein arm contacts, if ATP is present.
- The back-and-forth bending of a cilium or flagellum is produced by synchronized short “walks” by microtubules on opposite sides of the appendage. Long walks are prevented by the cross-connections between microtubules.
Intracellular Transportation
proteins and organelles can rapidly transported over long distances along microtubules, which serve as tracks that direct and facilitate the motion
Use Exon as example:
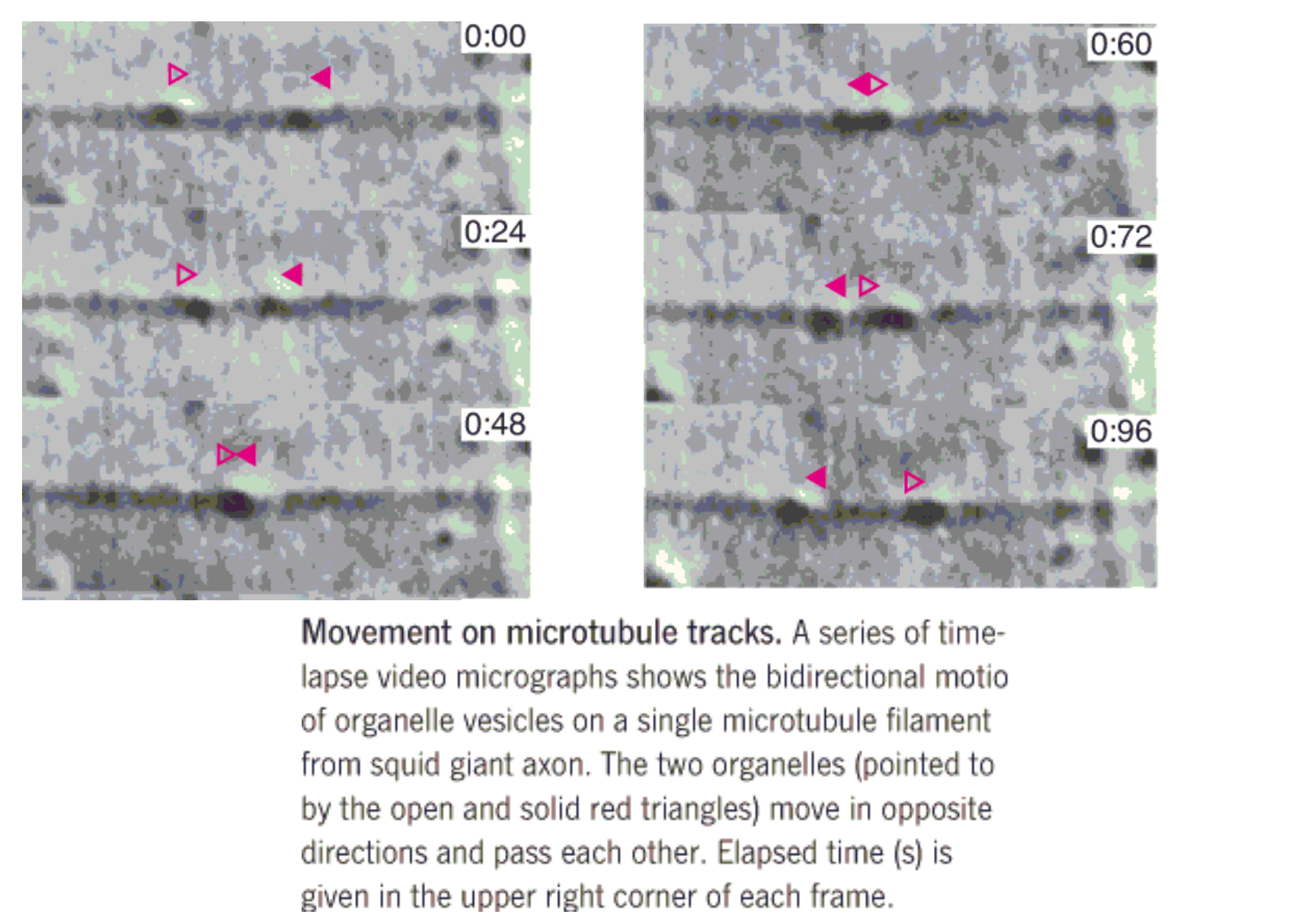
Transport along the microtubules occurs in both directions
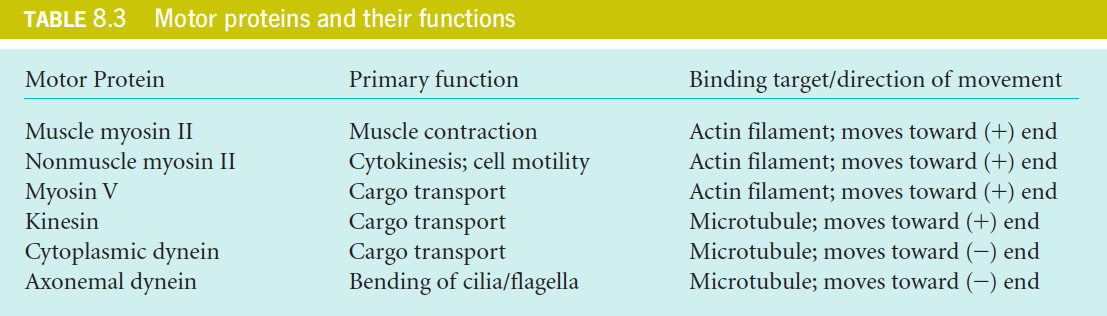
The ““molecular motors” are of two kinds.
- One, called cytoplasmic dynein (胞质动力蛋白), resembles (类似,像) the dynein involved in the motion of cilia and flagella
- responsible for transport from the plus end of the microtubule toward the minus end.
- The other, called kinesin(驱动蛋白), is used to transport objects in the opposite direction
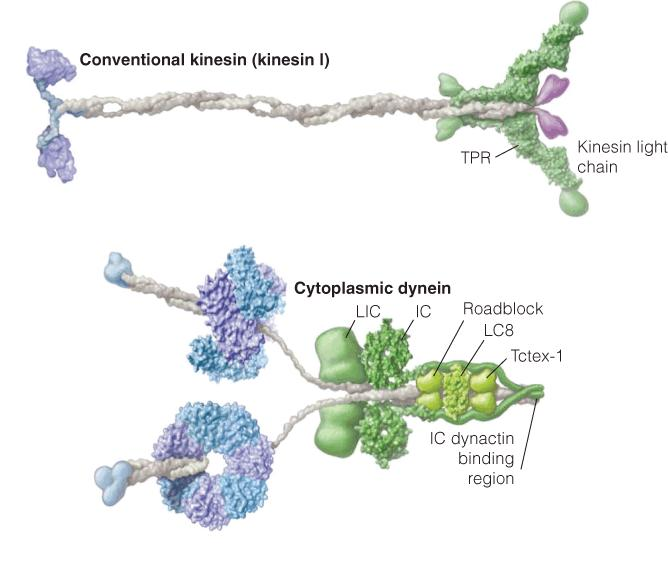
Kinesin Ⅰ(驱动蛋白Ⅰ) and dynein(动力蛋白)
Structural models of kinesin I and cytoplasmic dynein:
- The microtubule-binding portions are on the left and the cargo-binding portions are on the right.
- The motor domain of kinesin resembles the SI headpiece of muscle myosin. The motor domain of dynein binds ATP and contains six different subunits arranged in a circle. The microtubule-binding stalk and the linker to the cargo domain are covalently linked to the hexameric motor domain.
1. processively walking
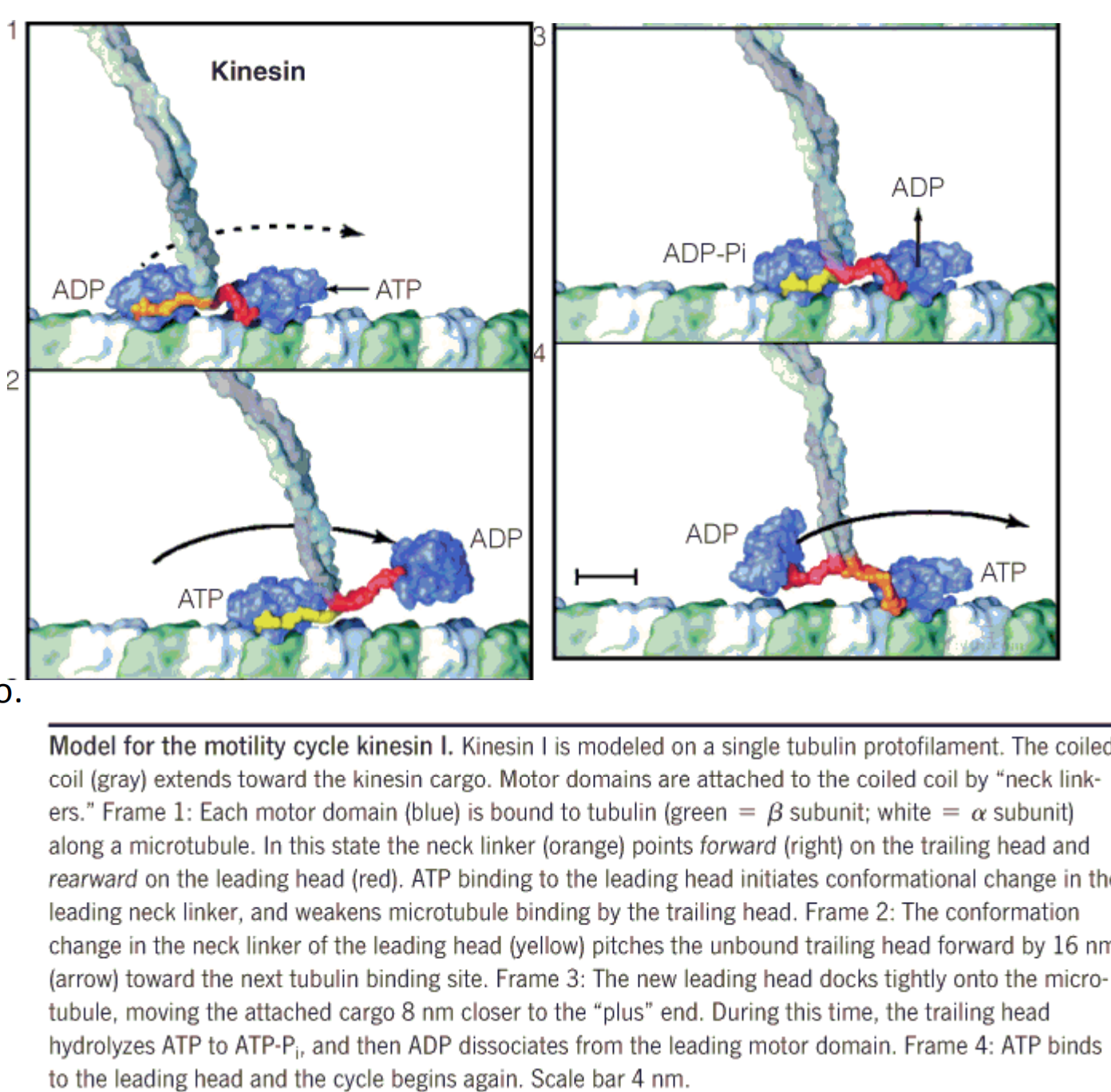
Model for the motility cycle kinesin I
- Kinesin I is modeled on a single tubulin protofilament.
- The coiled coil extends toward the kinesin cargo.
- Motor domains are attached to the coiled coil by “neck linkers.”
Myosin V and kinesin I
(1) Comparision
Kinesin bears similarity to the myosin family in both structure and function.
The helical tail of each molecule connects it to whatever “cargo” the dynein or kinesin is carrying, perhaps through associated proteins
Similarities in function between myosin and kinesin can be seen by comparing an ATP-hydrolysis cycle for each protein
A significant difference between myosin and kinesin is seen in their respective “duty ratios(负载比,占空率),” or the time each motor domain spends bound to its target.
- At any given time only a few myosin headpieces in a myosin filament will be attached to the actin microfilament, an individual myosin motor domain spends most of its time in the unbound state
- Kinesin doesn’t form oligomeric filaments; thus, it must always have one of its motor domains securely bound to the microtubule as it carries its cargo.
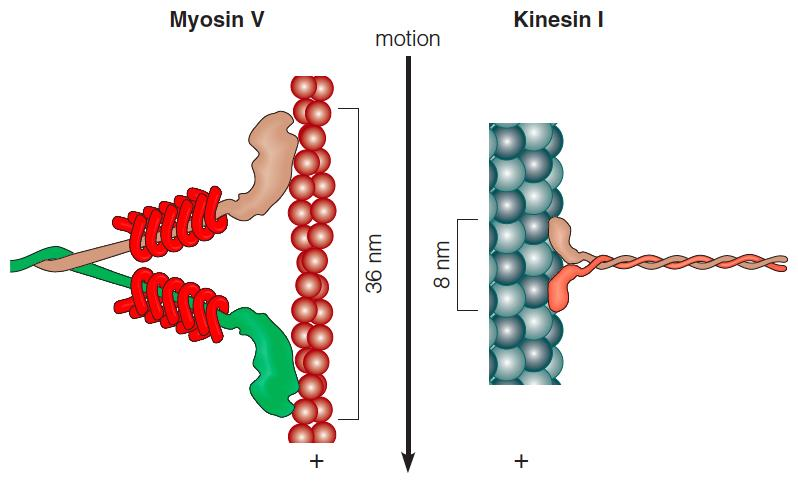
Like kinesin, myosin V is a processive(前进的) motor.
The motor domains of each protein are shown attached to their respective scaffold (脚手架): the actin filament or the microtubule.
The direction of movement is toward the + end of each filament type, and step sizes are indicated by the brackets.
The actin and microtubule has polarity, so the protein move at specific direction.
(2) Work Together
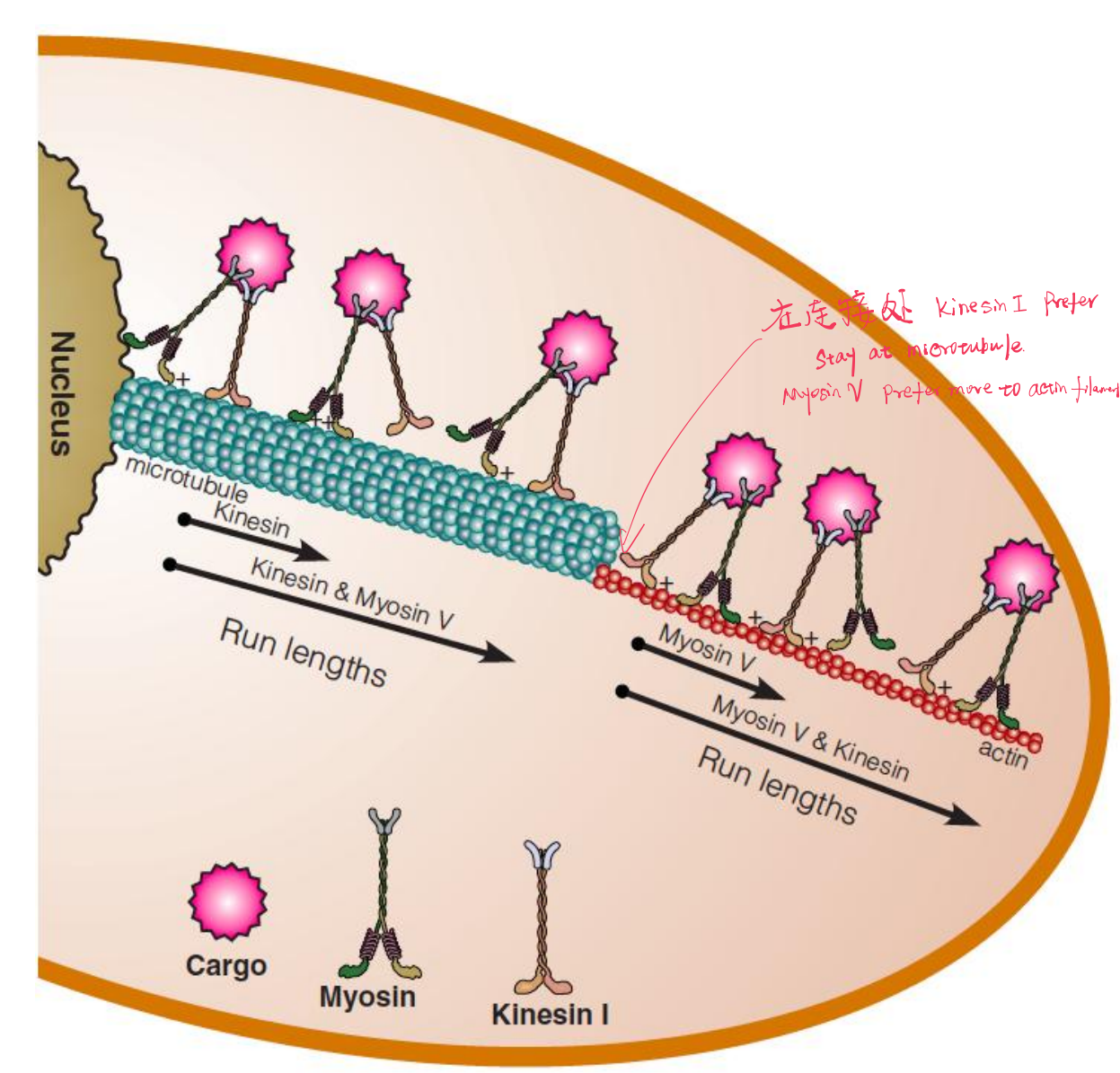
Myosin V and kinesin I work together to deliver cargo (货物):
- The distance a cargo is transported along a microtubule or microfilament is significantly greater when both carriers are present.
- Non-specific interaction between myosin V and tubulin, and kinesis and actin, keep the active carrier close to its target filament in the event it falls off too early.
Cargo bound to both carriers is transported farther on each filament type than when one or the other carrier is absent
九、Summery of Motor Proteins
十、Bacterial Motilities: Rotating Proteins
- Some bacteria move by the rotation of flagella, using molecular rotating motors set in the cell membrane.
- The bacterial flagellum (鞭毛) is a right-handed helical fiber, composed almost entirely of one fibrous protein, flagellin (鞭毛蛋白).
- It does not contain microtubules, actin, myosin, or any contractile system.
- Yet for many years it was assumed that the bacterial flagellum underwent in-plane bending motions, like those of sperm tails. Researchers were surprised to learn that, in fact, it rotates.
- This mechanism was demonstrated most simply when the flagellum of a bacterium was stuck to a glass plate by antiflagellin antibodies. Because the flagellum could no longer rotate, the bacterium did.
Structure of the bacterial flagellar motor from Salmonella enterica:
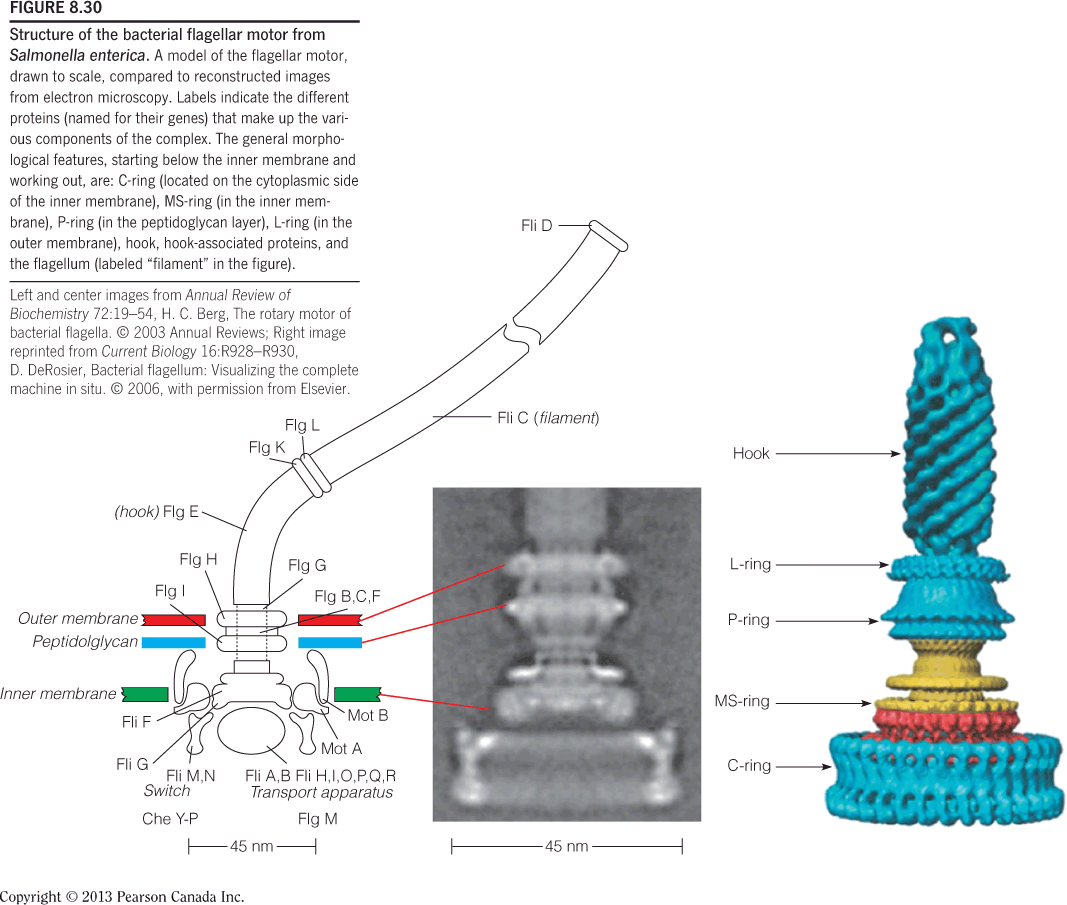
If the multiple flagella are all rotating counterclockwise (Figure 8.31a), their right-handed helical sense makes them push together. They tend to be drawn together in a bundle and propel the bacterium in a straight line, a movement known as running.
But if the flagella rotate clockwise (Figure 8.31b), they fly out from the surface and pull in all directions. The result is that the bacterium tumbles and thereby, changes direction.