L3 Isolation of cells and cell culture in vitro
一、Isolation of cells
Isolated and cultivated cells are commonly used to study cell biology
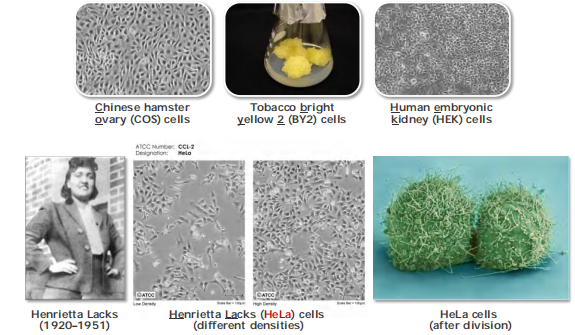
HeLa cells were the first immortalized human cell line (1951)
Virtually all cells can be grown in vitro (几乎所有的细胞都可以在体外培养)
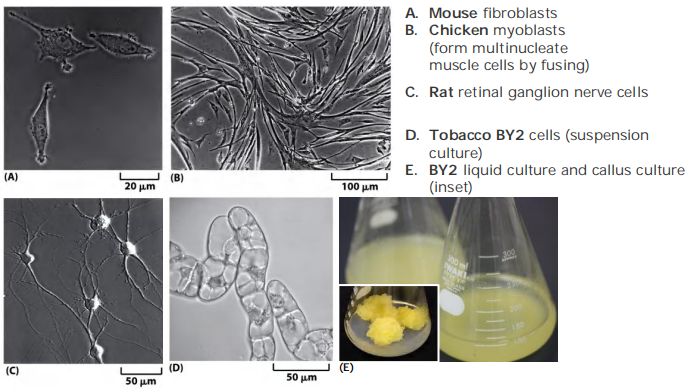
? Animal cells must be cultured with adhesion, plant cells can be cultured in suspension.
How to start a cell culture?
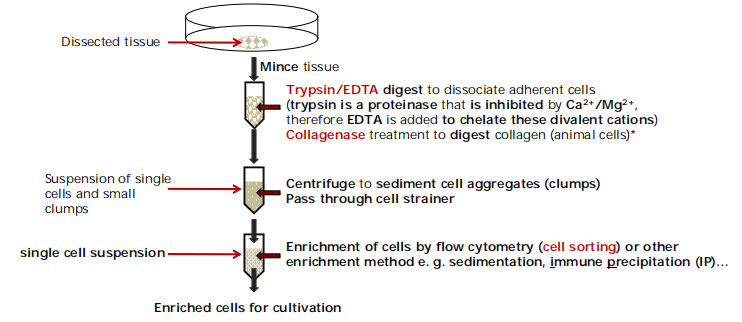
First you have to isolate primary cells from tissues
直接从组织中分离出来的细胞叫做元代细胞(Primary Cell)
1 | |
If you are to isolate plant cells for cell cultures, cellulase(纤维素酶) and macerozyme(核酶) are used instead of trypsin(胰蛋白酶) and collagenase(胶原酶) to digest the cell wall.
How to select specific cell types?
Growth/cultivation of single cell type that you are interested in. → Selection of specific cell types is necessary
Physical selection of the cells according to specific properties.
Fluorescence-activated cell sorting (FACS)
Immunoprecipitation of whole cells using antibody against specific cell-surface proteins (called marker, e.g., CD19) which is conjugated with beads (polydextran(聚葡聚糖) or magnetic).
- the antibody binds to a specific protein at the cell surface and thus attaches the whole cell to the bead.
- The cells are then collected by “pull-down”.
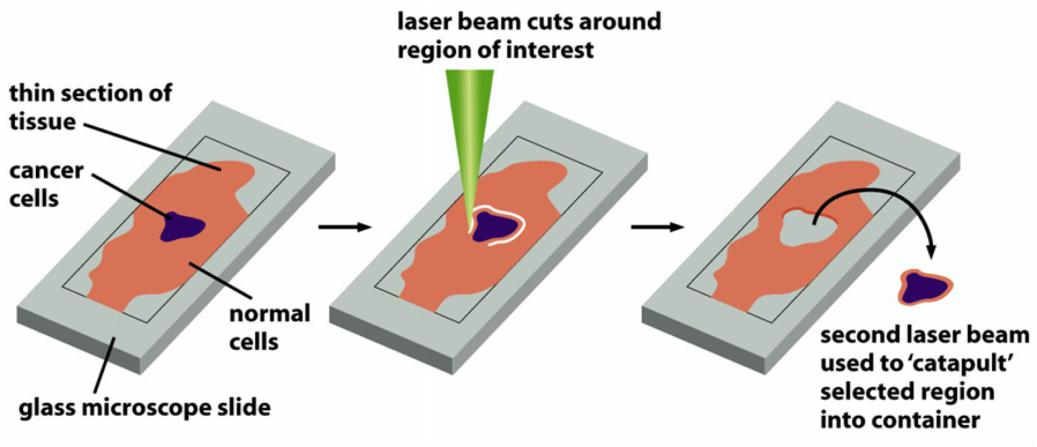
Laser capture microdissection
- excision of cells using a laser beam
Fluorescence-activated cell sorting (FACS)
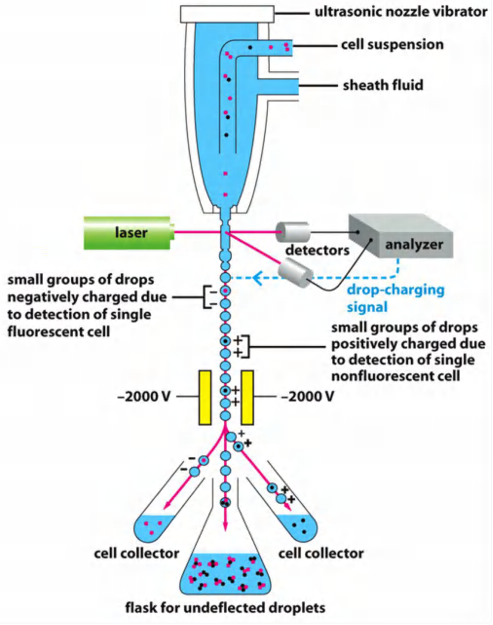
Prerequisite for sorting is the presence of a fluorescent marker in the cells (e.g. fluorescent dye or fluorescence-conjugated antibody)
Cells are guided by the sheath fluid into the center of the stream to get into the drops (1 cell/drop)
Detection of fluorescence (laser/analyzer)
Drops are electrically charged based on the result of analysis of fluorescence.
Sorting occurs then via deflection of the drop with the cell in an electrical field in different collection tubes
(Labeled cells can be negatively charged, non-labeled cells are positively charged)
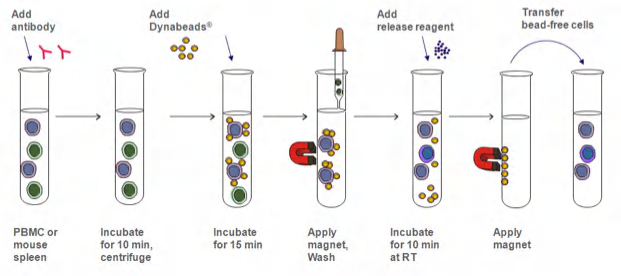
Magnetic beads (immunoprecipitation)
Cell sorting via immunoprecipitation using “magnetic beads”
Two strategies: positive isolation of cells and negative isolation of cells.
Positive isolation: Immobilize the cells you want
 and remove all other cells
and remove all other cells目标提取细胞可以被beads识别
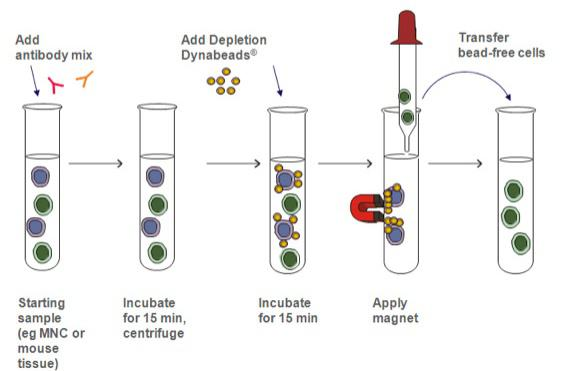
- Negative isolation: Immobilize the cells you want
 and remove all other cells
and remove all other cells - 目标提取细胞是不被beads识别的细胞
- Advantages: 目标提取的细胞没有被beads识别,因此保持了Natural property
Example: Isolation of T cells from thymus:
- Homogenize thymus tissue
- Add CD3-conjugated magnetic beads
- Immobilize cells by magnetic force
- Wash immobilized cells
- Mission accomplished!
Advantage: The cells you want to isolate remain “untouched”!
Laser capture microdissection
二、Cell culture
Growth & maintenance of cells in vitro
1. A basic cell culture media for mammalian cells usually contains:
- Buffer substance for pH regulation. Maintain Ph stability.
- Food source (e.g. glucose, glutamine, amino acids, nucleotides, etc.)
- Serum(血清)/growth stimulant (Some cells grows depends on growth factor)
- Minerals for metabolic functioning of the cells
- Antibiotic additives and pH indicator (Not necessary, cause the culture environment should be bacteria-free )
2. A basic cell culture media for plant cells usually contains
- Buffer substance for pH regulation
- Nitrogen source (e.g. NH4NO3)
- Minerals for metabolic functioning of the cells
3. Some commonly used media For Animal Cells
- Dulbecco’s modified essential medium (DMEM)
- RPMI-1640
- McCoy’s 5A
- F12, etc.
4. Commonly used media for plant cells
Murashige & Skoog (MS) medium in different concentrations:
- MS is perfect for most plants
- ½ strength is perfect for orchids
- some cell cultures like up to 2% sucrose
Plant medium is cheap
Immortalization of a cell line
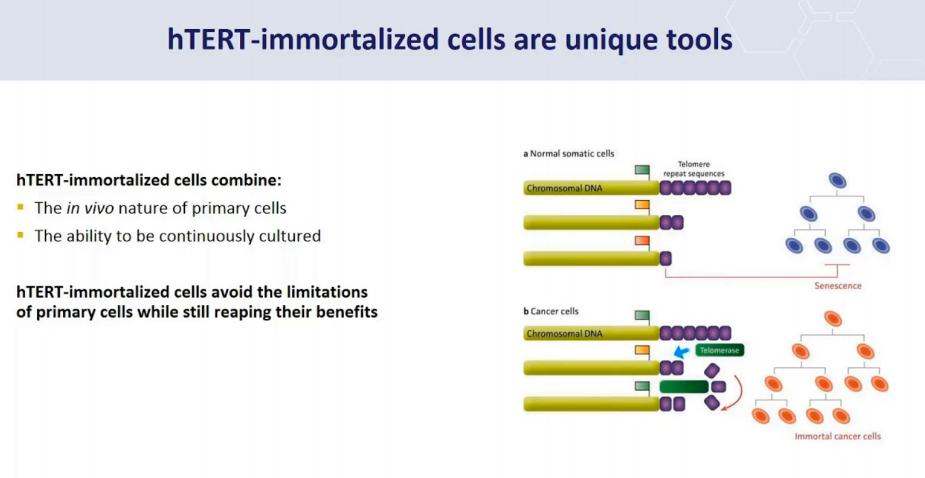
Immortalized cells are a population of cells which can escape normal cellular senescence (细胞衰老) and keep undergoing division. Thus, this kind of cells can grow in vitro for prolonged periods. This usually is due to some carcinogenic mutations.
Strategies to immortalization cells:
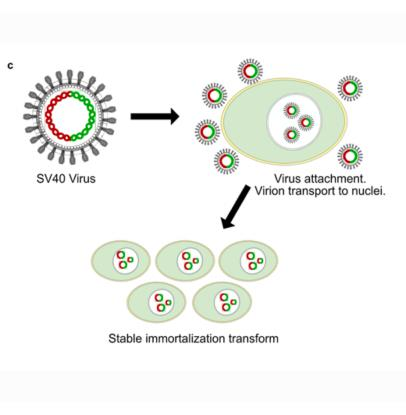
Virus to transform the cells, example is SV40:
Special Genes, example: hTERT:
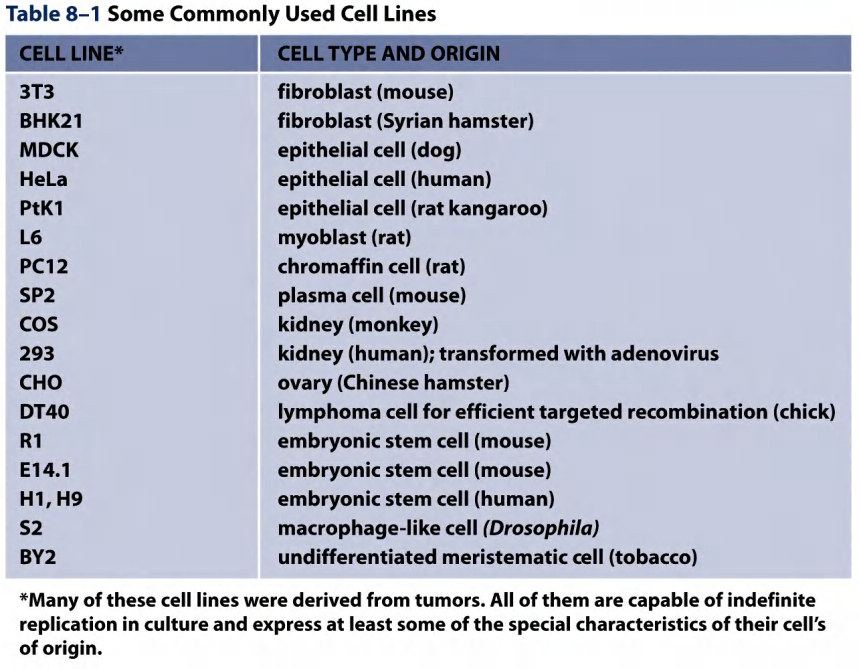
1. Commonly used cell lines
2. Authenticated Cell lines can be obtained from stock centers
ATCC Mission: To acquire, authenticate, preserve, develop, standardize, and distribute biological materials and information for the advancement and application of scientific knowledge.

ATCC serves by characterizing cell lines, bacteria, viruses, fungi and protozoa.
- 4,000 human and animal cell lines and 1,200 hybridoma.
- 18,000 strains of bacteria from 900 genera.
- 2,000 different types of animal viruses and 1,000 plant viruses.
- 49,000 yeast and fungi strains from 1,500 genera and 2,000 strains of protists.
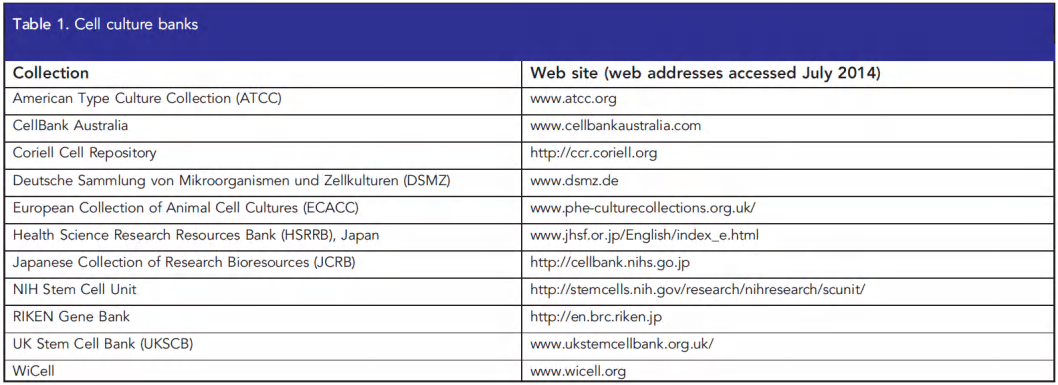
3. Generation of a “new” cell line by fusion of different cells
1 | |
Hybridoma cells: fusion of a differentiated cell with a tumor cell
Different types of cells can be fused together to display properties from the two sides…
Fusion with a tumor cell line results in immortal cells (immortalization)…
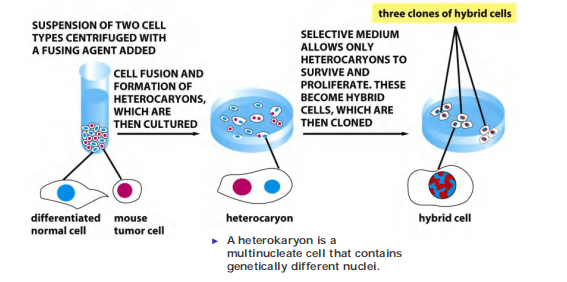
4. Hybridoma cells produce monoclonal antibodies in hybridoma cells
Generate antibody-secreting B lymphocytes by immunization of a mouse and fuse these cells with B lymphocyte tumor cells to generate a hybridoma line, an immortal, antibody-secreting cell line!!!
Form hybridoma:
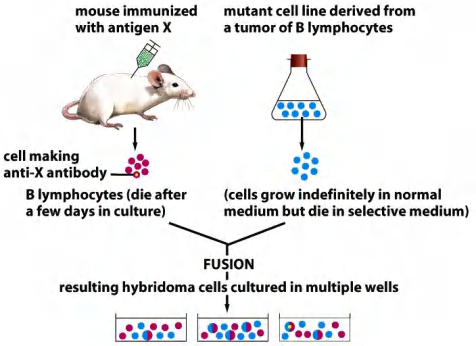
Next, screen the individual hybridoma to get the line that produces the best antibody…
All commercially available monoclonal antibodies are produced in hybridoma cells…
三、2D and 3D culture
3-D cell culture might reflect the in vivo condition more accurately.
In the in vivo situation, cells are embedded in the extracellular matrix (ECM), which are known to have effects on various essential cellular activities.
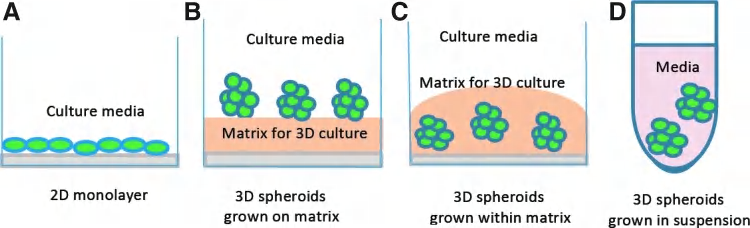
3D culture mimic the in vivo environment. (Animal cells cannot be cultured in suspension)
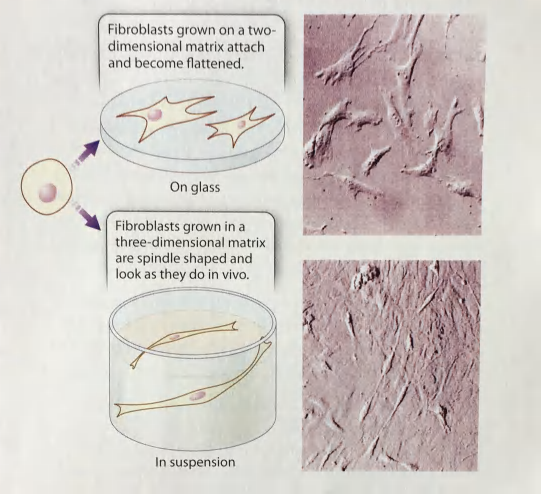
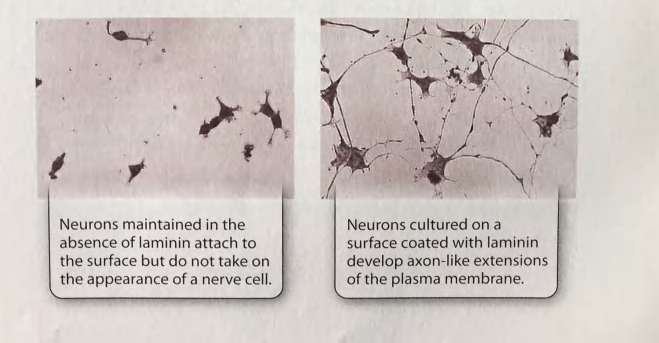
Cell shape determined by the structure and composition of the ECM
(Fibroblasts produce the ECM proteins and maintain the ECM environment)
1 | |
1 | |
Comparison between 2D and 3D culture
| 2D | 3D | |
|---|---|---|
| Cell interface to medium | All cells are equally exposed to media components | As in physiological conditions, there is gradient availability of media components. Upper layer of cells are highly exposed over the lower layer (Heterogeneous exposure) |
| Cell junction | Cell junctions are less prevalent and does not resemble physiological conditions | Cell junctions are prevalent and enable cell to cell communication. |
| Cell Differentiation | Moderately and poorly differentiated | Well differentiated |
| Drug metabolism | Drug metabolism not well observed | Enhanced drug metabolism with increased expression of CYP enzymes |
| Drug Sensitivity | Cells are sensitive and drugs show high efficacy | Cells often show resistance and drugs show low potency |
| Cell Proliferation 细胞增殖 | Higher proliferation rate than in natural environment | Proliferation rate may be high or low, it is based on cell type and 3D-cell culture technique. |
| Cell Shape | Flat and stretehed | Natural shape (ellipsoid/polarized) is retained |
| Response to Stimuli | Poor response to mechanical stimuli of cells | well-established responses to mechanical stimuli of cells |
| Viability | Sensitive to cytotoxin | Greater viability and less susceptible to external factor |
| Apoptosis 细胞凋亡 | Highly susceptible to drug-induced apoptosis | Enhanced resistance to drug-induced apoptotic stimuli |
Approaches commonly used to analyze culture cells
1. Imaging approaches
- Time-lapse live cell imaging
- Fluorescence-based imaging
- Immunofluorescence (IF) imaging
- EM, etc..
2. Biochemical approaches
Cell lysis and analyze proteins
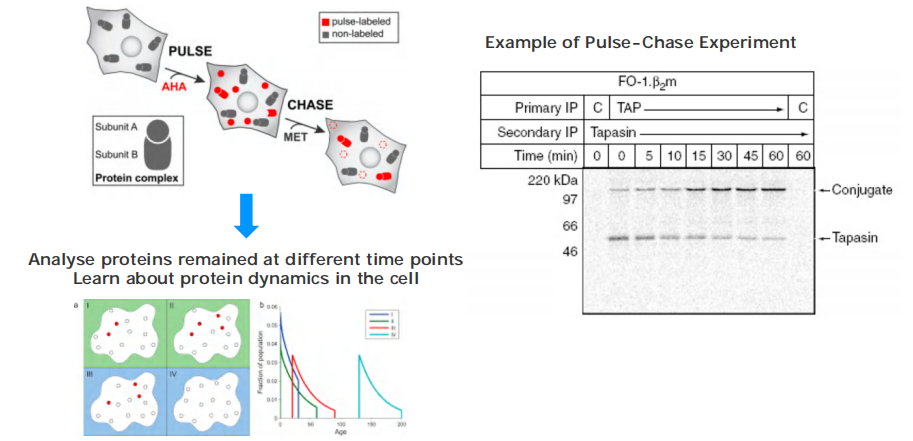
“Pulse-chase” experiments
3. Genetic manipulation of culture cells
- Mutagenesis (random)
- Gene-editing (knock-out or knock-in)
- Depletion of genes with specific siRNA (called knock-down)
- Ectopic expression (overexpression of genes)
- …
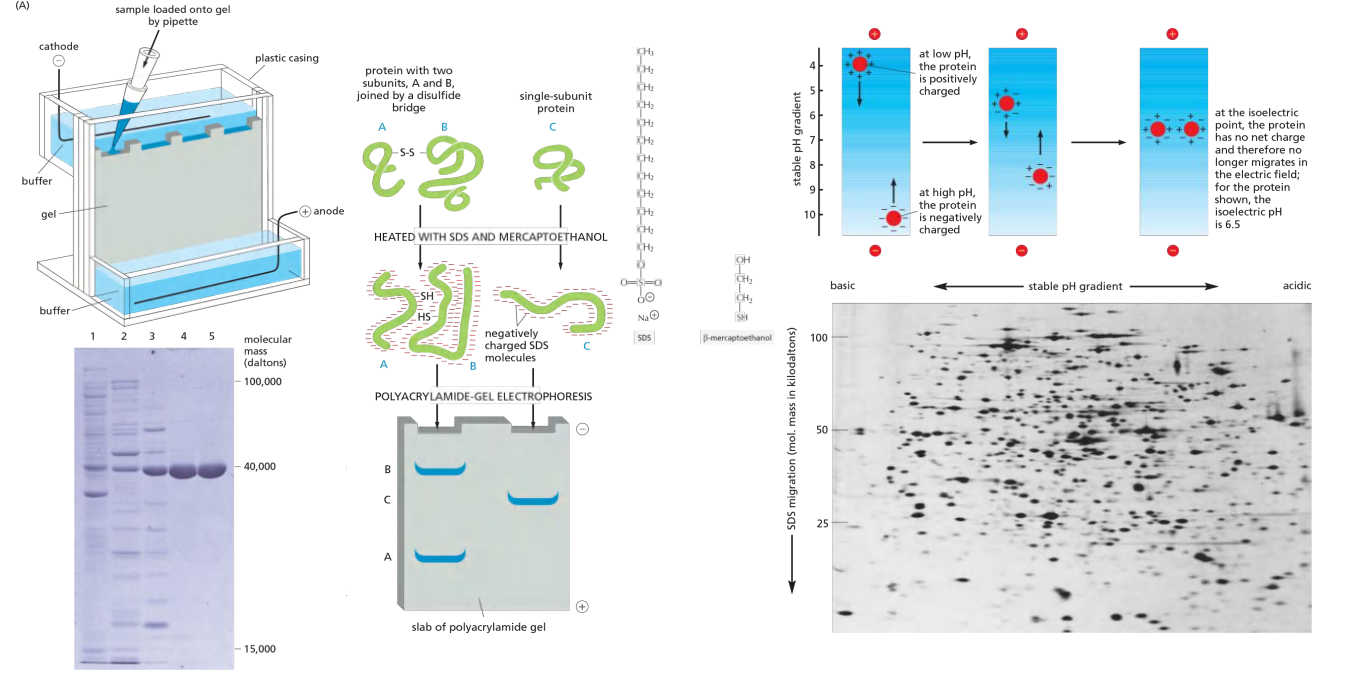
Protein analyses by electrophoresis
Pulse-chase experiments
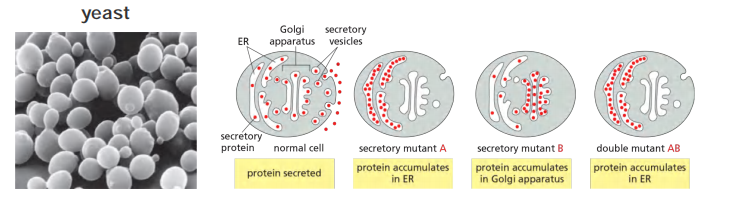
Using genetics in the cultured cells
Genetic manipulation of culture cells
Mutagenesis (random)
Gene-editing (knock-out or knock-in)
Depletion of genes with specific siRNA (called knock-down)
Ectopic expression (overexpression of genes)
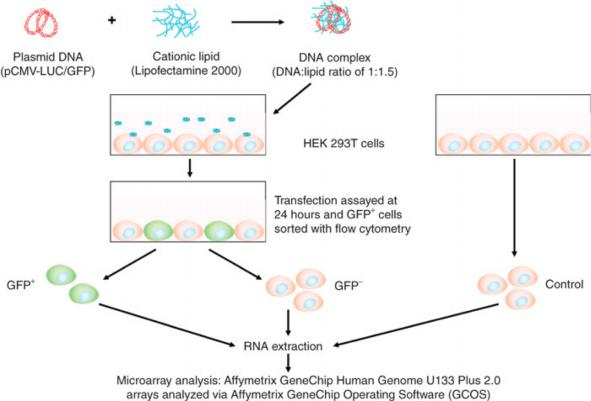
四、Transfection of the cell
Transfection/infection of culture cells
Transfection(转染): Delivery of exogenous gene (DNA, mRNA) into cultured cells
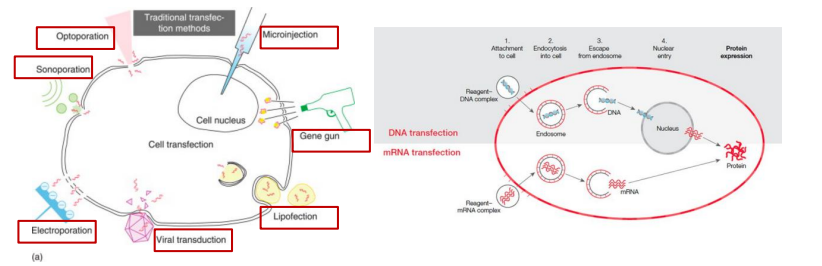
- Optoporation
- Microinjection
- Sonoporation
- Electroporation
- Electroporation
- Viral transduction: Using virus as a vector for the delivery is often called infection.
- Lipofection(脂质转染)
- Gene Gun
Control gene expression and observe effects
1. Inducible gene expression
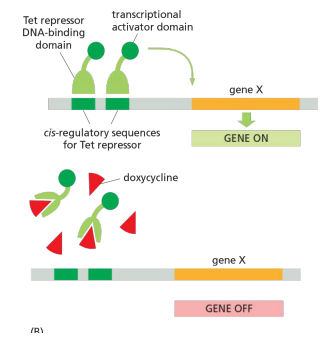
2. Analyze effects at cellular and molecular levels