L11 Sensory System
一、Organization of the sensory systems
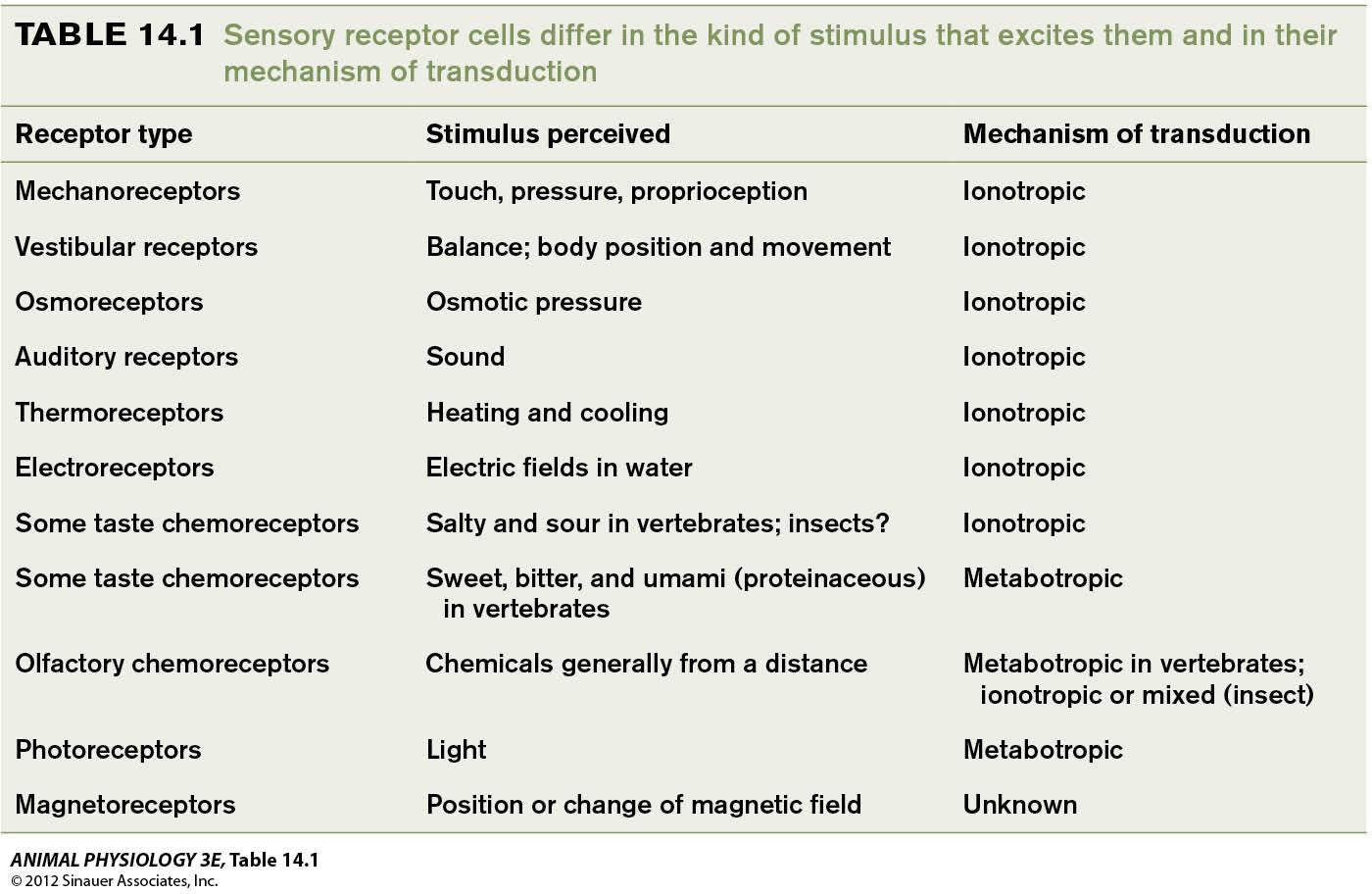
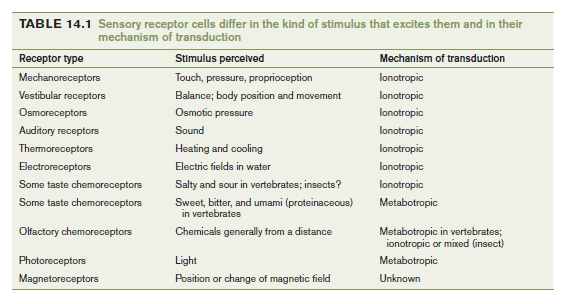
The 1st way to classify sensory receptors is by Sensory Modality
- Mechanoreceptor and touch
- Vestibular organs and hearing
- Chemoreception and taste
- Olfaction and smell
- Photoreception and vision
The 2nd way of classification is based on the form of Stimulus Energy that excites sensory receptors
- Electromagnetic energy (photoreceptors, electroreceptors and magnetoreceptors)
- Mechanical energy (auditory receptors, mechanoreceptors and vestibular receptors)
- Chemical energy (olfactory and taste receptors)
The 3rd classification is by mechanism of transduction
- Ionotropic transduction (Ionotropic receptors or ligand-gated channels)
- Metabotropic transduction
The 4th way of classification is by the location of the source of the stimulus energy relative to the body
- Exteroceptors
- interoceptors
Two kinds of sensory transduction mechanisms
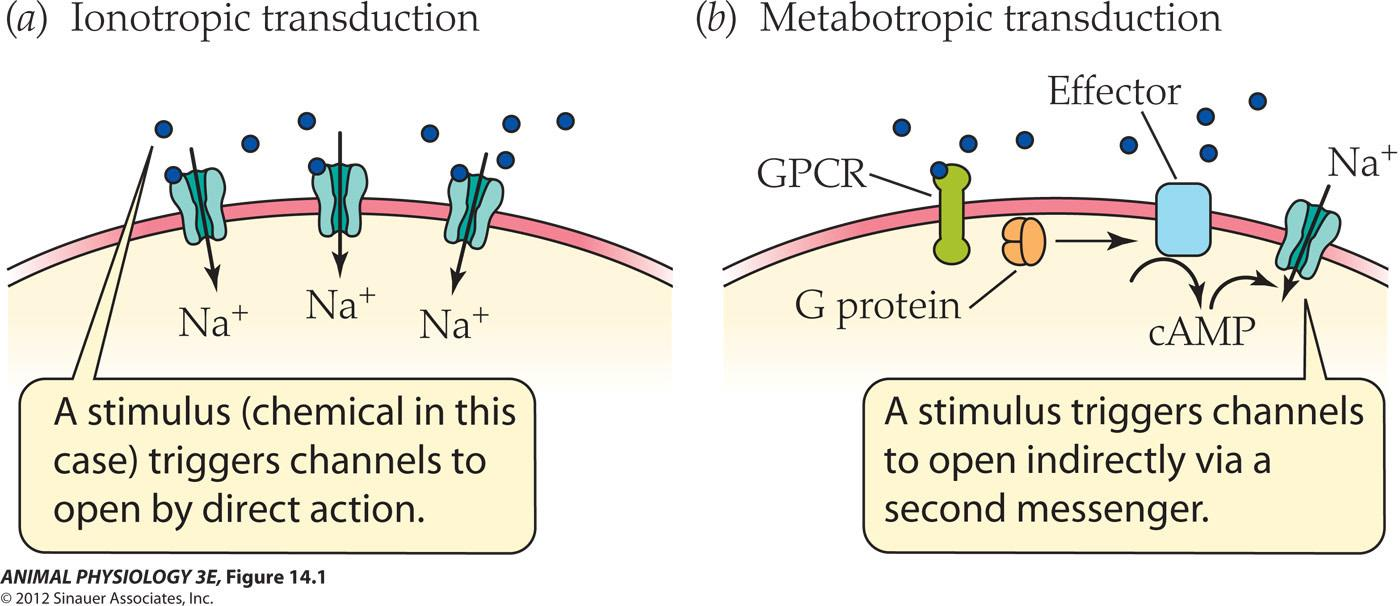
- Ionotropic transduction: A stimulus( chemical in this case) triggers channels to pen by direct action
- Metabotropic transduction: A stimulus triggers channels to open indirectly via a second messenger
The principle of labeled lines in sensory systems
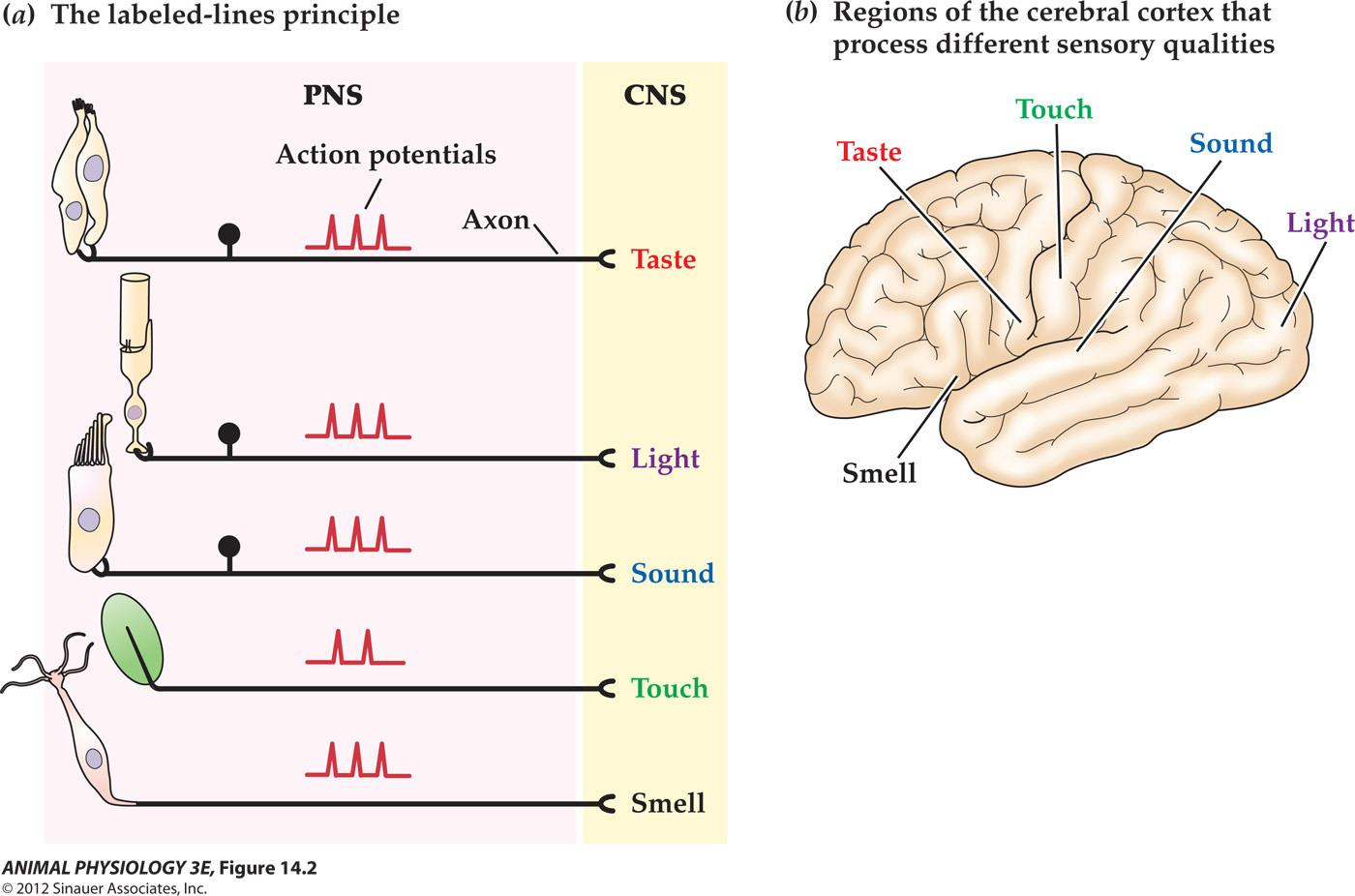
The principle of labeled lines in sensory systems
- In the peripheral nervous system (PNS), receptor cells sensitive to different kinds of stimuli send similar kinds of signals (action potentials) to the central nervous system (CNS). The CNS interpretation of the sensory modality depends on which lines (axons) convey the signals.
- Note that the sensory receptor cells for touch and smell are neurons with axons that enter the CNS, whereas the sensory cells that detect sound and taste have no axons but instead synaptically excite sensory neurons.
- In the CNS, different sensory pathways ultimately project to different regions of the cerebral cortex, providing the anatomical basis for the principle of labeled lines.
Localization of function in the human brain
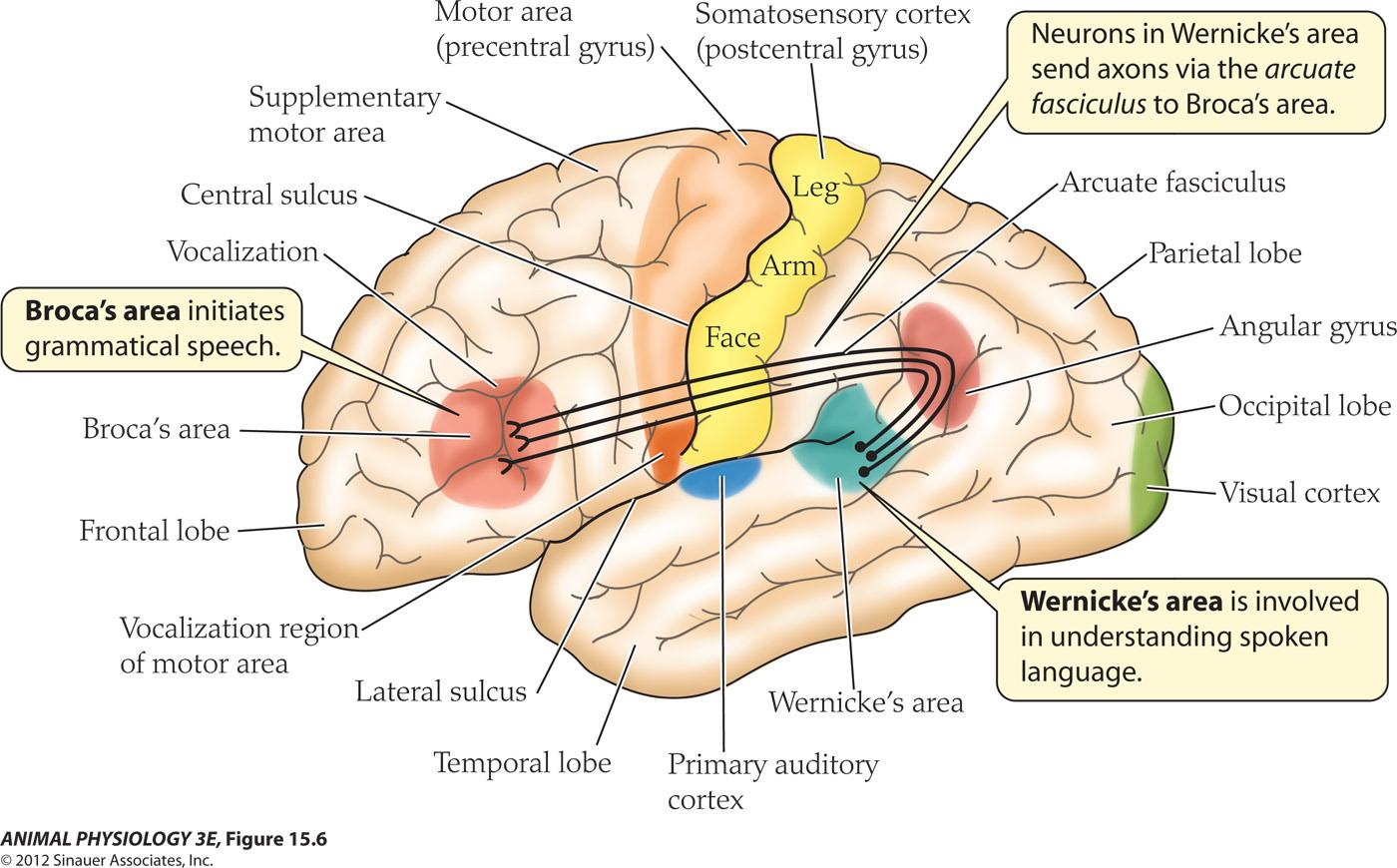
- Broca’s area initiates grammatical speech
- Wernicke’s area is involved In understanding spoken language
- Neurons in Wernicke’s area send axons via the arcuate fasciculus to Broca’s area
Summary
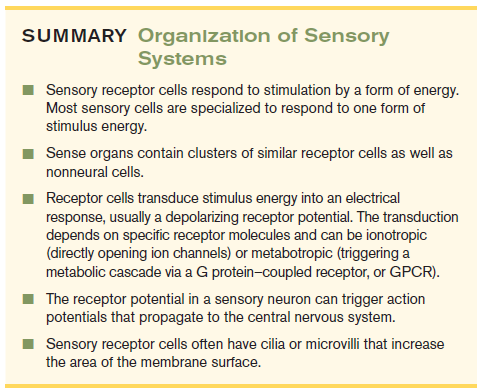
- Sensory receptor cells respond to stimulation by a form of energy Most sensory cells are specialized to respond to one form of stimulus energy
- Sense organs contain clusters of similar receptor cells as well as nonneural cells
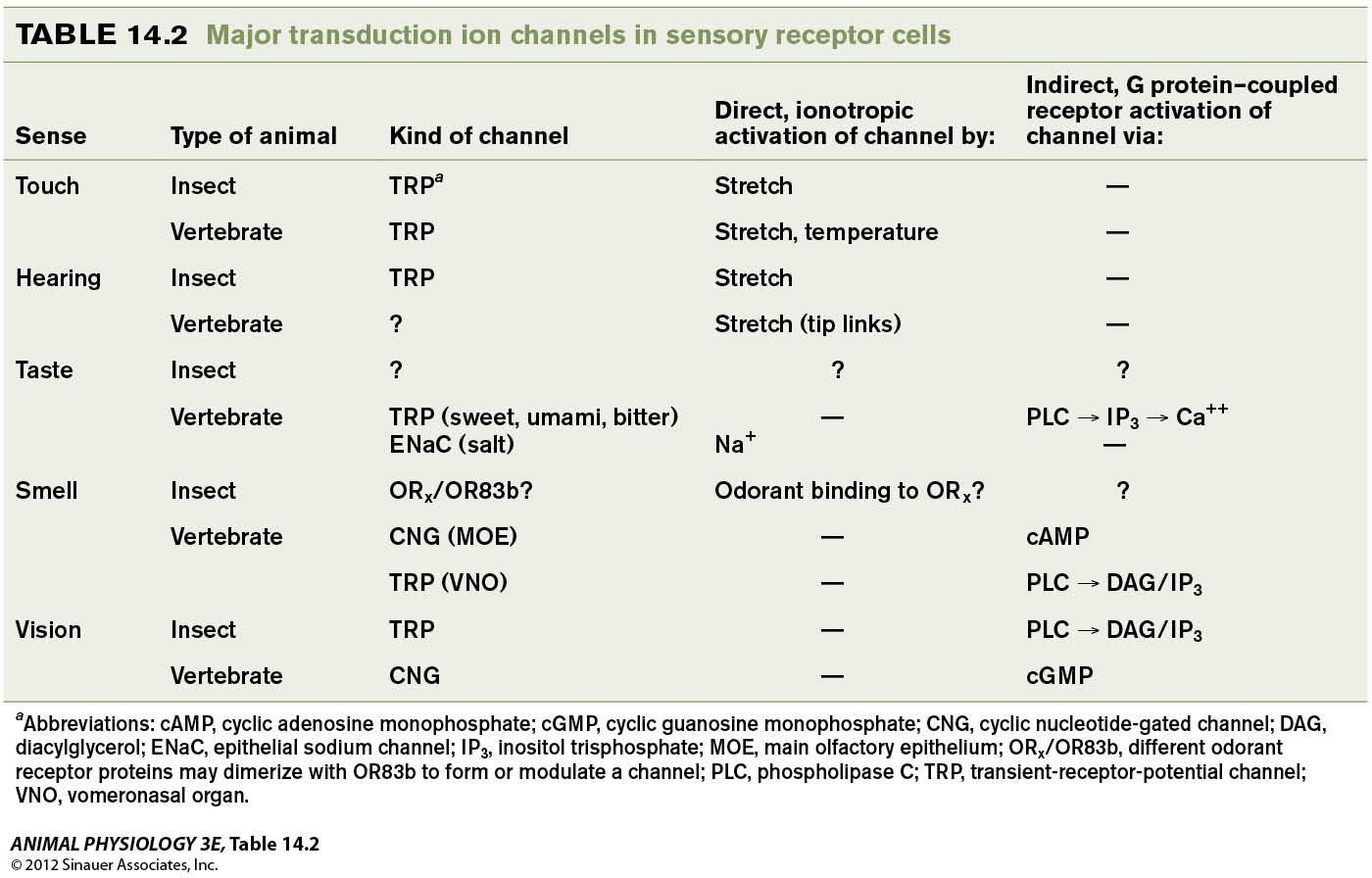
- Receptor cells transduce stimulus energy into an electrical response, usually a depolarizing receptor potential. The transduction depends on specific receptor molecules and can be ionotropic (directly opening ion channels)or metabotropic(triggering a metabolic cascade via a G protein-coupled receptor, or GPCR
- The receptor potential in a sensory neuron can trigger action potentials that propagate to the central nervous system
- Sensory receptor cells often have cilia or microvilli that increase the area of the membrane surface
二、Mechanoreception and touch
Insect cuticular mechanoreception 昆虫表皮机械感受:
Insect bristle sensilla exemplify mechanoreceptor responses
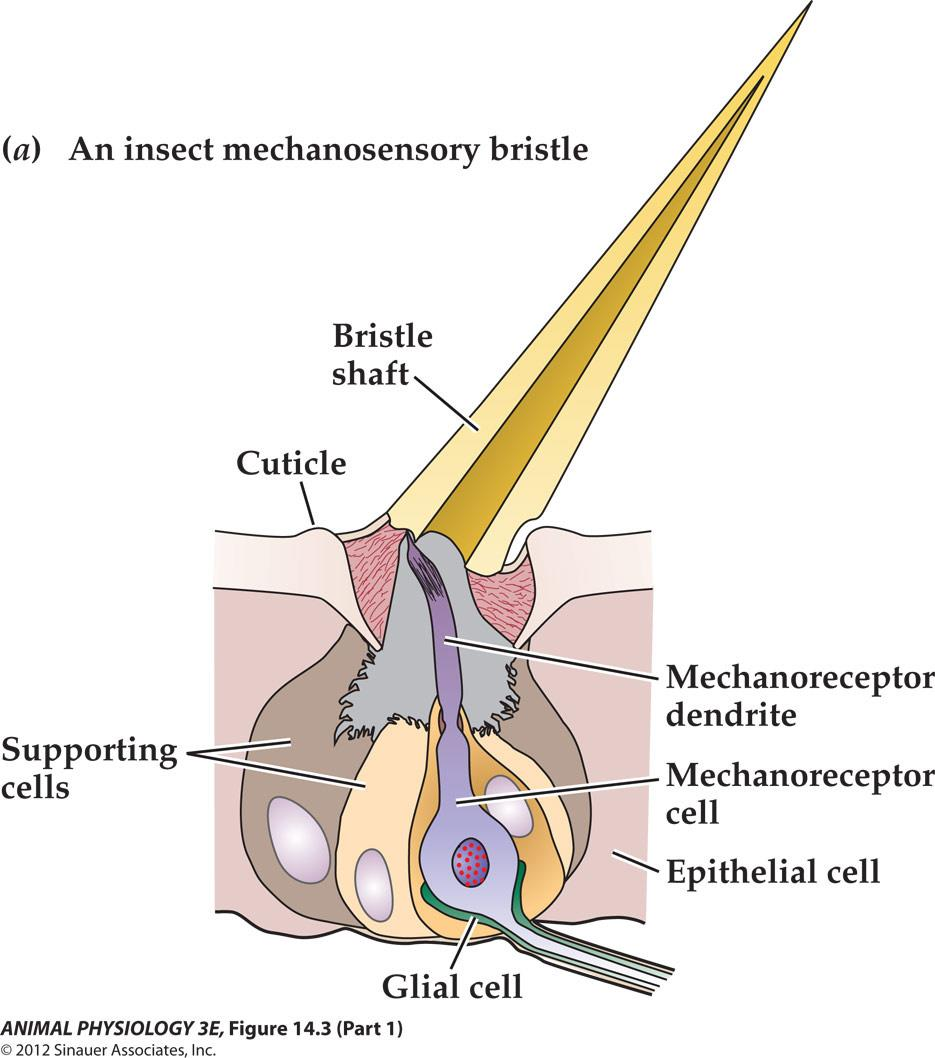
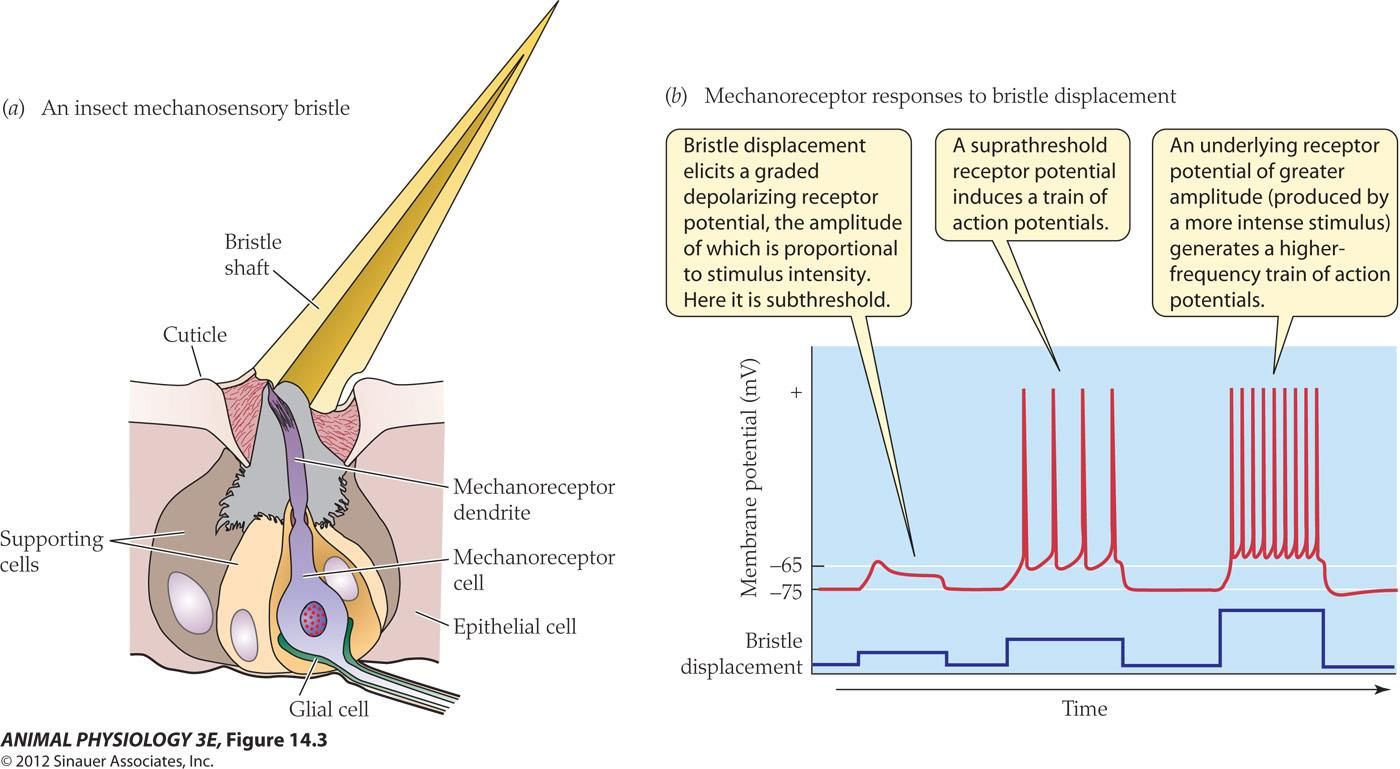
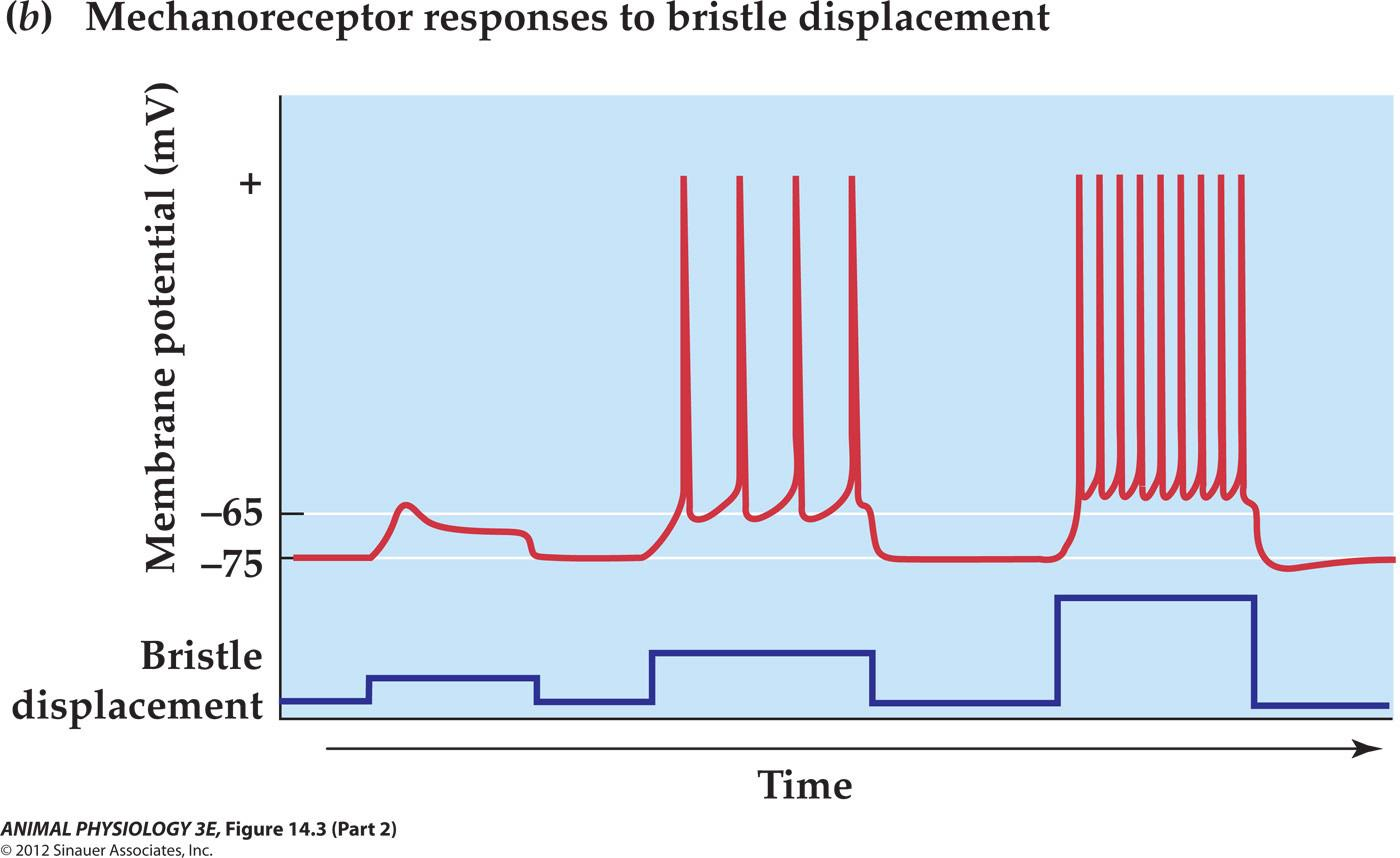
- Bristle displacement elicits a graded depolarizing receptor potential, the amplitude of which is proportional to stimulus intensity
- A suprathreshold receptor potential induces a train of action potentials.
- An underlying receptor potential of greater amplitude(produced by a more intense stimulus generates a higherequenacy train of action potentials
Stretch-activated channels
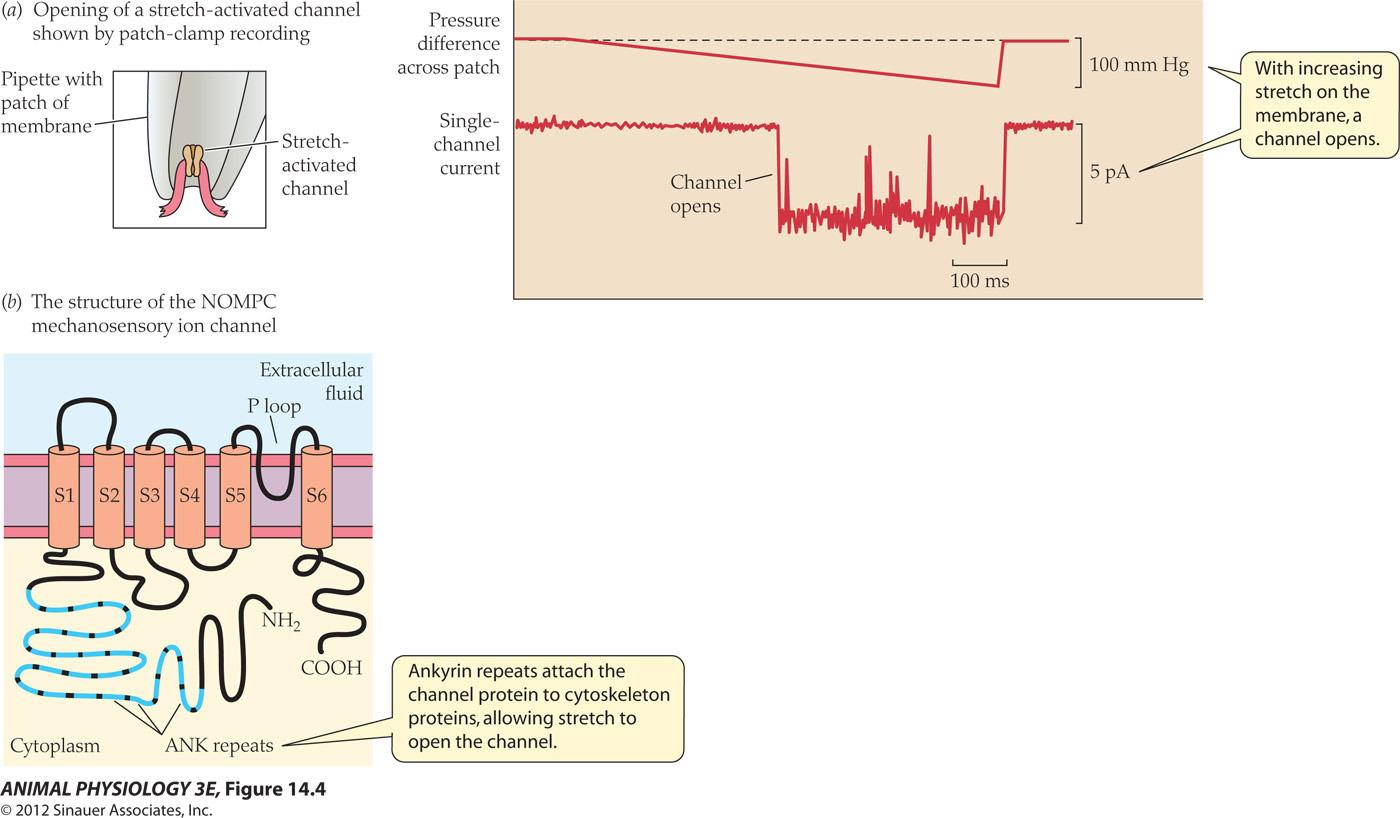
- (a) Patch-clamp recording from a patch of membrane isolated from a mammalian muscle fiber (see Figure 12.17). An increasing pressure difference across the patch (shown as millimeters of mercury [mm Hg]) distorts the membrane patch and opens the stretch-activated channel (single-channel currents shown in picoamps [pA], see Figure 12.17).
- (b) Protein subunit of the NOMPC ion channel of Drosophila (see page 364), which transduces mechanical deformation into an electrical receptor potential (see Figure 14.3). The protein is related to a voltage-gated K+ channel protein (see Figure 12.21) but without the voltage sensitivity; instead its ankyrin (ANK) repeats attach the protein to cytoskeletal components to provide mechanical sensitivity. Like the voltage-gated K+ channel protein, the NOMPC ion channel has six transmembrane αhelices (S1–S6) and a P loop. (Note: The structure of the mammalian channel shown in [a] may differ from that of the Drosophila channel shown in [b].) (a after Hamill 2006.)
Mechanoreceptor cells in mammalian skin
1. Touch receptors
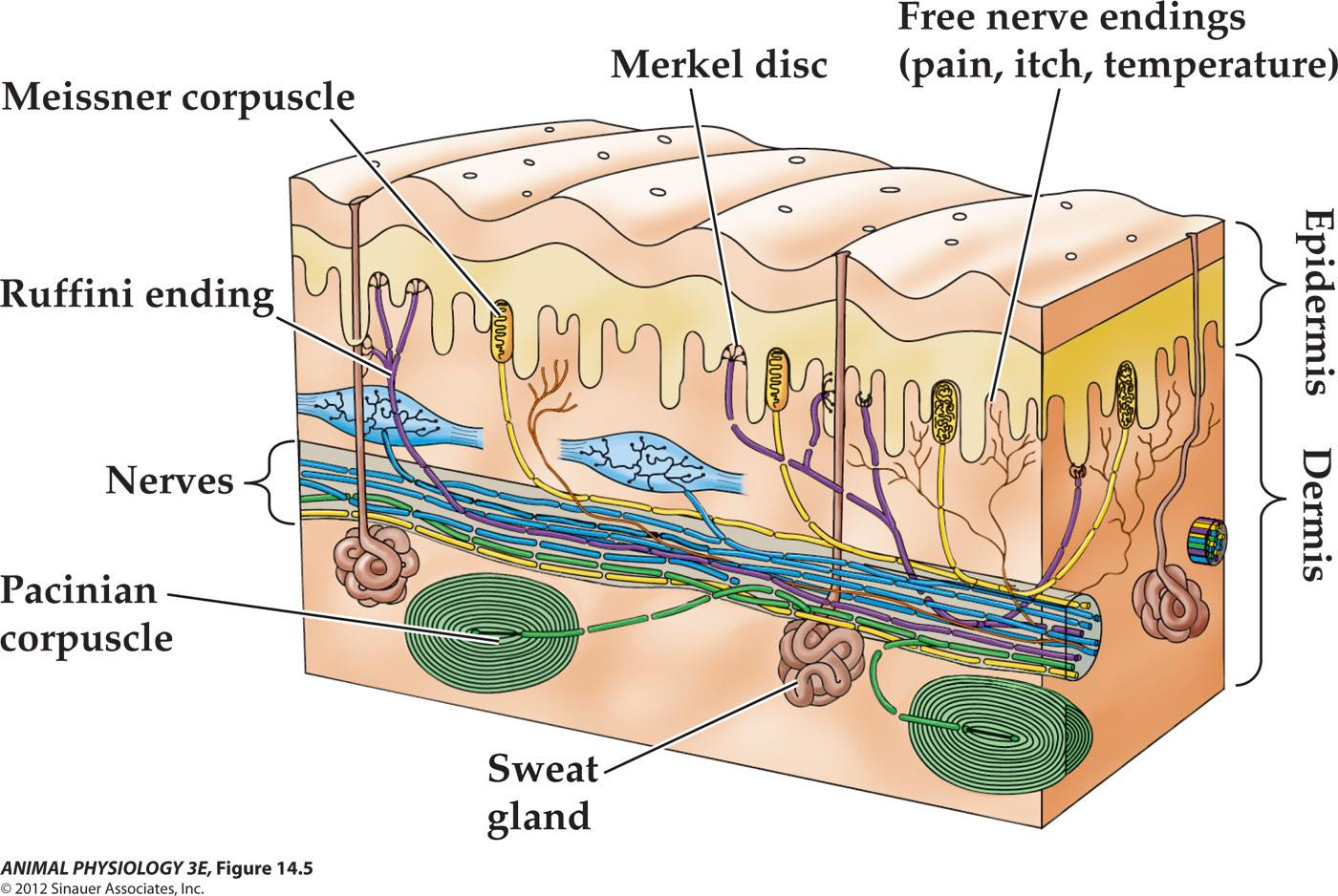
2. Tonic and phasic receptors
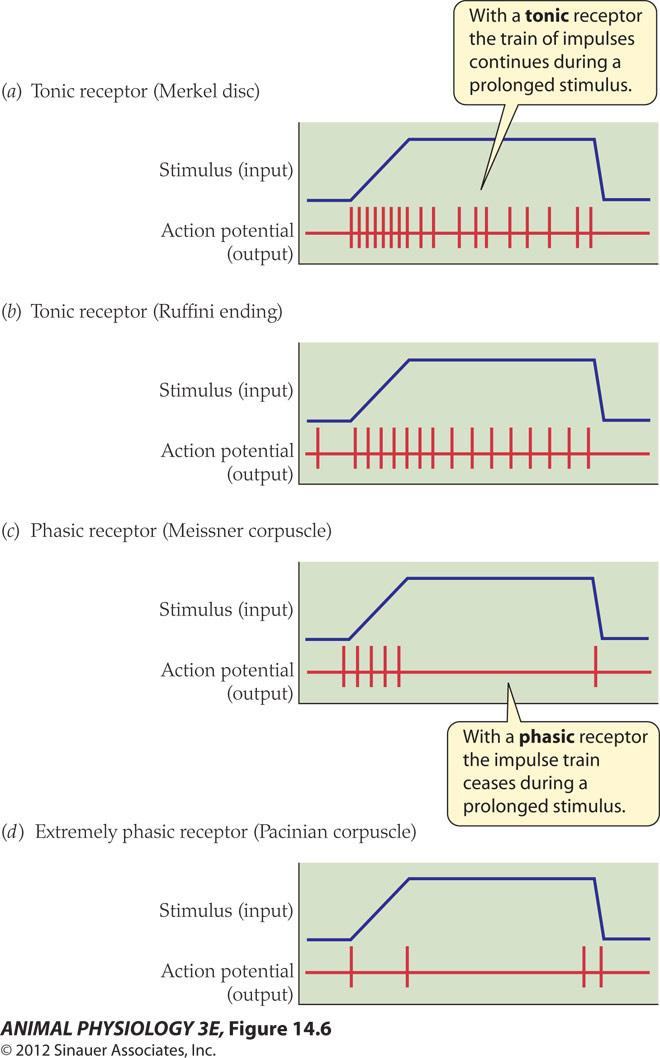
- With a tonic receptor the train of impulses continues during a prolonged stimulus.
- With a phasic receptor the impulse train ceases during a prolonged stimulus
For most sensory receptors, the frequency of action potentials in response to a continuous and constant stimulation decreases over time, a process termed sensory adaptation
Two basic types of responses can be recorded, called tonic (slowly adapting) and phasic (rapidly adapting).
What is the difference between phasic and tonic receptors?
三、Vestibular organs and hearing
Hair cells of the vertebrate acoustico-lateralis system
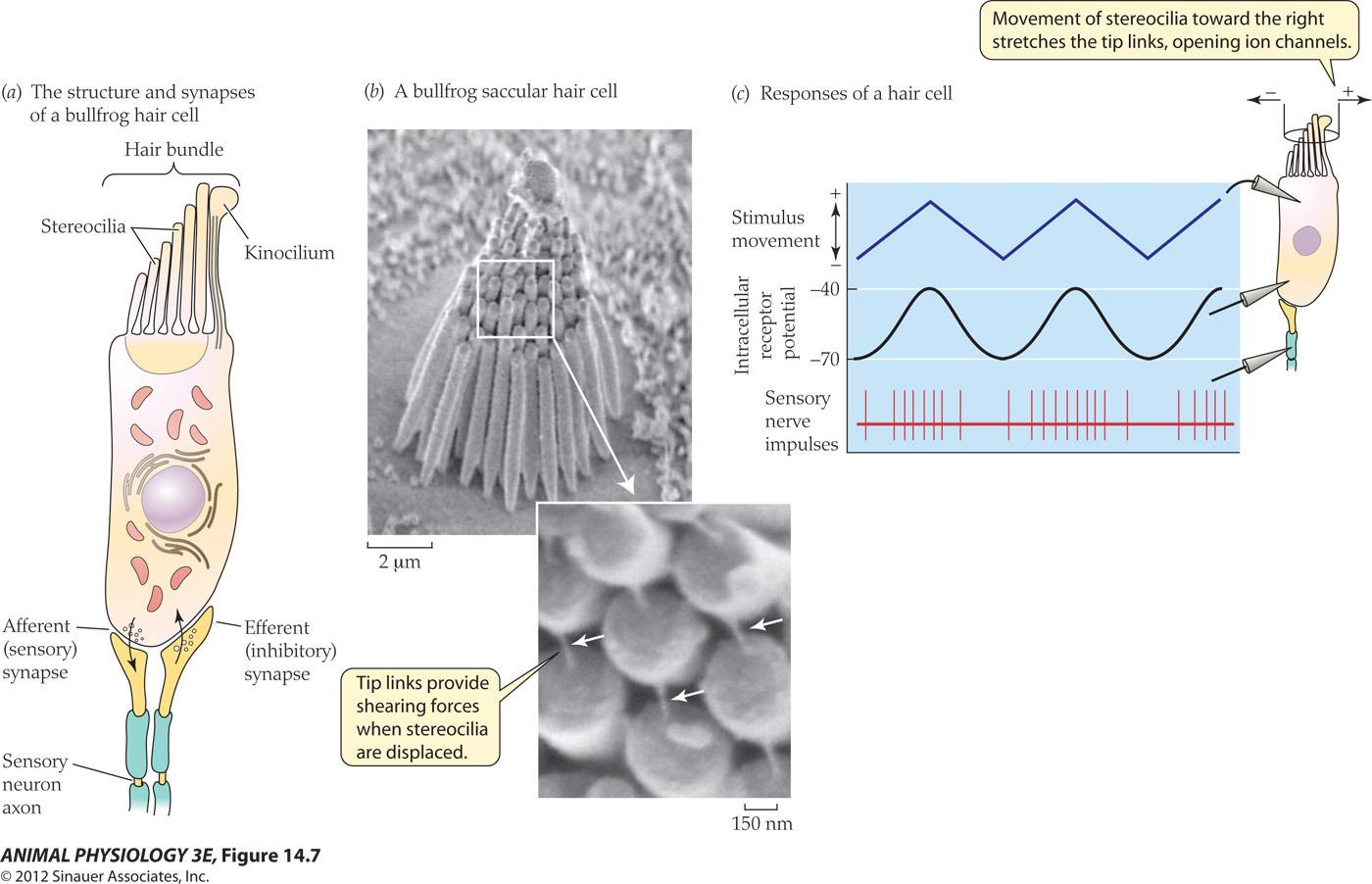
- (a) Hair cells contain stereocilia arranged in ranks from shortest to longest. A kinocilium (if present) is at the end with the longest stereocilia. Hair cells form synapses with afferent and efferent nerve axons.
- (b) This scanning electron micrograph shows the stereocilia and kinocilium of a bullfrog saccular hair cell. The inset shows tip links between adjacent stereocilia at high magnification (arrows).
- (c) Hair cells are depolarized and excited by movements of the stereocilia toward the kinocilium. Displacement of stereocilia away from the kinocilium Hyperpolarizes the hair cell, decreasing impulse frequency in the sensory axon. (Micrographs courtesy of Peter Gillespie; from Strassmaier and Gillespie 2002.)
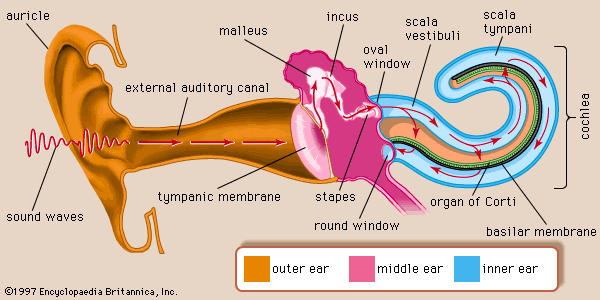
Anatomy of the Ear
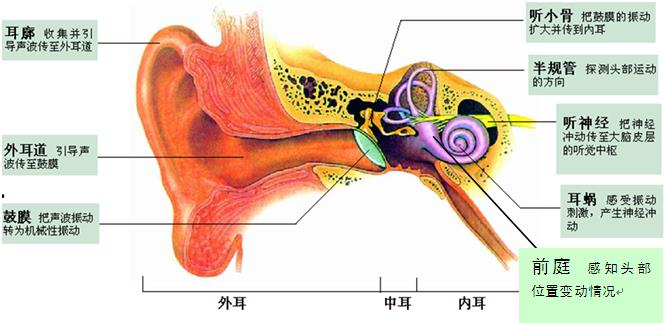
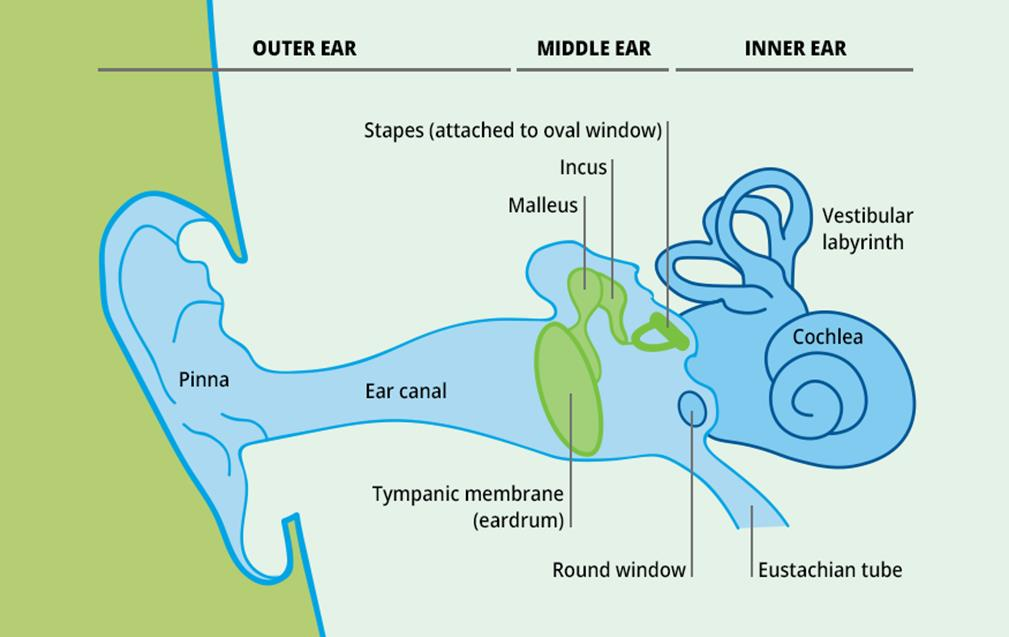
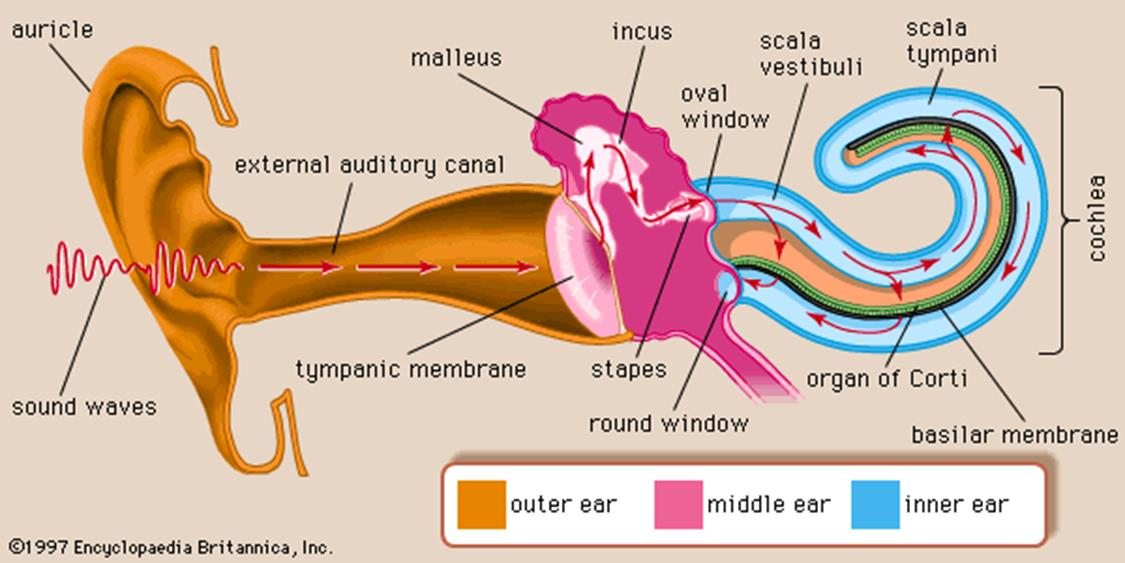
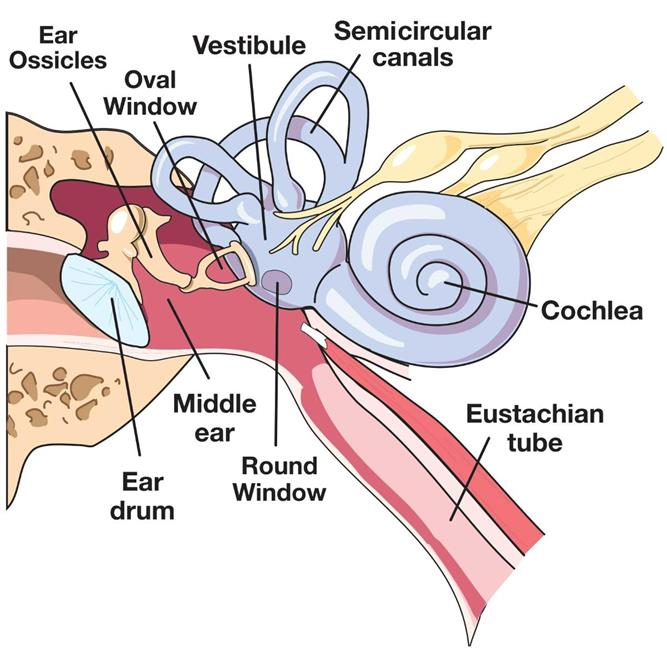
1. Anatomy of the mammalian ear
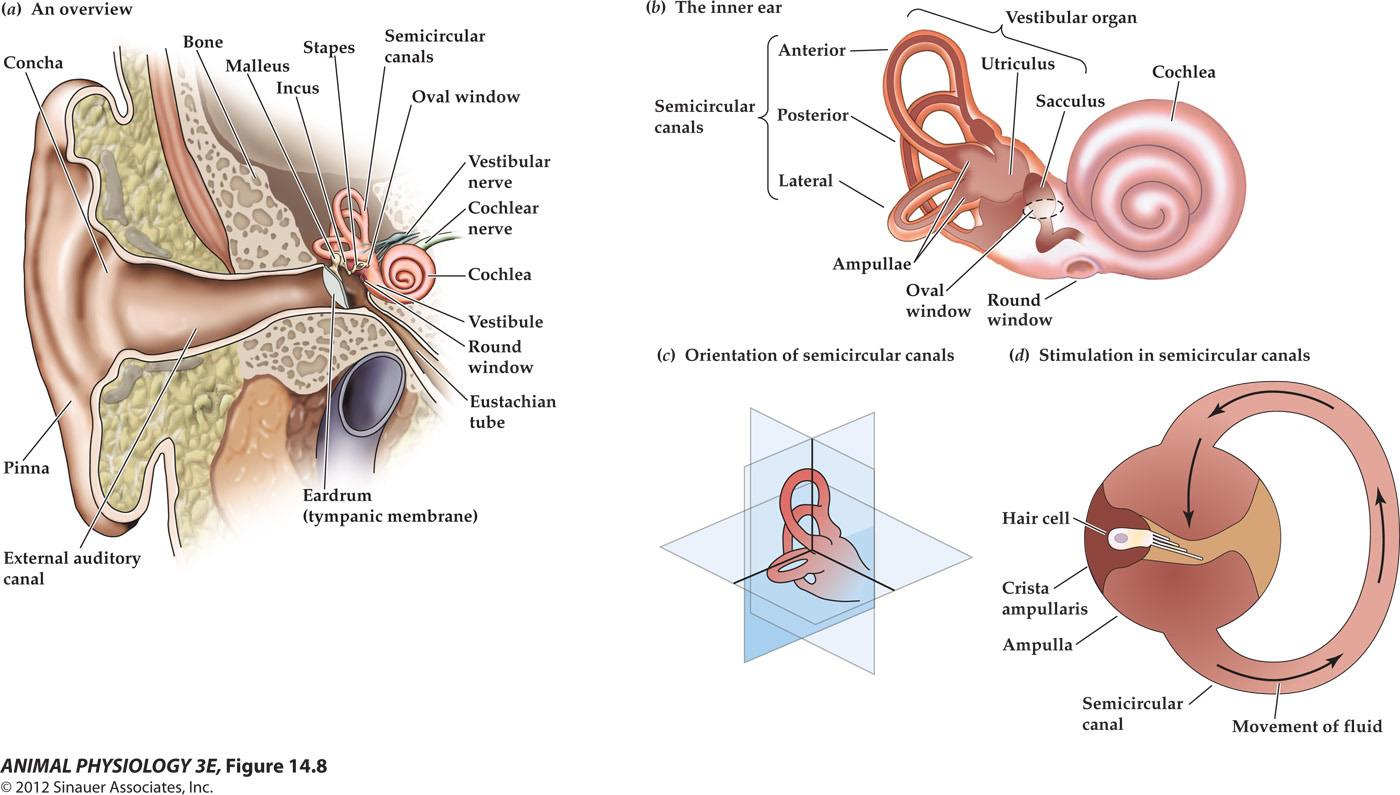
- (a) Structure of the human ear.
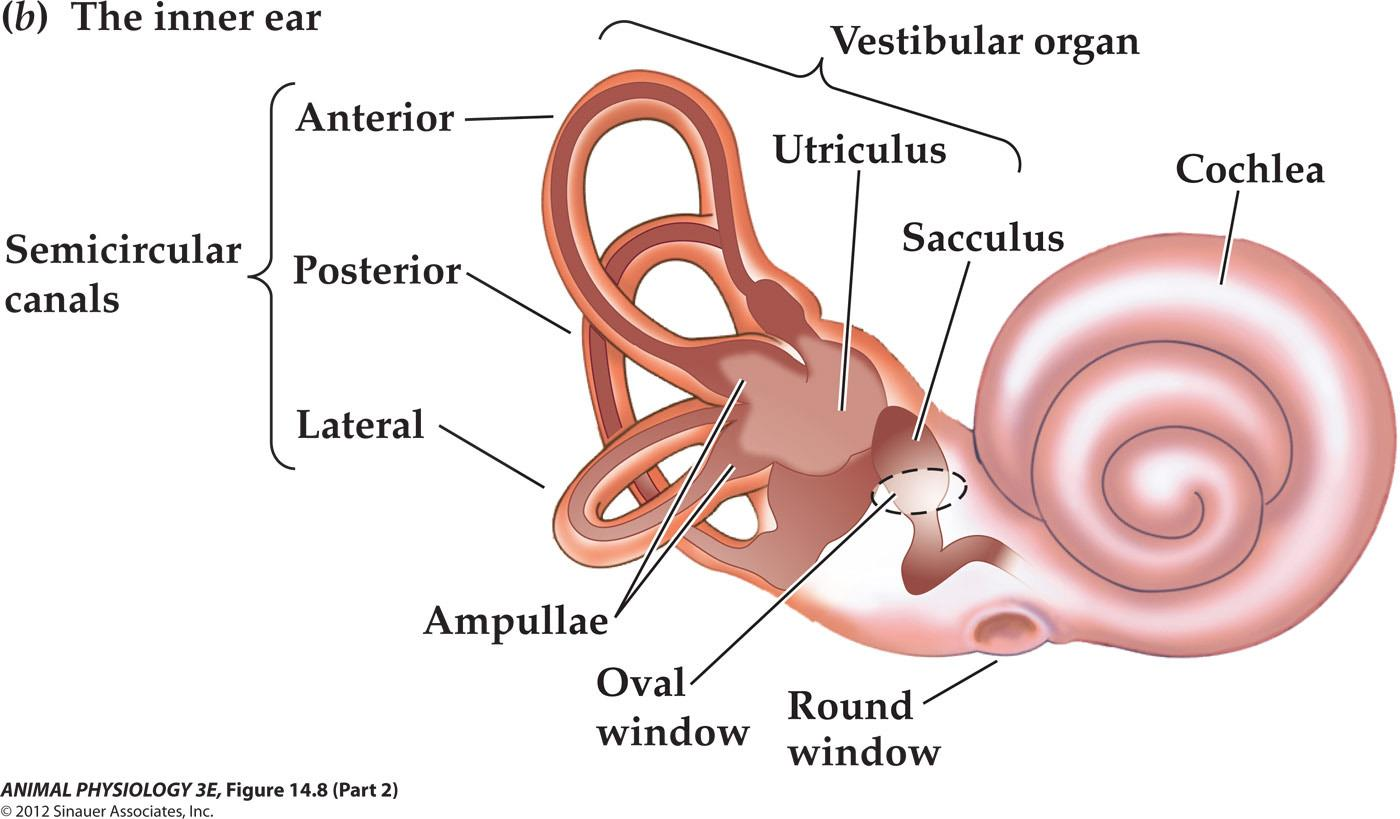
- (b) Components of the inner ear. The semicircular canal receptors are stimulated by head rotation. The utriculus and sacculus contain macular hair cells that are stimulated by linear motion of the head and by gravity. The cochlea contains auditory receptors.
- (c) The three semicircular canals of the inner ear are at approximately right angles to each other, so that any angular movement of the head stimulates at least one of them.
- (d) With rotation of the head, fluid movement in the canal stimulates the hair cells.
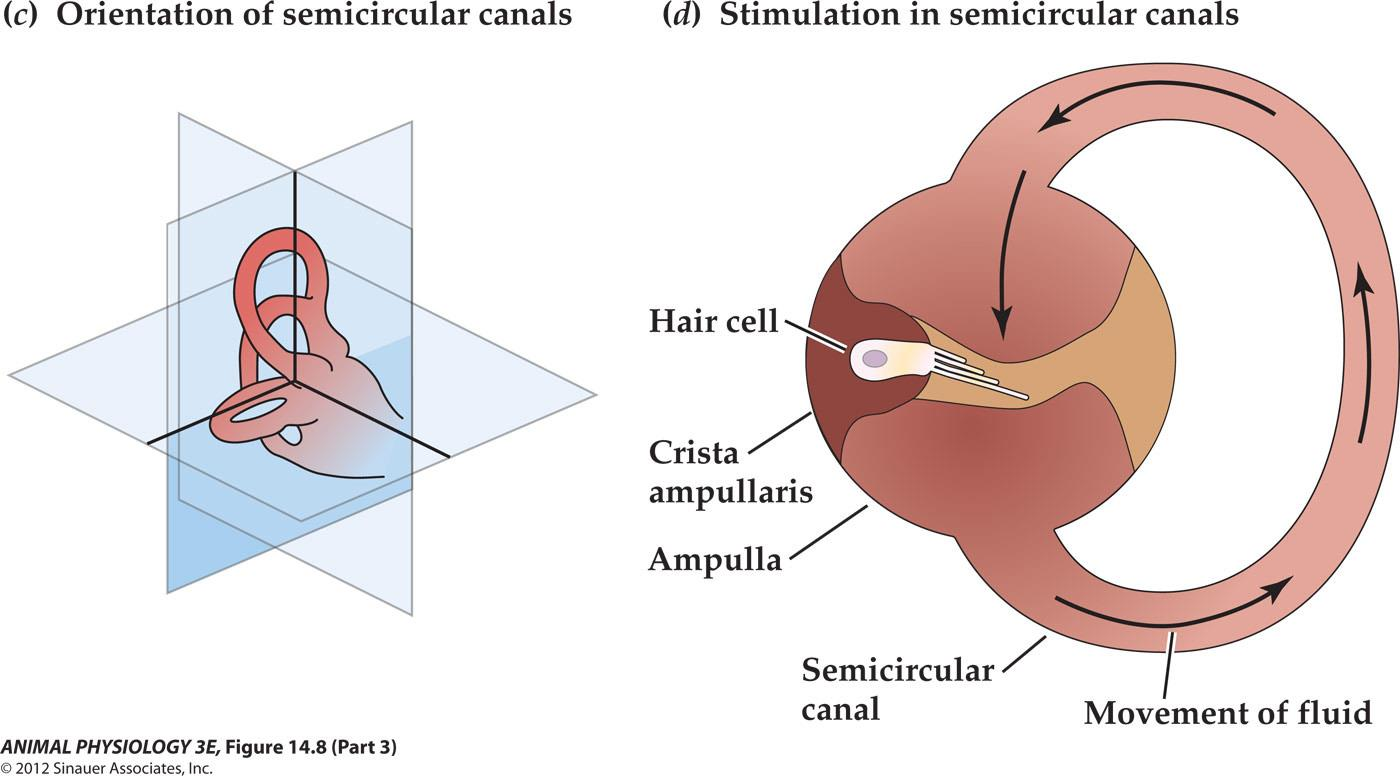
At the base of each canal, a region called the ampulla contains a cluster of hair cells in a structure called the crista ampullaris(壶腹嵴) (Figure 14.8d). Acceleration of the head causes fluid (endolymph) in the ampulla to slosh against the hair bundles of the hair cells, like water sloshing in a bowl when the bowl is suddenly moved. This movement of fluid pushes against the crista ampullaris and deflects the bundles of the hair cells, opening or closing mechanoreceptive channels as in the bullfrog hair cells
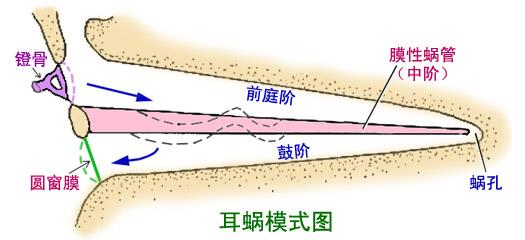
2. Anatomy of the cochlea
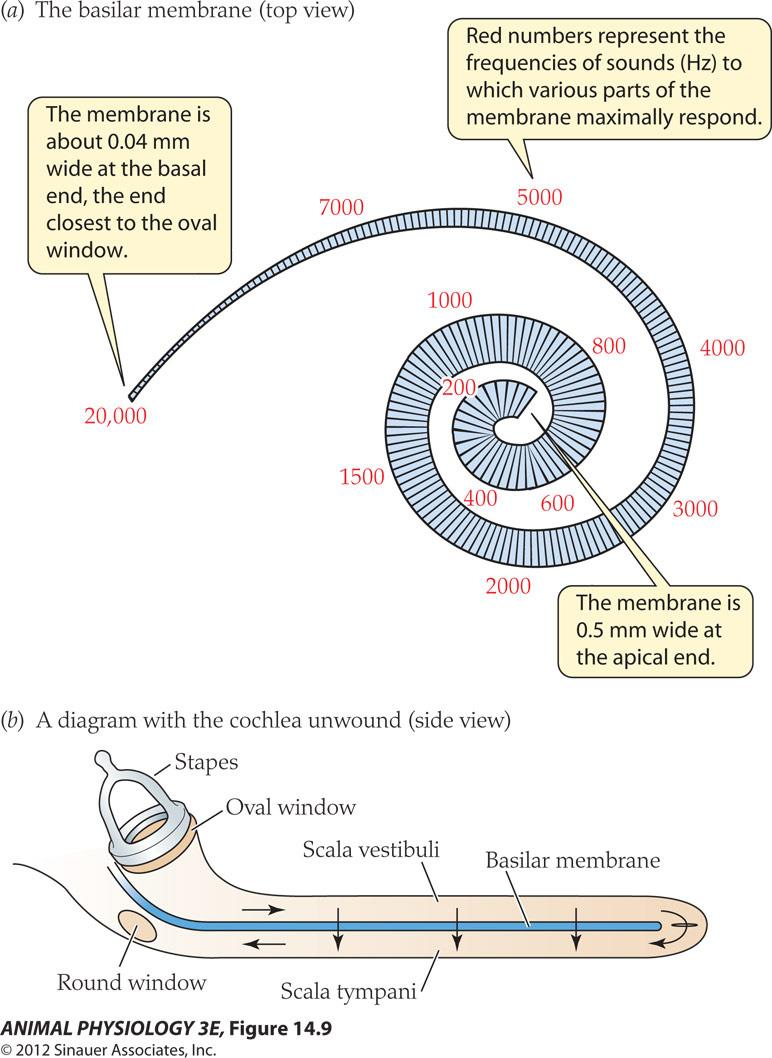
- The membrane is about 0.04 mm wide at the basal end. the end closest to the oval window
- Red numbers represent the frequencies of sounds( Hz)to which various parts of the membrane maximally respond
- The membrane is 0.5 mm wide at the apical end.
Amplitude of movement of the basilar membrane differs at different sound
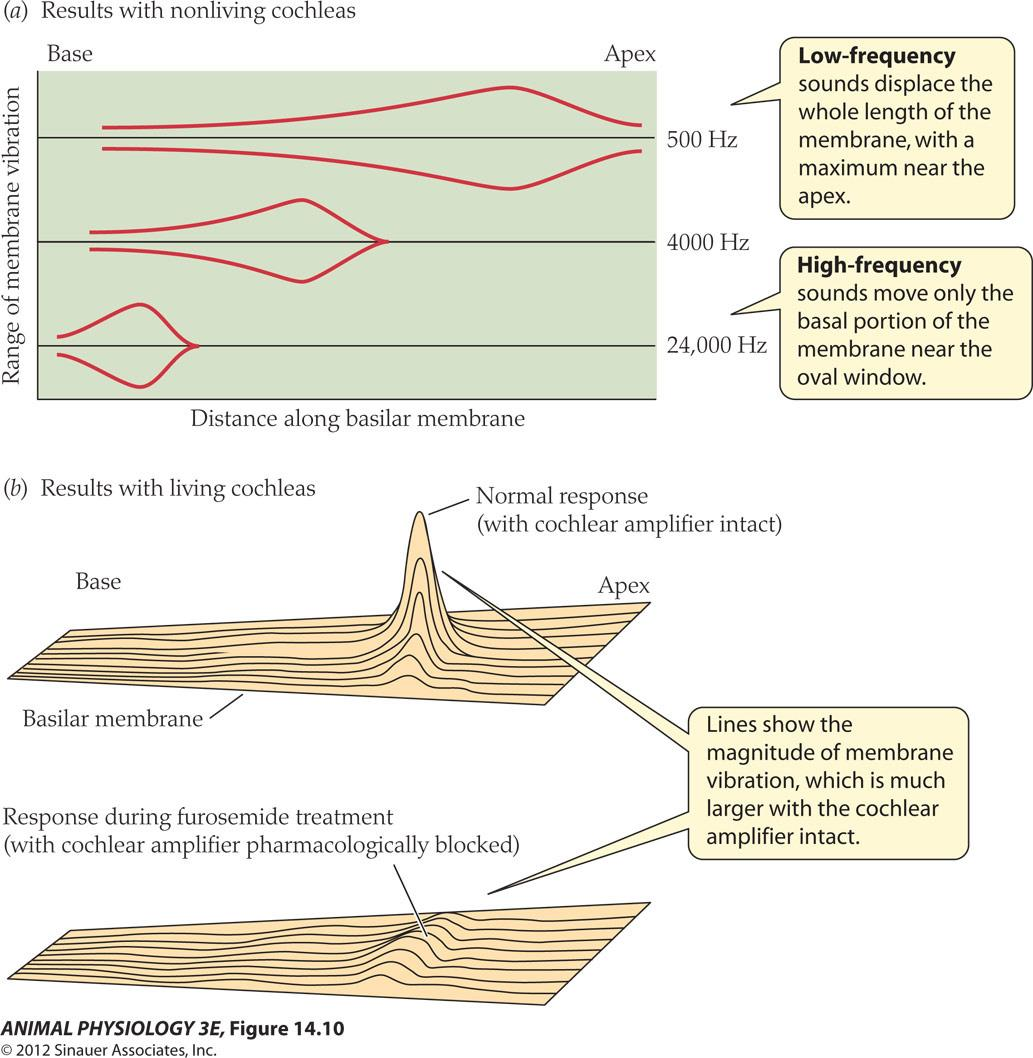
- Low-frequency sounds displace the whole length of the membrane with a maximum near the apex.
- High-frequency sounds move only the basal portion of the membrane near the oval window
- Lines show the magnitude of membrane vibration, which is much larger with the cochlear amplifier intact
Auditory interneurons and sound localisation
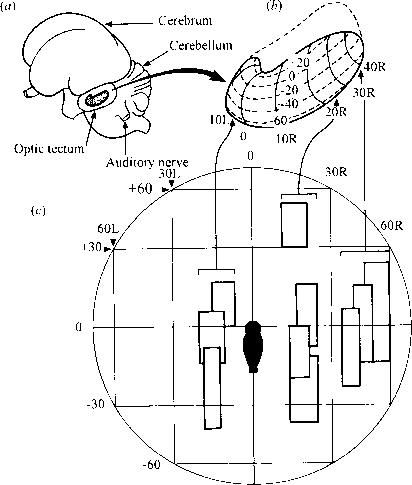
- The neuronal map of auditory space in the midbrain of the barn owl.
- (a) The left side of the owl’s brain, showing the location of the auditory midbrain (in bold outline) on the inner side of the optic tectum.
- (b) The left auditory midbrain enlarged from (a), with the co-ordinates of the neuronal map indicated in degrees of azimuth (L and R) and of elevation (+ and -) of auditory space.
- (c) A plot of auditory space in front of the owl in degrees of azimuth and of elevation, showing the receptive fields (bold rectangles) of ten neurons recorded in three separate electrode penetrations. The penetrations were made with the electrode parallel to the transverse plane at the positions indicated by the arrows linking (c) to (b). (Modified after Knudsen, 1981.)
1. The Sound Localization Strategy of the Barn Owl
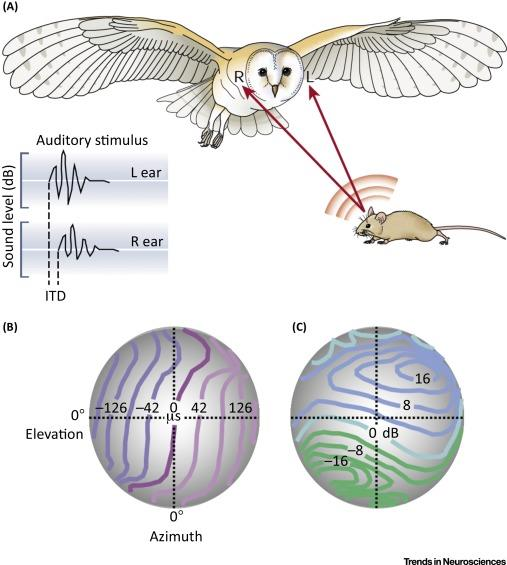
- (A) Barn owls can accurately localize prey based on interaural disparities even in total darkness. If, for instance, the sound origin is slightly to the left from the vertical and below the horizontal midline, it will arrive at the left ear slightly earlier (interaural time difference, ITD) and with a somewhat larger amplitude (interaural level difference, ILD).
- (B,C) Barn owls belong to the group of asymmetrical owls. The asymmetry of their skull does not significantly affect ITDs (B), which provide accurate information for the azimuthal position. However, the asymmetry does affect ILDs (C).
- As a consequence, iso-ILD-lines are strongly tilted and, therefore, provide information about the vertical position of a sound source.
A map of auditory space in the brain of a barn owl:
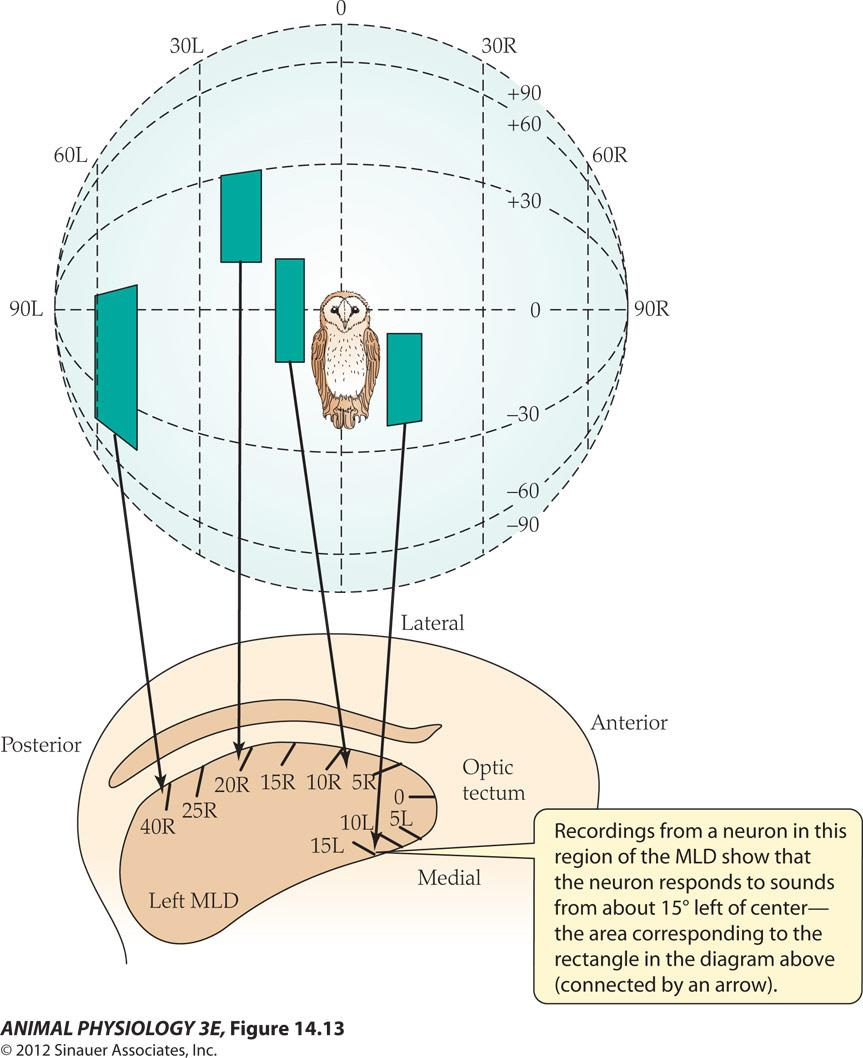
- Recordings from a neuron in this region of the MLD show that the neuron responds to sounds from about 15 left of center the area corresponding to the rectangle in the diagram above (connected by an arrow).
四、Chemoreception and taste
Drosophila taste sensillum and its responses to different stimulatory solutions
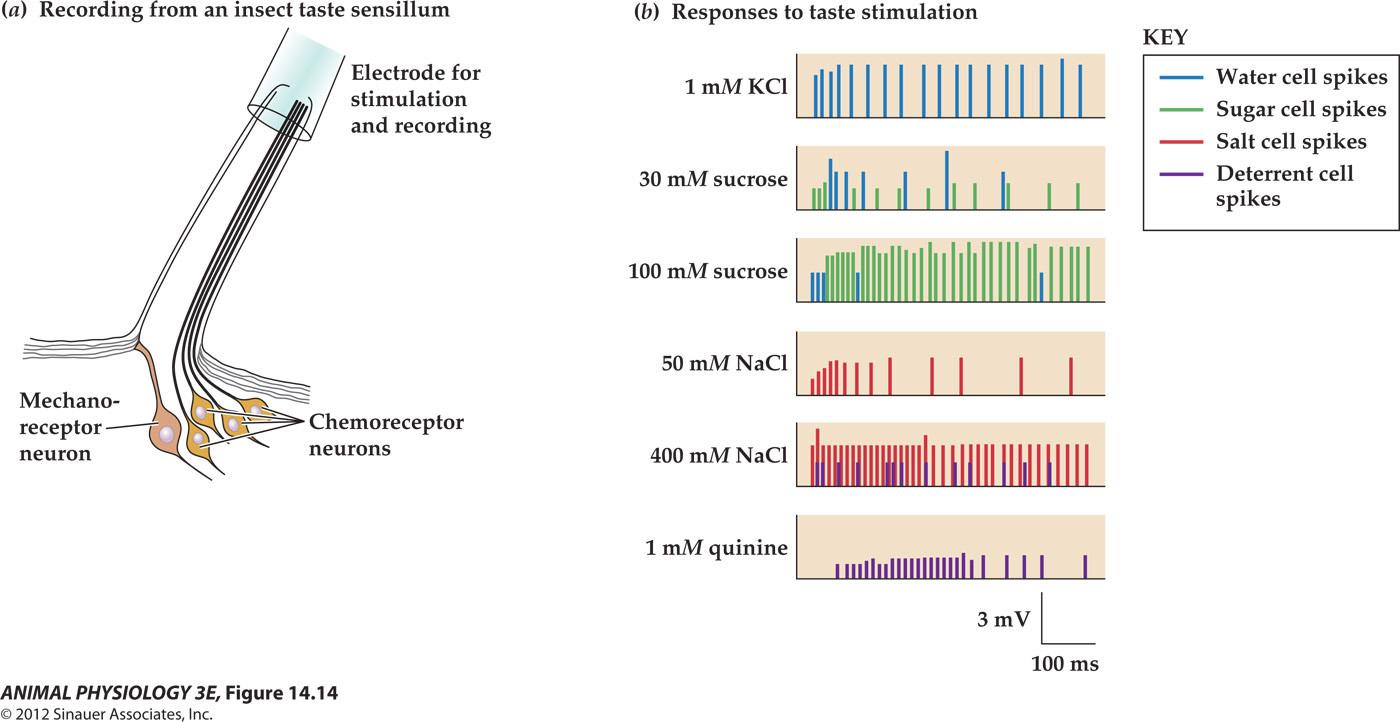
FIGURE 14.14 Drosophila taste sensillum and its responses to different stimulatory solutions
- (a) A typical taste sensillum on the leg tarsus contains four chemoreceptor cells and a mechanoreceptor cell. A pipette electrode both stimulates the sensillum and records action potentials from the sensory cells.
- (b) Each of the four chemoreceptor cells has spikes (extracellularly recorded action potentials) of a different size. Here the spike responses of different cells are rendered in different colors. The water cell responds best to water (with just enough salt to conduct charges), the sugar cell responds best to sugar solutions, and the salt cell responds best to increasing salt concentrations. The deterrent cell responds to quinine and other bitter alkaloids and also to high concentrations of salt
Mammalian taste buds
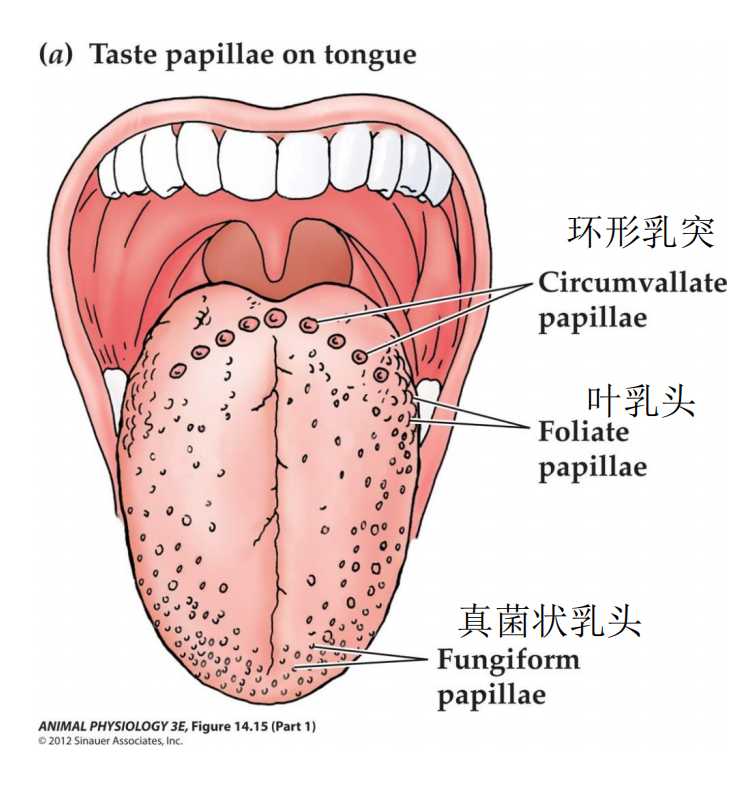
- (a) Taste buds are localized at taste papillae on the tongue.
- (b) Different kinds of taste papillae contain differing numbers of taste buds.
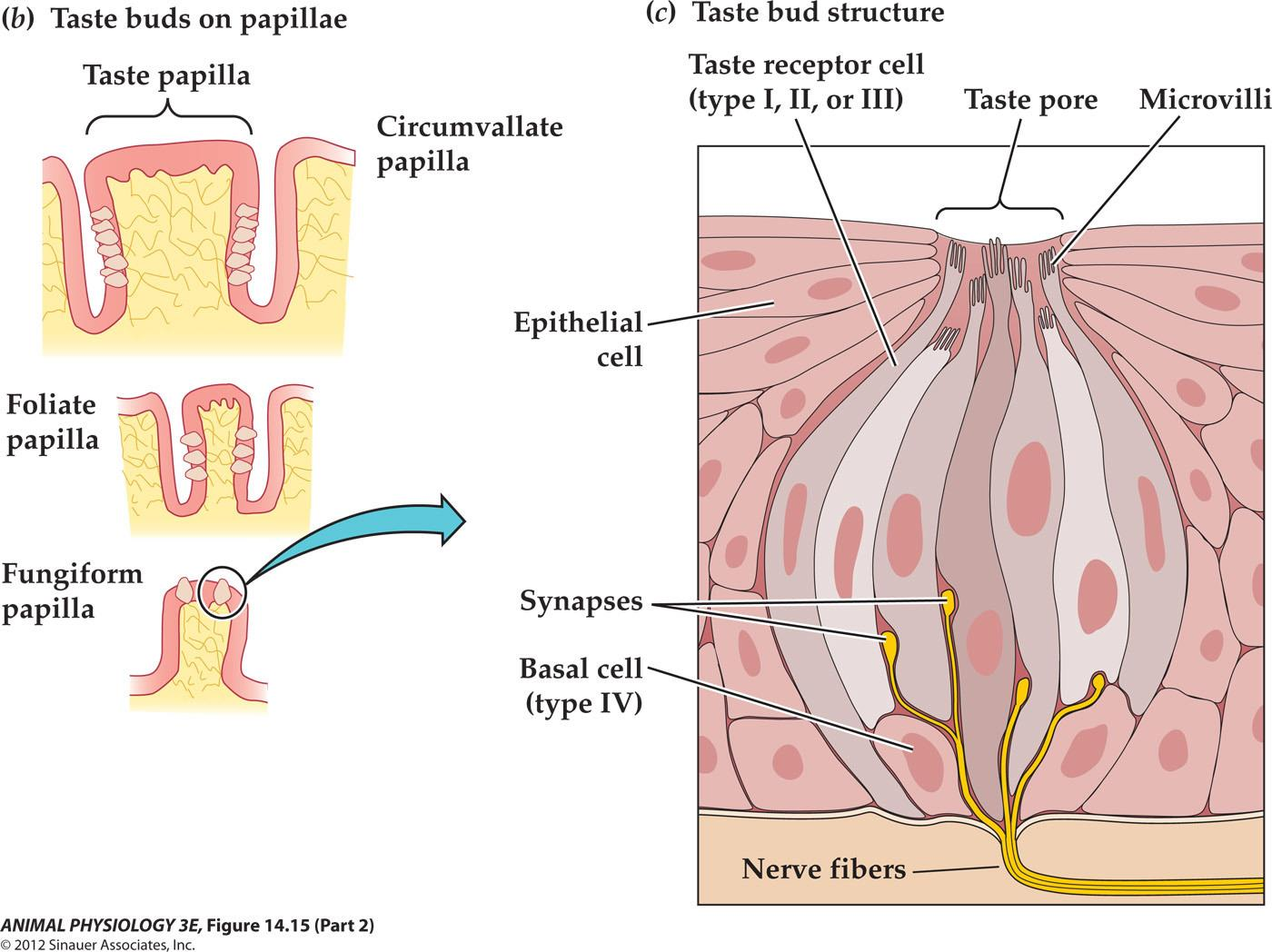
- (c) A taste bud contains many taste cells of different types; some form discrete synapses onto afferent sensory neurons. The taste cells of a taste bud extend microvilli through a taste pore to contact saliva. Receptor molecules in the microvillar membrane are exposed to taste stimuli at the surface of the tongue. (c from Kandel et al. 2000.)
Taste-transduction mechanisms differ for different tastes
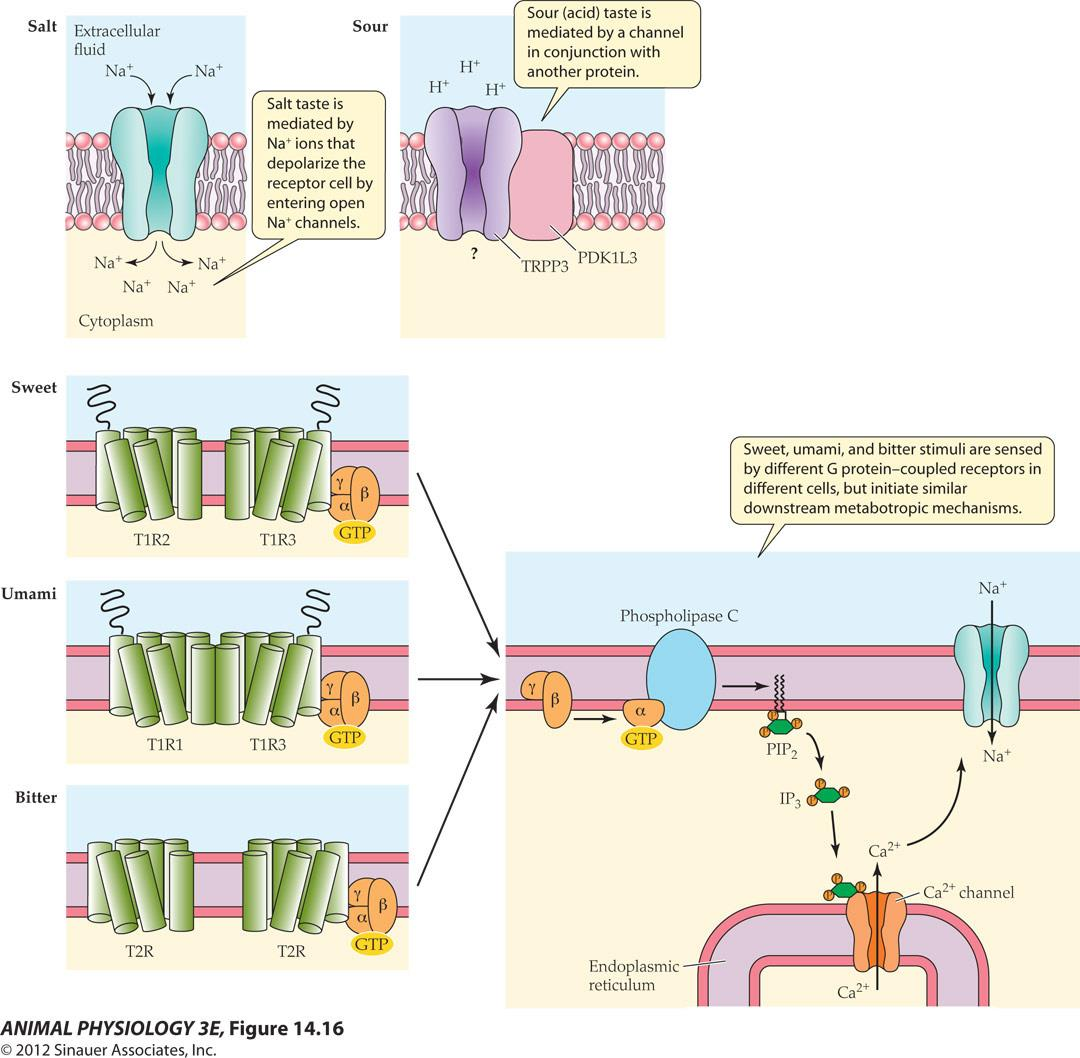
- Salt taste is mediated by Na ions that depolarize the receptor cell by entering open Na channels
- Sour(acid) taste is mediated by a channel in conjunction with another protein (TRPP3, PDK1L3)
- Sweet, umami(鲜), and bitter stimuli are sensed by different G protein-coupled receptors in different cells, but initiate similar downstream metabotropic mechanisms.
FIGURE 14.16 Taste-transduction mechanisms differ for different tastes
- The molecules for reception and transduction of different taste qualities are localized in different taste cells.
Type I cells mediate salt taste,
type III cells mediate sour taste, and separate subpopulations of type II cells mediate sweet, umami, and bitter taste.
For salt taste, sodium ions enter a taste bud cell through cation channels, directly depolarizing the cell. Sour taste is mediated by a channel (a complex of a TRP channel protein [TRPP3] and a related protein, PKD1L3). Sweet, umami, and bitter responses are mediated by GPCRs. Their signal transduction effects are similar to each other but occur in different cells.
In sweet taste, sugars bind to a particular dimer of GPCRs (T1R2 and T1R3), which acts via a G protein to activate phospholipase C to produce IP3; IP3 releases Ca2+ from intracellular stores to activate a TRP channel. In the taste quality umami, glutamate (monosodium glutamate [MSG]) and other amino acids stimulate another GPCR dimer. Bitter substances bind to a different family of GPCRs (the T2R family).
五、Olfaction and smell
Insect olfactory receptors detect pheromones and other chemicals
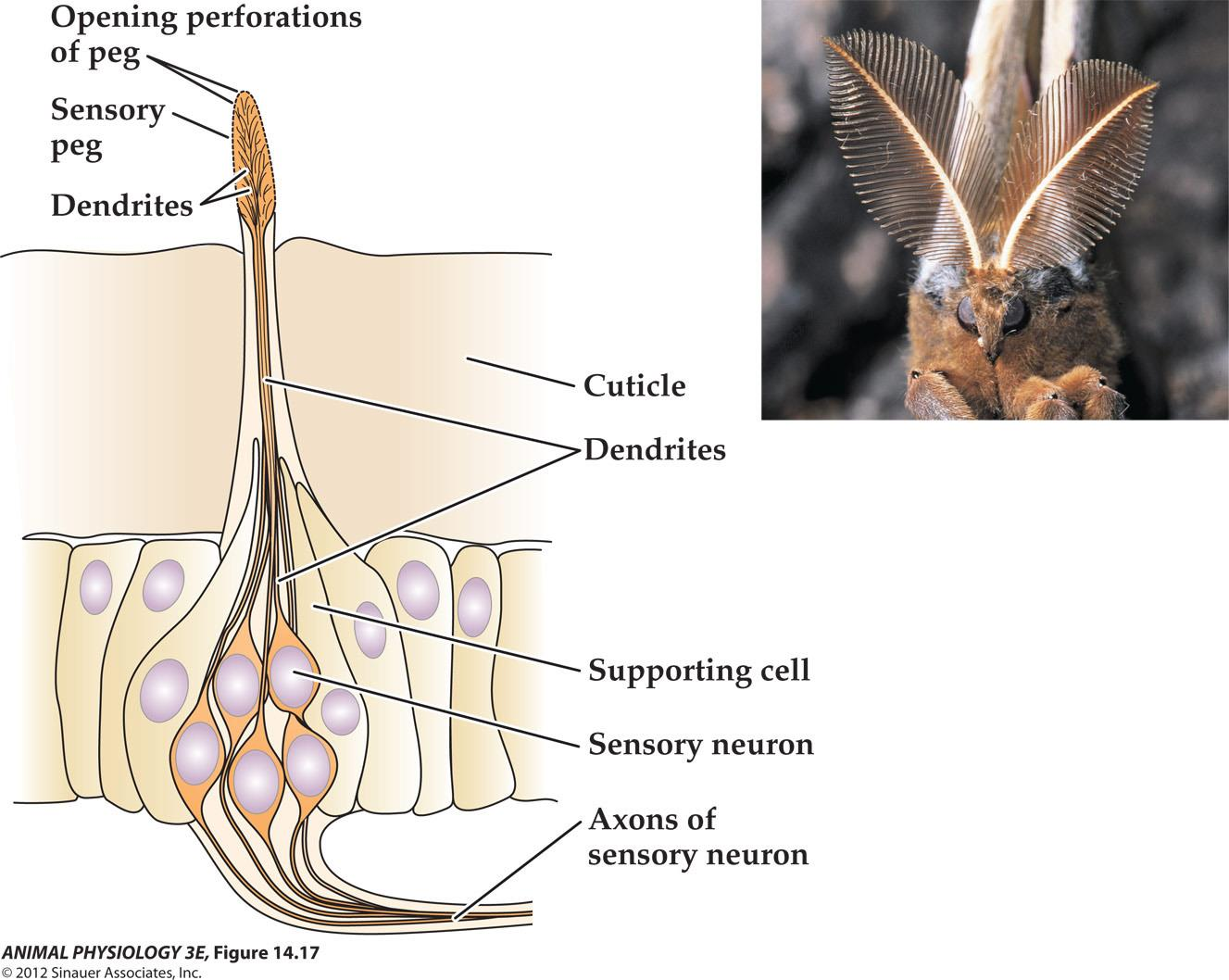
- FIGURE 14 17 Insect olfactory receptors detect pheromones and other chemicals Each pinnate antenna of a male Antheraea pol, phemus moth may have as many as 280,000 chemosensory neurons, many of which are specialized to detect a chemical signa(pheromone released by a female moth.
Vertebrate olfactory receptors
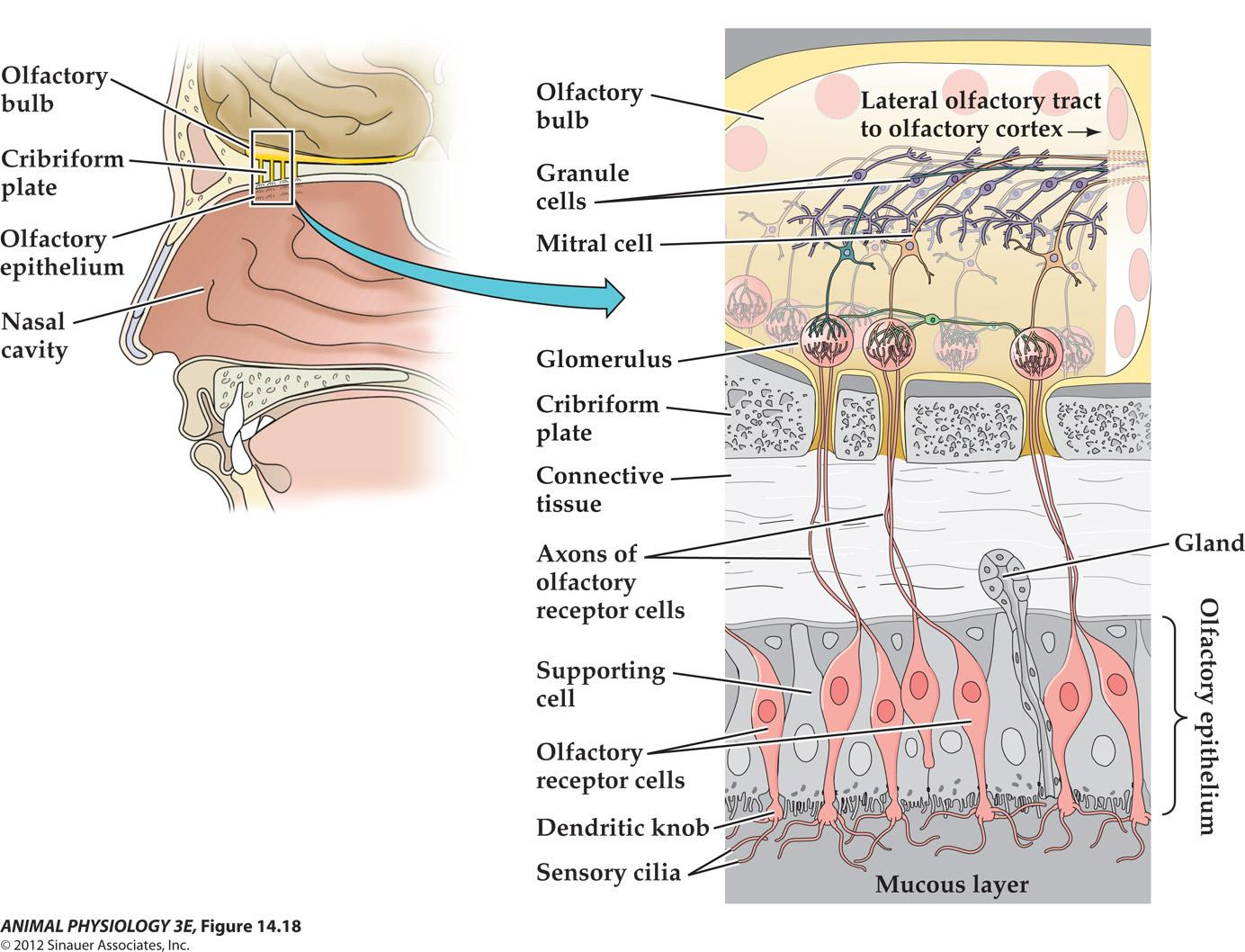
- FIGURE 14.18 Vertebrate olfactory receptors Olfactory receptor cells are small bipolar neurons, the sensory clia of which extend into the mucous layer of the nasal cavity. Ther arons perforate the bone of the cribriform plate to end in glomeruli of the olfactory bub of the brain Mitral cells and granule cells integrate olfactory information, and the mitral cell axons carry the information to the olfactory cortex.
1. Odorant excitation of a vertebrate olfactory receptor cell opens individual ion channels
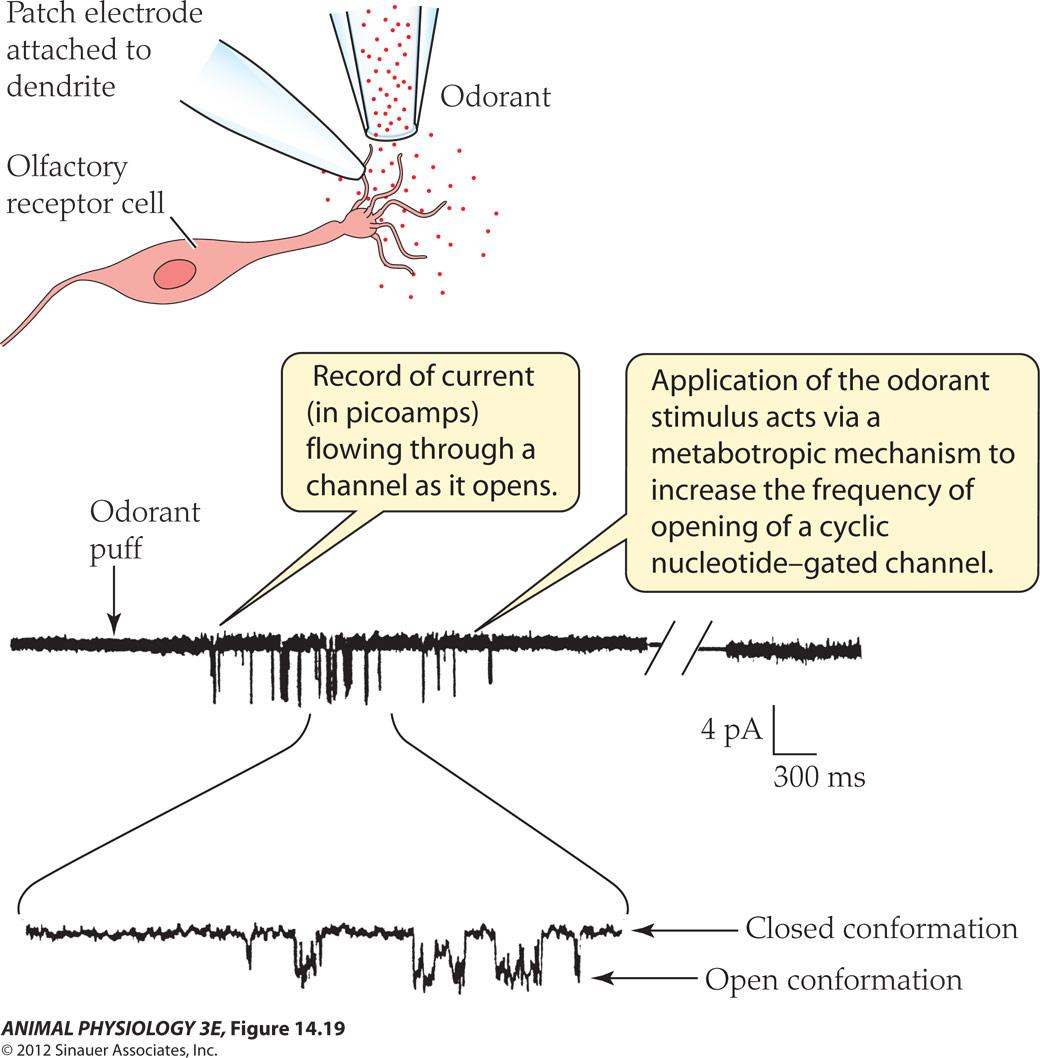
- FIGURE 14.10 Odorant excitation of a vertebrate olfactory receptor cell opens individual ion channels An individual olfac tory receptor cell was isolated from the ofactory epithelium of a salamander, and its response to odor stimulation was recorded with a patch electrode. The membrane patch remained attached to the cell A bref puff of odorant ed to opening of singe channels n the patch, as shown by recorded single-channel currents (After Firestein et al 1991. in Fain 2003.)
- Record of current (in picoamps flowing through a channel as it opens
- Application of the odorant stimulus acts via a metabotropic mechanism to increase the frequency of opening of a cyclic nucleotide-gated channels
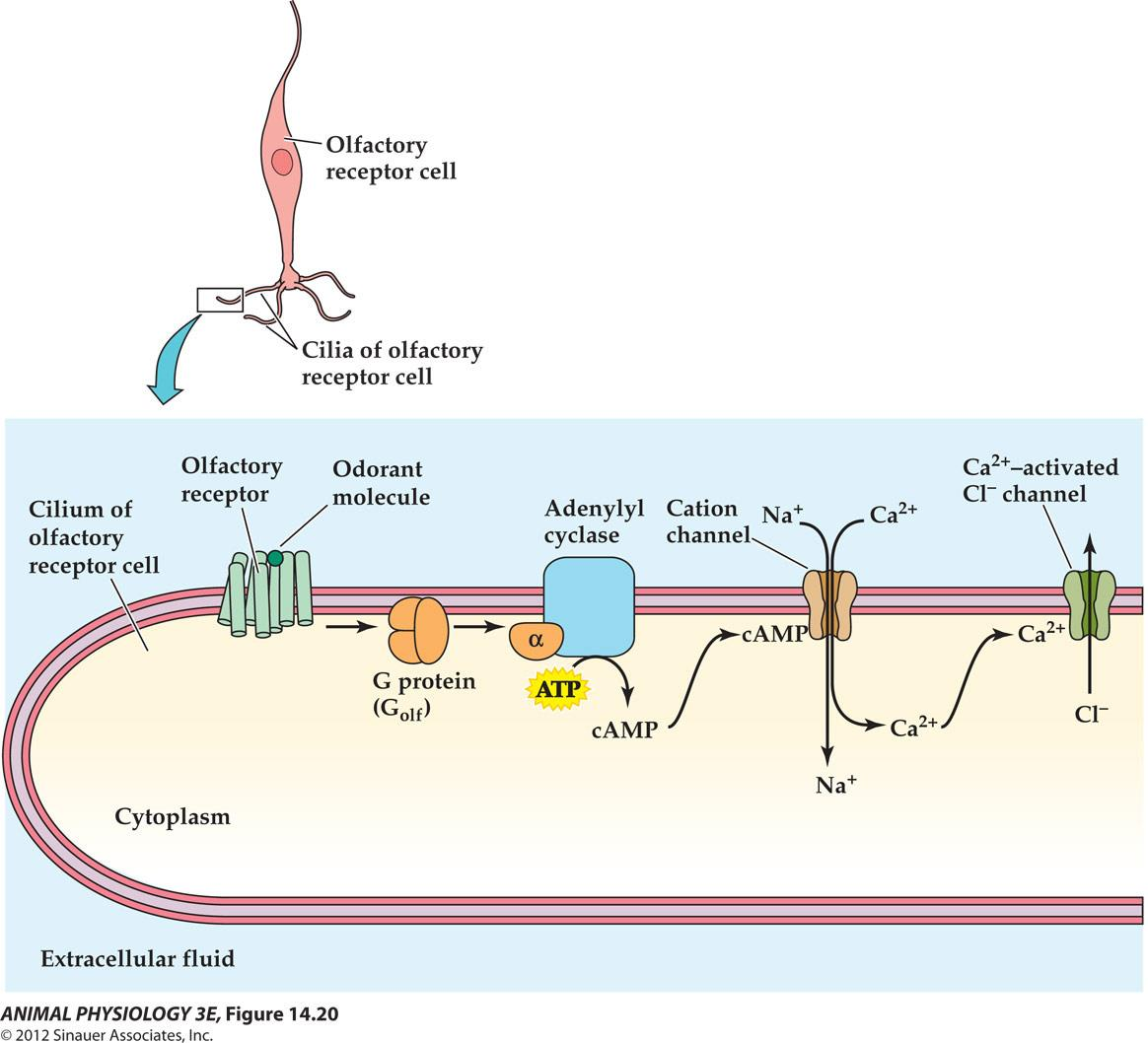
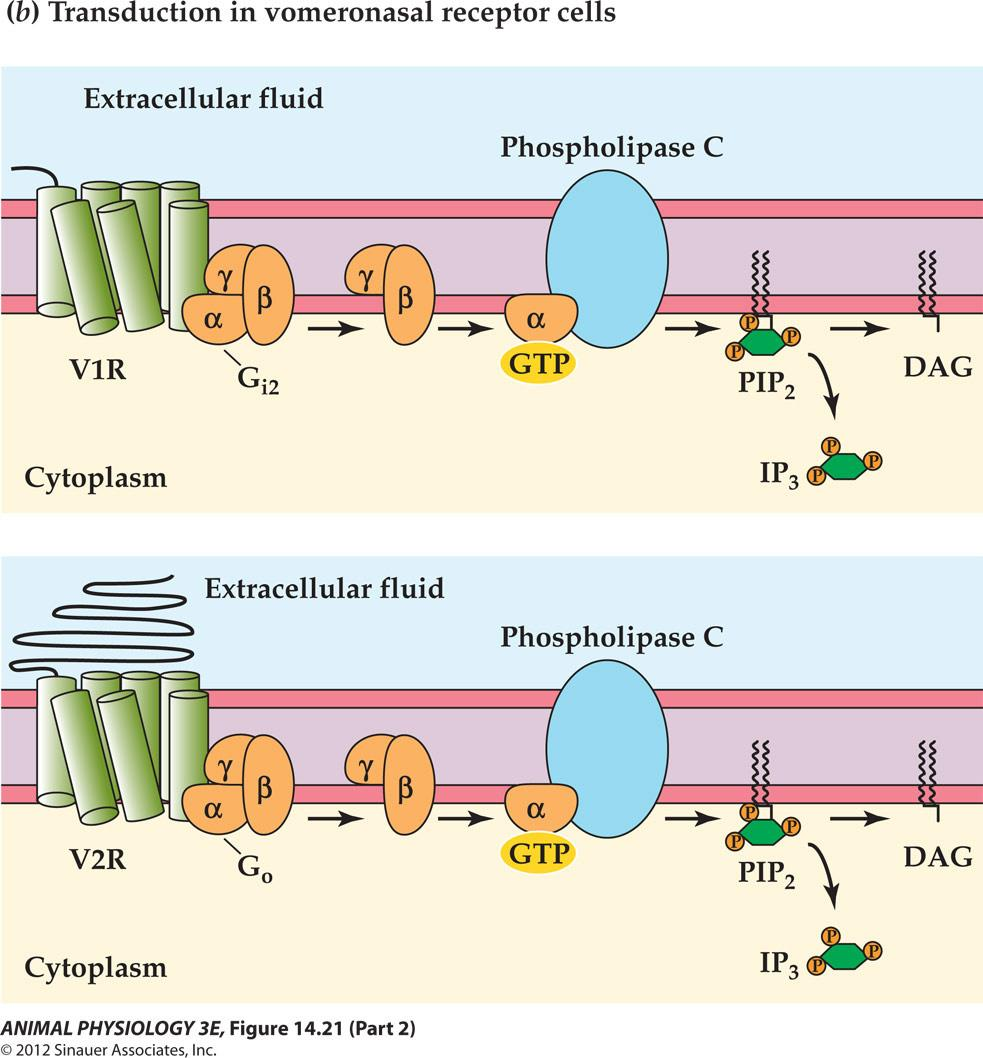
2. Olfactory transduction mechanisms in cilia membranes of olfactory receptor cells
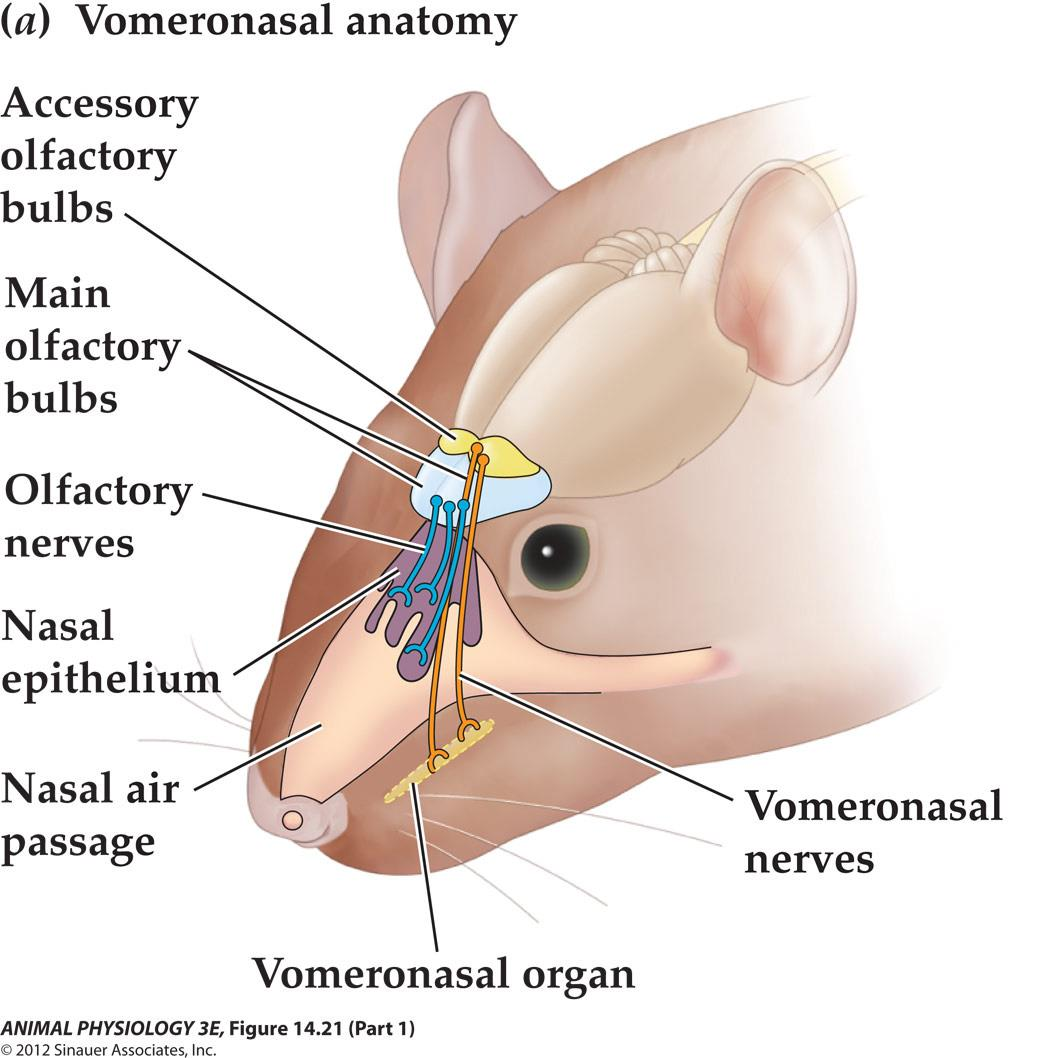
The vomeronasal organ is an accessory olfactory organ in most terrestrial vertebrates
YOU HAVE TWO NOSES
- 犁鼻器官 detects pheromones
六、Photoreception and vision
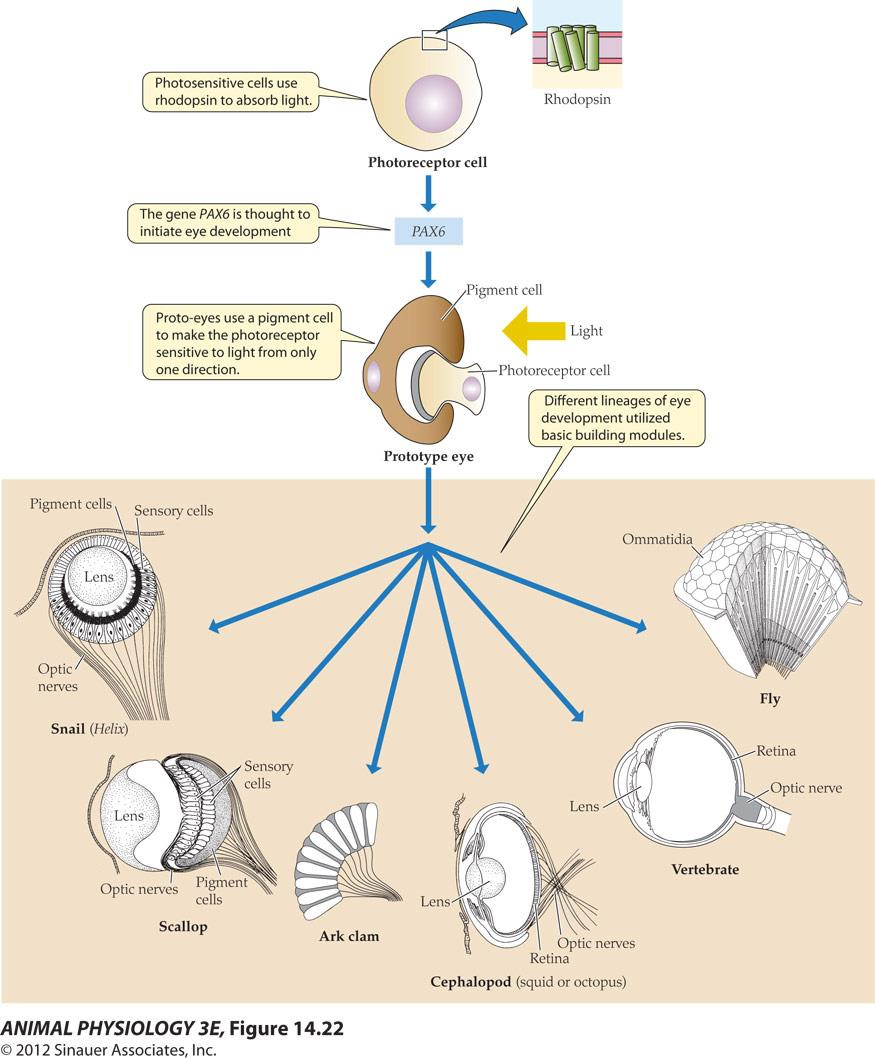
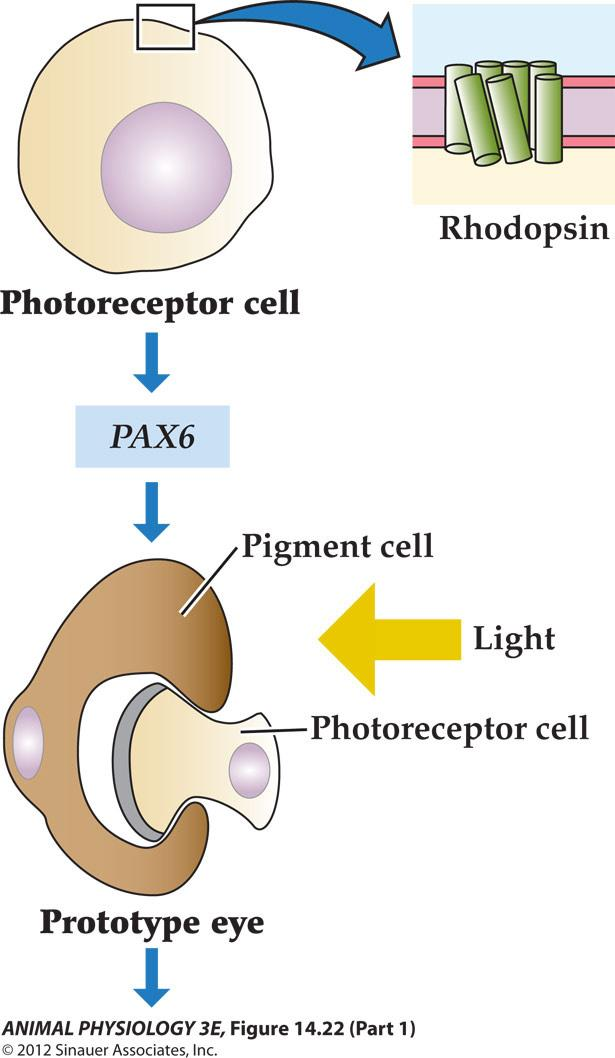
- Photosensitive cells use rhodopsin to absorb light.
- The gene PAX6 is thought to initiate eye development
- Proto-eyes use a pigment cell to make the photoreceptor sensitive to light from only one direction
- Different lineages of eye development utilized basic building modules
FIGURE 14.22 A hypothesis showing the evolution of eves A rhodopsin-based photoreceptor cell may have become a prototype eye by a developmental association with a screening pigment cell that provided directional selectivity, under control of PA6 or a related developmental gene. The prototype eye is thought to have evolved monoply letically into more complex eyes, including simple camera eyes (snail, scallop), complex camera eyes cephalopod, vertebrate), and compound eyes. The hypothesis is based on homologies of genes for photoreception and for eye development in different groups. (From Gehning 2005)
A hypothesis showing the evolution of eyes
Rhodopsin is a photopigment composed of two parts: retinal and opsin
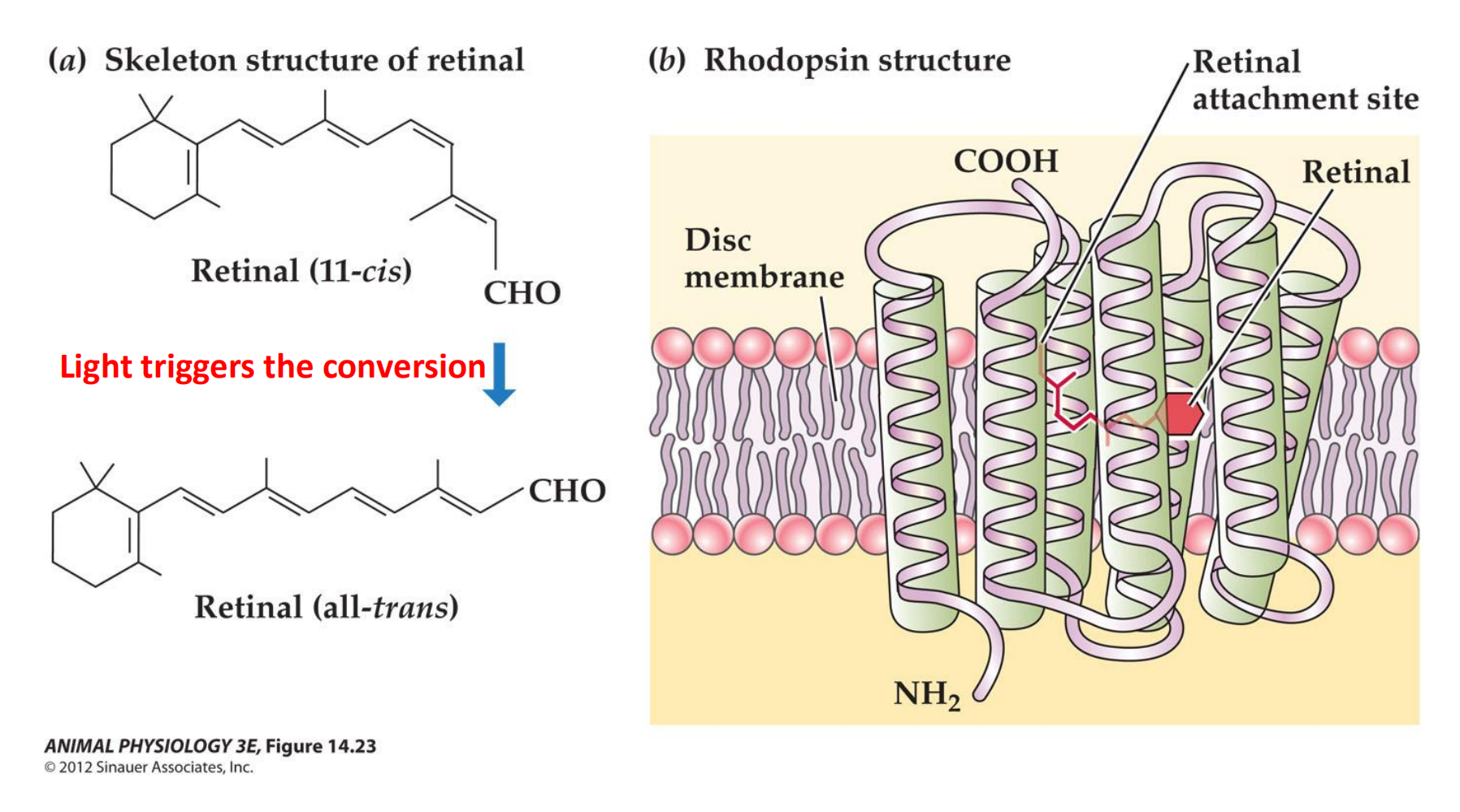
- Light triggers the conversion
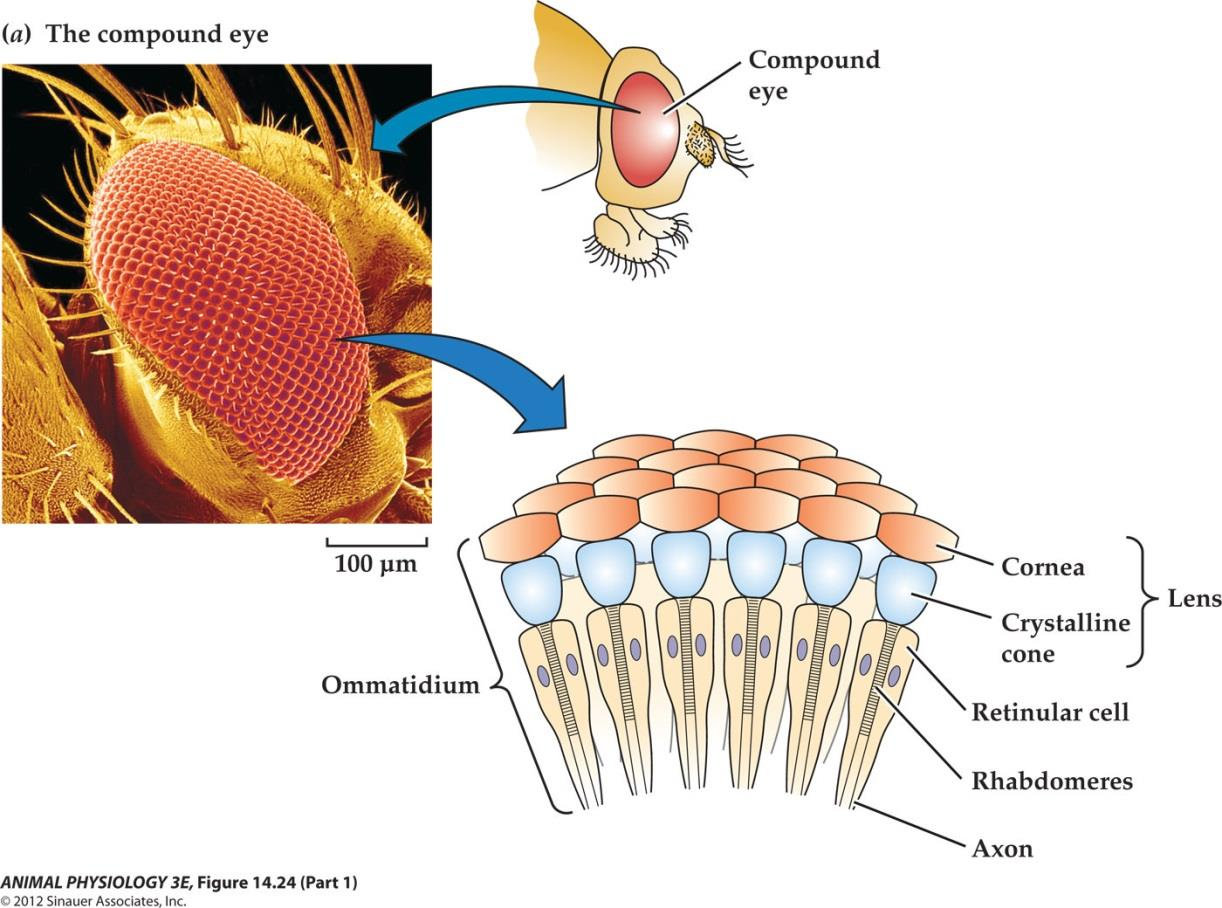
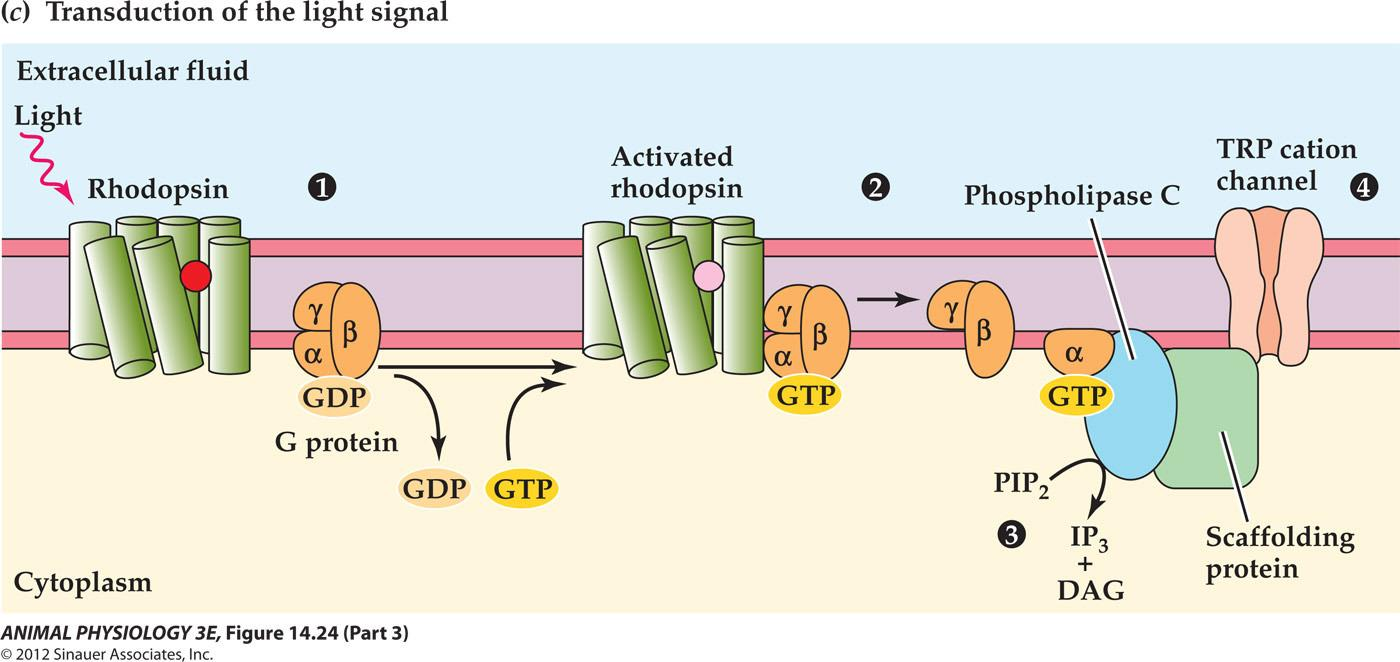
1. Photoreceptor transduction in the compound eye of the fruit fly Drosophila
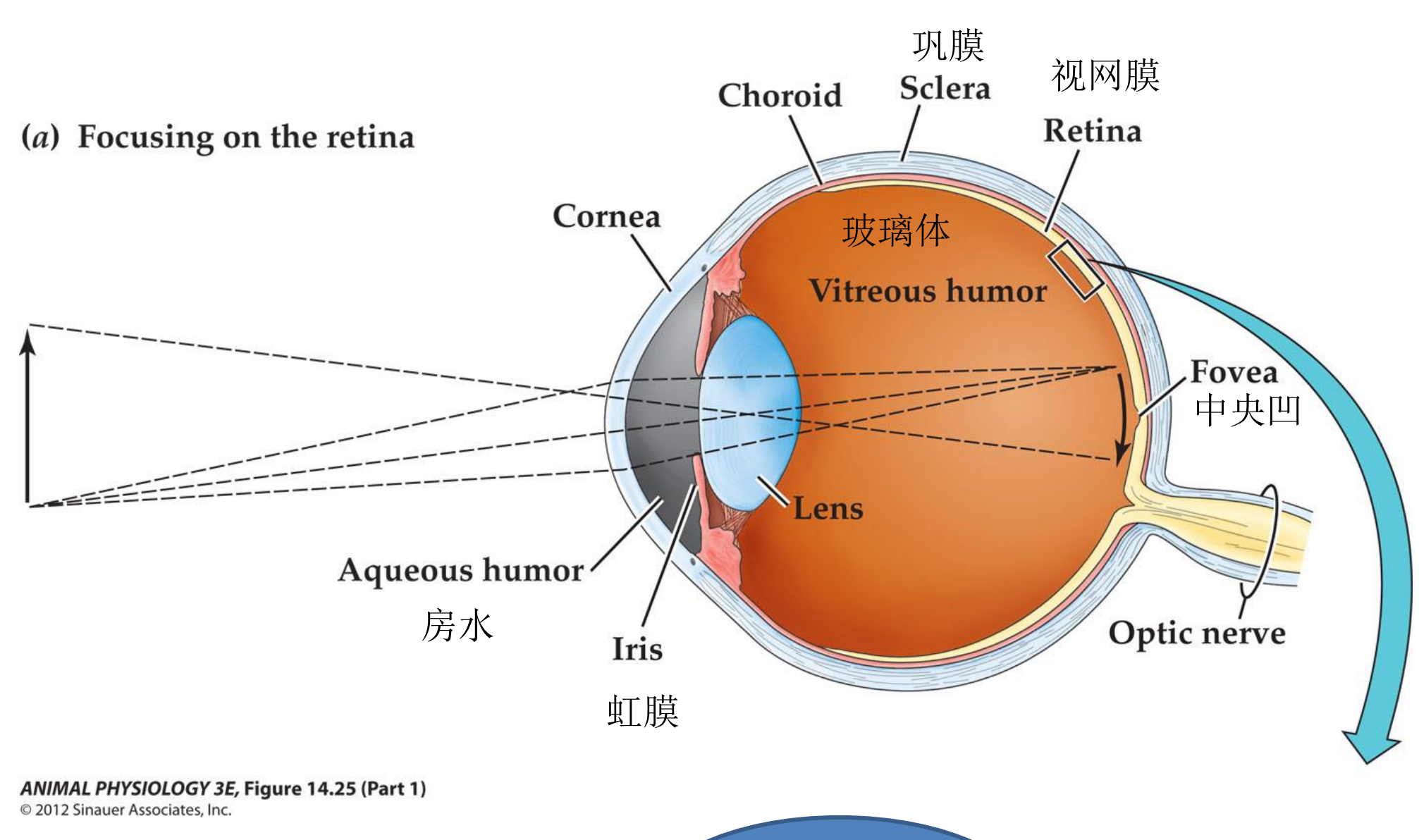
Structure of the mammalian eye and retina
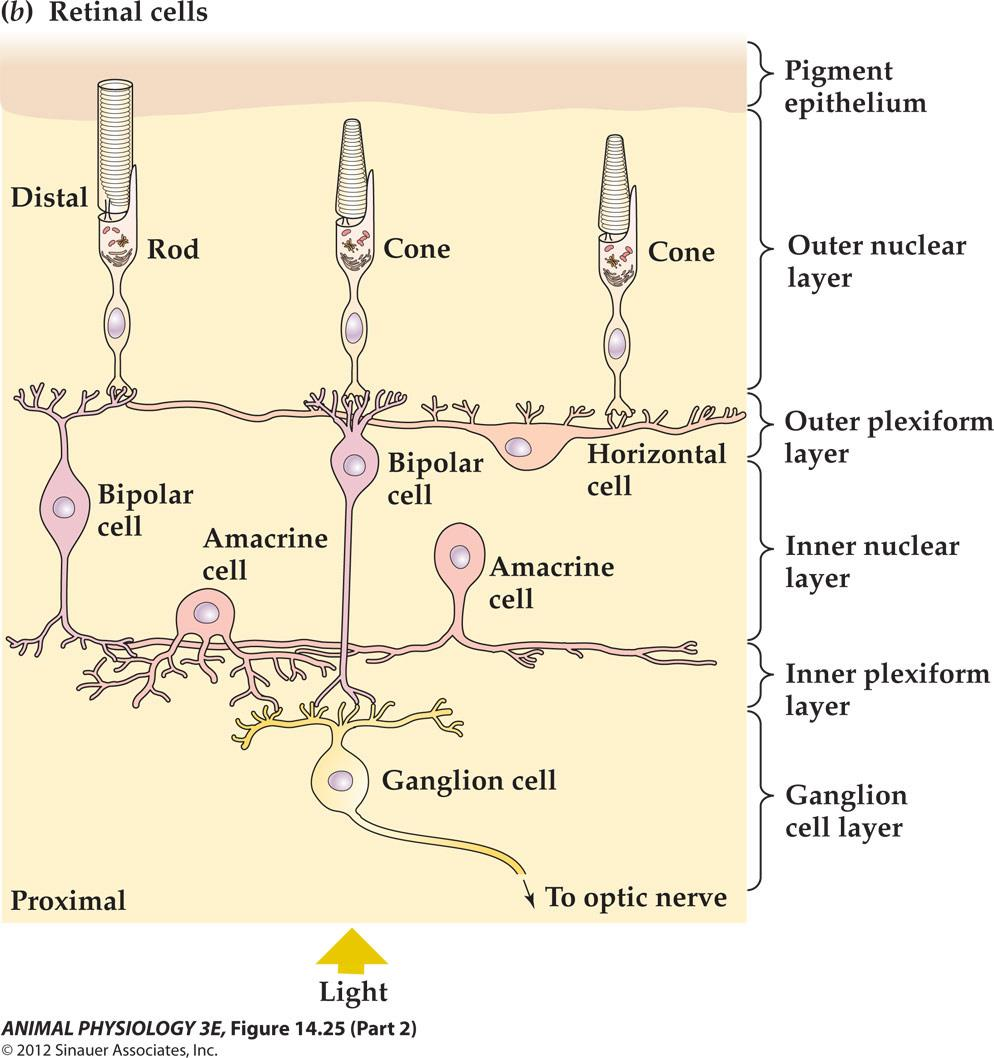
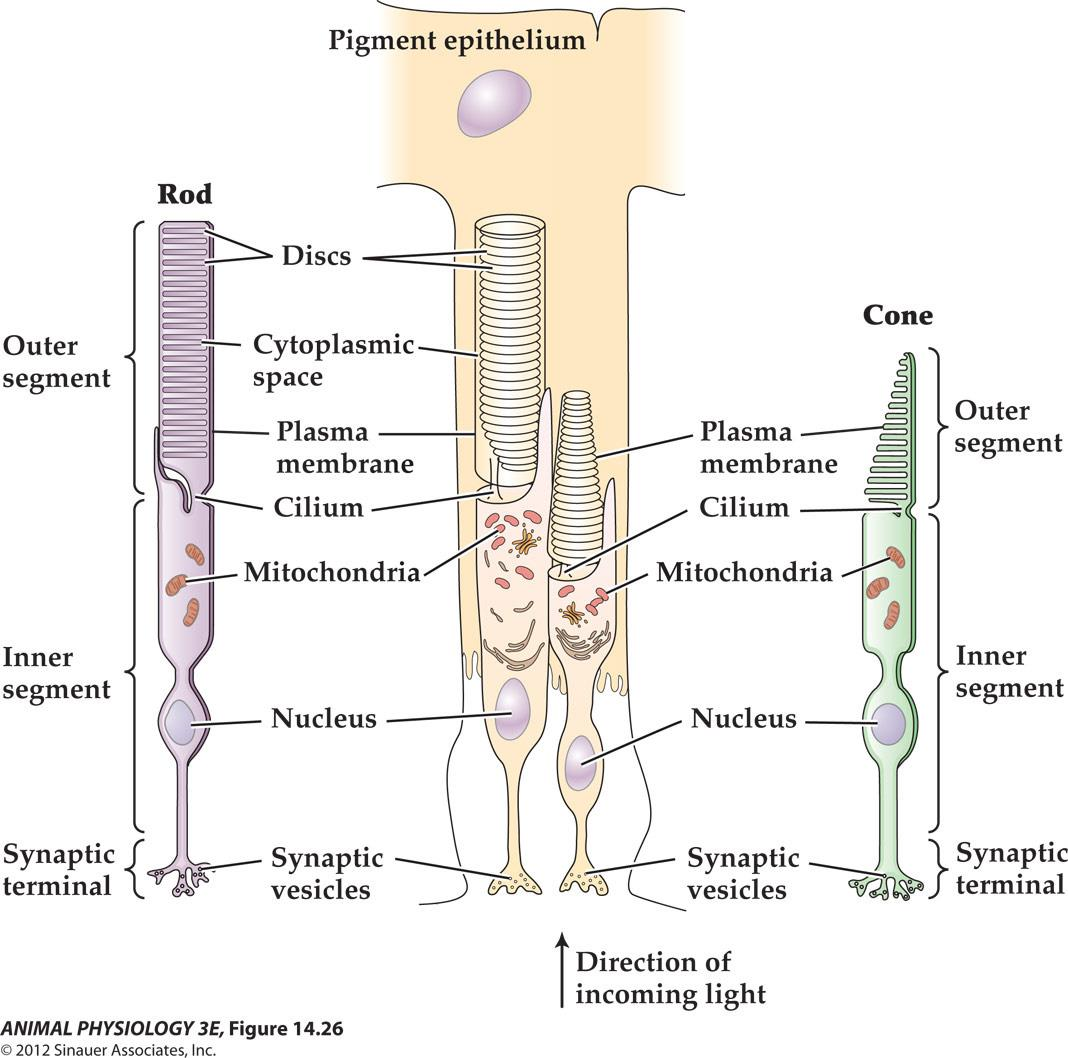
- FIGURE 14.26 Vertebrate photoreceptors Both rods and cones have an inner segment that contains the nucleus and synaptic terminal, and an outer segment that contains ordered lamellae bearing photopigment molecules In cones these lamellae are invaginations of the outer membrane, but in rods they are discs that are discontinuous with the outer membrane
1. Phototransduction closes cation channels in a rod outer segment
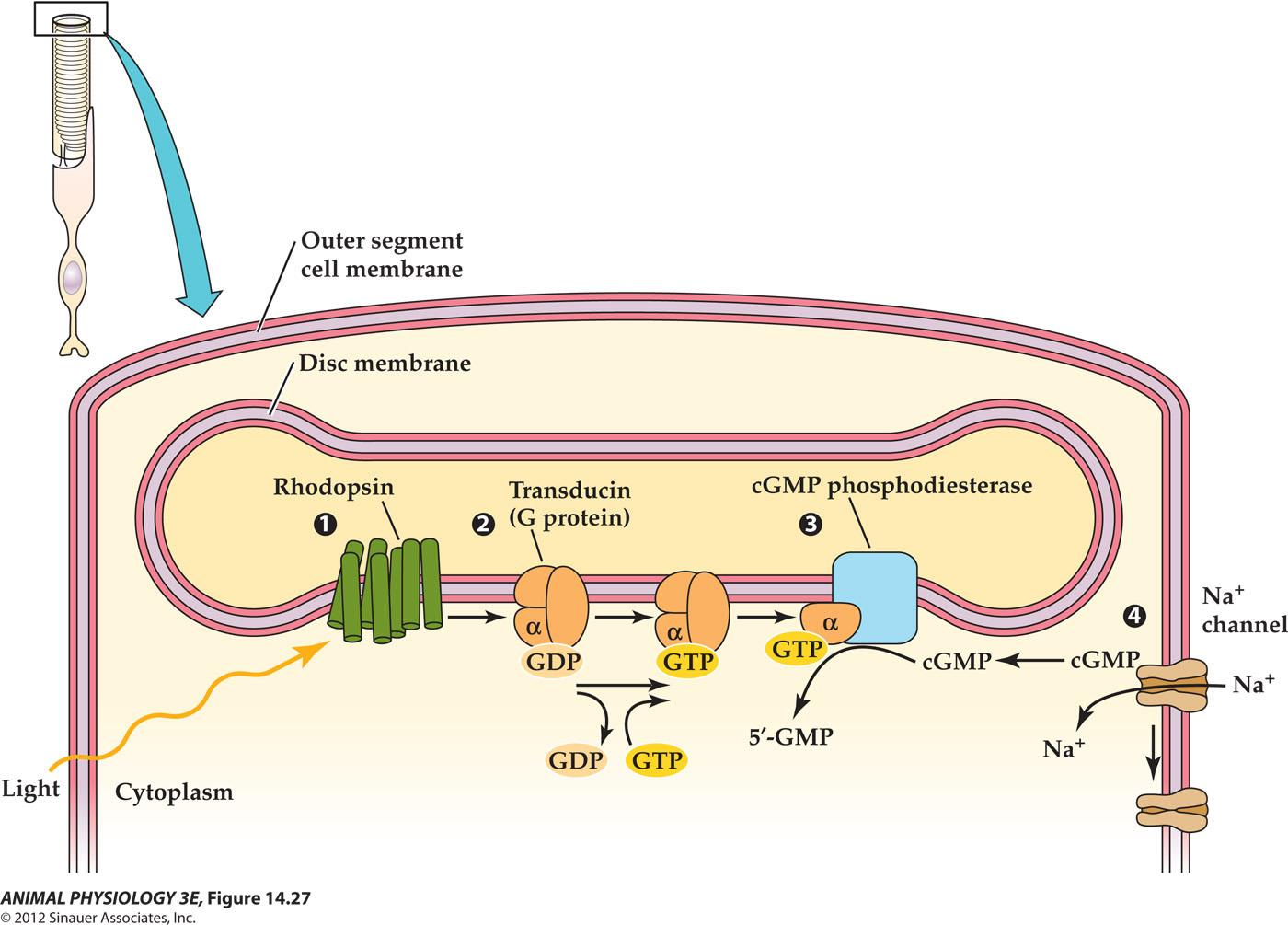
2. Light hyperpolarizes vertebrate photoreceptors
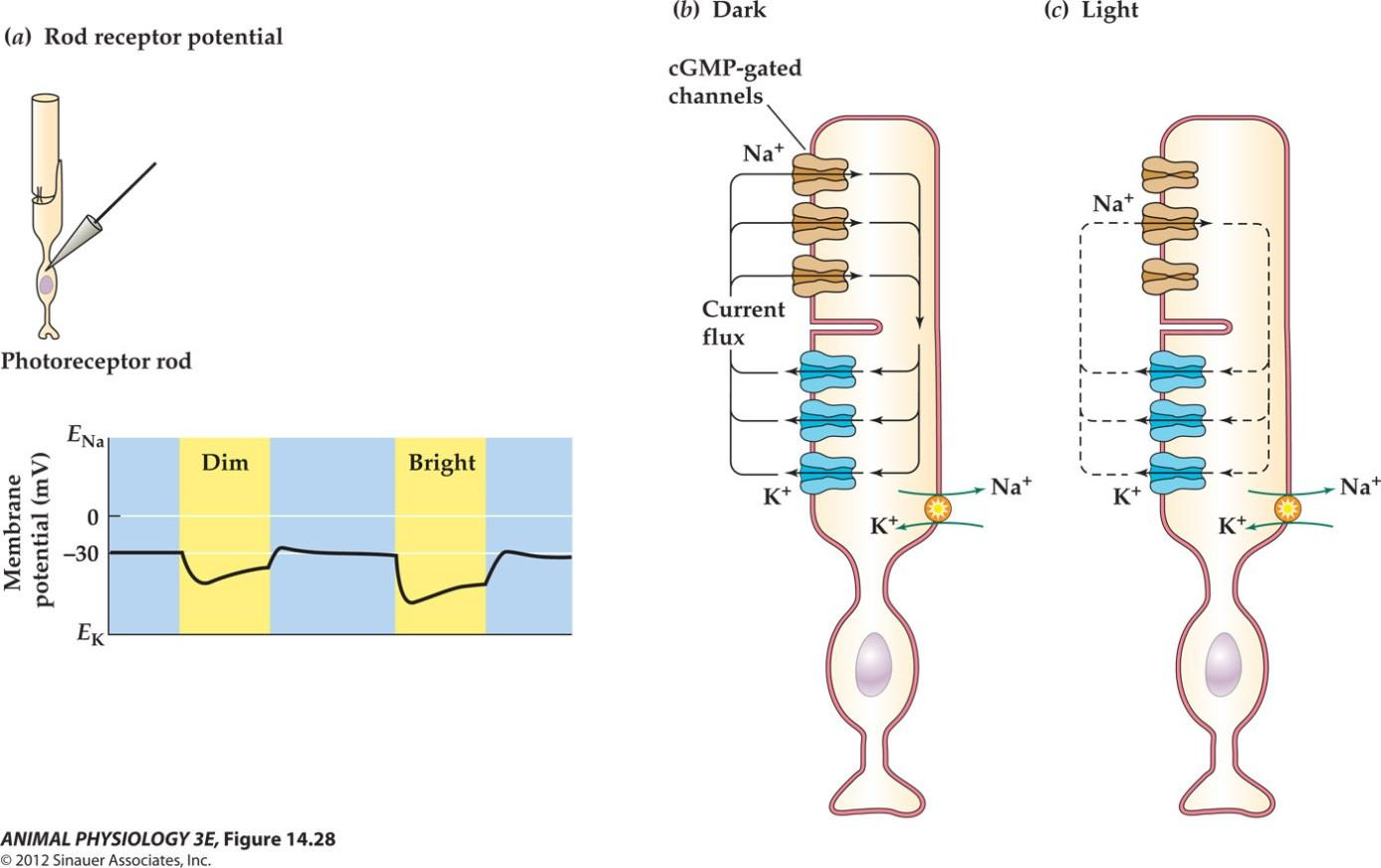
- FIGURE 14.28 Light hyperpolarizes vertebrate photoreceptors
- (a) Retinal rods and cones are relatively depolarized in the dark, and the receptor potential in light is a graded hyperpolarization. The brighter the light, the greater is the hyperpolarzation.
- (b)A dark current, carried largely by Na+ ions, enters the rod outer segment in the dark and depolarizes it.(c) Light acts to decrease the dark current by closing cGmp-gated Na channels, leading to hyperpolarzation
3. Central visual projections of a mammal
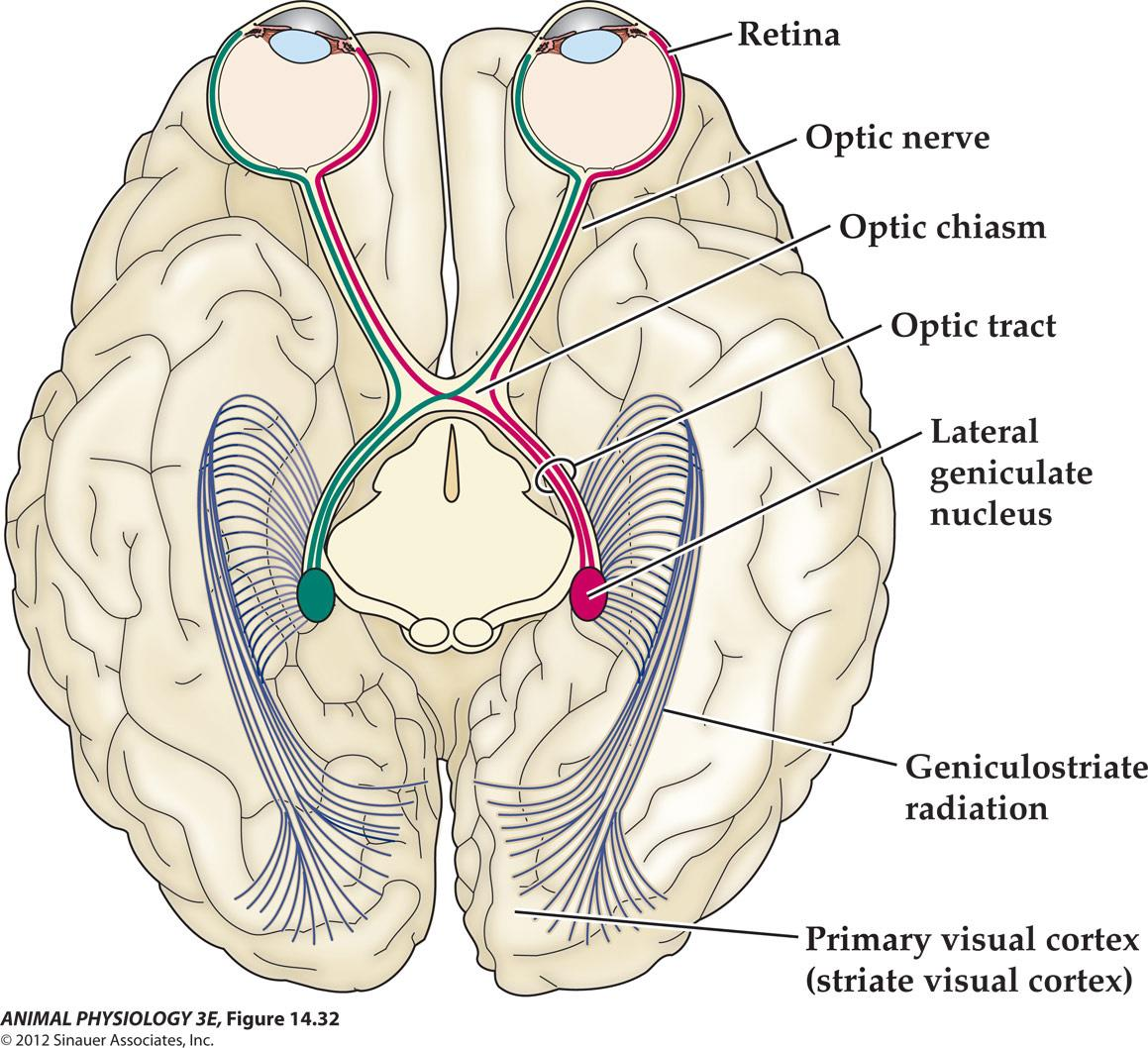
- FIGURE 14.32 Central visual projections of a mammal As shown in this cutaway view of the brain(seen from above), only part of the optic tract crosses the midline at the optic chiasm. Therefore, a stimulus in the visual field (the left of the visible world)projects to the right lateral geniculate nucleus(LGN)via both eyes(red path. way). Conversely, a stimulus in the light visual field projects to the left LGN and visual cortex(green pathway)
Different Receptors