L19 Control of cell division and cell growth
#一、The cell cycle control
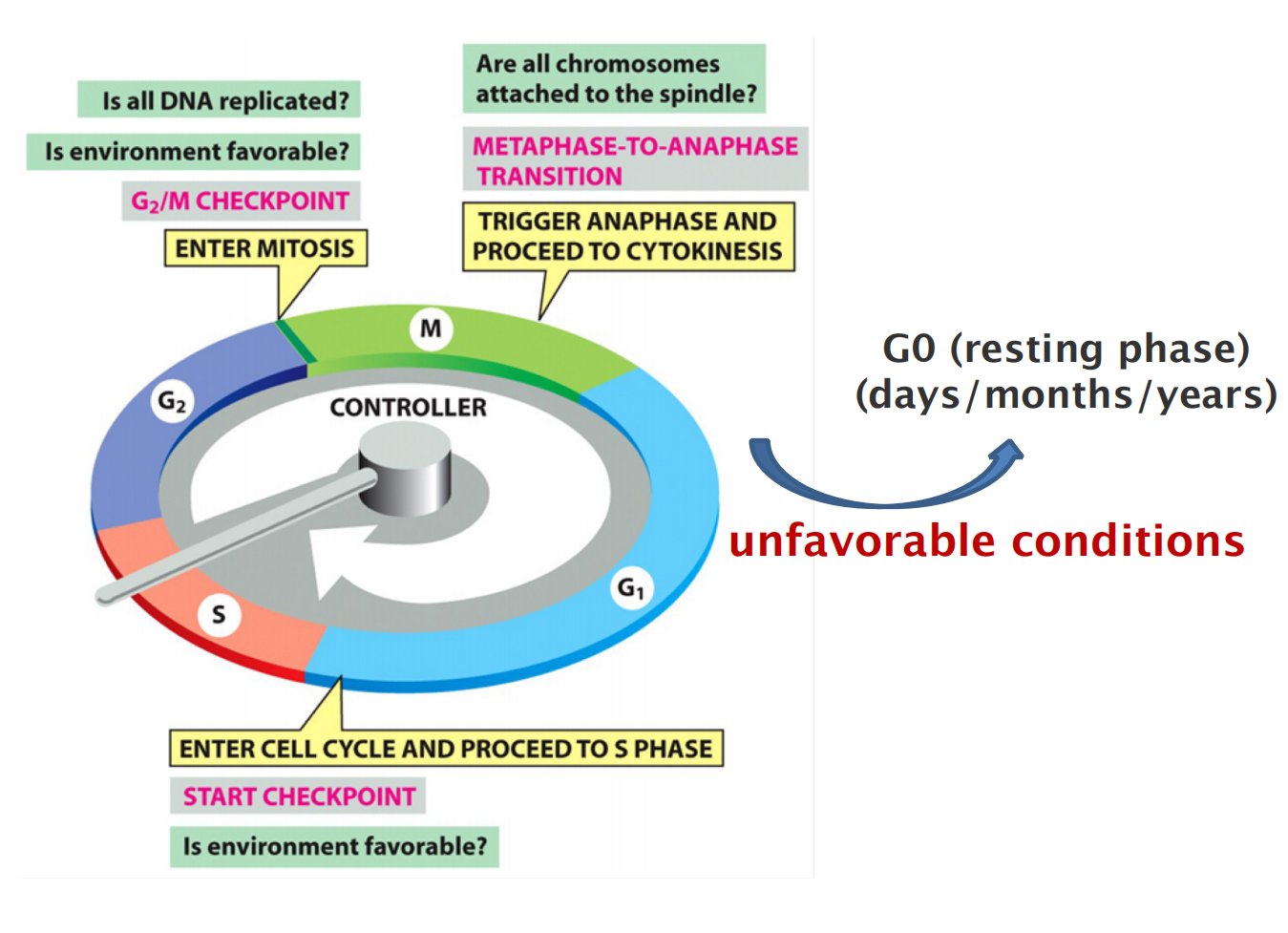
- Start marks the initiation of DNA replication and an irreversible commitment to cell division
- Internal and external factors regulate start
- Entry to start driven by activation of G1/S cyclin-CDK complexes
- Cell division of multicellular organisms needs stimulation by mitogens
Mitogens
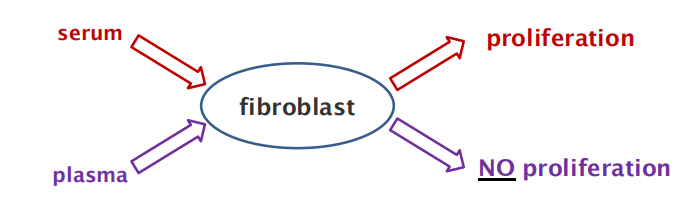
Both are derived from blood without cells, but serum is the supernatant after blood clotting, plasma is the supernatant prior to blood clotting.
What is the difference between serum and plasma?
- Platelet during blood clotting release the mitogen PDGF (platelet-derived growth factor)
Over 50 different mitogens have been identified yet: EGF, FGF, NGF, erythropoietin, etc.
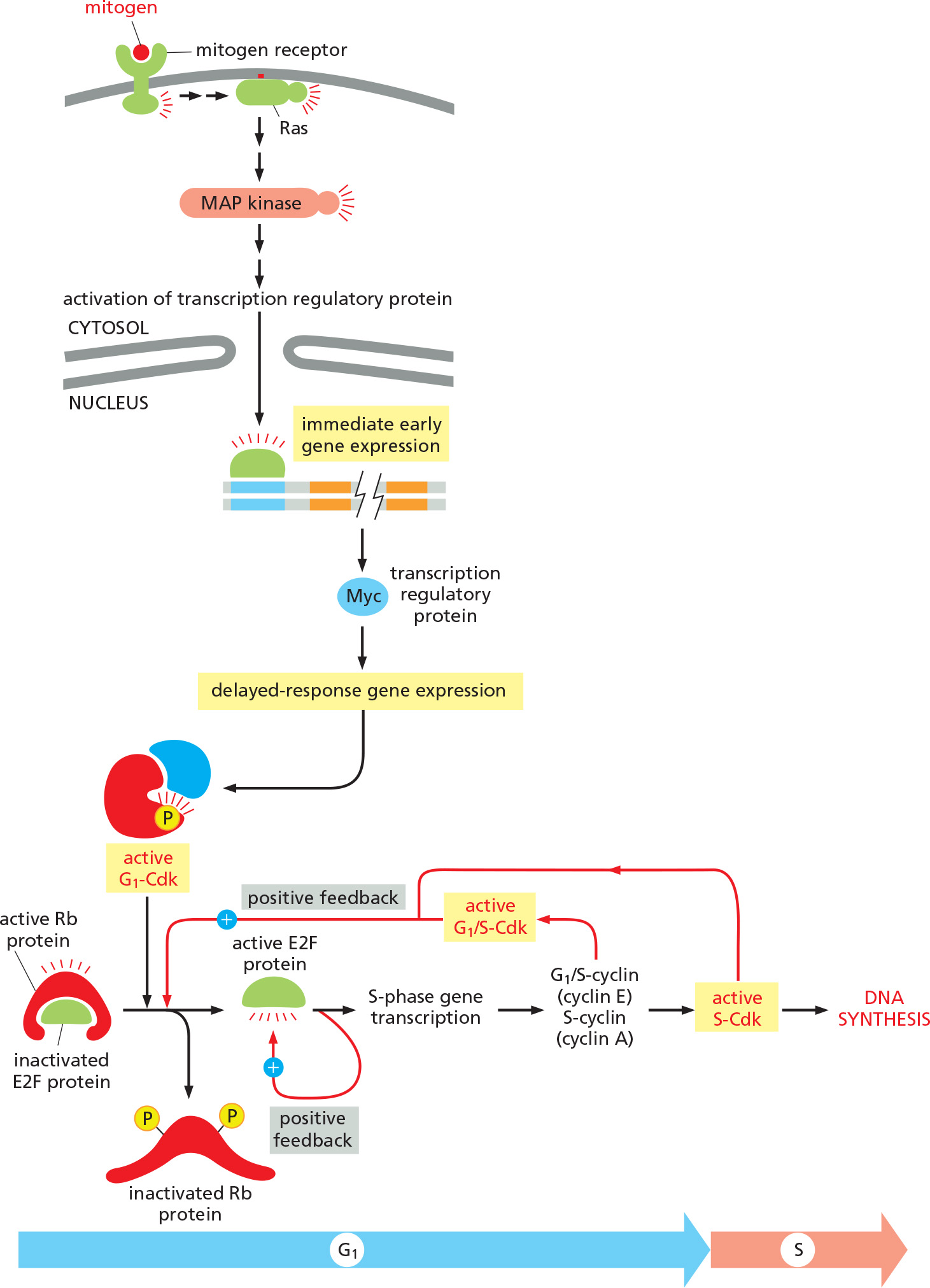
- mitogen activates receptor
- receptor activates Ras
- Ras activates the mitogen-activated protein (MAP)kinase cascade
- The MAP kinase cascade activates transcription factors and triggers immediate early gene expression
- one of these genes is the gene regulatory protein(transcription factor) Myc.
- the gene regulatory protein (transcription factor) Myc promotes expression of G1 cyclins (D cyclins)
- G1 cyclins (D cyclins) activate G1-Cdk and trigger cell cycle entry
- Functions by triggering a wave of G1/S –Cdk activity that relieves intracellular negative controls.
1. Mitogens triggers a wave of G1/S–Cdk activity
Mainly through phosphorylation on retinoblastoma (Rb) protein, liberating E2F proteins, which acts as transcription factors to simulate G1/S cyclins, etc.
G1-Cdk activates E2F proteins, which activate transcription of S-phase proteins like G1/S-cyclin & S-cyclin, resulting in further activation of G1-Cdk & S-Cdk and thus further inactivation(phosphorylation) of the Rb protein (positive feedback loop) and the cell cycle starts.
DNA damage block cell division
The control system blocks progression through each of these transitions if it detects problems inside or outside the cell.
The cell cycle control has major checkpoints:
- G1/S-phase transition checkpoint (START)
- G2/M-phase transition checkpoint
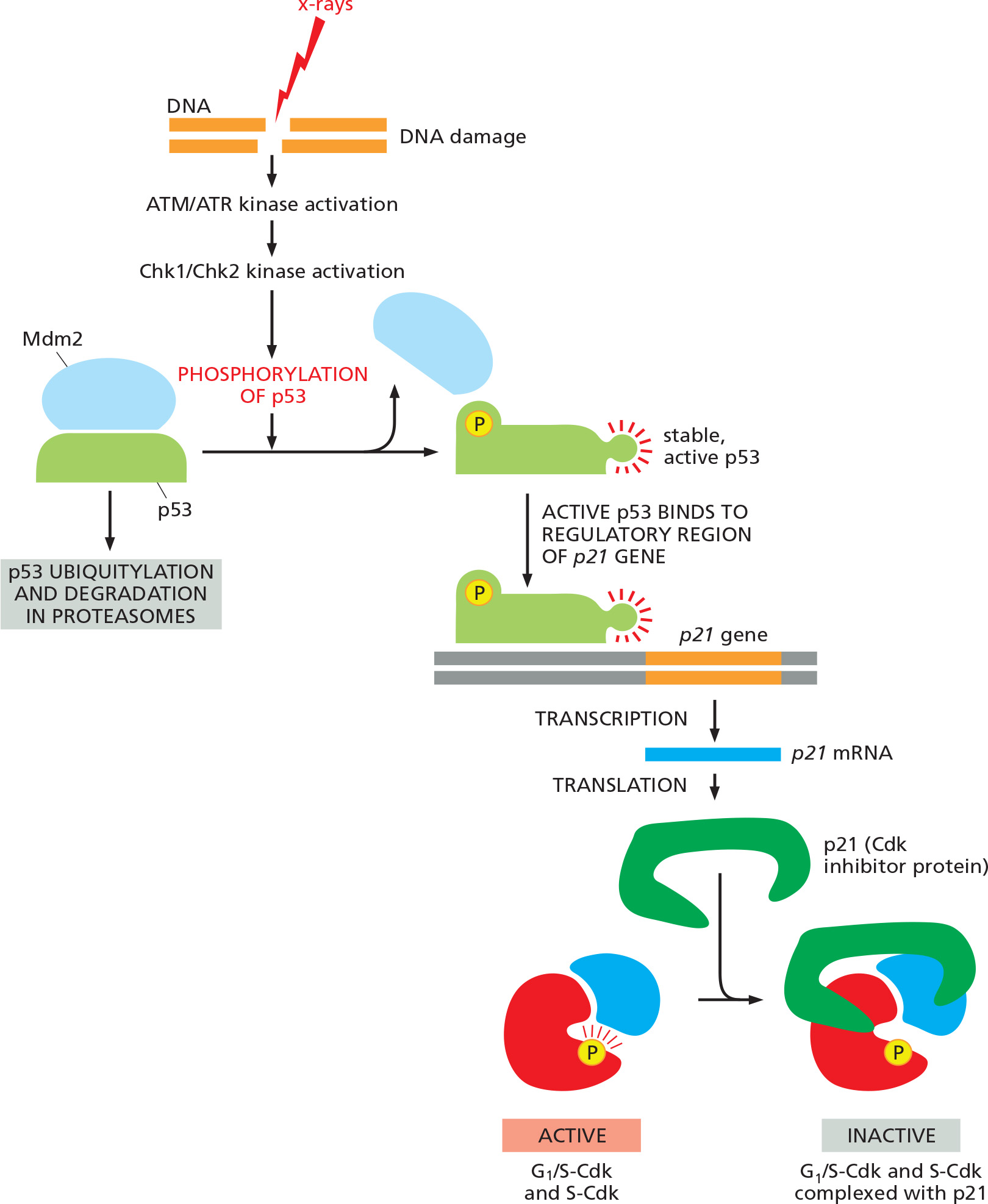
DNA damage response triggers cell cycle arrest: activation of the tumor suppressor p53.
- DNA damage activates ATM/ATR kinases.
- This triggers activation of the tumor suppressor p53, a transcription factor for p21.
The control system blocks progression through each of these transitions if it detects problems inside or outside the cell.
The cell cycle control has major checkpoints:
- G1/S-phase transition checkpoint (START)
- G2/M-phase transition checkpoint
- Metaphase-to-anaphase transition checkpoint: Spindle assembly checkpoint (SAC)
1. Detect DNA Damage Response
ATM mutation: Ataxia-telangiectasia (Louis–Bar syndrome)
- serious genetic disease
- hypersensitive to sunlight
- few live beyond 20s
- neurodegeneration
- Highly susceptibility to cancer
p53 mutation/loss:
- cancer,
- 50% of human cancer have p53 loss or mutation
Abnormal proliferation signals
Abnormal proliferation signals cause cell-cycle arrest or apoptosis, except in cancer cells
Hyperactivated form of Ras or Myc leads to either permanent cell-cycle arrest or apoptosis.
Excessive mitogenic stimulation often leads to the production of a cell-cycle inhibitor protein called Arf, which binds and inhibits Mdm2.
- Mdm2 normally promotes p53 degradation.
Mutations Arf or p53 or the proteins that help activate them are common in cancer cells.
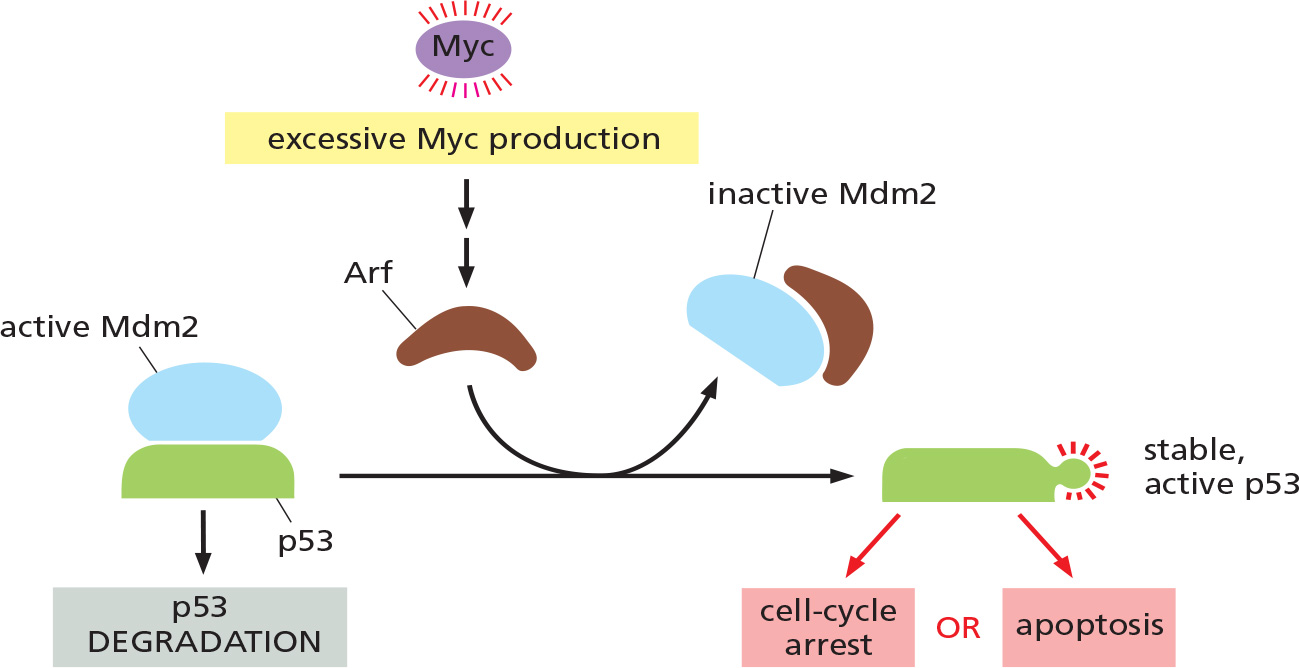
- It is no known how cells detect excessive mitogenic stimulation
#二、Control of cell growth
Coordination of cell growth and cell division:
Growth and division is not always coupled:
- terminally differentiated cells (muscle and nerve cells) grow but do not divide
- drosophilae fertilized eggs divide without growth.
Several coordination mechanisms must exist
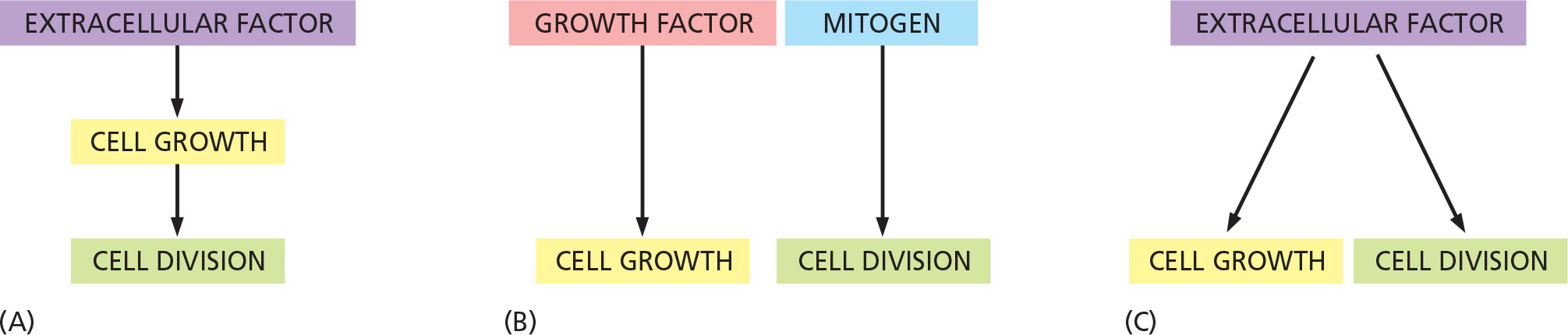
A) Extracellular factors (nutrition) determines both, growth rate and the rate of cell division
B) The rate of growth and cell division is controlled by separate extracellular factors
C) Extracellular factor determines growth rate and the rate of cell division via different signaling pathway