L13 Antiseizures And Drugs for Parkinson’s
一、Introduction
Epilepsy(癫痫)is a heterogeneous symptom complex—a chronic disorder characterized by recurrent seizures.
Seizures (癫痫发作) are finite episodes of brain dysfunction resulting from abnormal discharge of cerebral neurons: Disruption of the depolarization and repolarization mechanisms
- Approximately 1% of the world’s population has epilepsy, the 3rd most common neurologic disorder after dementia and stroke.
- The antiseizure drugs described in this chapter are also used in patients with seizures occurring as part of an acute illness such as meningitis. Seizures are occasionally caused by an acute underlying toxic or metabolic disorder, in which case appropriate therapy should be directed toward the specific abnormality, eg, hypocalcemia (low calcium in serum).
In most cases of epilepsy, however, the choice of medication depends on the empiric seizure classification.
Nature of Epilepsy
• Seizures may be partial or generalised depending on the location and spread of the abnormal neuronal discharge. The attack may involve mainly motor, sensory or behavioural phenomena. Unconsciousness occurs when the reticular formation is involved.
reticular formation:网状结构(英语: reticular formation)是脑部涉及到例如觉醒 /睡眠循环等动作的部分
Types of Epilepsy
Two major categories, namely partial and generalised seizures; there is some overlap and many varieties of each.
Generalised seizures 全身性癫痫
- Involve the whole brain, including the reticular system, thus producing abnormal electrical activity throughout both hemispheres. Immediate loss of consciousness.
Partial (Focal) seizures 部分(局灶性)癫痫发作
- The discharge begins locally, and often remains localised.
- Produce relatively simple symptoms without loss of consciousness.
Seizure Classification
1. Generalized Seizures
Generalized seizures are those in which there is no evidence of localized onset. The group is quite heterogeneous.
- Generalized tonic-clonic seizures (全身性强直阵挛性癫痫) are the most dramatic of all epileptic seizures and are characterized by tonic rigidity (muscle stiffness) of all extremities, followed in 15–30 seconds by a tremor (muscle contraction) that is actually an interruption of the tonus by relaxation
- The absence seizure (失神发作) is characterized by both sudden onset and abrupt cessation. Its duration is usually less than 10 seconds and rarely more than 45 seconds. Consciousness is altered. Absence attacks begin in childhood or adolescence and may occur up to hundreds of times a day.
- Atonic seizures (无张力癫痫) are those in which the patient has sudden loss of postural tone. If standing, the patient falls suddenly to the floor and may be injured
2. Partial Seizures
A localized onset of the attack can be ascertained, either by clinical observation or by electroencephalographic recording; the attack begins in a specific locus in the brain.
- Simple partial seizure, characterized by minimal spread of the abnormal discharge such that normal consciousness and awareness are preserved. lasting 60–90 seconds; residual weakness may last for 15–30 minutes after the attack
- The complex partial seizure also has a localized onset, but the discharge becomes more widespread (usually bilateral) and almost always involves the limbic system. Clinically, the patient may have a brief warning followed by an alteration of consciousness during which some patients stare and others stagger or even fall. After 30–120 seconds, the patient makes a gradual recovery to normal consciousness but may feel tired or ill for several hours after the attack.
- The secondarily generalized attack (继发性全身性发作), in which a partial seizure immediately precedes a generalized tonic-clonic seizure
二、Basic Pharmacology of Antiseizure Drugs
Drug mechanisms
1. Inhibition of Excitatory Synapse
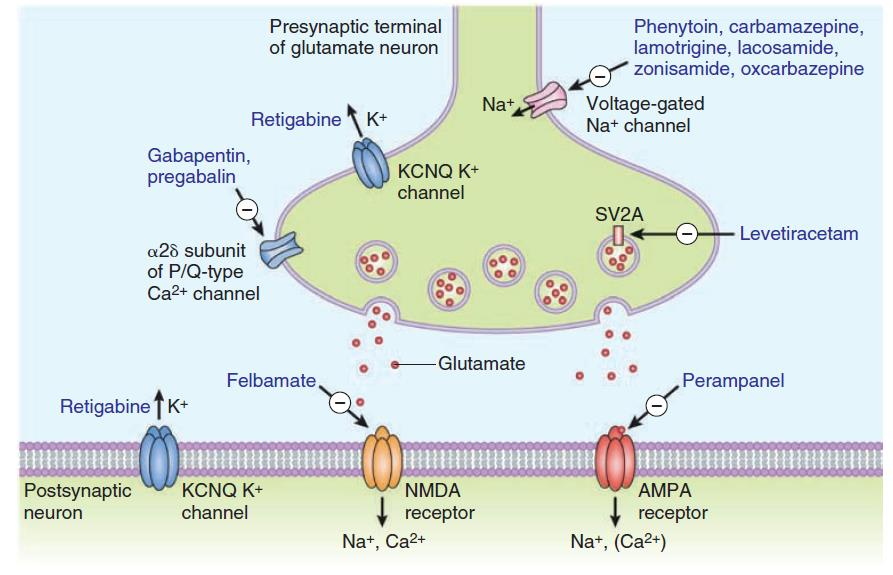 ### 2. Activation of Inhibitory Synapse (GABA)
### 2. Activation of Inhibitory Synapse (GABA)
 ### 3. Inhibition of sodium channel function
### 3. Inhibition of sodium channel function
phenytoin, carbamazepine, valproic acid, lamotrigine
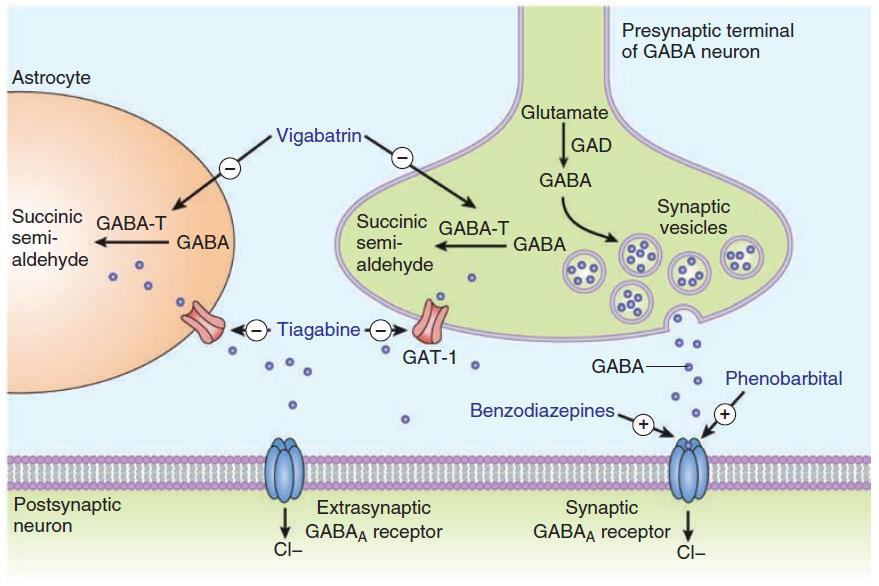
4. Enhancement of GABA actions
- Increase GABA actions at receptor (benzodiazepines, phenobarbital)
- vigabatrin inhibits GABA transaminase
- Tiagabin blocks GABA uptake
5. Inhibition of Calcium P/Q-type channels
 Gabapentin, pregabalin
Gabapentin, pregabalin
Drugs Used in Partial Seizures & Generalized Tonic-Clonic Seizures
The classic major drugs for partial and generalized tonic-clonic seizures are phenytoin (and congeners), carbamazepine, valproate, and the barbiturates.
The availability of newer drugs—lamotrigine, levetiracetam, gabapentin, oxcarbazepine, pregabalin, topiramate, vigabatrin, and zonisamide is altering clinical practice in countries where these compounds are available
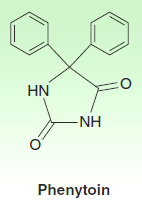
1. Phenytoin
Phenytoin is the oldest nonsedative antiseizure drug.
 **Mechanism of Action**
**Mechanism of Action**
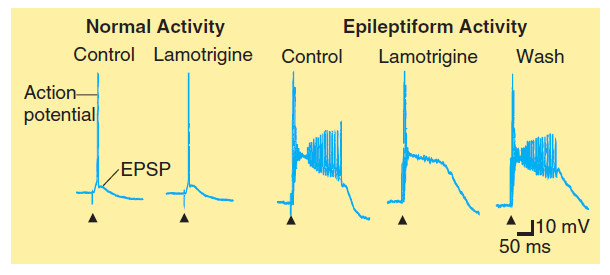
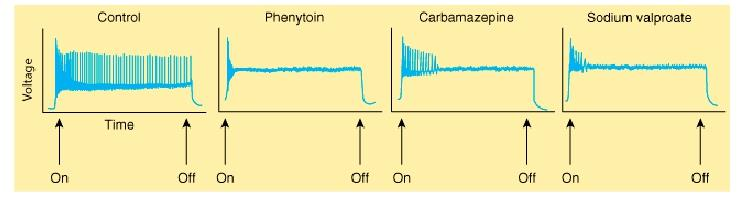
- It is a use-dependent effect on Na^+^ conductance, arising from preferential binding to—and prolongation of—the inactivated state of the Na^+^ channel.
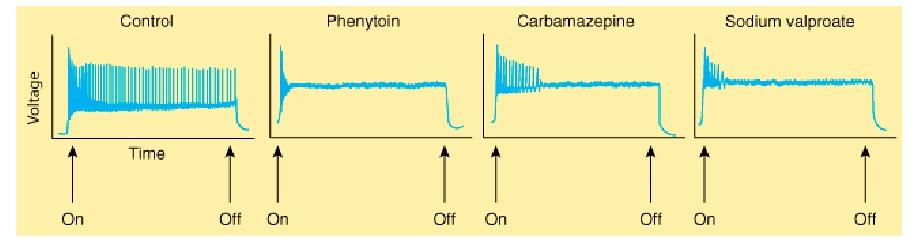 **Clinical Use**
**Clinical Use**
- Phenytoin is effective against partial seizures and generalized tonic-clonic seizures. In the latter, it appears to be effective against attacks that are either primary or secondary to another seizure type.
Pharmacokinetics
- Absorption of phenytoin sodium from the gastrointestinal tract is nearly complete in most patients. Intramuscular injection is not recommended for phenytoin.
- Phenytoin is highly bound to plasma proteins. Drug concentration in cerebrospinal fluid is proportionate to the free plasma level. Phenytoin accumulates in brain, liver, muscle, and fat.
Drug Interactions & Interference with Laboratory Tests
- Since phenytoin is 90% bound to plasma proteins, other highly bound drugs, such as phenylbutazone and sulfonamides, can displace phenytoin from its binding site. In theory, such displacement may cause a transient increase in free drug
- A decrease in protein binding—eg, from hypoalbuminemia—results in a decrease in the total plasma concentration of drug but not the free concentration. Intoxication may occur if efforts are made to maintain total drug levels in the therapeutic range by increasing the dose.
- Phenytoin has been shown to induce microsomal enzymes responsible for the metabolism of a number of drugs. Autostimulation of its own metabolism, however, appears to be insignificant.
2. Carbamazepine
Drug for bipolar depression. It was initially marketed for the treatment of trigeminal neuralgia but has proved useful for epilepsy as well.
Mechanism of Action
- blocks sodium channels
 ### 3. Phenobarbital
### 3. Phenobarbital
The oldest of the currently available antiseizure drugs
Mechanism of Action
- Enhancement of inhibitory process
- Diminution of excitatory transmission
Clinical Use
- partial seizures, generalized tonic-clonic seizures
Therapeutic Levels, & Dosage
- From 10 mcg/mL to 40 mcg/mL
- Documentation of effectiveness is best in febrile seizures, and levels below 15mcg/mL appear ineffective for prevention of febrile seizure recurrence.
- The upper end of the therapeutic range is more difficult to define because many patients appear to tolerate chronic levels above 40 mcg/mL
4. Valproic Acid & Sodium Valproate
Mechanism of Action
- Effect on Na^+^ currents.
- Blockade of NMDA receptor-mediated excitation
- Effects on GABA
- To increase levels of GABA in the brain after administration of valproate, by facilitating glutamic acid decarboxylase (GAD), the enzyme responsible for GABA synthesis, has been described. An inhibitory effect on the GABA transporter GAT-1 may contribute.
- At very high concentrations, valproate inhibits GABA transaminase in the brain, thus blocking degradation of GABA.
Drug Interactions
- Valproate displaces phenytoin from plasma proteins. In addition to binding interactions, valproate inhibits the metabolism of several drugs, including phenobarbital, phenytoin, and carbamazepine, leading to higher steady-state concentrations of these agents.
三、Degenerative Diseases of the Nervous System
Chronic neurological conditions associated with progressive loss of neurons.
- evidence of inflammation?
- evidence of cellular necrosis?
Examples:
- Parkinson’s disease.
- Alzheimer’s disease.
- Motor neuron disease (ALS)
Parkinson’s disease
1. Introduction
2^nd^ most common neurodegenerative disease
- Mean onset = 57 years of age.
- Affects 1-2% of population over 60 years of age.
Etiology is unknown
Disease progression is highly variable
Can be early onset in some cases.
2. Parkinsonism
Tremors – hands and head develop involuntary movements when at rest; pin‐rolling sign (finger and thumb)
Muscle rigidity – arthritis‐like stiffness, difficulty in bending or moving limbs; poker face
Bradykinesia (运动迟缓) – problems chewing, swallowing or speaking; difficulty in initiating movements and controlling fine movements; walking becomes difficult (shuffle feet)
Postural instability (姿势不稳) – humped over appearance, prone to falls
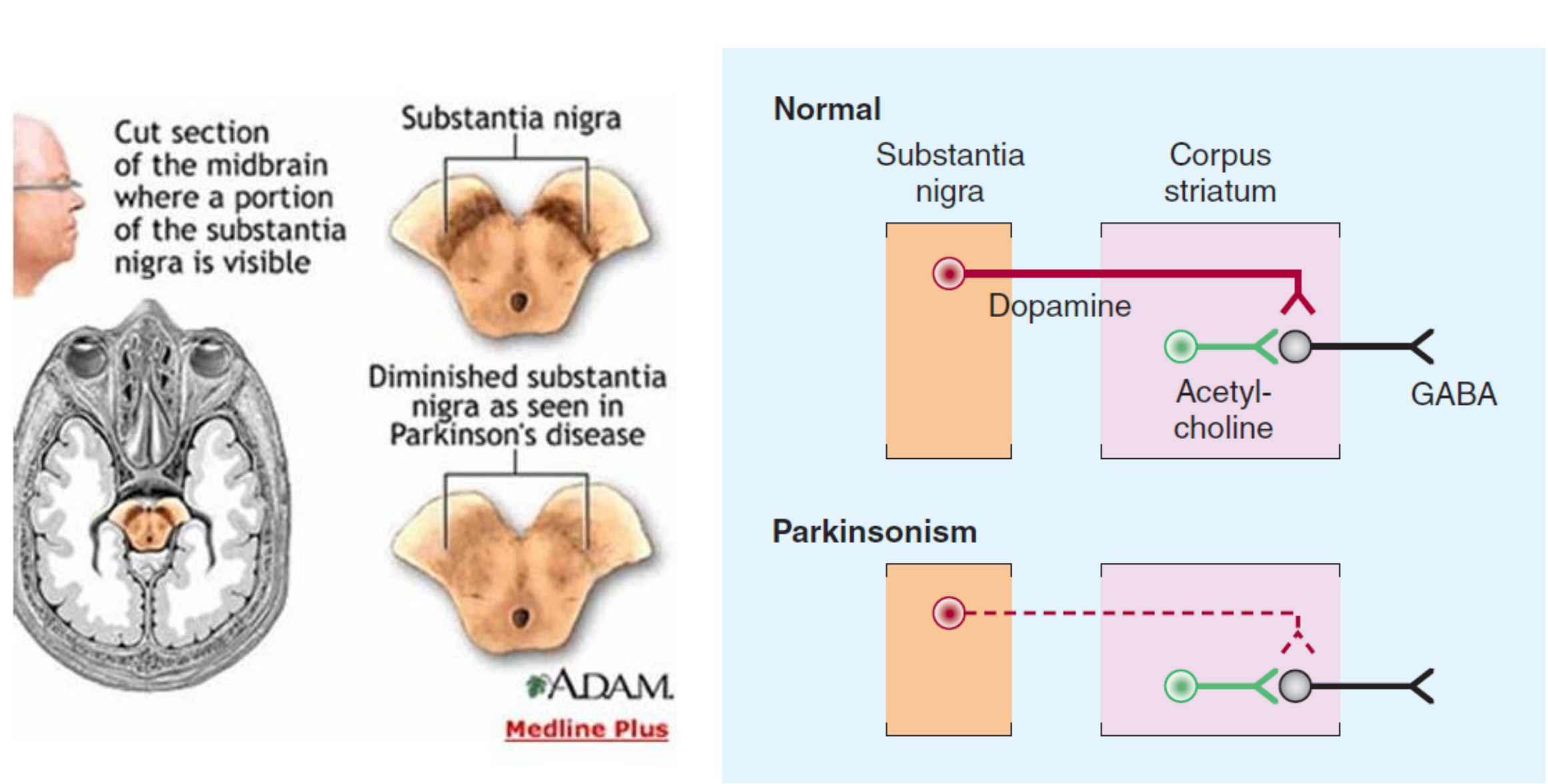
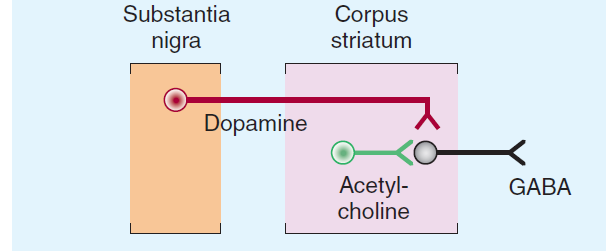
3. The Substantia Nigra (黑质) in Parkinson’s Disease
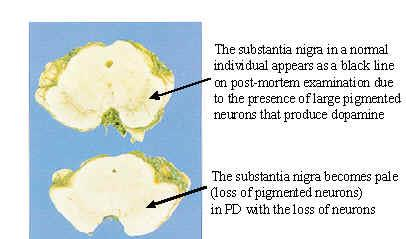 + Symptoms related to selective loss of pigmental neurons in the midbrain. Substantia nigra pars compacta – Dopaminergic neurotransmission to caudate nuclei (i.e., striatum) and putamen.
+ Symptoms related to selective loss of pigmental neurons in the midbrain. Substantia nigra pars compacta – Dopaminergic neurotransmission to caudate nuclei (i.e., striatum) and putamen.
4. Drug Treatment of Parkinson’s Disease
 ### 5. Pathogenesis
### 5. Pathogenesis
Familial PD: first mutation discovered was in gene that coded for synuclein; several further gene loci discovered.
Sporadic PD: no significant gene association as opposed to AD with the apolipoprotein E association
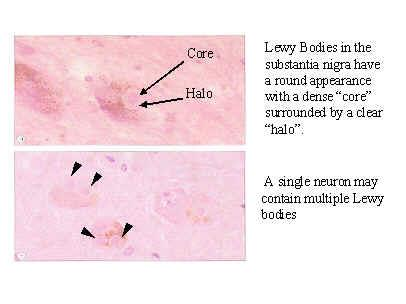
 **Lewy Bodies – H&E Section**
**Lewy Bodies – H&E Section**
 **Lewy Bodies –** **Immunoperoxidase staining**
**Lewy Bodies –** **Immunoperoxidase staining**
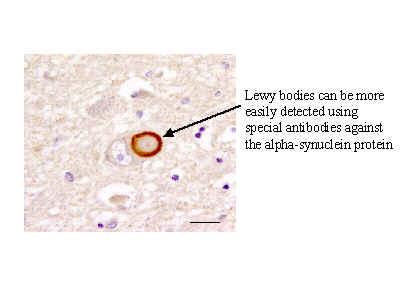 α-synuclein – abnormally deposited in the CNS in Parkinson’s Disease, leading to the formation of Lewy bodies (the pathological hallmark of PD)
α-synuclein – abnormally deposited in the CNS in Parkinson’s Disease, leading to the formation of Lewy bodies (the pathological hallmark of PD)
Reactive protofibrils of α-synuclein increased by catecholamines (i.e., dopamine).
- Cytoplasmic oxidation of dopamine -> hydroxydopamine leads to formation of Lewy bodies causing dopaminergic cell death
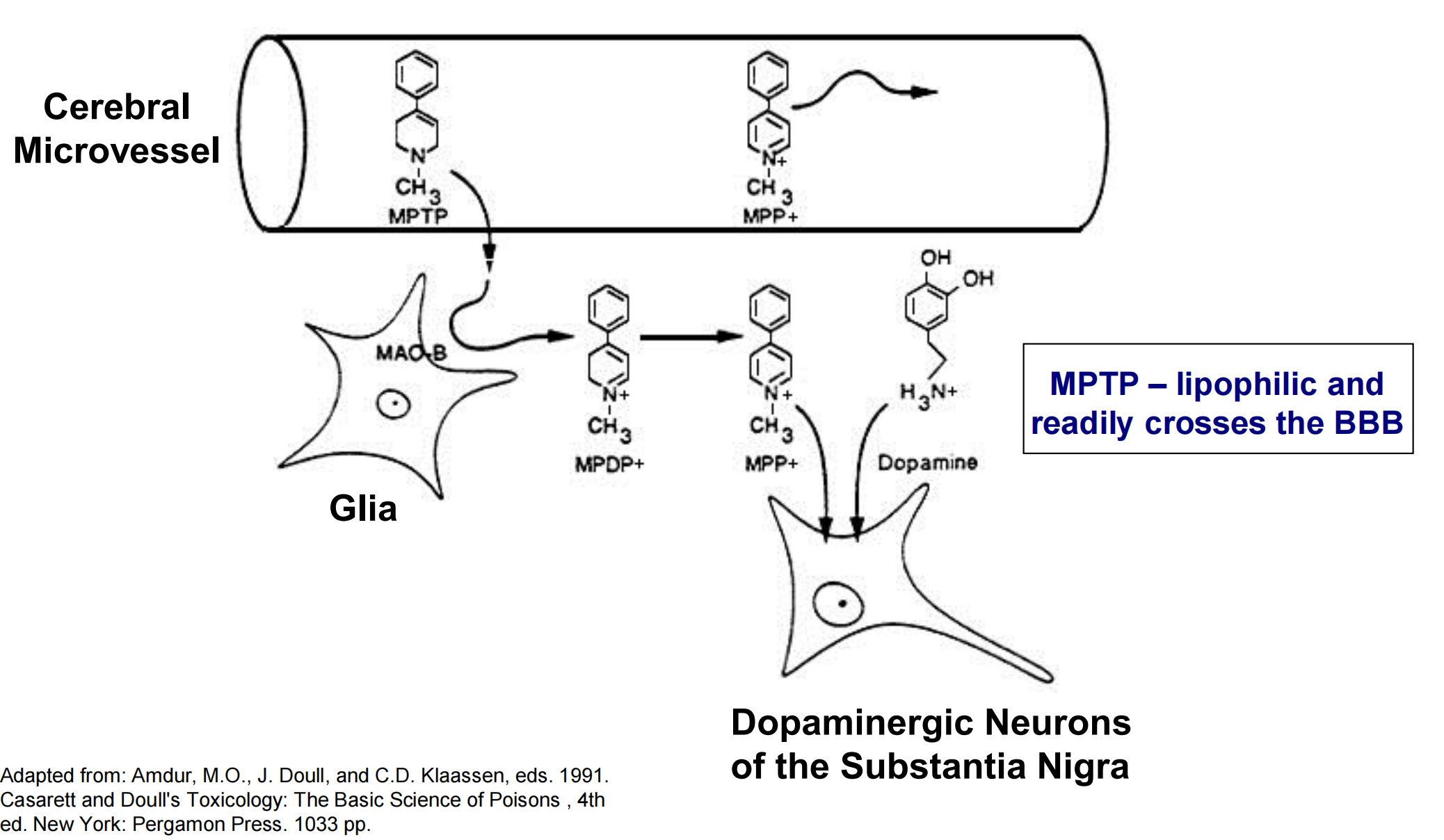
6. MPTP and Dopaminergic Neurons
MPTP – induces oxidative damage to dopaminergic neurons.
- Effect identified in 1976 due to incorrect synthesis of MPPP, an analogue of pethidine (Demerol – opioid analgesic).
- Symptoms of Parkinson’s disease observed within 3 days.
Effect on dopaminergic neurons is indirect.
- MPTP itself is not a neurotoxin
- Enzymatically converted (via MAO-B (Monoamine oxidases B)) in the CNS to MPP+, which selectively targets dopaminergic neurons in the substantia nigra
- MPP+ high-affinity substrate for dopamine reuptake transporters localized to the pre-synaptic membrane of neurons in the substantia nigra.
 ### 7. Oxidative stress and Parkinson’s Disease
### 7. Oxidative stress and Parkinson’s Disease
Dopamine metabolism results in reactive oxygen species (oxidative deamination of dopamine by MAO -> H2O2).
Glutathione (primary CNS antioxidant) levels are depressed in Parkinson’s disease.
- Renders neurons more susceptible to ROS toxicity
- Observed in workers exposed to insecticides/pesticides.
Coenzyme Q10 study: 1200 mg/day may slow progression.
8. Summary

 ## Pharmacological Treatment of Parkinson’s Disease
## Pharmacological Treatment of Parkinson’s Disease
Goals:
- Primary = restore dopamine receptor function
- Secondary = inhibition of muscarinic cholinergic receptors
Several types of drugs:
- Levodopa
- Dopamine Receptor Agonists
- Monoamine Oxidase Inhibitors (MAOIs).
- Catechol-O-Methyltransferase (COMT) inhibitors.
- Muscarinic Cholinergic Receptor Antagonists.
- Amantidine
 Other Drugs
Other Drugs
- Dopamine Receptor Agonists
- Enzyme inhibitors
- Dopamine Facillitators
 ### 1. Levodopa
### 1. Levodopa
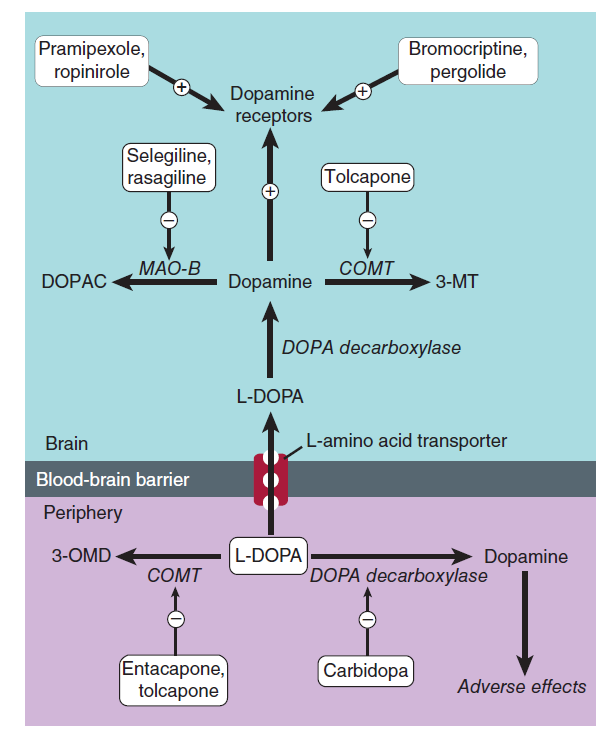
Prodrug – immediate metabolic precursor of dopamine.
- Levodopa can cross the blood-brain barrier while dopamine cannot.
- CNS – enzymatically converted to dopamine by L-aromatic amino acid decarboxylase.
1-3% of Levodopa actually enters the brain.
- Primarily due to extracerebral metabolism
- Extracerebral metabolism can be reduced by administering a non-BBB permeating peripheral L-aromatic amino acid decarboxylase inhibitor.
Sinemet® = levodopa + carbidopa
Mechanism of Action: restoration of synaptic concentrations of dopamine.
- Activation of post-synaptic D2 receptors = inhibit adenylyl cyclase = promote voluntary movement via indirect pathway.
Therapeutic Effectiveness
- Best results obtained in first few years of treatment.
- 80% of patients show marked initial improvement (primarily in terms of resolution of muscle rigidity and bradykinesia)
- 20% show virtually normal motor function.
- Over time, levodopa therapy becomes less effective
- Progressive loss of dopaminergic neurons
- Downregulation of D1/D2 receptors on post-synaptic terminals.
- Some patients require reduced doses of levodopa to prevent side effects
2. Dopamine Receptor Agonists
Ropinirole (Requip®) – D2 receptor agonist.
- Effective as monotherapy in patients with mild disease.
Apomorphine – potent D1/D2 agonist
- Given via subcutaneous injection to provide temporary relief of “off” periods of akinesia.
- Short period of effectiveness ( ~ 2 h).
- Associated with several side effects (i.e., dyskinesias, drowsiness, sweating, hypotension).
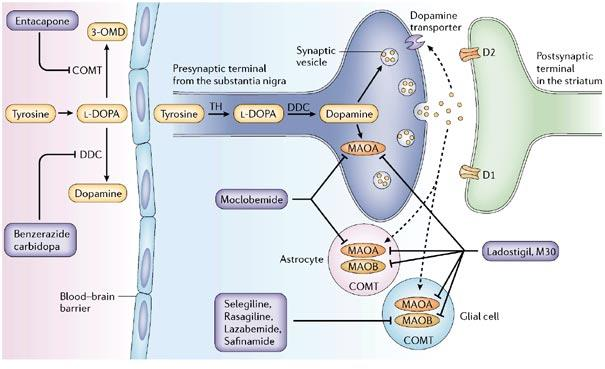
3. Monoamine Oxidase Inhibitors (MAOIs)
Two types of MAO have been characterized.
- MAO-A – primarily metabolizes NE and 5-HT.
- MAO-B – primarily metabolizes dopamine
Selegiline (Eldepryl®)
- Selective, irreversible inhibitors of MAO-B.
4. Selegiline – MAO-B Inhibitor
Therapeutic Effectiveness
- Effective in early Parkinson’s disease (as monotherapy or in combination with levodopa)
- Enables reduction in levodopa dose or may smooth the “on-off” fluctuations associated with levodopa
- Metabolite = Desmethylselegiline – neuroprotective.
Adverse Effects
- Selectivity for brain MAO-B makes selegiline less likely to produce ADRs involving peripheral tyramine (i.e., wine, cheese, and chopped liver syndrome).
- Tyramine = catecholamine releasing agent
- Blocks MAO-A at high doses.
- Hypertensive crisis due to peripheral accumulation of NE.
- Fatal hyperthermia – may occur when administered in conjunction with meperidine, cocaine, or fluoxetine.
5. Catechol-O-Methyltransferase (COMT) Inhibitors
Inhibition of L-aromatic amino acid decarboxylase is associated with compensatory activation of COMT.
- Increased plasma levels of 3-OMD = poor response to levodopa (competition for active transporter in the gut and at the BBB?)
Adjunctive therapy in patients treated with levodopa.
Entacapone
Selective COMT inhibitors – diminish peripheral metabolism of levodopa.
May also reduce “on-off” fluctuations
Adverse Effects:
- Related to increased plasma concentrations of levodopa.
- Include dyskinesias, nausea, and confusion.
- Other side effects: diarrhea, abdominal pain, orthostatic hypotension, sleep disorders, orange urine discoloration.
- Tolcapone – potentially hepatotoxic.
6. Acetylcholine‐Blocking Drugs
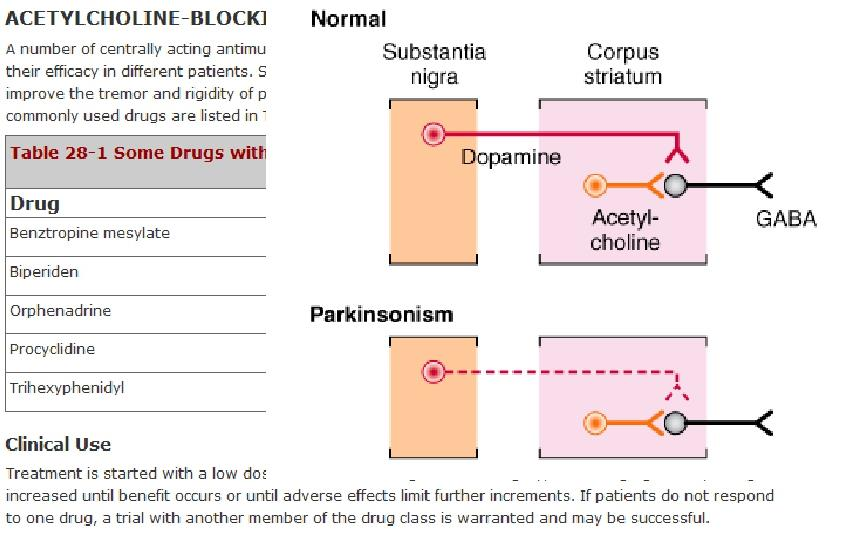 ### 7. Muscarinic Cholinergic Receptor Antagonists
### 7. Muscarinic Cholinergic Receptor Antagonists
Muscarinic Receptors – localized to striatal neurons.
- Mediate cholinergic tremor
- May cause presynaptic inhibition of dopamine release
Trihexyphenidyl (Artane®) and Benztropine (Cogentin®)
- Therapeutic Effectiveness
- Useful in patients administered neuroleptics as anti-dopaminergic properties of these drugs antagonize effects of levodopa
- Improve muscle rigidity and tremor but have little effect on bradykinesia.
- Adverse Effects
- Characterized as “atropine-like” = dry mouth, inability to sweat, impaired vision, urinary retention, constipation, drowsiness, confusion.
8. Amantidine (Symmetrel®) Dopamine facillitator
Antiviral drug with anti-Parkinsonian properties.
Mechanism of action is unclear
- Potentiates dopaminergic function by modifying synthesis, release, or reuptake of dopamine. Act on NMDA receptor
Therapeutic Effectiveness
- Less effective than levodopa
- Therapeutic benefits are short-lived.
Adverse Effects
- Primarily CNS = restlessness, depression, irritability, insomnia, agitation, excitement, hallucinations, confusion
- Overdoses = acute toxic psychosis
- Others = headache, edema, postural hypotension, heart failure, GI disturbances.