L3 Construction of neural circuits
一、Cell Polarity
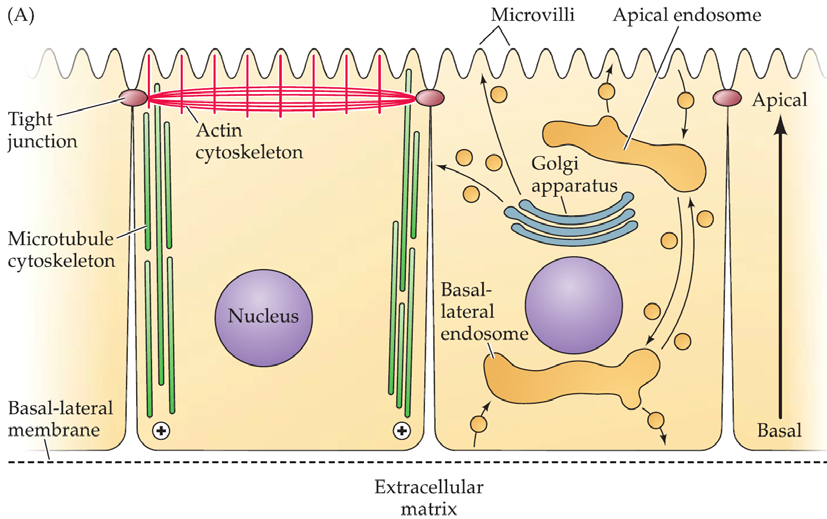
Neurons are especially elaborate examples of polarized epithelial cells, a fundamental cell class found in most tissues.
The apical(顶上的) domain has a distinctive actin cytoskeleton, and membrane extensions (villi) that increase the surface area for taking in and releasing specific molecules
There are tight junctions.
The Golgi apparatus is oriented toward the apical membrane.
The basolateral(基底外侧) domain makes contact with the extracellular matrix
- It has specialized adhesion contacts.
- The plus ends of microtubules are oriented toward the basal membrane.
- Its surface is specialized for intercellular communication.
First Step of Polarization Is Neural Circuit Formation
Neuronal polarization: the first step in neural circuit formation
Distinguishing the apical domain (the eventual axon, adapted for secretion) from the basal domain (the dendrites, which will eventually become specialized for receiving signals).
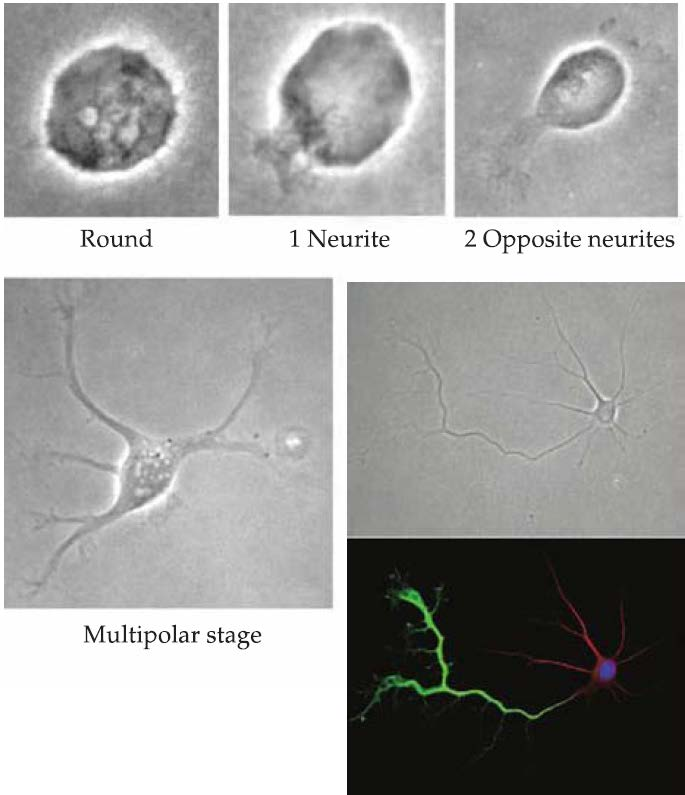
- Once neurogenesis is complete, outgrowth of neuronal processes begins.
- Initially, a number of apparently equivalent small extensions (referred to as neurites(神经突), since at first they have neither axonal nor dendritic identities) protrude from the immature neuron.
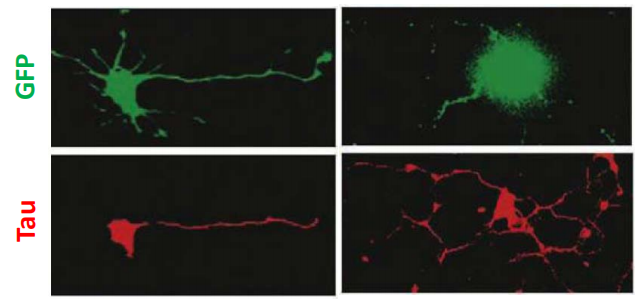
- Microtubule and actin components of the cytoskeleton as well as other proteins are redistributed among the neurites so that a single process is identified as the axon(the apical domain).
- The remaining processes become dendrites (the basal domain).
Regulation of cell polarity
Members of the PAR family, are distributed preferentially in the nascent axon.
- PAR proteins interact with cytoskeletal elements and signaling molecules, including Rho and other protein kinases; signal transduction molecules activated by secreted Wnts; neurotrophins(神经营养因子); and cell surface-bound cell adhesion molecules.
- When the function of PAR proteins or related signaling molecules is disrupted, the specification of a single axon does not occur.
- Multiple axons
Axon
1. Introduction
Once the axon has been specified, it navigates over millimeters or even centimeters, through complex embryonic terrain, to find appropriate synaptic partners.
“The growing fibers are clearly endowed with considerable energy and have the power to make their way through the solid or semi-solid protoplasm of the cells of the neural tube. But we are at present in the dark with regard to the conditions which guide them to specific points.“ ( **–**Ross G. Harrison, 1910)
Harrison got his BA and PhD from Johns Hopkins.
He was considered for a Nobel prize for his work on nerve-cell outgrowth.
2. Axon growth cones
Axon growth cone: a specialized structure at tip of extending axon.
- Growth cones are highly motile.
- They explore the extracellular environment, determine the direction of growth, and then guide the extension of the axon in that direction.
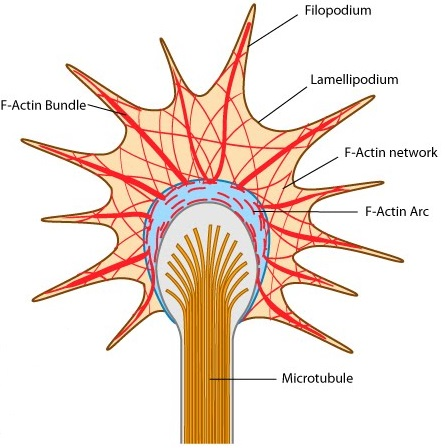
- Lamellipodium (片状脂质体): a sheetlike expansion of the growing axon at its tip.
- Filopodia (丝状伪足): numerous fine processes that extend from each lamellipodium.
- Filopodia rapidly form and disappear from the terminal expansion, like fingers reaching out to sense the environment.
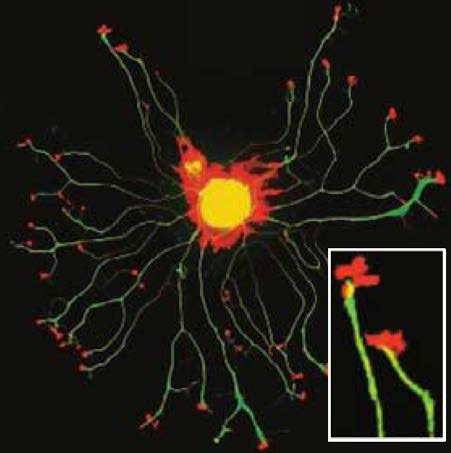
Growth cone motility
Growth cone motility reflects rapid, controlled rearrangement of the cytoskeleton.
The force to move the axon is generated by ATP-dependent modification of the actin and microtubule cytoskeletons.
actin cytoskeleton: regulating changes in lamellipodial and filopodial shape for directed growth.
microtubule cytoskeleton: responsible for the elongation of the axon itself
1. Molecular basis of growth cone motility
The molecular composition of both the actin and microtubule cytoskeletons changes in distinct regions of the growing axon, suggesting a great deal of dynamism within growing neural processes.
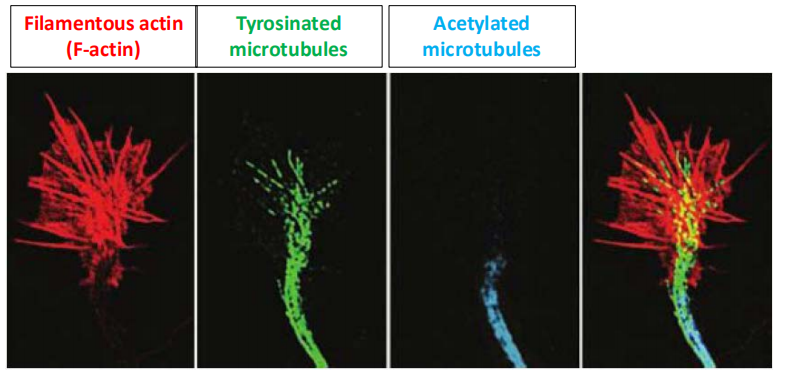
2. Actin and tubulin
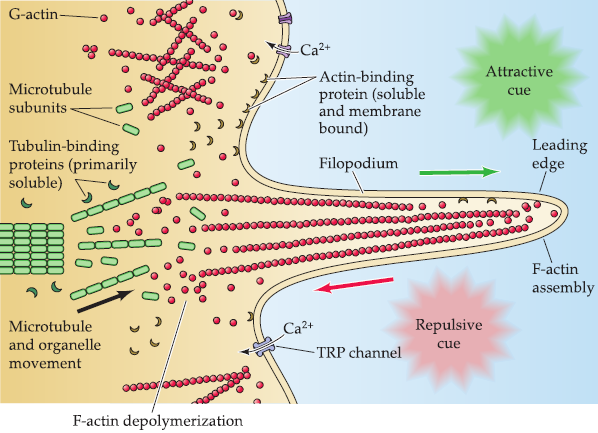
Actin is the primary molecular constituent of a network of cellular filaments found in the lamellipodia and filopodia of growth cones.
Tubulin is the primary molecular constituent of the microtubules that run parallel to the axis of the axon and give it both structural integrity and a means for transporting proteins from the nerve cell body to the axon terminal

Actin and tubulin are found in two forms in the growth cone and axon:
- freely soluble monomers in the cytoplasm.
- polymers that form filaments (actin) or microtubules (tubulin).
Polymerization and depolymerization
The dynamic polymerization and depolymerization of actin at the membrane of the lamellipodiurn, as well as within the filopodium sets the direction of growth cone movement, in part by generating local forces that orient the growth cone toward or away from substrates.
The polymerization and depolymerization of tubulin into microtubules consolidates the direction of movement of the growth cone by stabilizing the axon shaft.
The interface between the actin and microtubule cytoskeletons regulates the balance of active growth versus stability.
1. Proteins regulating polymerization and depolymerization
Binding to these molecules and catalyzing posttranslational modifications, or by recruiting enzymes that modify the primary molecular elements of the cytoskeleton.
Actin-binding proteins
Found throughout the growth cone cytoplasm.inside
Either bind actin directly, or modify actin monomers by phosphorylation and other posttranslational modifications.
Microtubule-binding proteins
More concentrated in the axon shaft.
Modulate posttranslational modifications of monomeric and polymerized tubulin.
Other microtubule-binding proteins (“motors“) are essential for moving molecules and organelles (“cargo”) up and down neuronal processes.
二、Mechanisms regulating polymerization and depolymerization
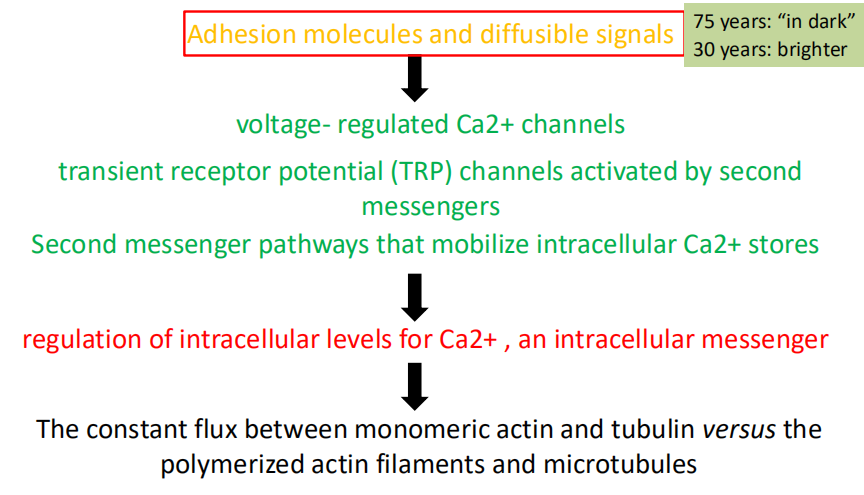
- Adhesion molecules and diffusible signals
- Voltage-regulated Ca2+ channels; transient receptor potential (TRP) channels activated by second messengers; Second messenger pathways that mobilize intracellular Ca2+ stores
- Regulation of intracellular levels for Ca2+, an intracellular messenger
- The constant flux between monomeric actin and tubulin versus the polymerized actin filaments and microtubules
Non-diffusible signals for axon guidance
- the extracellular matrix molecules and their integrin receptors
- the Ca2+ -independent cell adhesion molecules (CAMs)
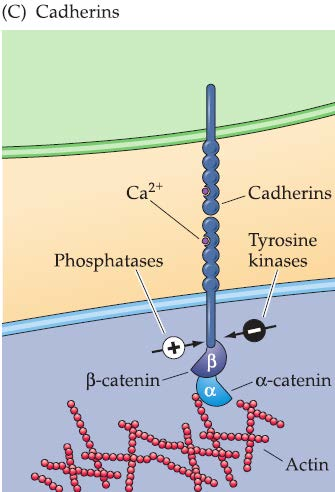
- the Ca2+-dependent cell adhesion molecules, or cadherins(钙粘蛋白)
- the ephrins(麻黄素) and Eph receptors
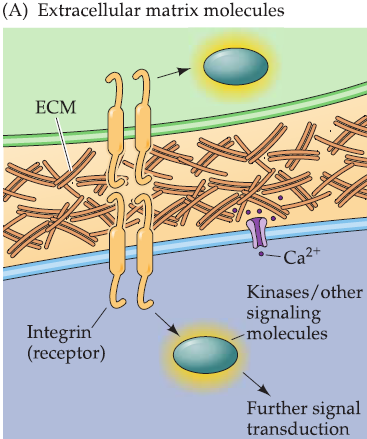
1. Extracellular matrix cell adhesion molecules
the first to be associated with axon growth
laminins, collagens, and fibronectin(层粘连蛋白,胶原,纤连蛋白)
- Secreted by the cell itself or by its neighbors
- Forming polymers and creating a durable local extracellular substance, rather than diffusing away from the cell after secretion.
Cell surface receptors: integrins(整合素)
- lntegrins do not have kinase activity or any other direct signaling capacity.
- Triggers a cascade of events-perhaps via interactions between the cytoplasmic domains of integrins with kinases and other signaling molecules, as well as Ca2+ channels-that can stimulate axon growth and elongation.
2. CAMs and cadherins
Found on growing axons and growth cones as well as on surrounding cells and targets.
Both CAMs and cadherins have dual functions as ligands and receptors, usually via hemophilic (“like with like”) binding.
- CAMs, especially the L1 CAM: bundling, or fasciculation, of groups of axons.
- Cadherins: important determinants of final target selection in transition from growing axon to synapse.
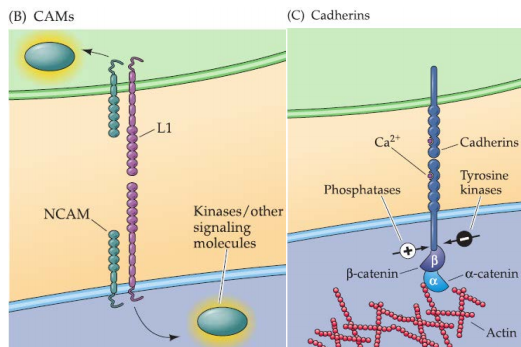
Both CAMs and cadherins rely on a somewhat indirect route of signal transduction.
Ca2+-independent CAMs interact with cytoplasmic kinases to initiate cellular response.
Ca2+-dependent cadherins engage the APC/β-catenin pathway
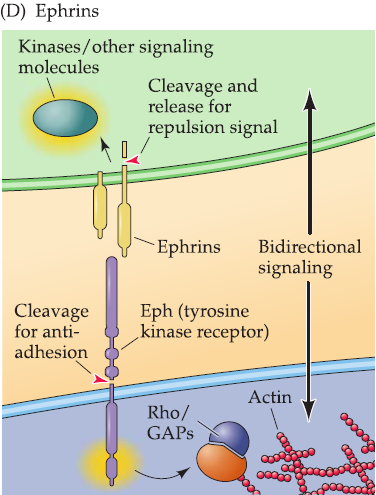
3. ephrins
Ephrin ligands and their tyrosine kinas receptors (Eph receptors, or Ephs).
Bidirectional signaling.
- Both affect target cell and signal-generating cell
4. Local translation
- what does local means: soma between axon?
5. m6A modification and axon growth
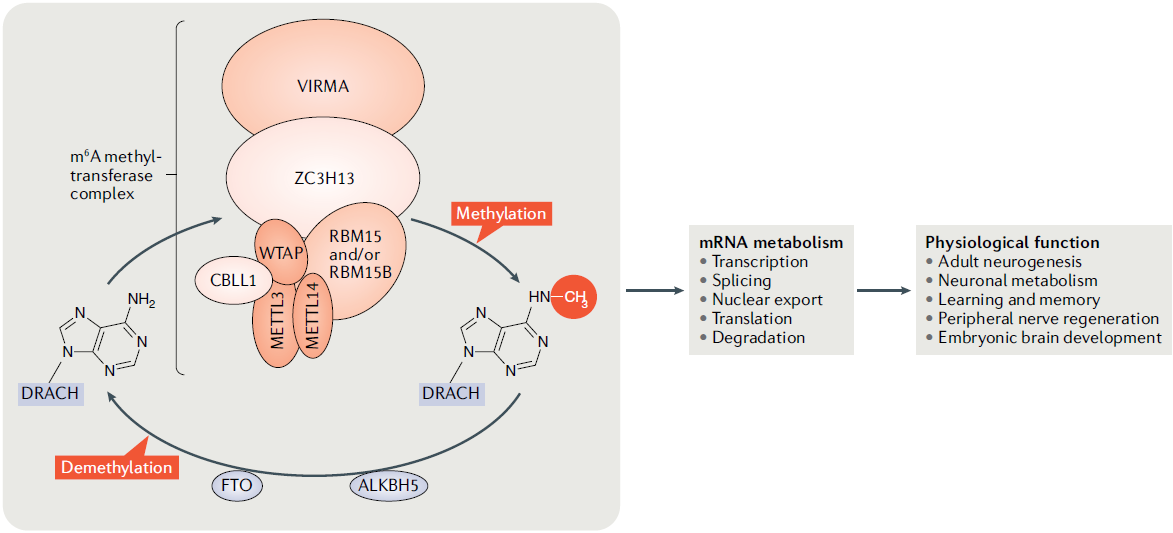
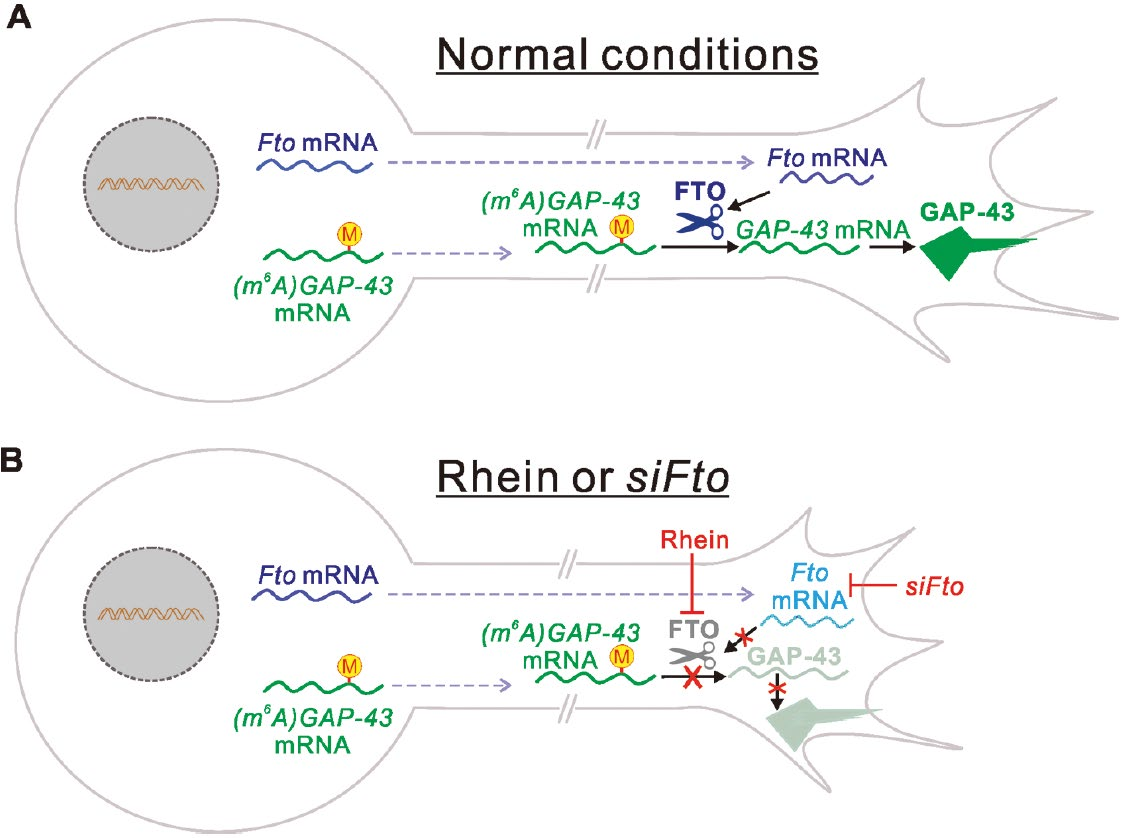
Human developmental or neurological disorders related to adhesive interactions and signal transduction
X-linked hydrocephalus. 脑积水
MASA (an acronym for mental retardation, aphasia, shuffling gait, and adducted thumbs).
Kallman’s syndrome (which compromises reproductive and chemosensory function).
X-linked spastic paraplegia. 半身不遂
Chemoattraction and chemorepulsion
A growing axon must eventually find an appropriate target while avoiding inappropriate ones.
Ramon Cajal proposed that target-derived signals, most likely released by the target cells themselves, selectively attract growth cones to useful destinations—chemoattraction.
chemorepulsion: opposite to the chemoattraction
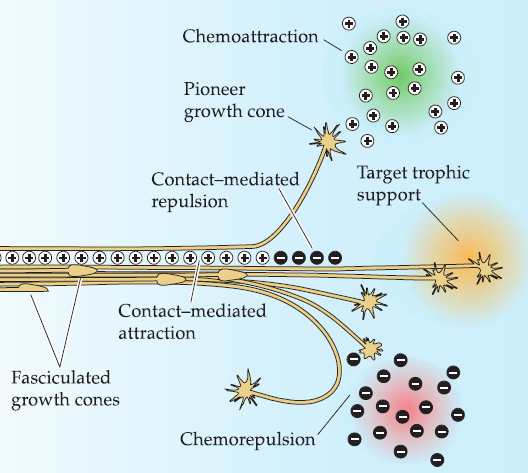
1. Difficulties in identifying chemoattractive and chemorepulsive signals
Small amounts of such factors expressed in the developing embryo.
Difficulties in distinguishing tropic molecules (which guide growing axons toward a source) from trophic(营养的,有关营养的) molecules (which support the survival and growth of neurons and their processes once an appropriate target has been contacted).
Solved by laborious biochemical purification and analysis of attractive or repulsive activities from vertebrate (chick) embryos and genetic analysis of axon growth in both Drosophila and C. elegans.
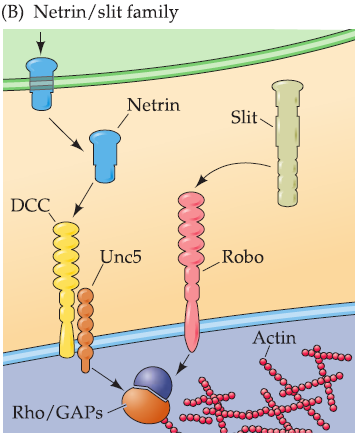
2. Netrins & Slits
Sanskrit, ‘’to guide“: One of the first families of chemoattractant molecules to be identified.
- Chick: proteins with chemoattractant activity following biochemical purification.
- C. elegans: influenced axon growth and guidance.
Unc: “uncoordinated,“ which describes the behavioral phenotype of the mutant worms.
DCC (deleted in colorectal cancer) receptor mediates netrin-dependent chemoattraction.
Unc5 receptor mediates netrin–dependent chemorepulsion.
- Netrin receptors have repeated amino acid motifs in their extracellular domains; a transmembrane domain; and an intracellular domain with no known enzymatic activity.
- The Rho/GAP family of signaling proteins, all of which modulate second messenger-mediated cytoskeletal modification, provide a final step in netrin signaling.
Slit and its receptor robo: chemorepulsion.
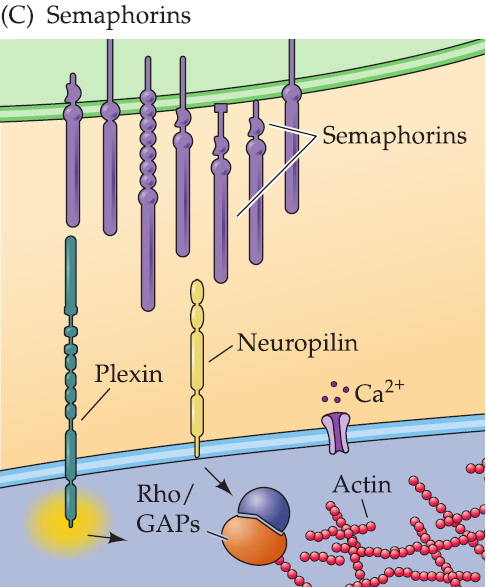
3. Semaphorins(信号素)
Semaphorins are bound to cell surfaces or to the extracellular matrix, where they prevent the extension of nearby axons.
Their receptors are transmembrane proteins (including the plexins and a protein called neuropilin) whose cytoplasmic domains have no known catalytic activity.
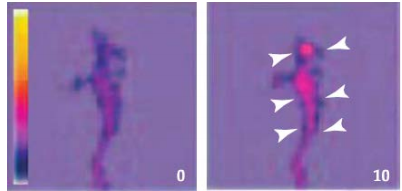
Semaphorin signaling leads to changes in Ca2+ concentration that presumably activate intercellular kinases and other signaling molecules to modify the growth cone cytoskeleton.
Semaphorin treatment of cultured neurons results in growth cone collapse, which is Ca2+ signaling-dependent.
4. Commissural(合缝处的) axon guidance
Netrin
mediate the pre-cross attraction
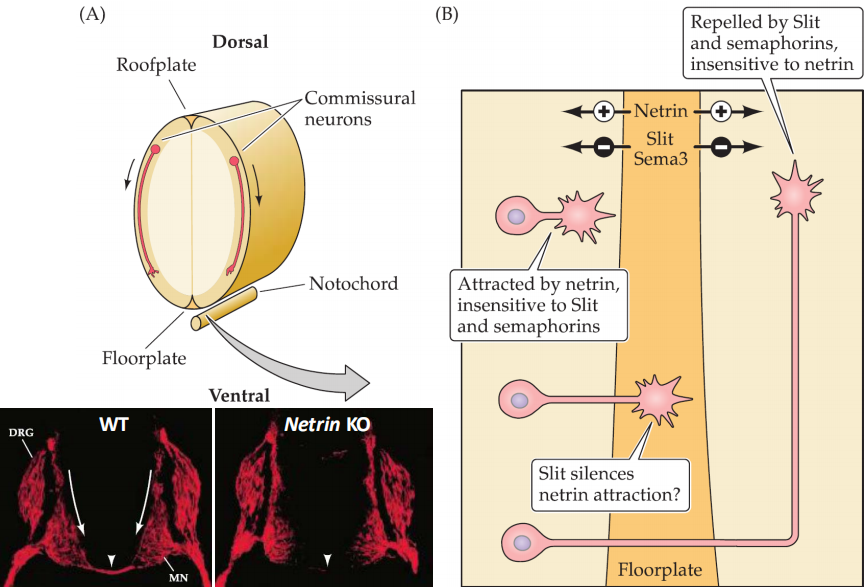
- The commissural neurons should firstly attracted to the floorplate
- Then cross the floorplate and leave the floor plate by silencing the netrin attraction and then make neurons insensitive to netrin (Repelled by Slit and semaphorins)
Robo3
Mediate the post-cross repulsion
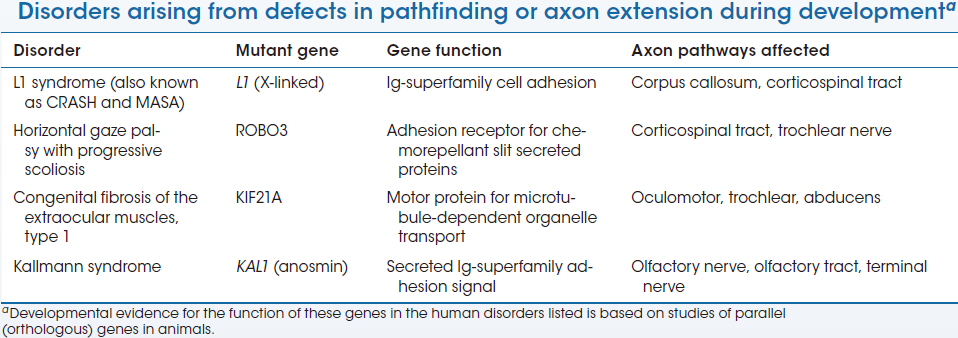
Axon Guidance Disorder
There is now a “short list” of rare but highly informative mutations in genes known to regulate growth cone pathfinding or axon extension during development.
These mutations disrupt both CNS axon tracts and peripheral nerves, resulting in mild and specific motor or sensory deficits as well as more global difficulties, including intellectual disability and social and cognitive impairments.
Selective synapse formation
Once an axon reaches its target region, additional cell-cell interactions dictate which target cells to innervate from among a variety of potential synaptic partners.
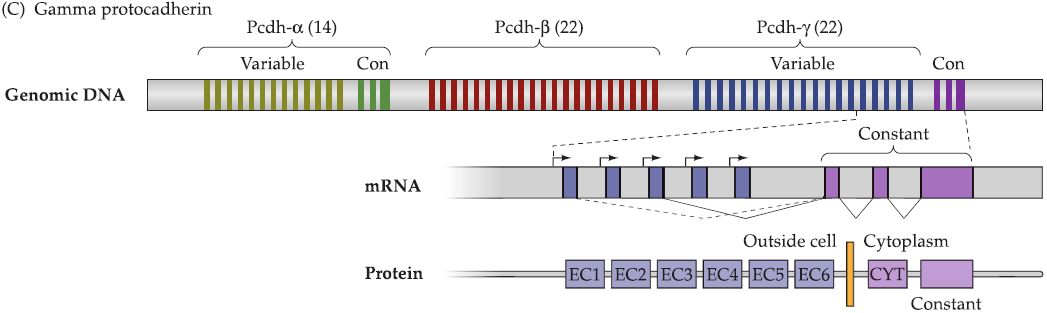
1. Protocadherins (原钙粘蛋白)
Formation of protocadherins
protocadherins: resemble the general cadherin family of cell adhesion molecules
- single gene encodes a large number of protocadherins.
- three regions (α, β, γ) consisting of multiple alternatively spliced exons that encode the extracellular and transmembrane domains of individual protocadherinvariants (there are total 58).
- a conserved domain encoding the intracellular portion of all protocadherin isoforms.
- Protocadherin isoforms on opposing cells bind to each other with varying affinity based upon their degree of similarity (high binding) or divergence (lower binding).
protocadherins are not uniformly expressed at neighboring synaptic sites in cultured neurons.
DSCAM1 in fly
DSCAM1: named for its similarity to the mammalian down syndrome cell adhesion molecule.
DSCAM1 has 38,000 isoforms based on the numbers of exons in the gene and predicted splicing.
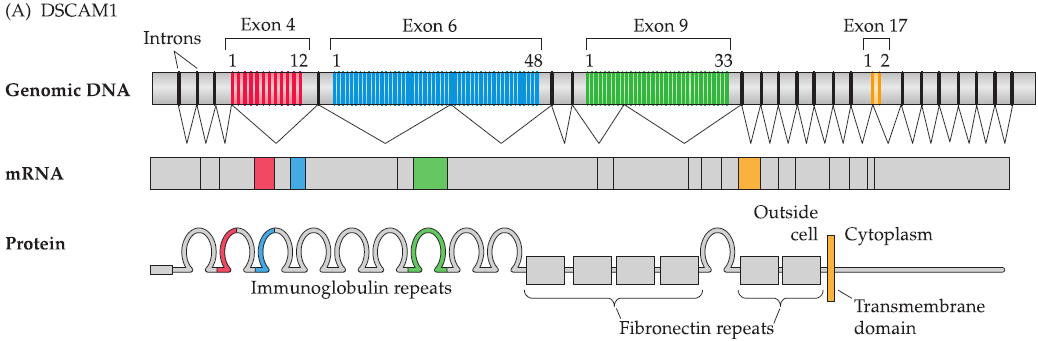
- Use alternative splicing to generate different proteins
2. Molecular mechanisms involved in synapse formation
Step 1: Initiation
Initiation: local regulation between the presumptive pre- and postsynaptic membranes mediated by members of the cadherin and protocadherin family of Ca2+ cell adhesion molecules.
- Initial accumulation of synaptic vesicles as well as transport vesicles that contain molecular components that contribute to the presynaptic active zone.
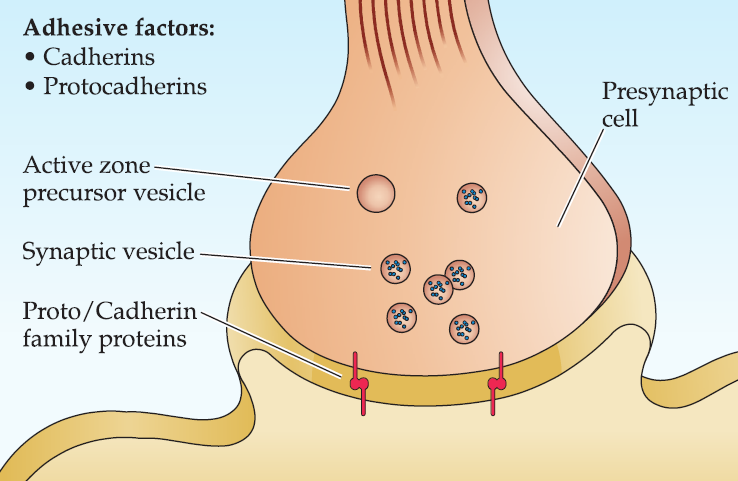
Step 2
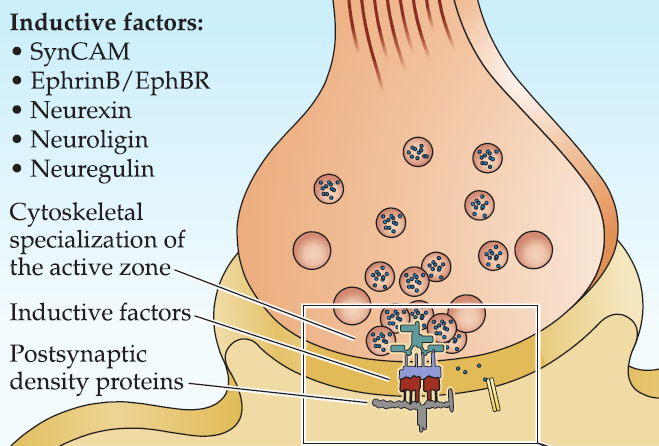
Additional adhesion molecules such as SynCAM, neurexin and neuroligin, ephrinB and EphB, are recruited. Adhesive signaling between these molecules initiates differentiation of the presynaptic active zone and the postsynaptic density.
- The presynaptic terminal also releases molecules (e.g., neuregulin) that influence the expression and clustering postsynaptic receptors and associated proteins.
Step 3
The interaction of neurexin(轴突蛋白) (a presynaptic transmembrane adhesion protein) with neuroligin(神经连接蛋白) (a postsynaptic adhesion protein) is central for recruiting and retaining cytoskeletal elements that localize synaptic vesicles to the presynaptic terminal and mediate their fusion.
- Neurexin have a specialized transmembrane domain that helps localize synaptic vesicles, docking proteins, and fusion molecules contributed by active zone vesicles in the presynaptic terminal.
- Neuroligin, upon binding neurexin, is essential for localizing neurotransmitter receptors and postsynaptic proteins to the postsynaptic specialization.
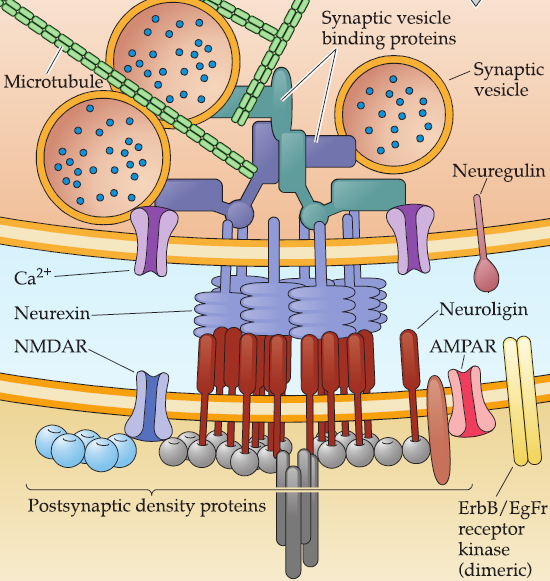
(1) Neurogulin1 (Nrg1)
Nrg1 is a transmembrane protein usually made in presynaptic cells and released following proteolytic cleavage of the ectodomain (outside portion) of the protein.
Neuregulin is released via local proteolytic cleavage and binds to dimeric ErbB receptor kinases or to dimeric ErbB/EGF(epidermal growth factor) receptor kinases.
Nrgl signaling is thought to elicit increased synthesis and insertion of neurotransmitter receptors at a nascent postsynaptic site.
Regulation of neuronal connections by trophic interactions
Trophic interaction (Greek trophe, meaning, “nourishment”): long-term dependency between neurons and their targets.
- Once synaptic contacts are established and the initial distribution of synapses is set, neurons become dependent on the presence of their targets for continued survival as well as the further growth and differentiation of axons and dendrites.
- In the absence of synaptic partners, the axons and dendrites of developing neurons typically atrophy and often die.
- This mechanism is used to make sure a efficient formation of synapse.
Neurotrophic factors (also called neurotrophins): signaling molecules provided by target cells.
- expression is limited to neurons and a few non-neural neuronal targets such as muscles.
Why do developing neurons depend so strongly on their targets?
- production of an initial surplus of nerve cells (on the order of two- or threefold); the final population is subsequently established by the death of those neurons that fail to interact successfully with their intended targets.
1. Target-derived trophic support regulates survival of related neurons
The pioneering neuroembryologists Viktor Hamburger and Rita Levi-Montalcini carried a series of studies and showed that targets play a major role in determining the size of the neuronal populations that innervate them:
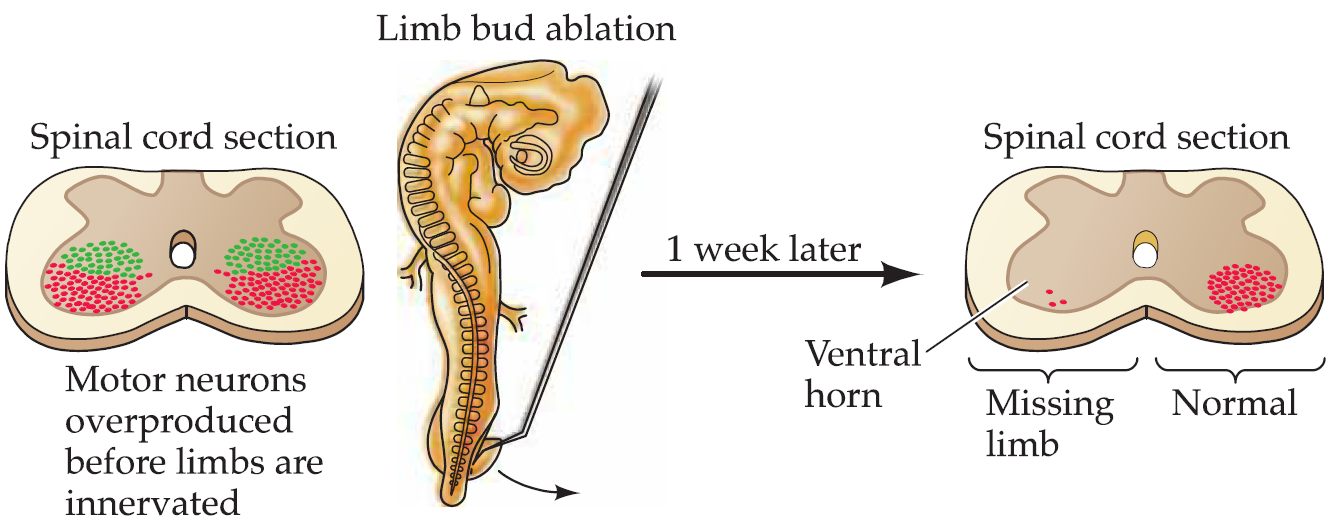
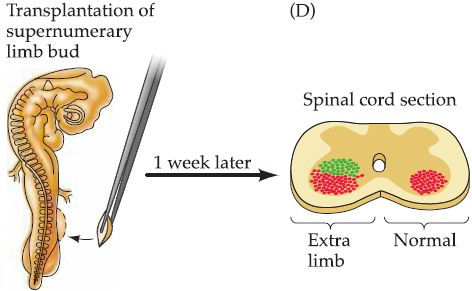
2. Competitive interactions and formation of neuronal connections:Synaptic refinement
Once the size of a neuronal population is established by trophic regulation, trophic interactions continue to modulate formation of synaptic connections.
Many fundamental ideas about the ongoing modification of developing brain circuitry have come from simpler, more accessible parts of the nervous system, most notably the vertebrate neuromuscular junction and autonomic ganglion cells.
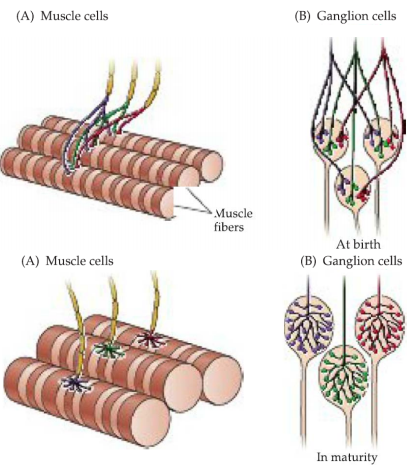
Adult skeletal muscle fibers and neurons in some classes of autonomic ganglia (parasympathetic neurons) are each innervated by a single axon.
Initially, however, each of these target cells is innervated by axons from several neurons-–polyneuronal innervation.
inputs are gradually lost during early postnatal development until only one remains—synapse elimination.
- overall number of synaptic contacts in the peripheral nervous system increases steadily during the course of development.
尽管innervation在发育的过程中减少,但是总体的synaptic connection增加
Patterns of electrical activity in the pre- and postsynaptic partners are thought to influence this competition for target space and neurotrophic support.
- acetylcholine receptors (AChR): curare (箭毒马鞍子,箭毒)
- presynaptic action potentials: TTX
Convergence: every target cell is innervated-and continues to be innervated-by the right number of inputs and synapses.
Divergence: every innervating axon contacts the right number of target cells with an appropriate number of synaptic endings.
- This regulation of convergence (the number of inputs to a target cell) and divergence (the number of connections made by a neuron) in the developing nervous system is another key consequence of trophic interactions among neurons and their targets.
3. Molecular basis of trophic interactions
Trophic interactions regulate three essential steps in the formation of mature neural circuits:
- Survival of a subset of neurons from a considerably larger population.
- Formation and maintenance of appropriate numbers of connections.
- Elaboration of axonal and dendritic branches to support these connections.
Neurotrophic protein: NGF as a model system
- NGF was discovered by Levi-Montalcini as an “activity“ that elicited robust growth of neuronal processes both in the animal and in cell culture.
- Purified as a protein from a rich biological source, the salivary glands of the male mouse
Neurotrophins: NGF, BDNF, NT-3, NT-4/5
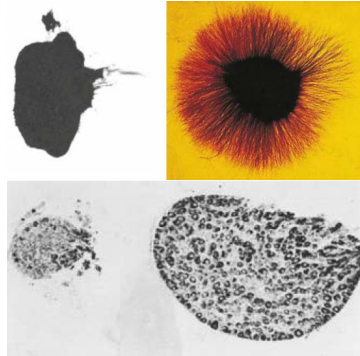
Neurotrophins have distinct effects on different target neurons
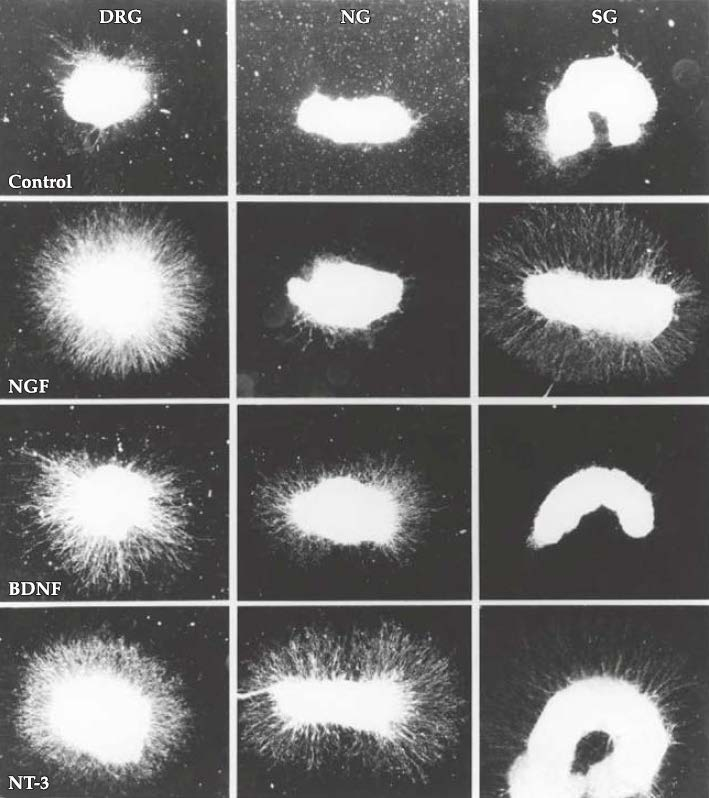
NG: nodose ganglia
SG: sympathetic ganglia
Why different neurotrophins has different effects? And why different neurons respond differently to different neurotrophins?
- Different neurons express different receptors
Different neurotrophins are selectively available in different targets
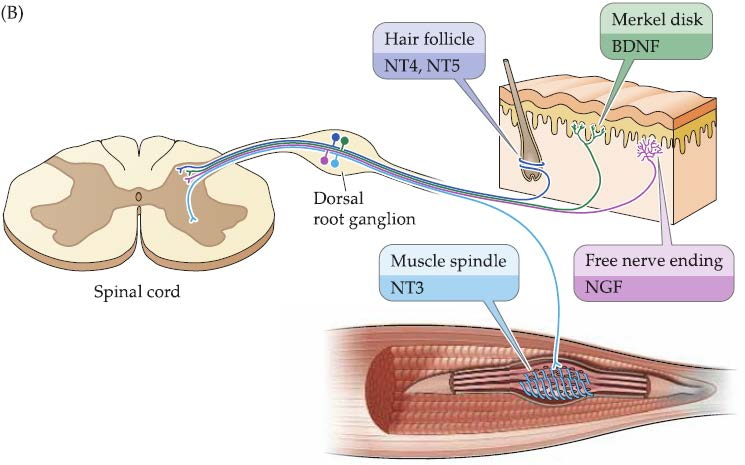
Secreted molecules with neurotrophic influences
CNTF (ciliary neurotrophic factor): cytokine in inflammation and immune responses
LIF (leukemia inhibitory factor): cytokine
GDNF (glial-derived neurotrophic factors) and related proteins: kidney development and spermatogenesis
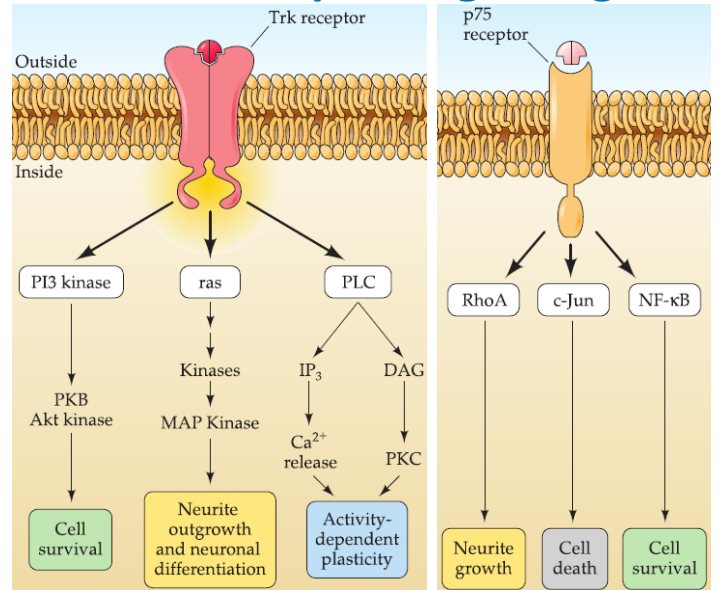
Neurotrophin signaling
Neurotrophic factors are key regulators for three distinct cellular mechanisms:
- neural process growth/retraction
- synapse stabilization/elimination
- cell survival/death
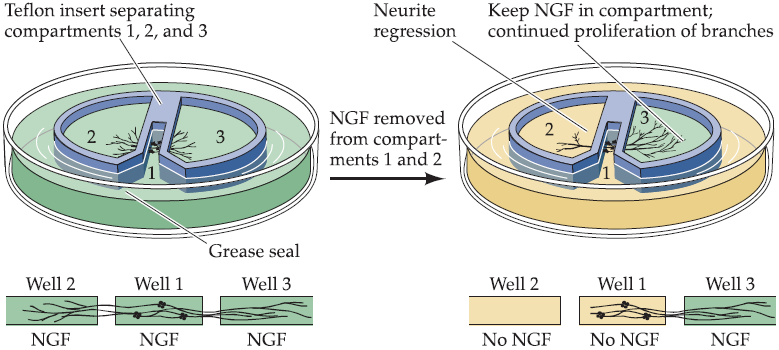
NGF acts locally to stimulate neurite growth:
- “Local” means: relaying the neurotrophic signal from the axon terminal to the cell body
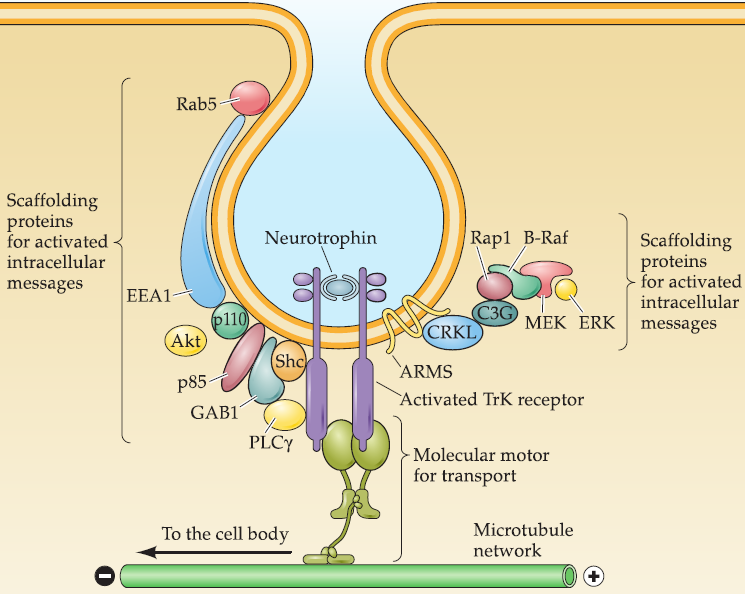
Neurotrophins bound to their transmembrane receptors (primarily the Trk subset of receptors) are selectively internalized by assembling a signaling endosome that includes ligand/receptor complex with several scaffolding proteins that bind one of three Intracellular effectors.
This signaling endosome- can also bind molecular motors that engage the microtubule cytoskeleton.
This signaling endosome is transported back to the cell body to activate downstream targets, including modifying gene expression.
1. Neurotrophin receptors and their specificity
Tyrosine kinase (Trk) receptors: a single transmembrane protein with a cytoplasmic tyrosine kinase domain.
- TrkA: NGF.
- TrkB: BDNF and NT-4/5.
- TrkC: NT-3
- some degree of cross-activation between factors and receptors: NT-3 to TrkB.
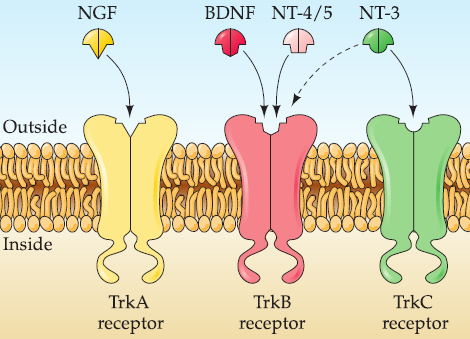
- Trk receptors have high affinity for processed ligands.
p75 receptors: activated by all neurotrophins.
- receptor has high affinity for unprocessed neurotrophinsbut low affinity for the processed ligands.
All neurotrophins are secreted in an unprocessed form that undergoes subsequent proteolytic cleavage.
Trk and p75 receptors are expressed only in subsets of neurons, and neurotrophins are available from different classes of targets.
- selective binding between ligand and receptor likely accounts for some of the specificity of neurotrophic interactions.
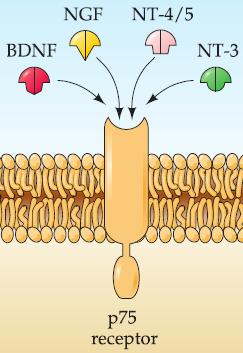
2. Neurotrophin signaling process