L09 protein function
从有氧呼吸入手,如果只依靠diffusion的话,无法满足正常机体的氧气需求。
Insects solve the problem by having tracheae(导管), tubular networks that lead from the body surface down into the tissues. 一些生物选择了Oxygen Transport Proteins.
一、Protein Functions
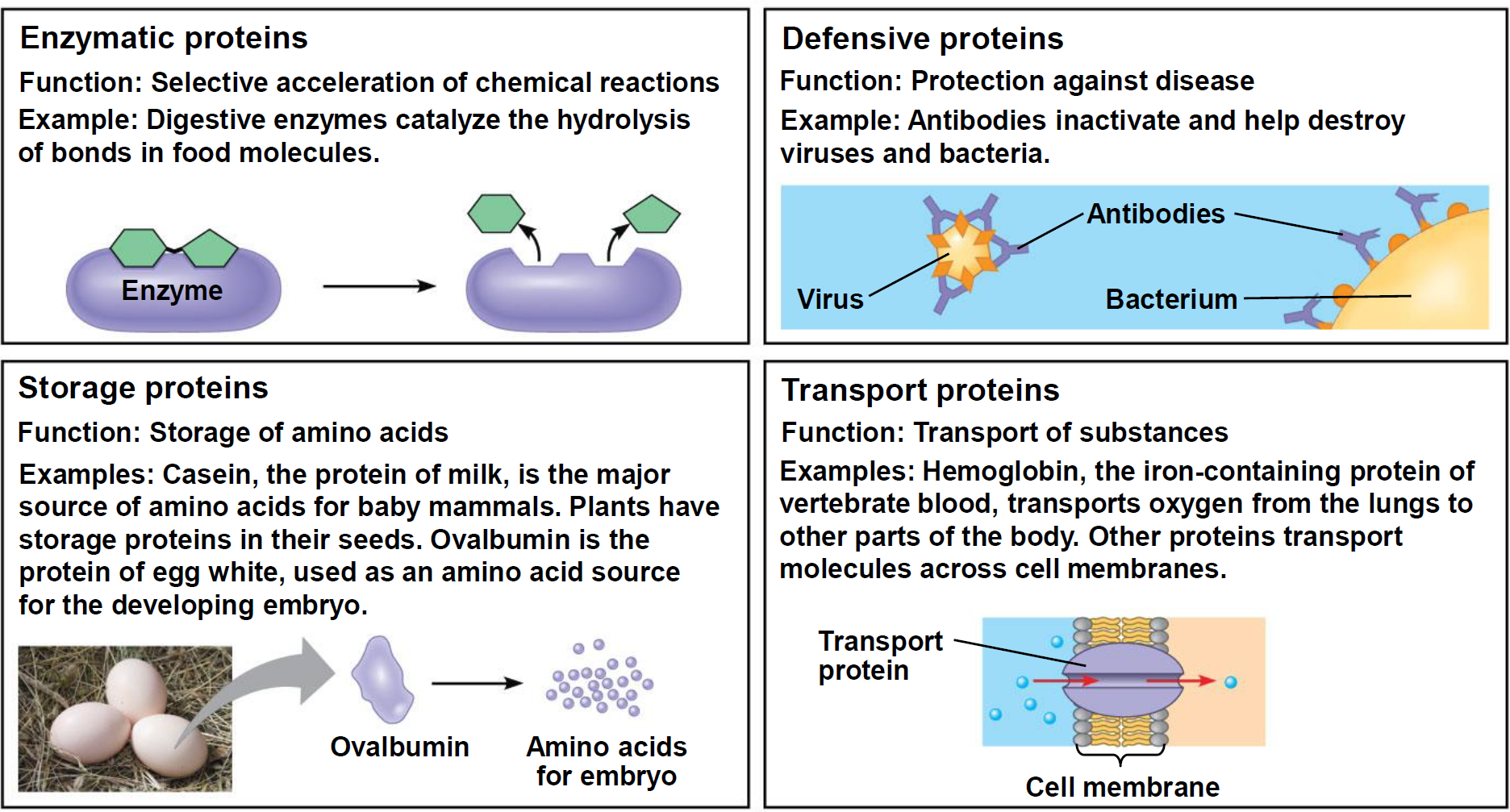
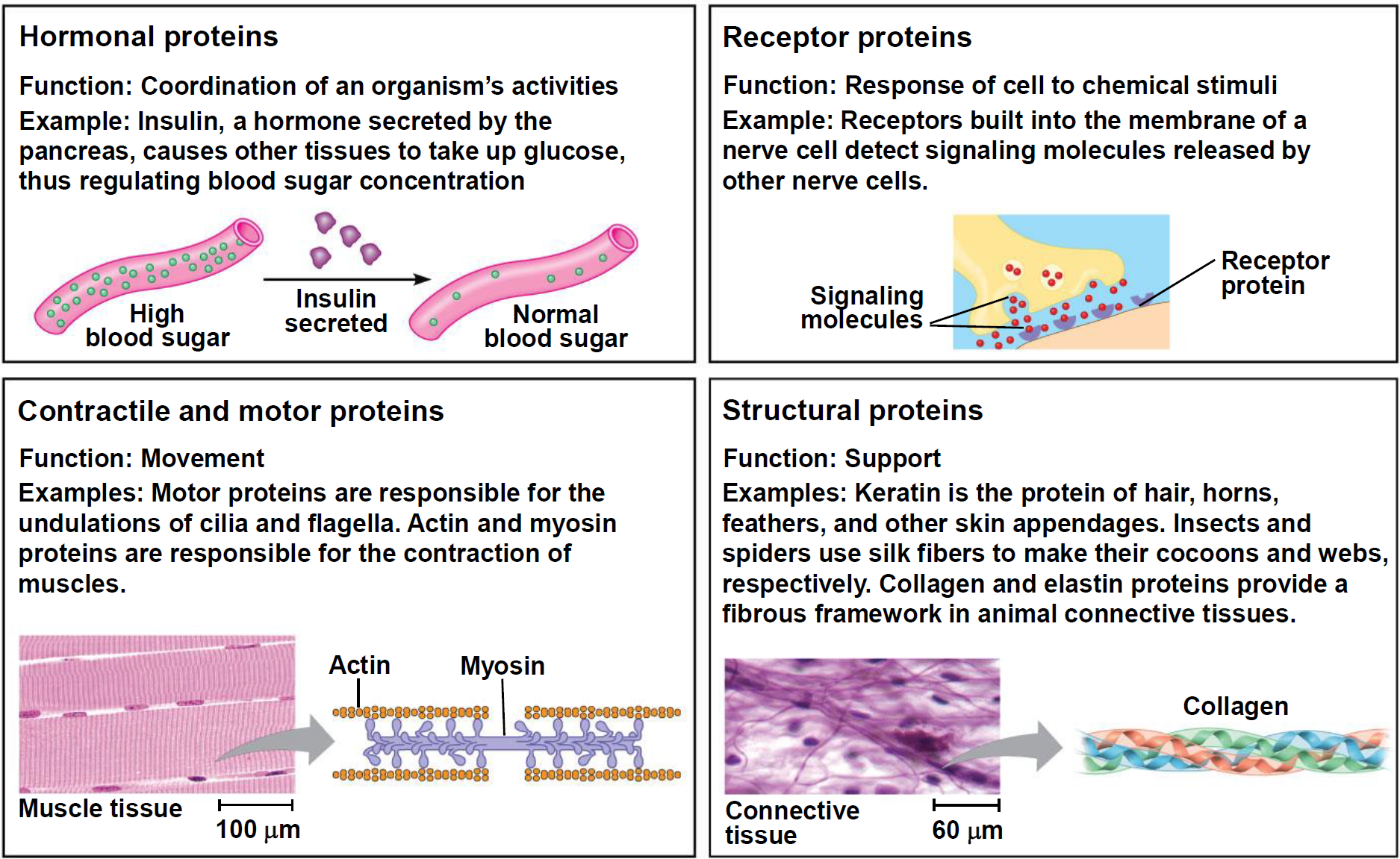
- Hormonal proteins 激素蛋白: Coordination of an organism’s activities
- Insulin, a hormone secreted by the pancreas, causes other tissues to take up glucose, thus regulating blood sugar concentration
- Receptor proteins: Response of cell to chemical stimuli
- Receptors built into the membrane of a nerve cell detect signaling molecules released by other nerve cells.
- Contractile and motor proteins 收缩和运动蛋白: Movement
- Motor proteins are responsible for the undulations of cilia and flagella. Actin and myosin proteins are responsible for the contraction of muscles.
- Structural proteins: Support
- Keratin(角蛋白) is the protein of hair, horns, feathers, and other skin appendages. Insects and spiders use silk fibers to make their cocoons and webs, respectively.
- Collagen and elastin proteins provide a fibrous framework in animal connective tissues.
- Enzymatic proteins 酶蛋白: Selective acceleration of chemical reactions
- Digestive enzymes catalyze the hydrolysis of bonds in food molecules.
- Defensive proteins: Protection against disease
- Antibodies inactivate and help destroy viruses and bacteria.
- Storage proteins: Storage of amino acids.
- Casein(干酪素,酪蛋白), the protein of milk, is the major source of amino acids for baby mammals.
- Plants have storage proteins in their seeds.
- Ovalbumin(卵白蛋白,卵清蛋白) is the protein of egg white, used as an amino acid source for the developing embryo.
- Transport proteins: Transport of substances
- Hemoglobin, the iron-containing protein of vertebrate(脊椎动物的) blood, transports oxygen from the lungs to other parts of the body.
- Other proteins transport molecules across cell membranes.
二、Globins in oxygen transport
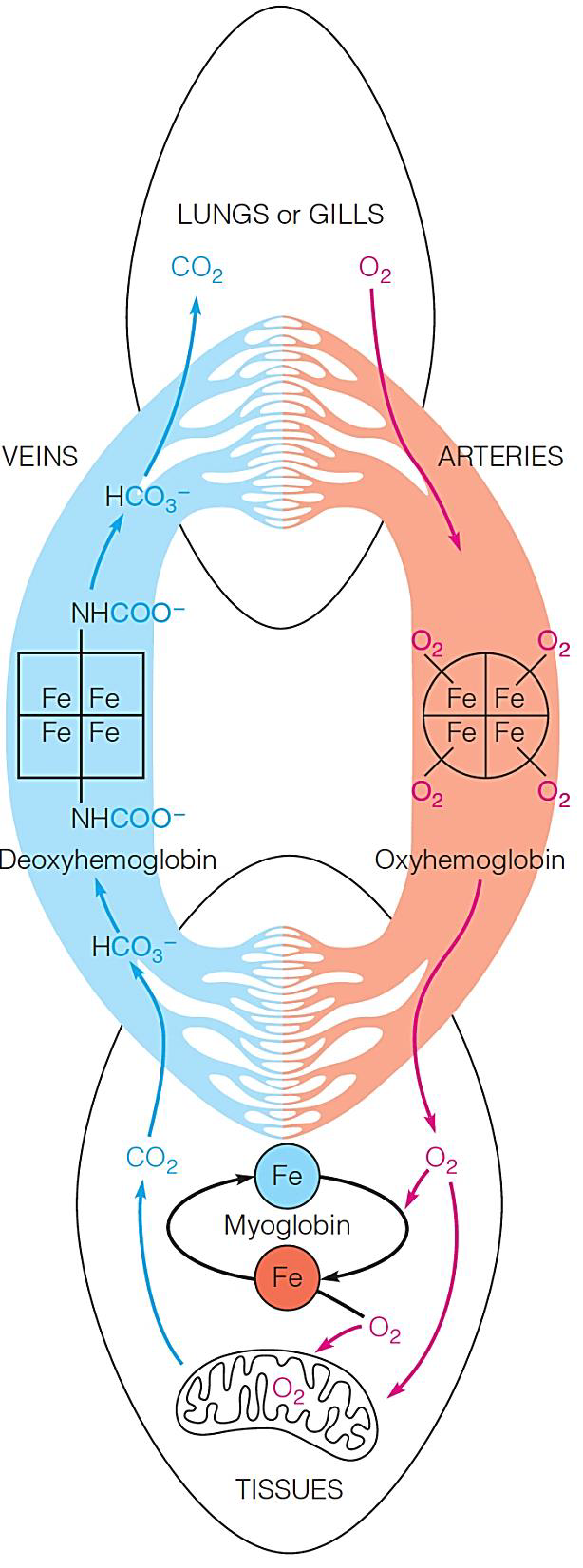
Vertebrate animals use hemoglobin and myoglobin to provide their tissues with a continuous $O_{2}$ supply.
Hemoglobin(血红蛋白,Hb,Hgb) transports $O_{2}$ from the lungs or gills(鳃) to the respiring tissues, where it is used for aerobic metabolism in the mitochondria.
Inside cells, dissolved $O_{2}$ diffuses freely or is bound to myoglobin(肌红蛋白), which aids transport of $O_{2}$ to the mitochondria.
Myoglobin can also store $O_{2}$ for later use (as in deep-diving mammals).
$CO_{2}$ produced by oxidative processes in the tissues is carried back to the lungs or gills by hemoglobin and released.
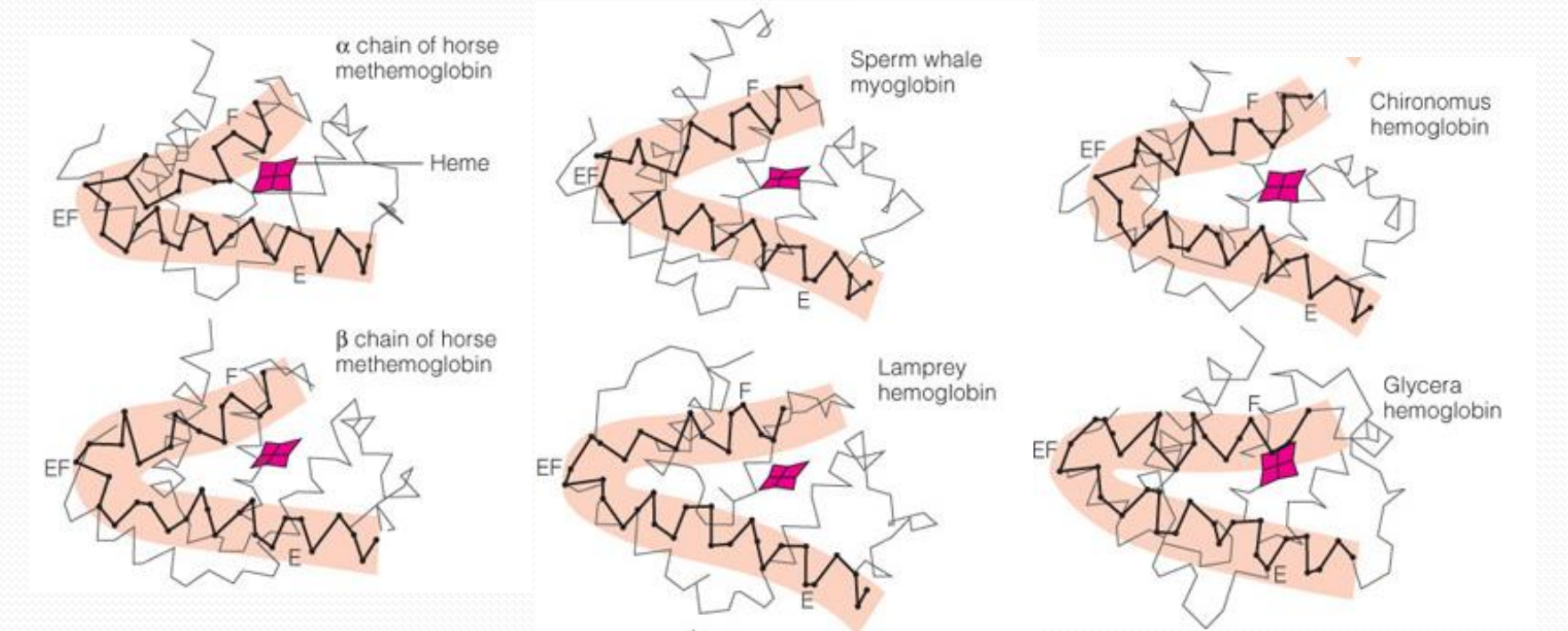
Structures of myoglobin and hemoglobin
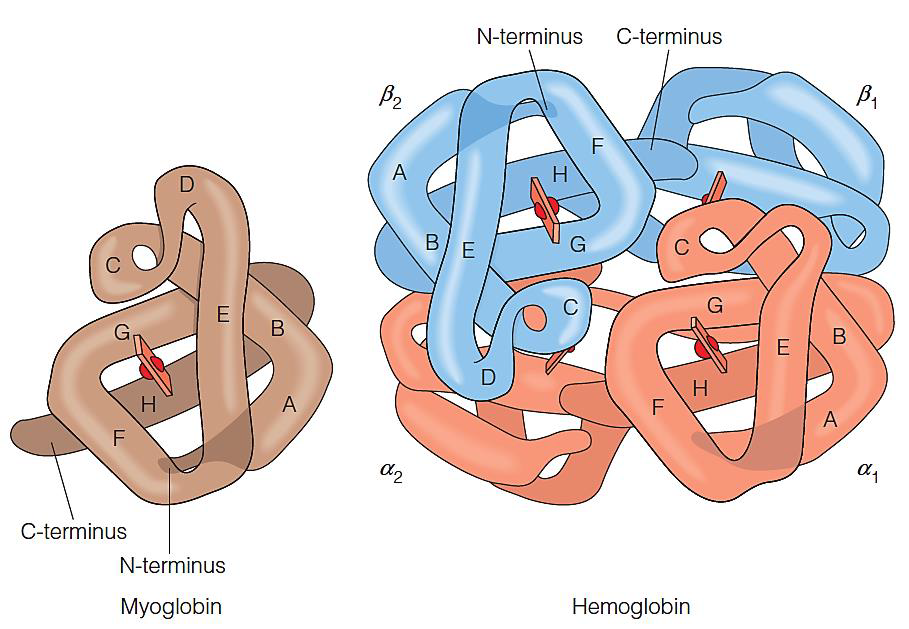
1. Myoglobin(Mb)
153 amino acid residues
Monomer of 8 helixes
Carry oxygen in muscle
2. Hemoglobin(Hb)
Tetramer: $\alpha_2\beta_2$
Two α subunits
two β subunits
7 helices in the α subunit and 8 helices in the β subunit
Each subunit shares similar fold with myoglobin
Each subunit contains a heme(亚铁血红素) group
3. Hb subunits are structurally similar to Mb
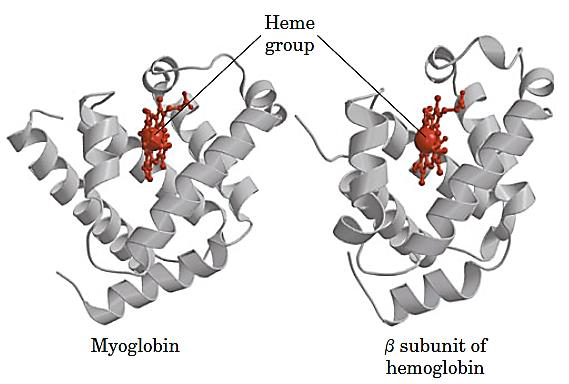
The heme and oxygen binding sequences are identical:
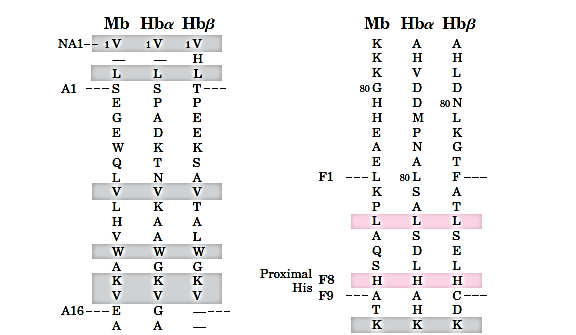
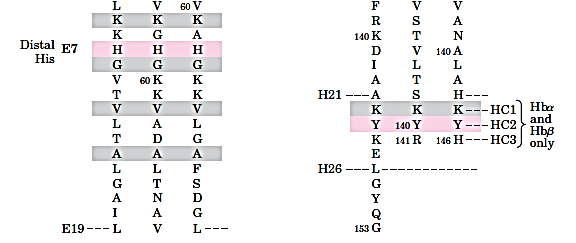
The oxygen binding of Mb and Hb
Oxygen Binds Reversibly to Heme:
Oxymyoglobin - oxygen bearing myoglobin
Deoxymyoglobin - oxygen-free myoglobin
1. Prosthetic group 辅基
也称辅因子(英语:cofactor)指与酶(酵素)结合且在催化反应中必要的非蛋白质化合物。某些分子如水和部分常见的离子所扮演的角色和辅因子相当类似,但由于含量不受限制且普遍存在,因此不归类为辅因子。
- Apoprotein(脱辅基蛋白): Without prosthetic group
- Holoprotein(全蛋白): With prosthetic group
一个Oxygen Transfer Protein应该bind $O_2$ reversibly and protect it from reaction with any other substance that can reduce $O_2$.
The globins 通过provide 一个$O_2$可以reversiblly bind的site来实现这个功能。
The Oxygen Binding Site
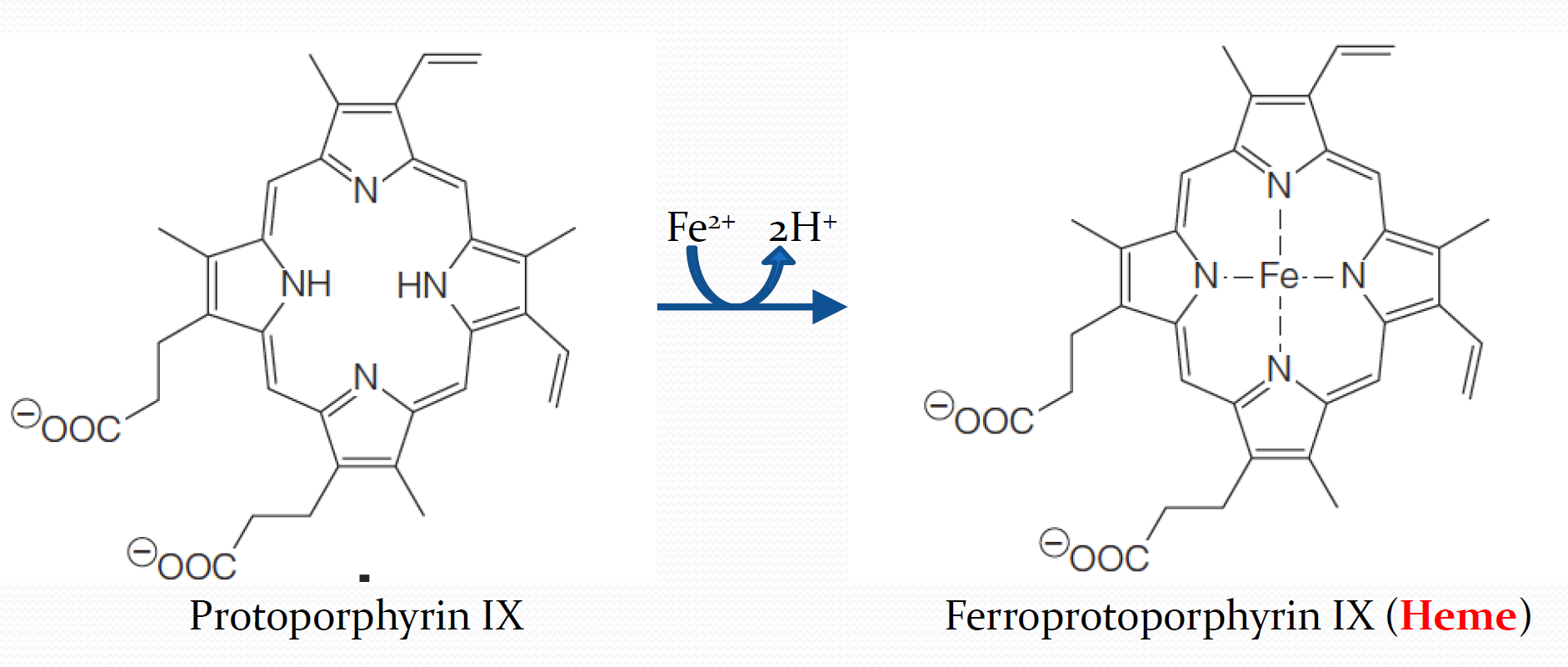
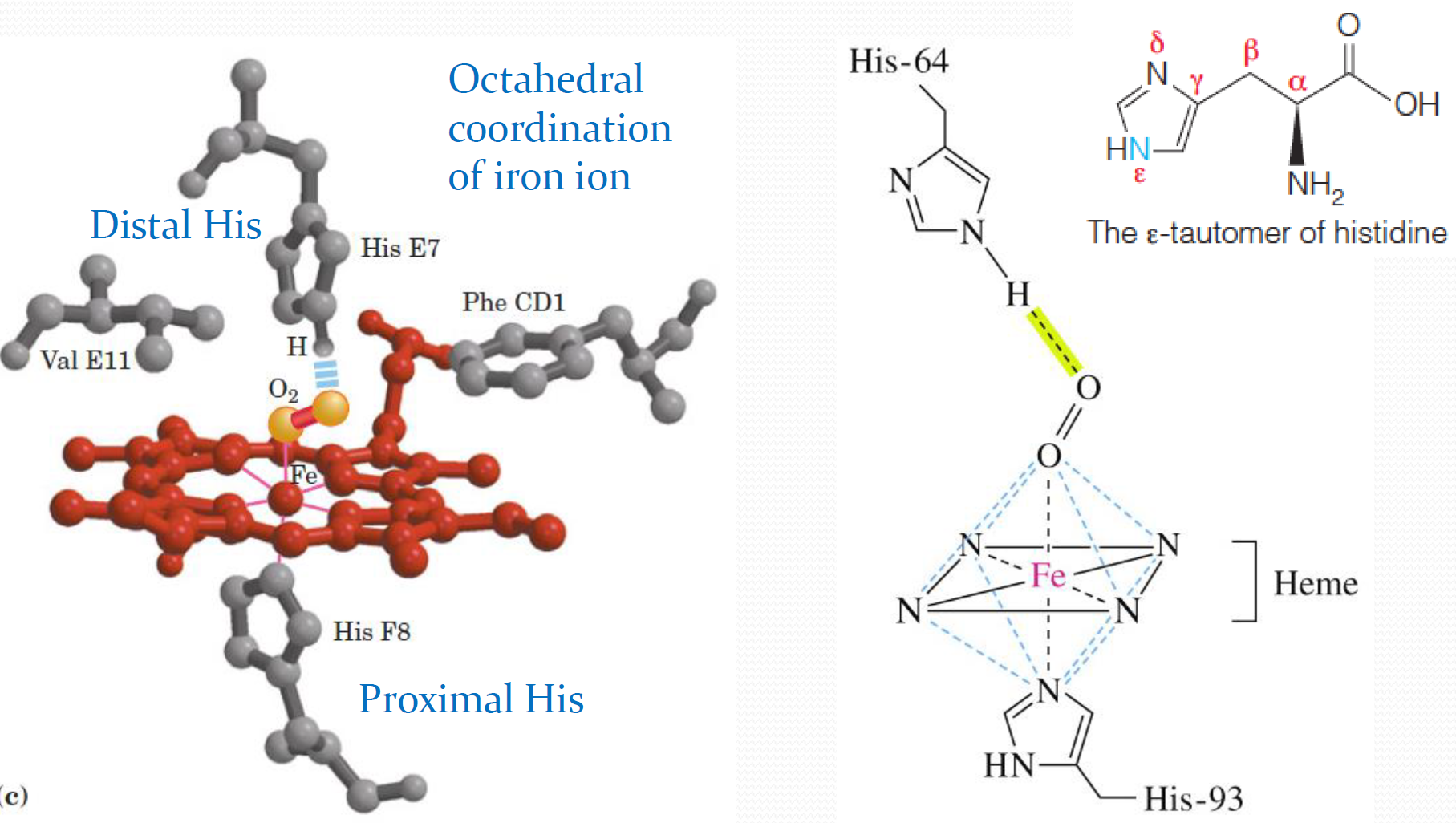
Various iron-containing proteins can hold Fe(II) in a number of possible ways. Throughout the myoglobin–hemoglobin family, the iron is chelated by a tetrapyrrole(四吡咯) ring system called protoporphyrin IX (Figure 7.4a)
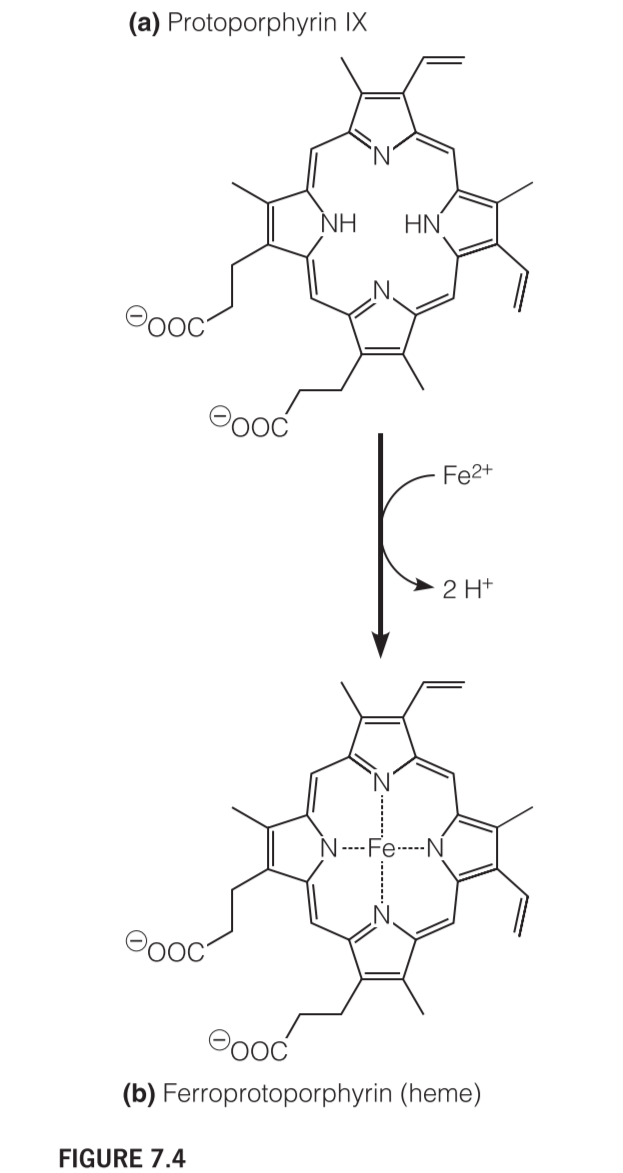
2. heme
The complex of protoporphyrin IX with Fe(II) is called heme
The Structure of heme
This prosthetic group is bound in a hydrophobic crevice(裂缝,裂隙) in the myoglobin or hemoglobin molecule
Ferrous iron($Fe^{2+}$)is normally octahedrally(八面体地) coordinated, which means it should have 6 ligands,or binding groups, attached to it.
As shown in Figure 7.5a, the nitrogen atoms of the porphyrin ring account for only four of these ligands. Two remaining coordination sites are available, and they lie along an axis perpendicular to the plane of the heme.
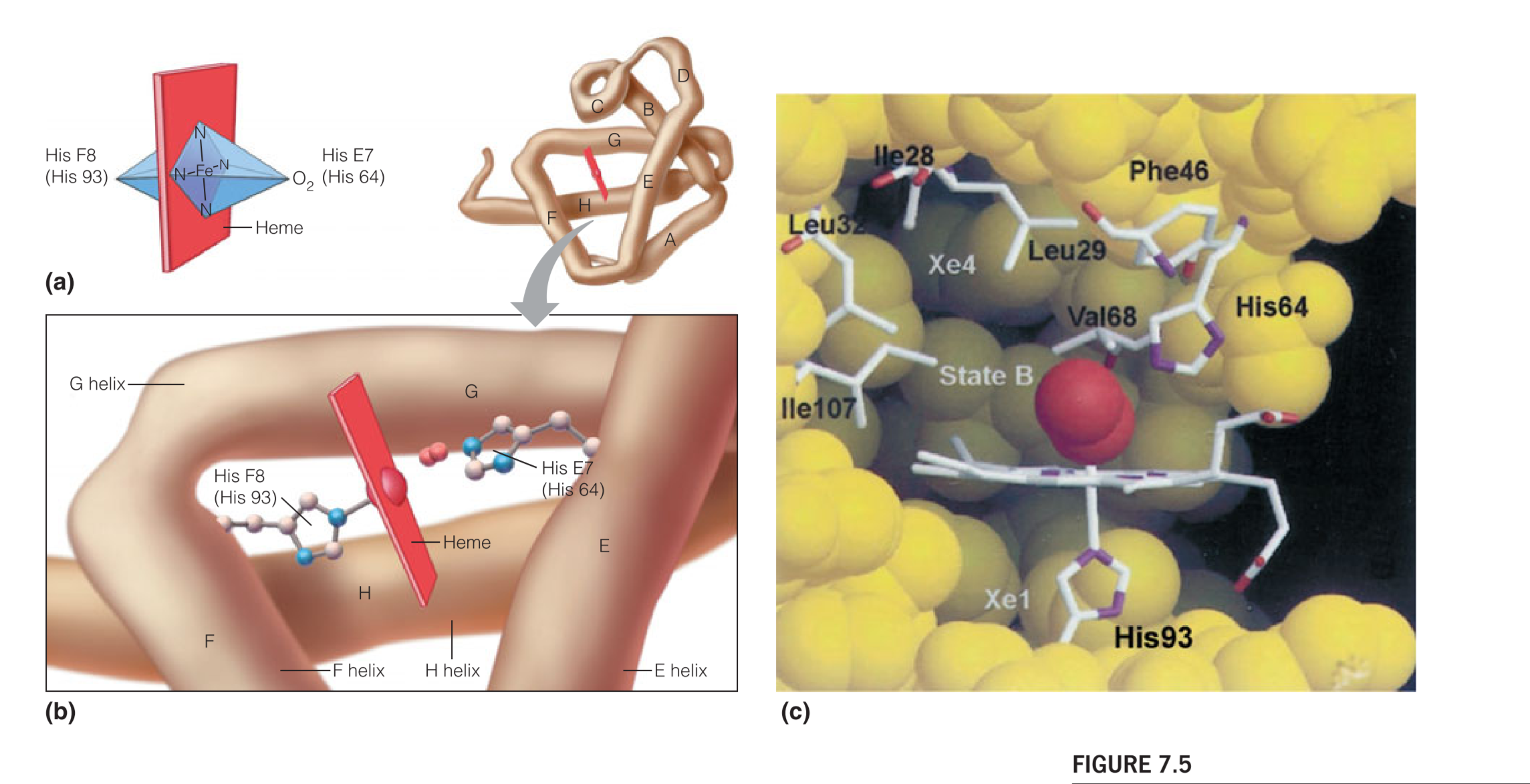
In both the deoxygenated and the oxygenated forms of myoglobin, one of these remaining coordination sites is occupied by the $\varepsilon$-nitrogen of histidine residue number 93
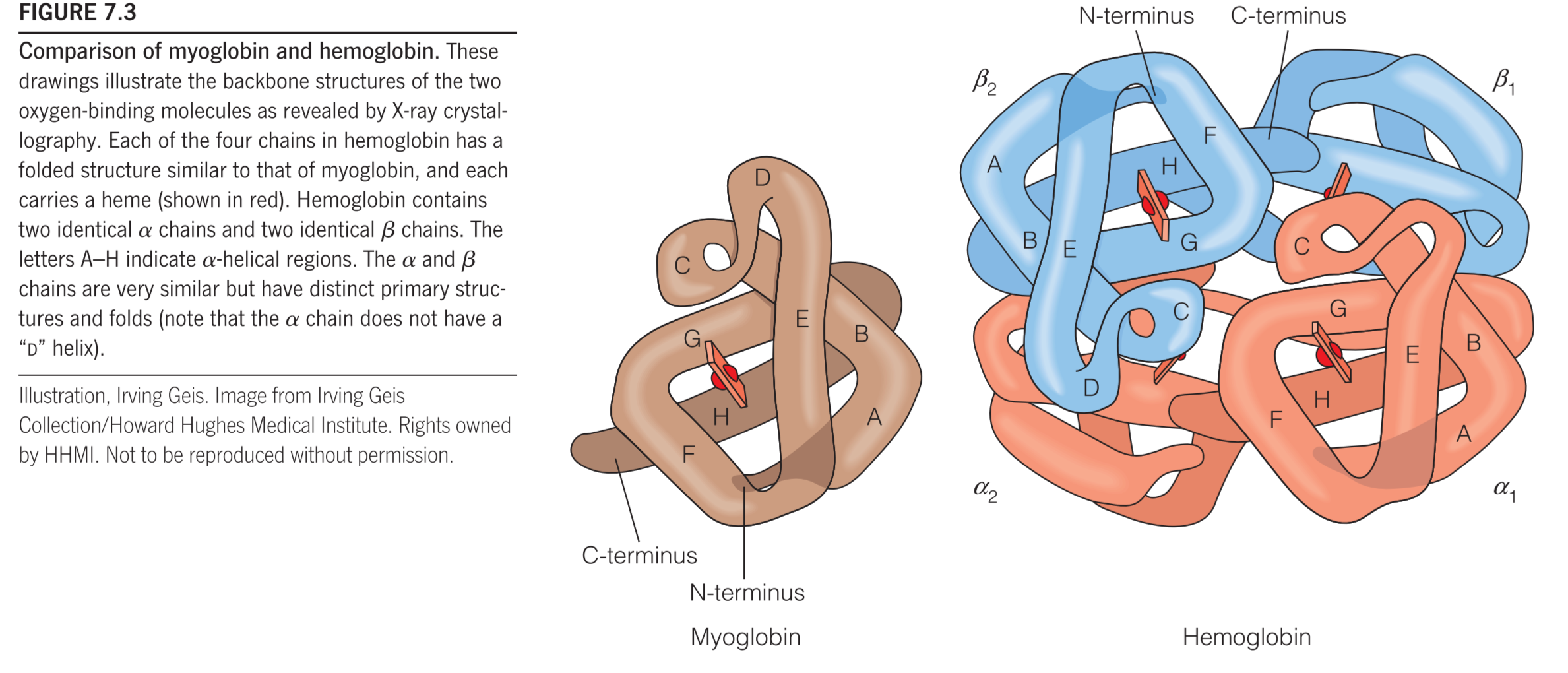
The eight helical segments in the globins are designated A through H (as shown in Figure 7.3), and residue 93 is located in the F helix.(Fig 7.5b)
Using a nomenclature(命名法) that allows for meaningful comparisons between the homologous sequences of different globins, this residue is called histidine F8 (It is the eighth residue in the F helix)
Because its directly contact with the $Fe^{2+}$, it is also called the proximal(近端的) histidine
In deoxymyoglobin, the remaining coordination site, on the other side of the iron, is unoccupied. When oxygen is bound, making oxymyoglobin, the molecule occupies this site.
The reversibility of oxygen binding
(1)distal(末端的) histidine
由于电子轨道较好的重合,使得$O_2-Fe^{2+}$更偏向于具有$O_2-Fe^{3+}$的性质。
Stabilization of this special kind of bond is achieved by a hydrogen bond between the bound $O_2$ and another important His residue located in the $O_2$ binding pocket – The so-called distal(末端的) histidine (His 64, or E7; see Figure 7.5c)
The H-bond between His E7 and $O_2$ selectively increases the affinity of Mb for $O_2$ vs. CO, which does’t make similar bond to E7. 但是即便如此,CO与Mb的亲和性还是比$O_2$高了200 times,如果没有E7 H-bond,亲和性大概会高6000 times.
因此,distal histidine对于促进氧气与Mb结合具有很关键的作用。
(2)Ways to Keep $Fe^{2+}$ Stable
A similar mode of oxygen binding, with histidines at the homologous F8 and E7 positions, is found in each subunit of hemoglobin.
The iron [Fe(II)] is easy to be oxidized [Fe(III)] in the free Heme
But the iron is stable in the Mb or Hb molecule, the stability of Fe(II) is critical to the reversible oxygen binding
The hydrophobic environment and the distal(末梢的,末端的) histidine (base) prevent the iron oxidation, which is acid-based.
The globins provide environments for ferrous heme in which the first step of an oxidation reaction (the binding of oxygen) is permitted, but the final step (oxidation) is blocked.
In fact, the ferrous heme in myoglobin and hemoglobin can become oxidized to the ferric ($Fe^{3+}$) state, to form metmyoglobin (metMb) or, respectively, methemoglobin.(metHb)
The oxidized iron [Fe(III)] exist in metMb or metHb, which do not bind oxygen, a water molecular occupy the oxygen binding site instead.
Methemoglobin (正铁血红蛋白) (pronounced “met-hemoglobin”) is a hemoglobin in the form of metalloprotein, in which the iron in the heme group is in the Fe³⁺ (ferric) state, not the Fe²⁺ (ferrous) of normal hemoglobin.Methemoglobin cannot bind oxygen, which means it cannot carry oxygen to tissues. It is bluish chocolate-brown in color.
For this reason red blood cells contain enzymes that can reduce the in methemoglobin to and thereby restore its binding activity.
The binding of other ligands
Diatomic gases:
- Carbon monoxide (CO)
- For the heme alone, extremely higher binding affinity compared to O2 (>6000 folds.
- For myoglobin, moderate higher binding affinity (~200 folds.
- The distal His do not form a H-bond with CO
- Nitric oxide (NO)
Analysis of oxygen binding (Mb)
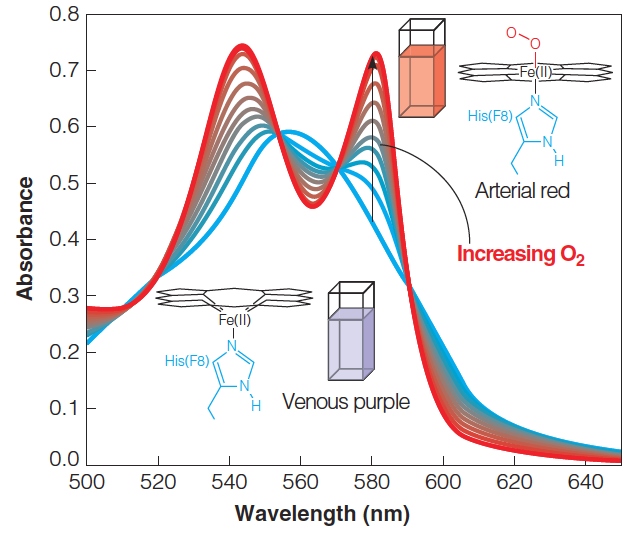
1. Monitor the oxygen binding by spectroscopy(光谱)
2. Oxygen-binding curve for myoglobin
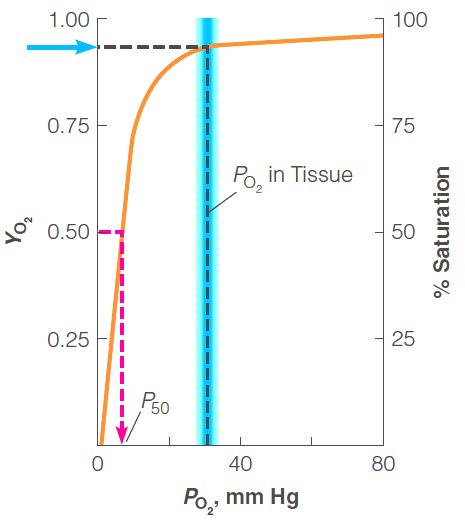
The free oxygen concentration is expressed as $P_{O2}$, the partial pressure of oxygen
The proportion of myoglobin binding sites that are occupied is expressed as a fraction,$Y_{O2}$
The value of $P_{50}$, the partial pressure of oxygen at 50% saturation
在$P_{50}$的点,可以反映当前蛋白$K_d$, 即反映与氧气的binding affinity:
$$
K_d = \frac{[O_2][Mb]}{[Mb \cdot O_2]} = \frac{\bcancel{[Mb]}[O_2]}{\bcancel{[Mb \cdot O_2]}} = [O_2] \varpropto P_{50}
$$
And
$$
Y_{O2} = \frac{P_{O_2}}{P_{50} + P_{O_2}}
$$
3. Kinetics of oxygen binding and release
$K_{on}$ and $K_{off}$
The distal His acts as a “gate” in ligand binding and realse
Indications from CO release by myoglobin
Dynamic motions of myoglobin facilitate ligand binding and release
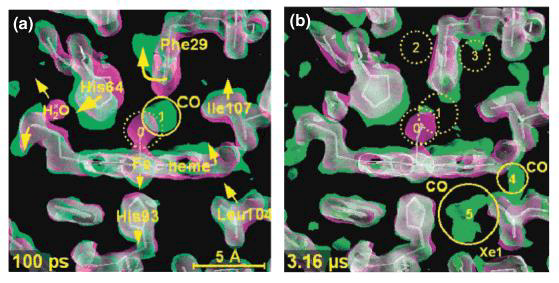
How does the oxygen bind and release
1. Diversity in hemoglobin structures
The hemoglobin is multisubunit protein
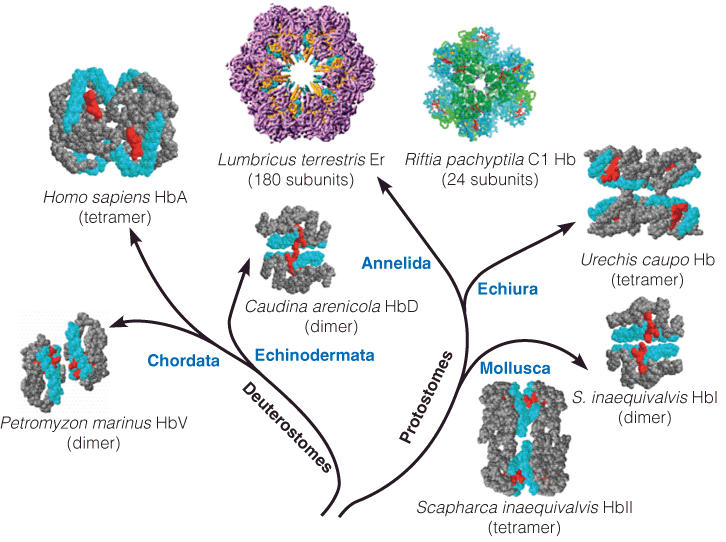
2. How does Hb achieve oxygen binding and release?
The key point is binding affinity
- High $O_2$ binding affinity: Transport
- Low $O_2$ binding affinity: Release
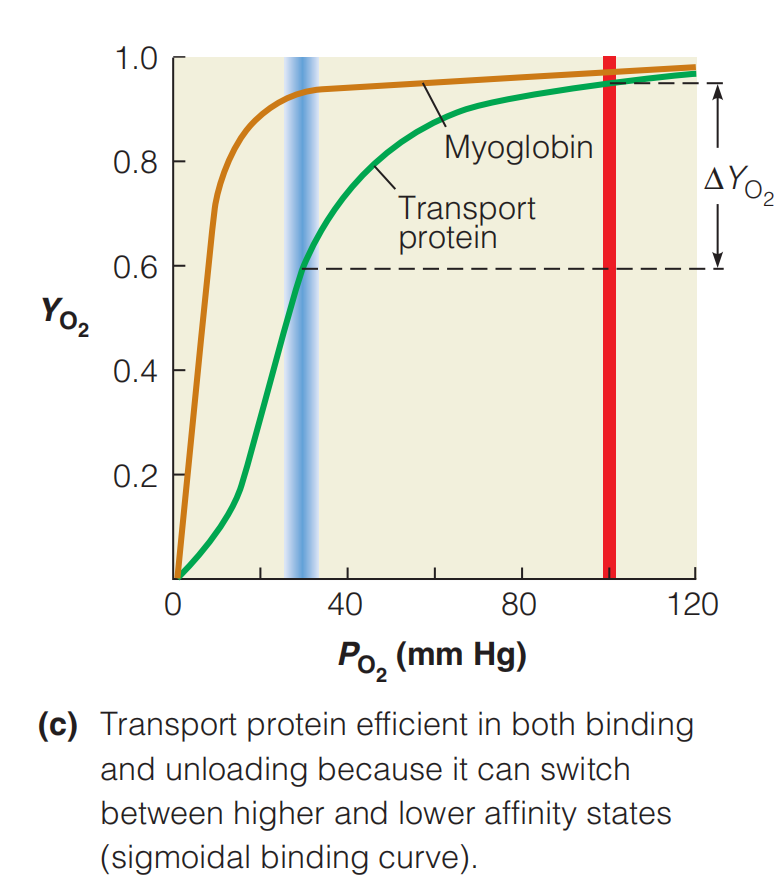
To achieve optimum $O_2$ delivery to tissues, an ideal oxygen transport protein would be nearly saturated at 100 mm Hg, deliver sufficient $O_2$ to tissues at rest, yet maintain a significant $O_2$ reserve for periods of high demand.
If the transport protein had a hyperbolic(双曲线的) binding curve like that of myoglobin, such behavior would be impossible to achieve.
- With such a binding curve, the protein would be inefficient in supporting either basal or extreme metabolism.
This problem has been solved through the evolution of transport proteins, such as hemoglobin, that have the sigmoidal(反曲) binding curve shown in Figure above.
A sigmoidal curve is very efficient because it allows nearly full saturation of the protein in the lungs or gills, as well as optimal release in the capillaries.
3. Cooperative binding (Hb)
More oxygen is bound, the affinity for oxygen becomes greater.
This behavior must mean that a cooperative interaction exists among $O_2$-binding sites.
Cooperativity – Communication Between Binding Sites
Cooperativity in binding requires communication between multiple binding sites
Hill equation and Hill coefficient (h)
$$
\log (\frac{Y_{o_2}}{1-Y_{O_2}}) = h\log{P_{O_2}}-h\log P_{50}
$$
Hill coefficient (h) is affected by
• The number of ligand-binding sites
• The energy of their interaction
Hill plot
Hill plot, a plot of the Hill equation indicates
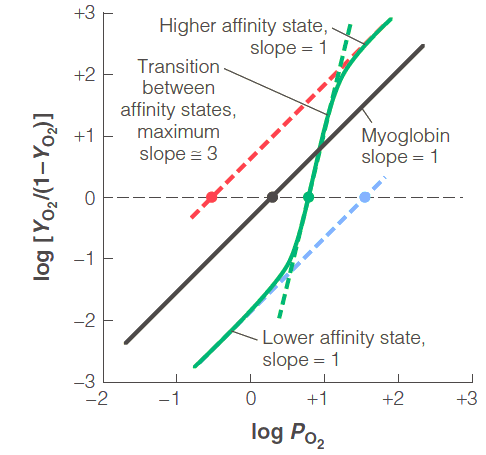
positive cooperativity (h > 1)
negative cooperativity (h < 1)
no cooperativity (h = 1)
(1) Myoglobin, h = 1
(2) Hemoglobin, h differs
- In the higher and lower affinity states, h = 1
- In the transition state, h = 3
4. Allosteric effect 变构效应
The cooperativity is one kind of allosteric effect
The cooperative binding of oxygen by hemoglobin is one example of what is referred to as an allosteric effect
Proteins that are regulated by allosteric effects are called allosteric proteins, including many enzymes
In allosteric binding, the uptake of one ligand by a protein influences the affinities of remaining unfilled binding sites
Allosteric effector 别构剂,别构效应物
Binds to a proteins’ allosteric site
A site that different from proteins’ active site
• Activator
• Inhibitor
(1) Allosteric effectors of Hemoglobin
Proton — pH
$$
Hb\cdot 4O_2 + 4H^+ \rarr Hb\cdot 4H^+ + 4O_2
$$
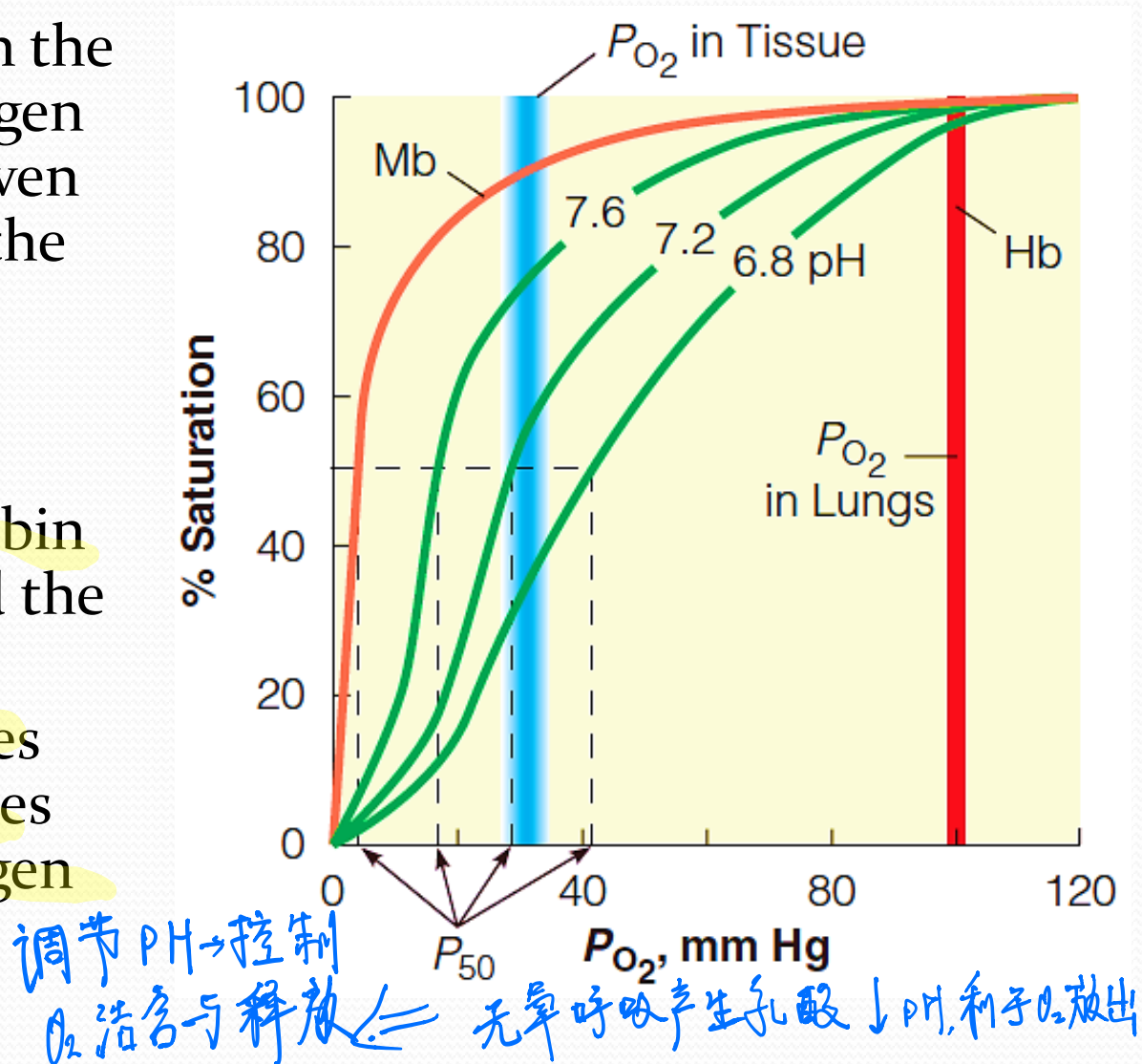
A pH drop in the blood in the capillaries lowers the oxygen affinity of Hb, allowing even more efficient release of the last traces of oxygen
Lowering the pH stabilizes the deoxyHb and decreases the affinity of Hb for oxygen
- Accumulation of $CO_2$
- Hypoxia (低氧;组织缺氧;氧不足) in muscle
$CO_2$
CO2 transport from the tissues to the lungs
Dissolve in blood to form bicarbonate ions (碳酸氢根离子)
A small portion bind to the N-terminal amino groups of hemoglobin to form carbamates (氨基甲酸酯)
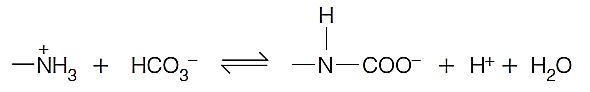
This carbamation reaction allows hemoglobin to aid in the transport of $CO_2$ from tissues to lungs or gills,
the protons released on carbamate formation contribute to the Bohr effect
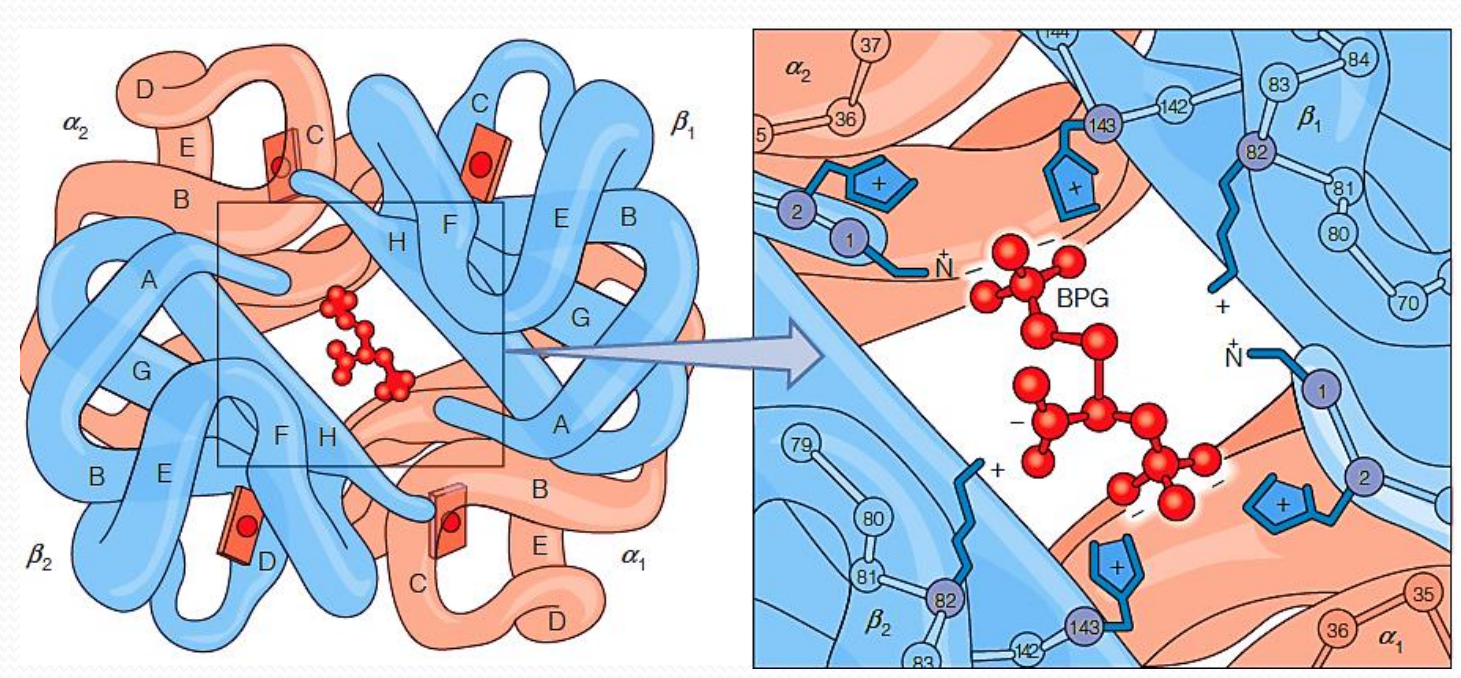
2,3-Bisphosphoglycerate (2,3-双磷酸甘油酸酯)
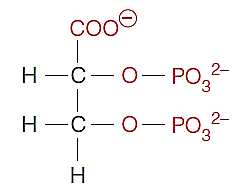
Found in mammals
lowers the affinity of deoxyHb for oxygen
Negatively charged 2,3-BPG is bound to positively charged groups of deoxyHb, stabilizing deoxyHb
Bohr effect
The response of hemoglobin to changes in pH is called the Bohr effect.
Mb has little Bohr effect.
(1) Chemical basis of Bohr effect
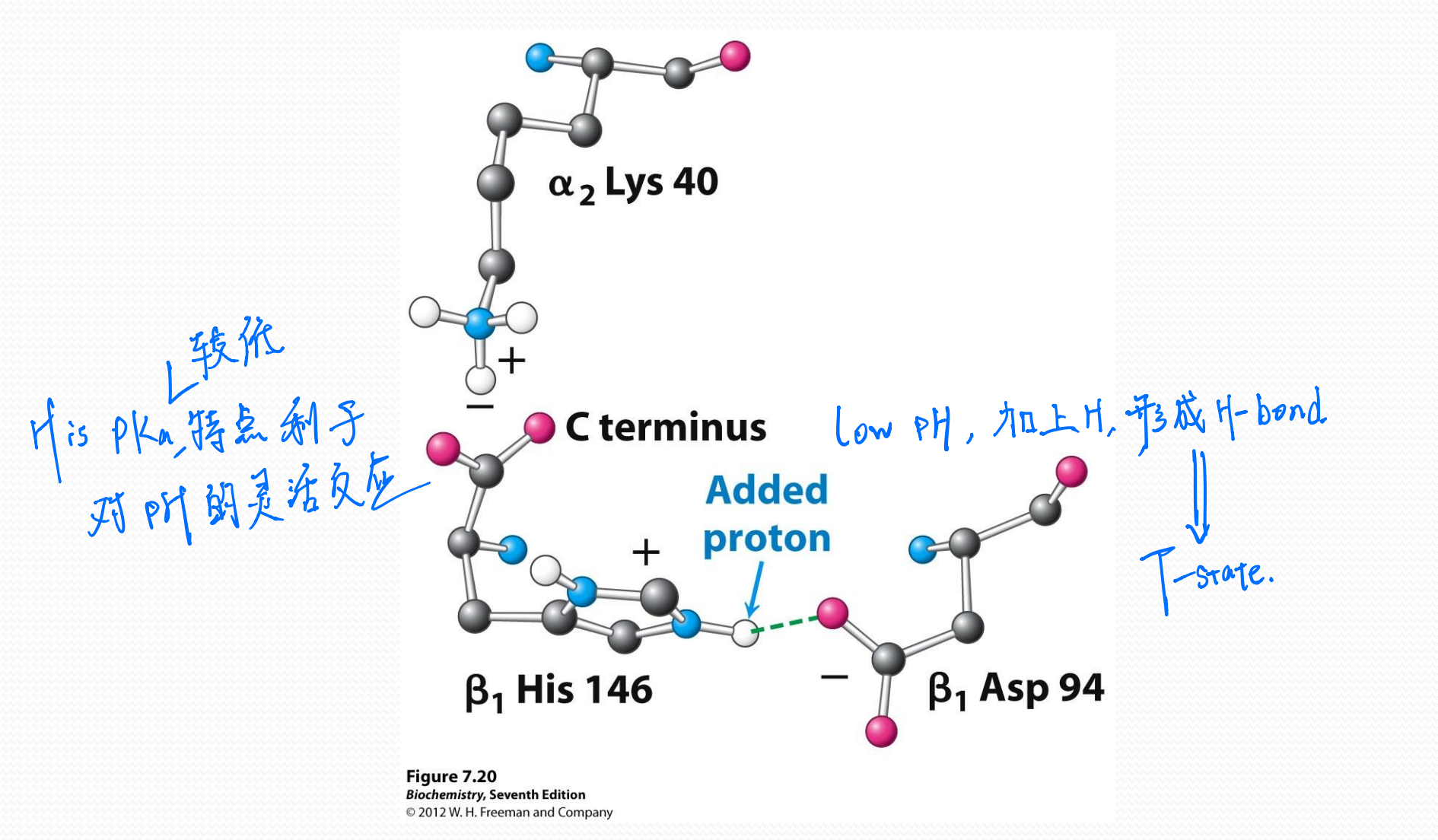
Models for the allosteric change in Hb
Hemoglobin switches between two conformational states with lower and higher $O_2$-binding affinities
The low affinity state (higher $P_{50}$)
- T state (Tense state)
The high affinity state (lower $P_{50}$)
- R state (Relax state)
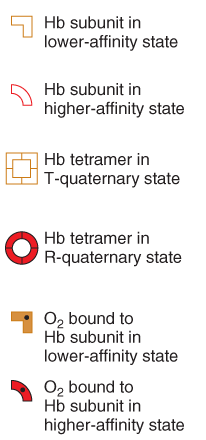
(1) Sequential model
(2) Symmetry model (化学平衡)
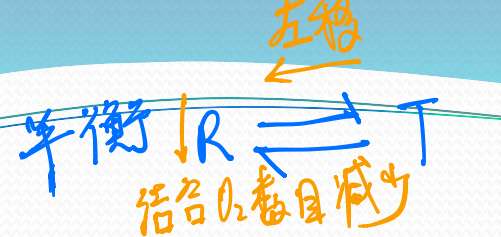
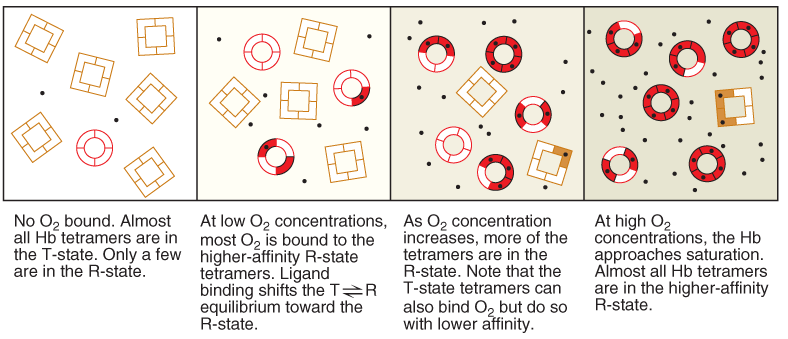
5. The change in hemoglobin quaternary structure during oxygenation
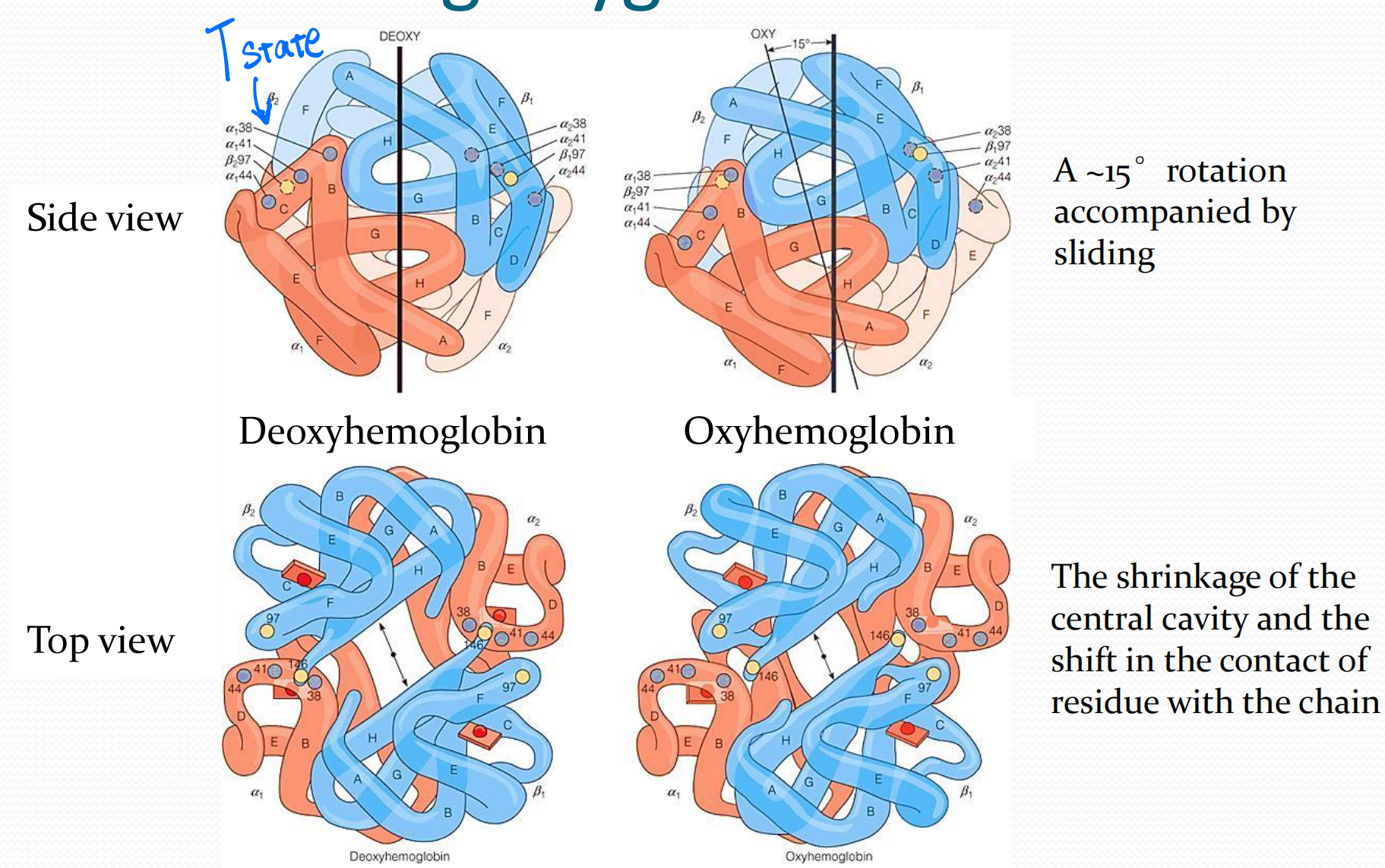
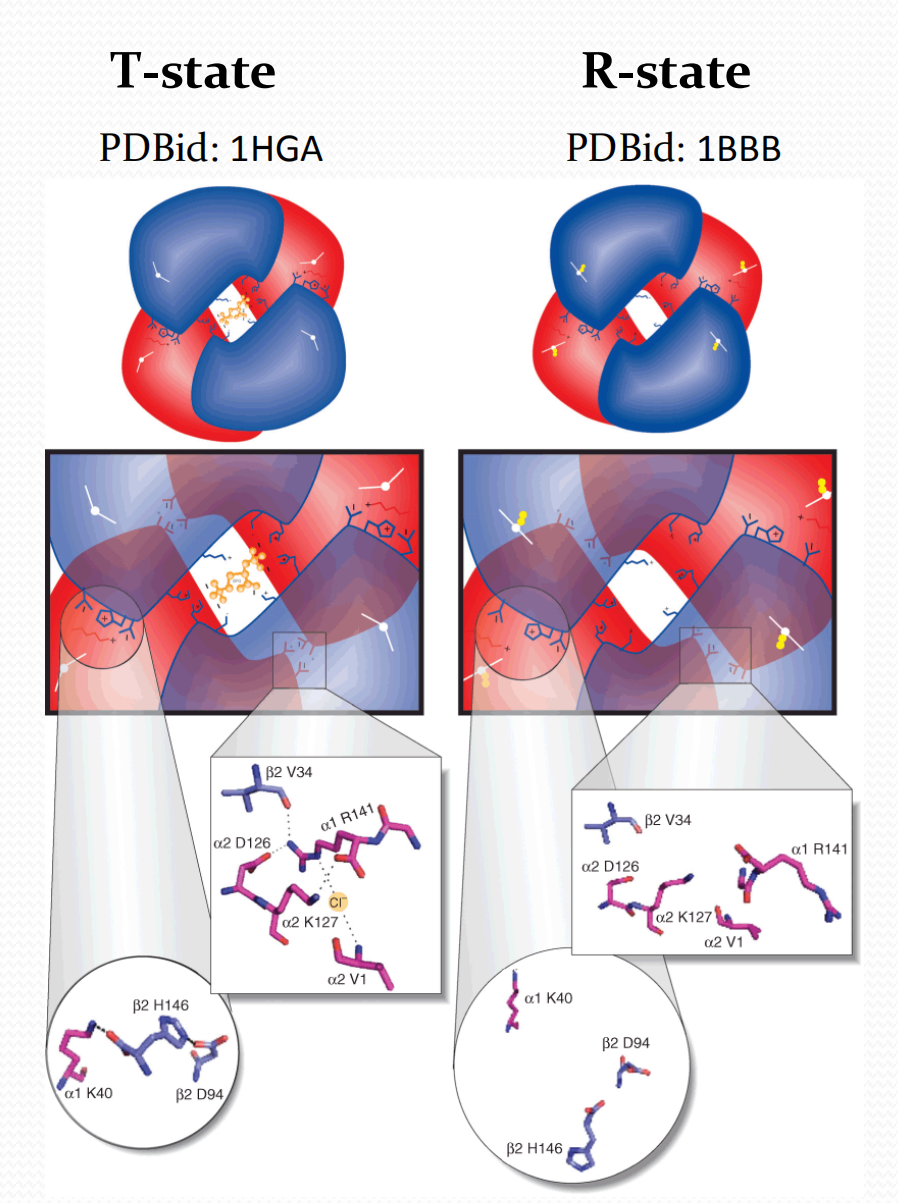
Intra-group interactions weaker in R-state.
Key salt bridge interactions disrupted during the switch between T and R state hemoglobin quaternary structures
6. The molecular basis of the allosteric change in Hb
- In the deoxy state, heme has a dome shape
- The binding of oxygen to deoxyhemoglobin causes conformational changes in the heme
- Binding of the $O_2$ ligand pulls the iron into the heme plane, flattening the heme and causing strain
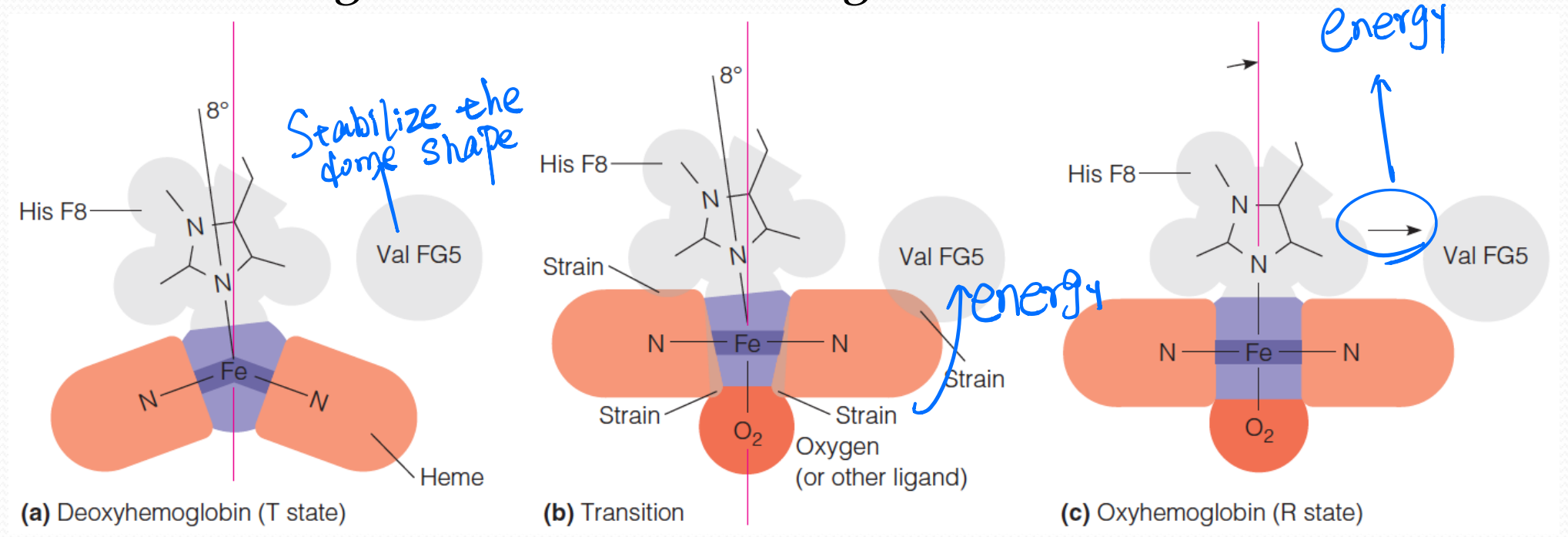
7. The molecular basis of the allosteric change in Hb
A small movement of the heme iron upon $O_2$-binding is translated into a larger movement of the F helix by the covalent connection between the F helix and the proximal histidine
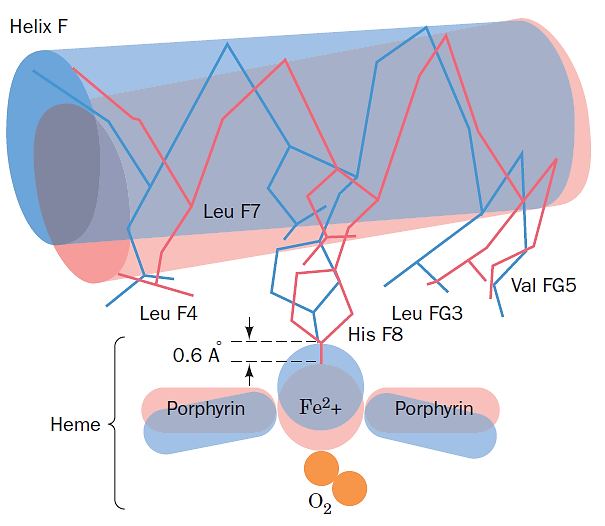
8. Other functions of Globins
Nitric oxide (NO) signaling
NO scavenging(清除)
- Cytoglobin 胞红蛋白
- Neuroglobin 神经球蛋白
Apoptosis 细胞凋亡
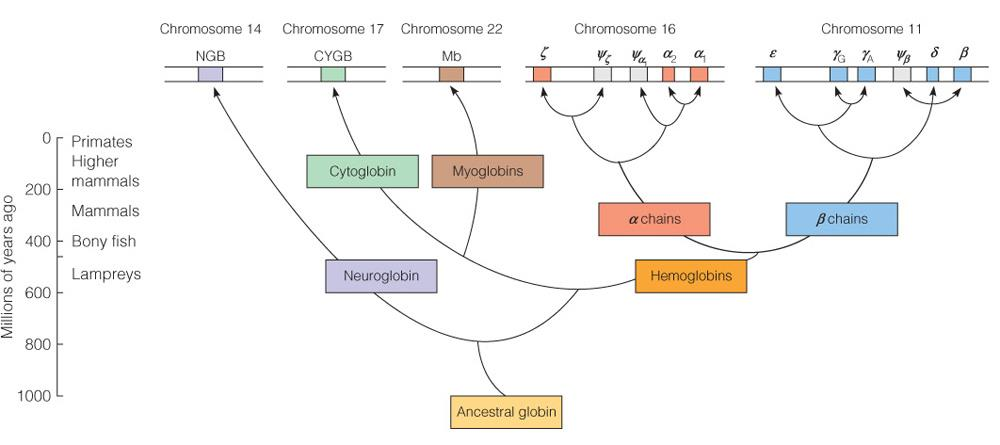
三、Protein evolution
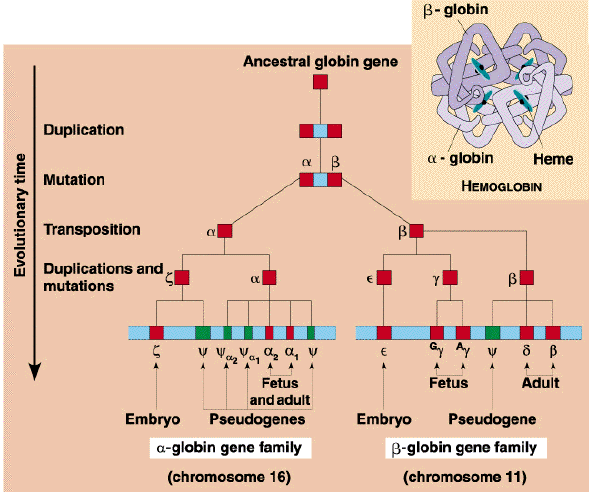
How did the first globin arise?
How can globins be so diversified through evolution?
How did functions of globins evolve?
Structure evolution
Protein evolution mechanisms
Proteins that are related by a common evolutionary origin are called homologs
Paralogs (within an organism,旁系同源物)
Orthologs (in different species,直系同源物)
1. Divergent evolution 发散进化
2. Convergent evolution 趋同进化/趋同演化
3. Coevolution 共进化
Ways Contributes to protein evolution
- Gene duplication, the way to generate paralogs
- Exon recombination, fusion of initially independent genes
- Multi-domain proteins
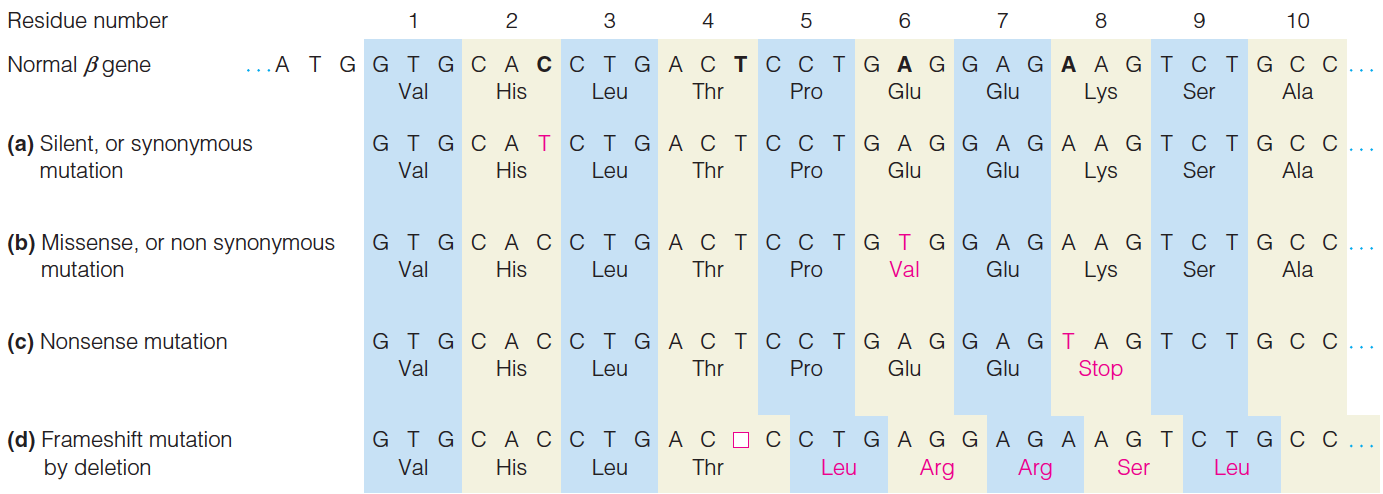
- Mutations:
- Silent (synonymous) mutation 沉默(同义词)突变
- Missense (nonsynonymous) mutation 错义(非同义)突变
- Nonsense mutation 无义突变
- Frameshift mutation 移码突变
- In frame deletion mutation 框架内缺失突变
Evolution of the globin protein family
Conservation in the globin evolution
Hemoglobin’s function is affected by some mutations
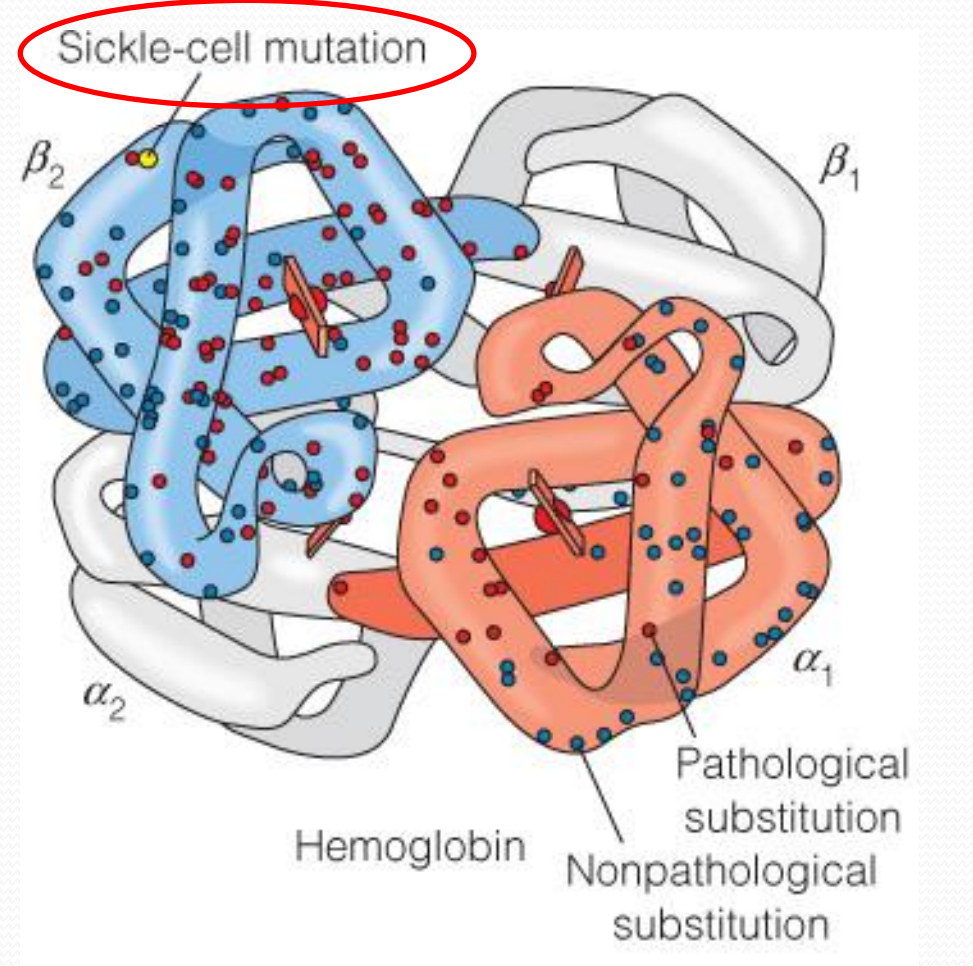
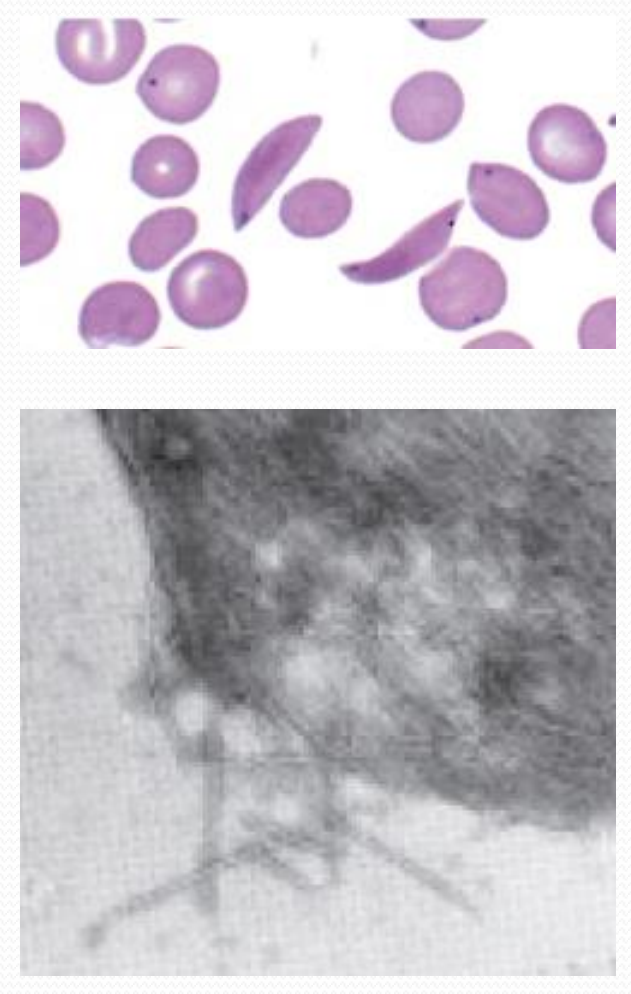
1. Hemoglobin and sickle-cell disease
Erythrocytes in sickle-cell disease
Hemoglobin fibers spill out of a sickled cell
Evolutionary molecular engineering
Directed evolution
- Radom mutation
- Site-directed mutagenesis
- DNA shuffling
- DNA combination
- Library screen
- Phage display
- Radom mutation
Aims
High stability
- Charged residues
New functions
- Enzyme design
四、Immunoglobins
Immune response
1. Adaptive immune response
Humoral
- B lymphocytes (B-cell)
- Antibody (immunoglobulin)
Cellular
- T lymphocytes (T-cell)
- T-cell receptor (immunoglobulin-like)
2. Features of adaptive immune response
Versatile, but highly specific for each case
Memory
Antigenic determinants (epitopes) 抗原表位
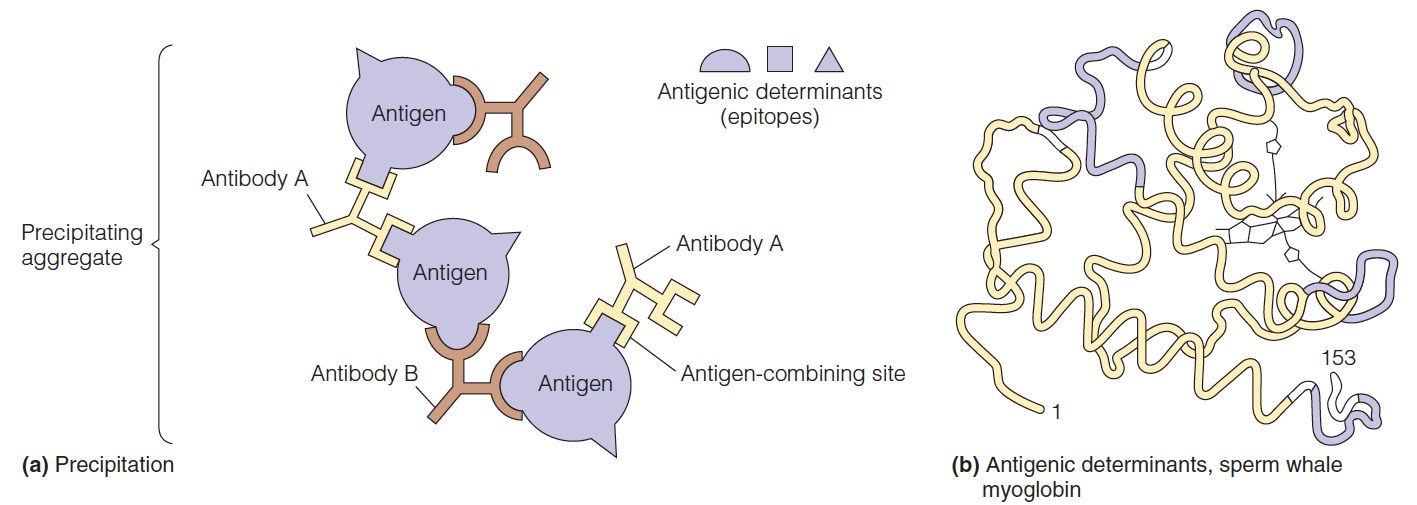
Some antigenic determinants involve portions of chain that are far apart in the primary sequence but close together in the tertiary structure, a so-called discontinuous epitope(非连续表位).
The clonal selection theory
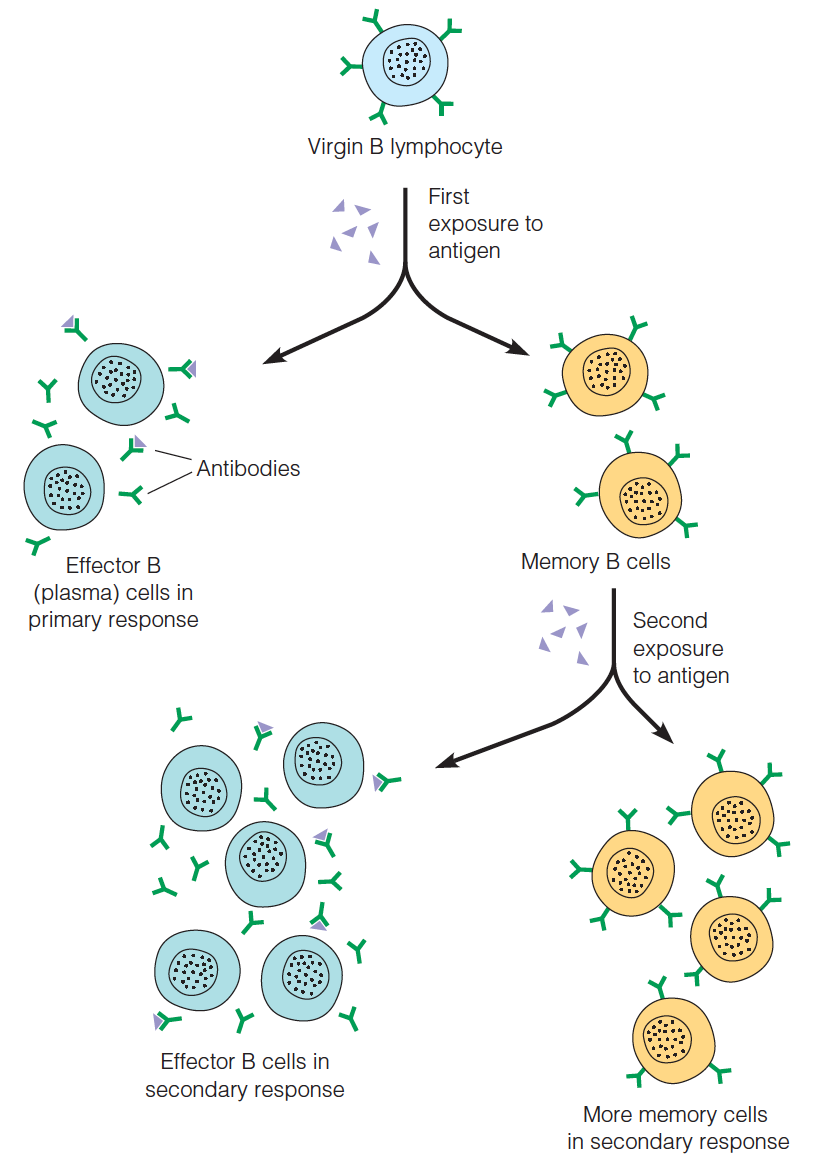
Memory in humoral immunity
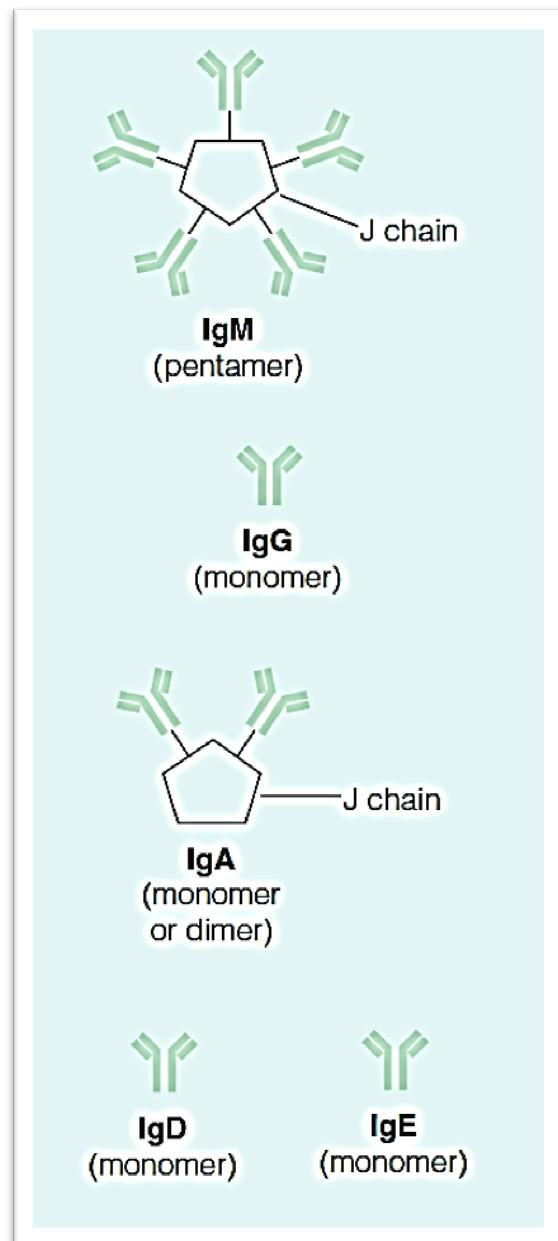
Immunoglobulin (Ig)
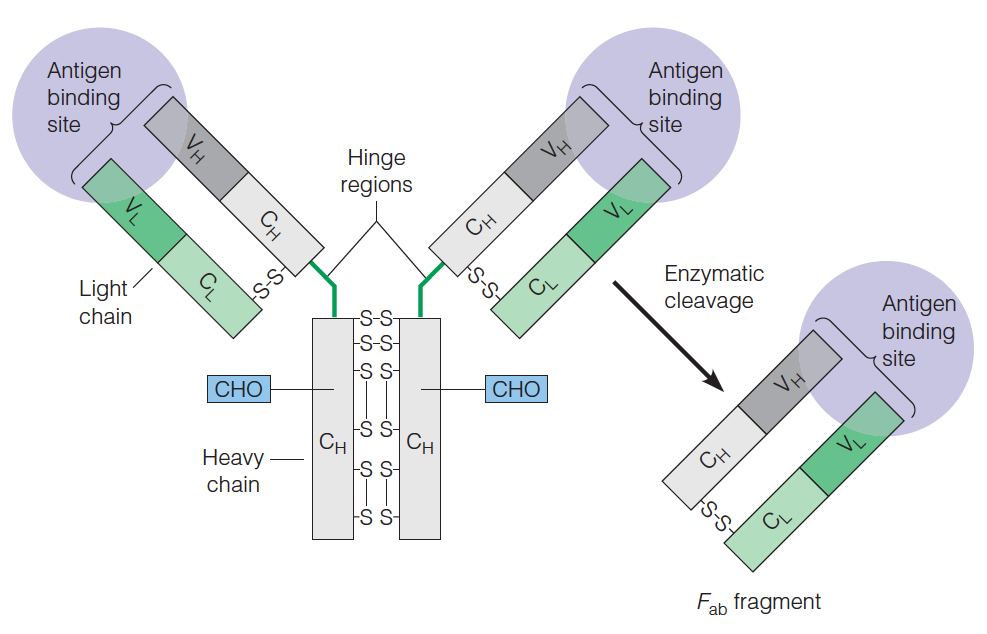
Each immunoglobulin monomer (e.g.,IgG) consists of four chains:
Two identical heavy chains (53 kDa each)
Two identical light chains (23 kDa each)
Held together by disulfide bonds
Immunoglobulin molecules contain both constant and variable regions.
- The variable regions are the antigen binding sites.
1. IgG antibody and Fab
igG
The diversity as well as the exquisite(精致的) specificity of antigen binding sites is determined by the hypervariable complementarity determining regions (CDR,高变互补决定区) from both the light and the heavy chains.
Structure of igG:
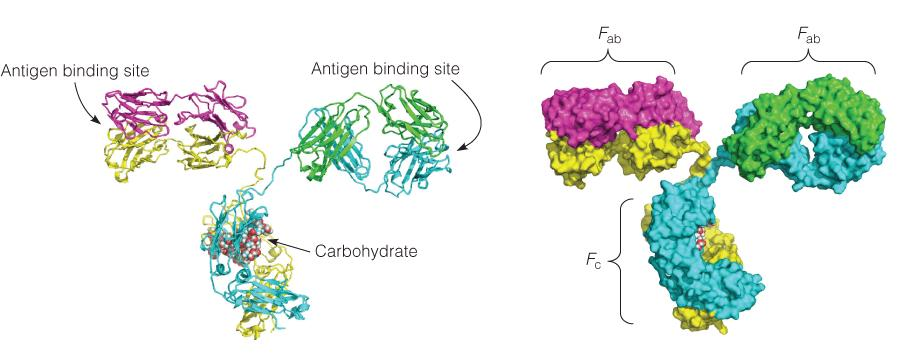
Antigen binding by Fab
CDR are loops
Recognition happens on the antibody surface and mediated by CDR
Antigens contain epitopes on their surface
- Neuraminidase (神经氨酸酶) (purple)
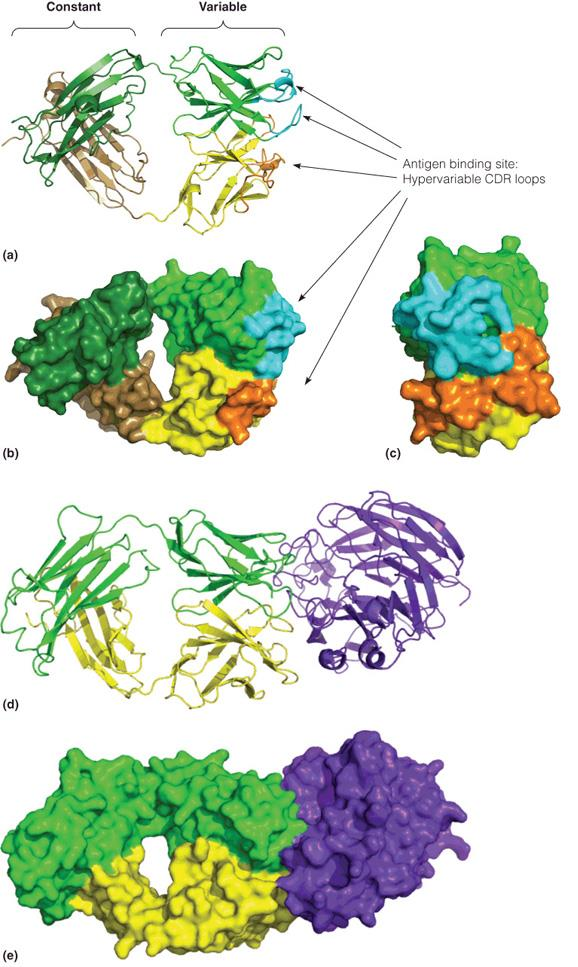
2. The immunoglobulin fold
The Ig fold is a common structure in domains of many proteins
- immunoglobulin superfamily (免疫球蛋白超家族)
Two antiparallel sheets are stacked face to face and covalently bonded by a disulfide bond
IgG contains 12 Ig domains, four of which is within Fab
Detect target ig
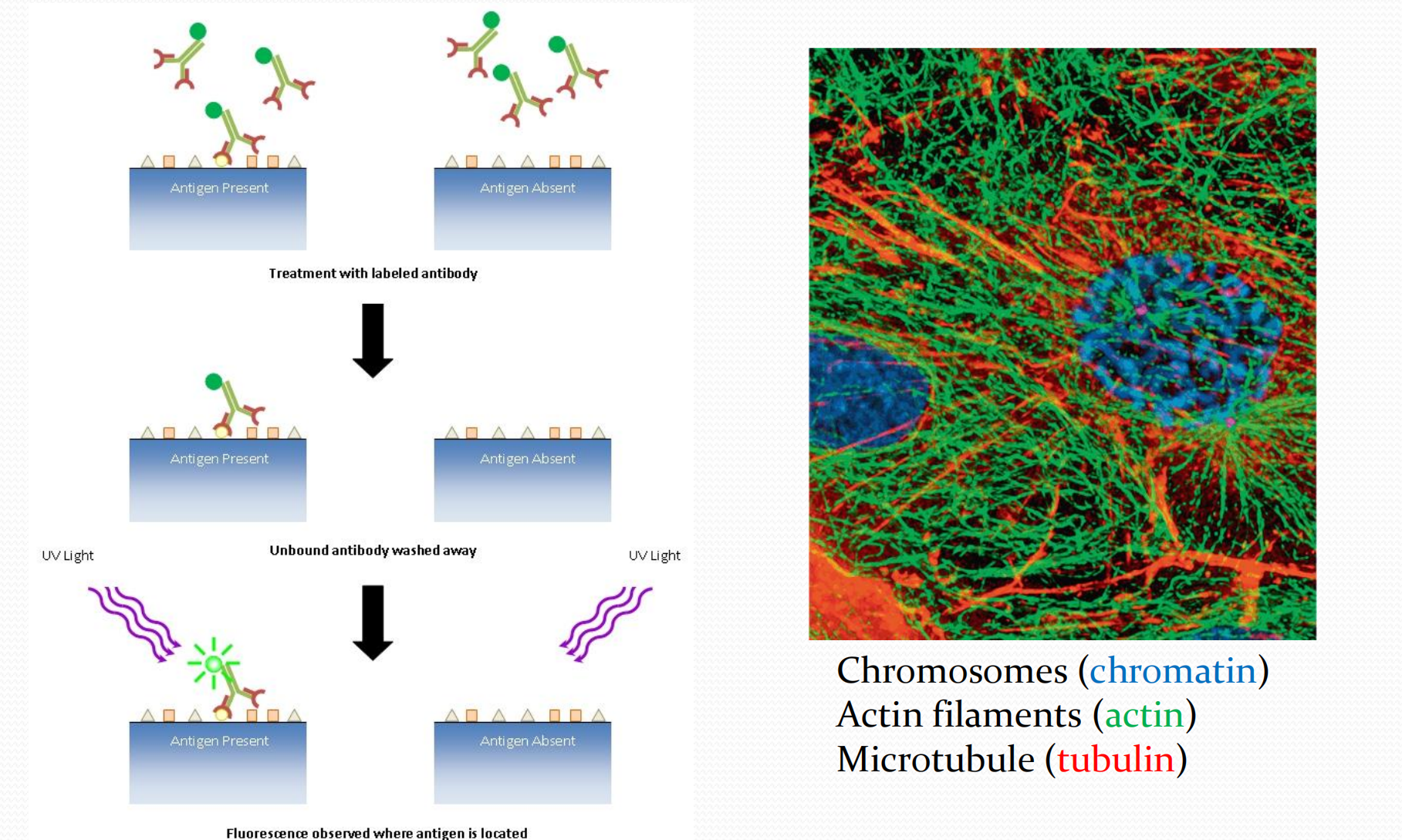
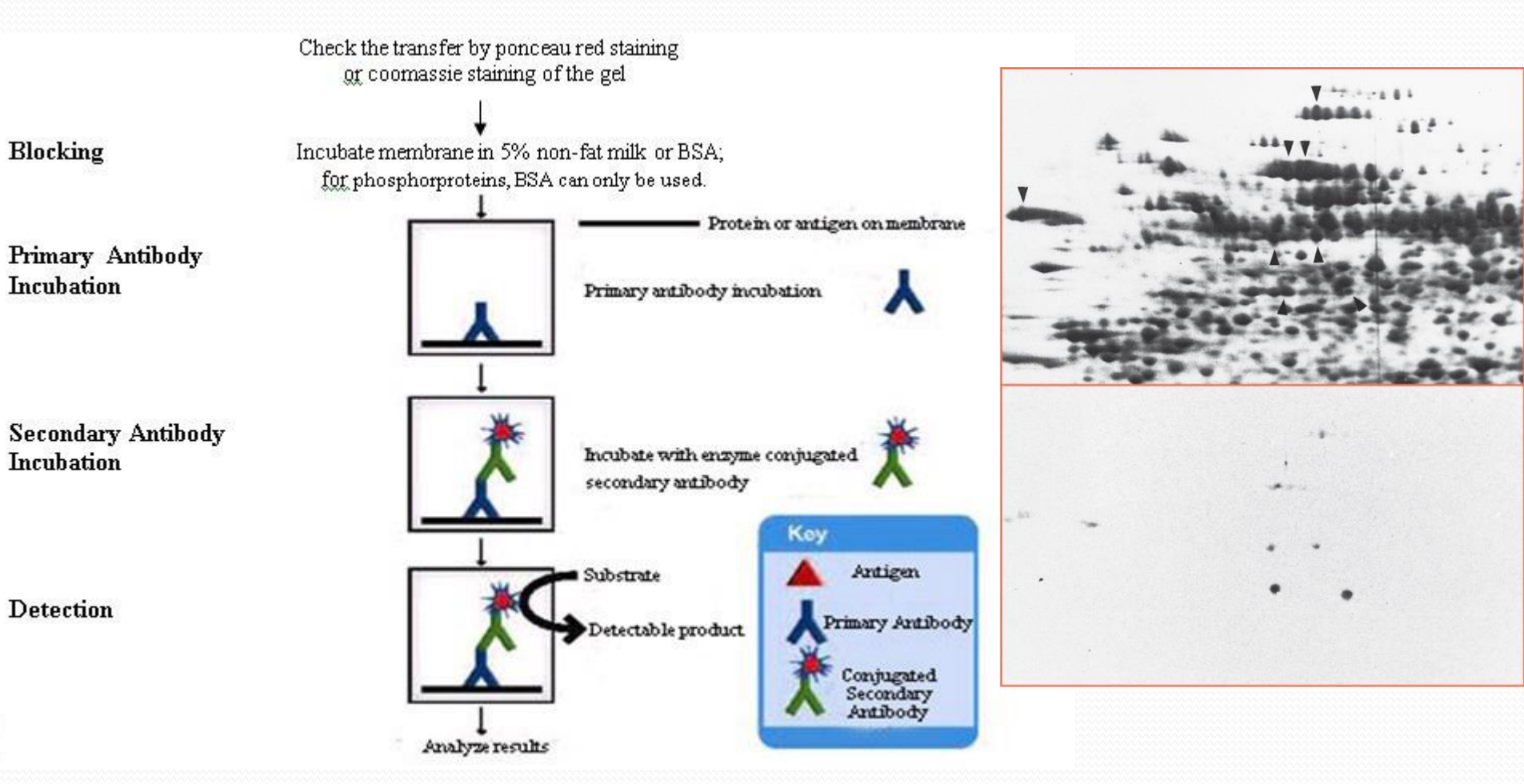
1. Western blot
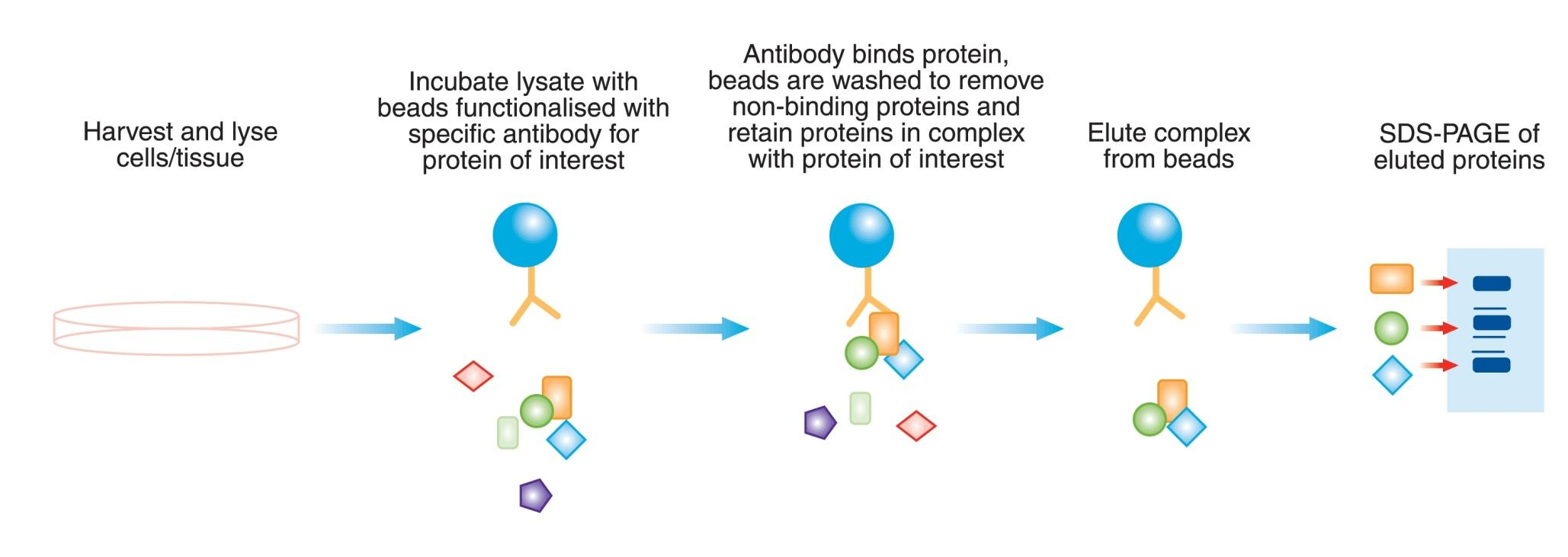
2. Co-immunoprecipitation (Co-IP)
3. Protein cellular localization