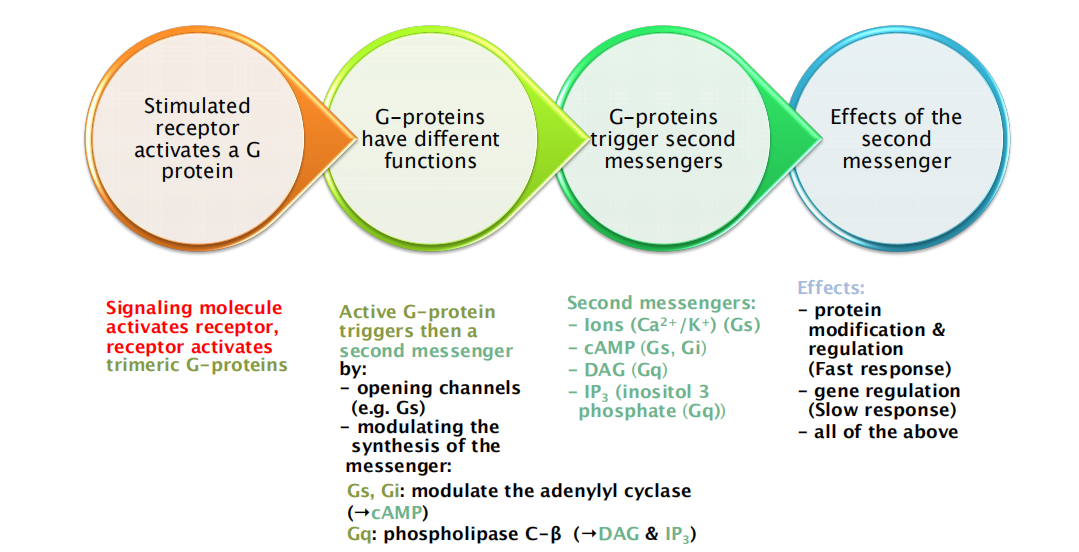
L16 Cell communication via G-protein coupled receptor
一、Structure of GPCRs and G-proteins
Trimeric G Proteins Relay Signals From GPCRs
G-protein-coupled receptors (GPCRs) form the largest family of cell-surface receptors, and they mediate most responses to signals from the external world, as well as signals from other cells, including hormones, neurotransmitters, and local mediators
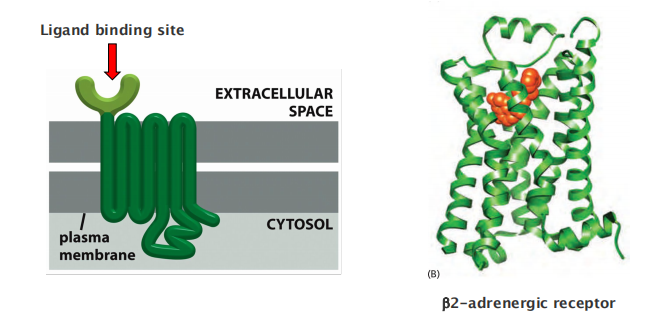
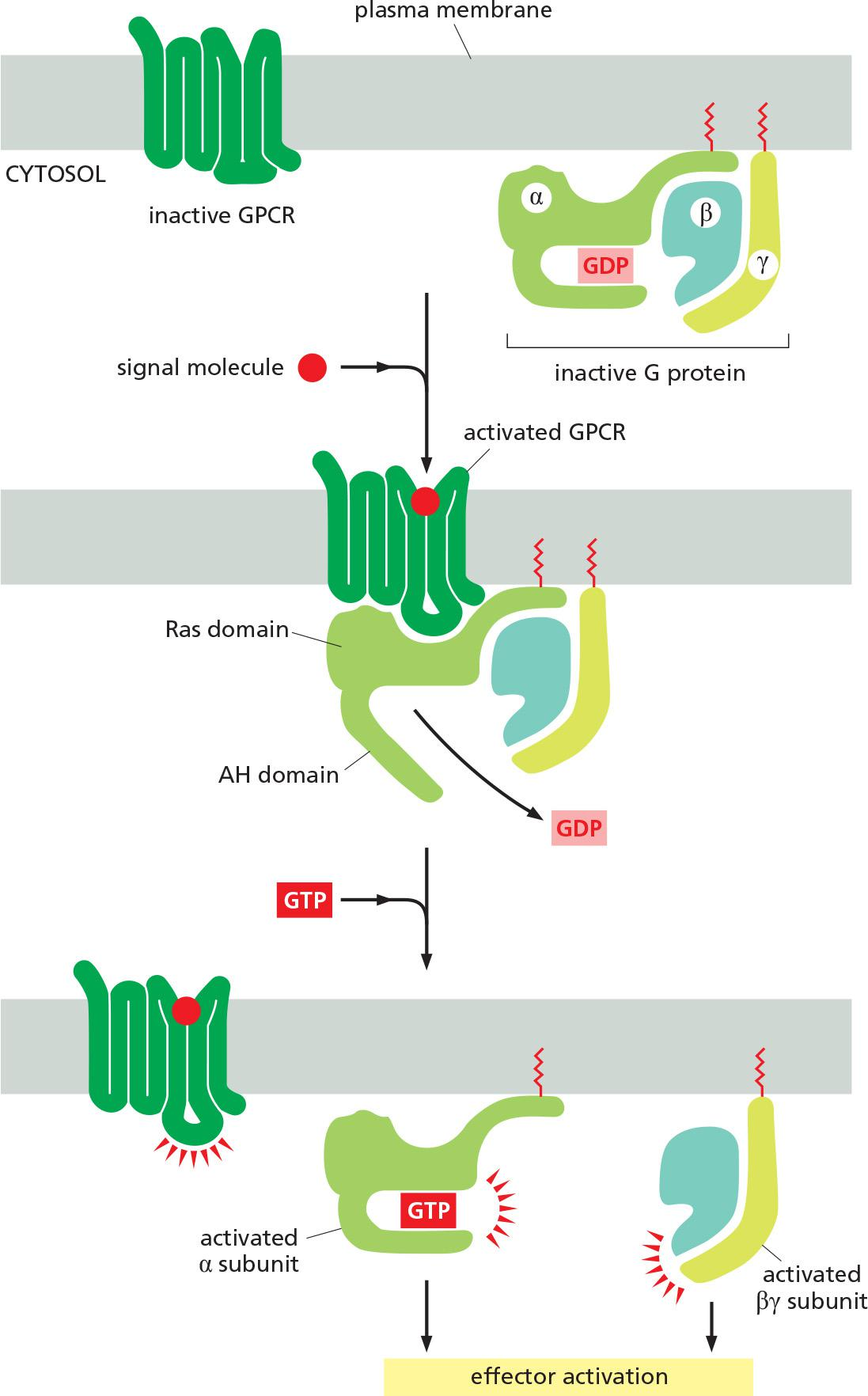
All GPCRs have a similar structure. They consist of a single polypeptide chain that threads back and forth across the lipid bilayer seven times, forming a cylindrical structure, often with a deep ligand-binding site at its center (Figure 15–21). In addition to their characteristic orientation in the plasma membrane, they all use G proteins to relay the signal into the cell interior.
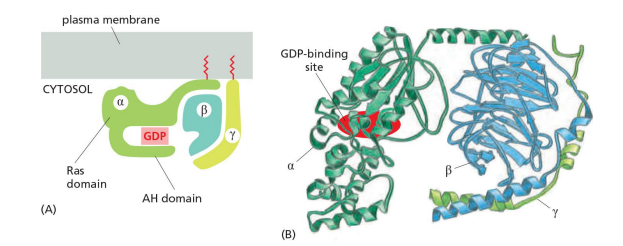
- G proteins are composed of three protein subunits—α, β, and γ. In the unstimulated state, the α subunit has GDP bound and the G protein is inactive (Figure 15–22).
- When a GPCR is activated, it acts like a guanine nucleotide exchange factor (GEF) and induces the α subunit to release its bound GDP, allowing GTP to bind in its place.
- GTP binding then causes an activating conformational change in the Gα subunit, releasing the G protein from the receptor and triggering dissociation of the GTP-bound Gα subunit from the Gβγ pair—both of which then interact with various targets, such as enzymes and ion channels in the plasma membrane, which relay the signal onward
regulator of G protein signaling (RGS). RGS proteins act as α-subunit-specific GTPase-activating proteins (GAPs) (see Figure 15–8), and they help shut off G-protein-mediated responses in all eukaryotes.
~ 50% of known drugs are working with GPCRs
They are seven-pass transmembrane proteins structurally similar to bacteriorhodopsin
- One single polypeptide, 7-pass transmembrane in a serpentine(像蛇般蜷曲的,蜿蜒的) manner
The largest cell surface receptor family with >800 GPCRs in humans.
- The receptors that respond to sight, smell, taste, and neurotransmitters, etc.
~ 150 GPCR known ligands
All GPCRs need trimeric GTP-binding proteins to relay signals
Trimeric G-protein
Subunit $\alpha$ binds to GTP/GDP and has GTPase activity
- β subunit is bound by α and γ
- β and γ form a functional subunit
二、GPCR activation and deactivation
Activation of a trimeric G-protein by stimulating GPCRs
Ligand binding activates GPCR, which then acts as a GEF to exchange GDP for GTP and thus activates the G-protein
- “Activated trimeric G-proteins” dissociate into an active GTP-bound $\alpha$ subunit and an active β-γ complex.
α subunit and/or the β-γ complex bind to target proteins (effectors)
Deactivation of a G-protein by GTP hydrolysis
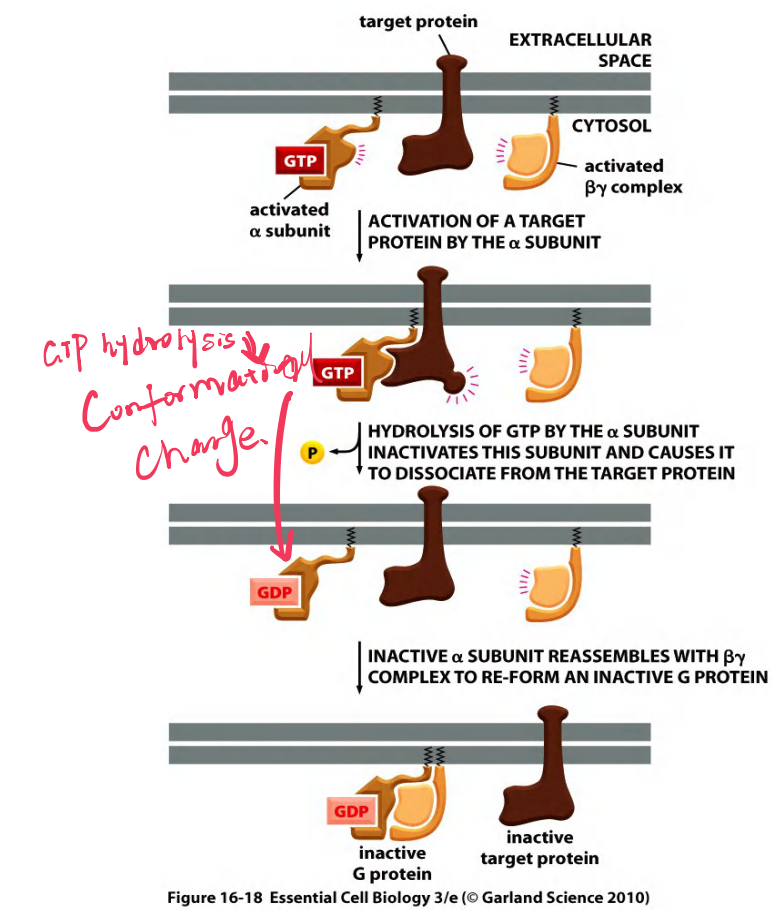
α subunit hydrolyzes GTP and deactivate itself and the β-γ complex.
RGS (regulator of G-protein signaling) acts as α-subunit-specific GTPase activating protein (GAP) to promote GTP hydrolysis.
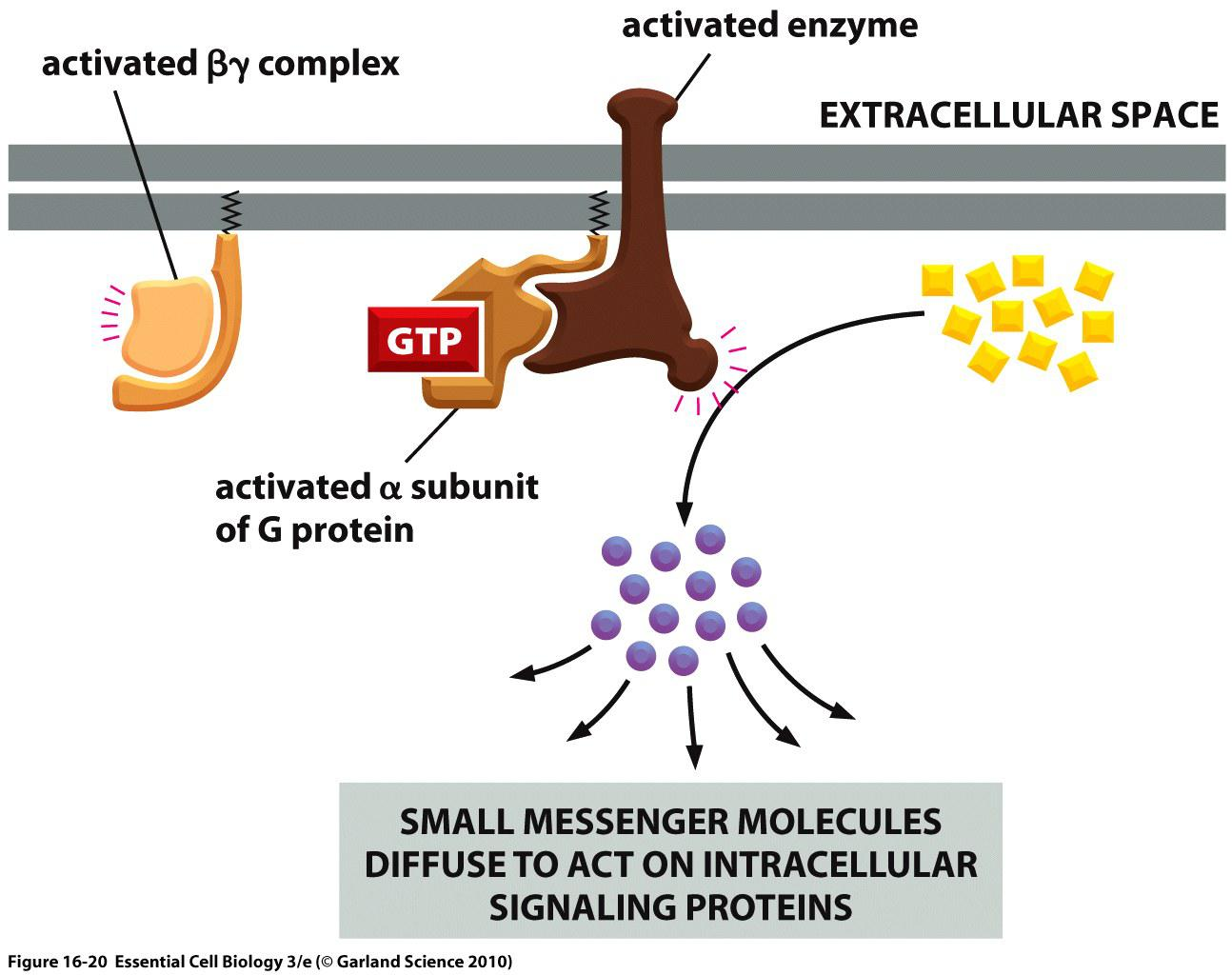
Activated G protein relays GPCR signal via small second messenger molecules
“First messengers”: signaling molecules that activate the receptor
“Second messengers”: small molecules that are produced by the activated enzymes.
Small molecules rapidly diffuse away from their source, thereby amplifying and spreading the intracellular signal
Small messengers:
- cAMP (produced by adenylyl cyclase)
- DAG (diacylglycerol) and IP
3(inositol 1,4,5 triphosphate) (produced by phospholipase C-β) - Ca2+ (levels are controlled by gated channels)
- cAMP/cGMP (to trigger gated ion channels in smell and vision)
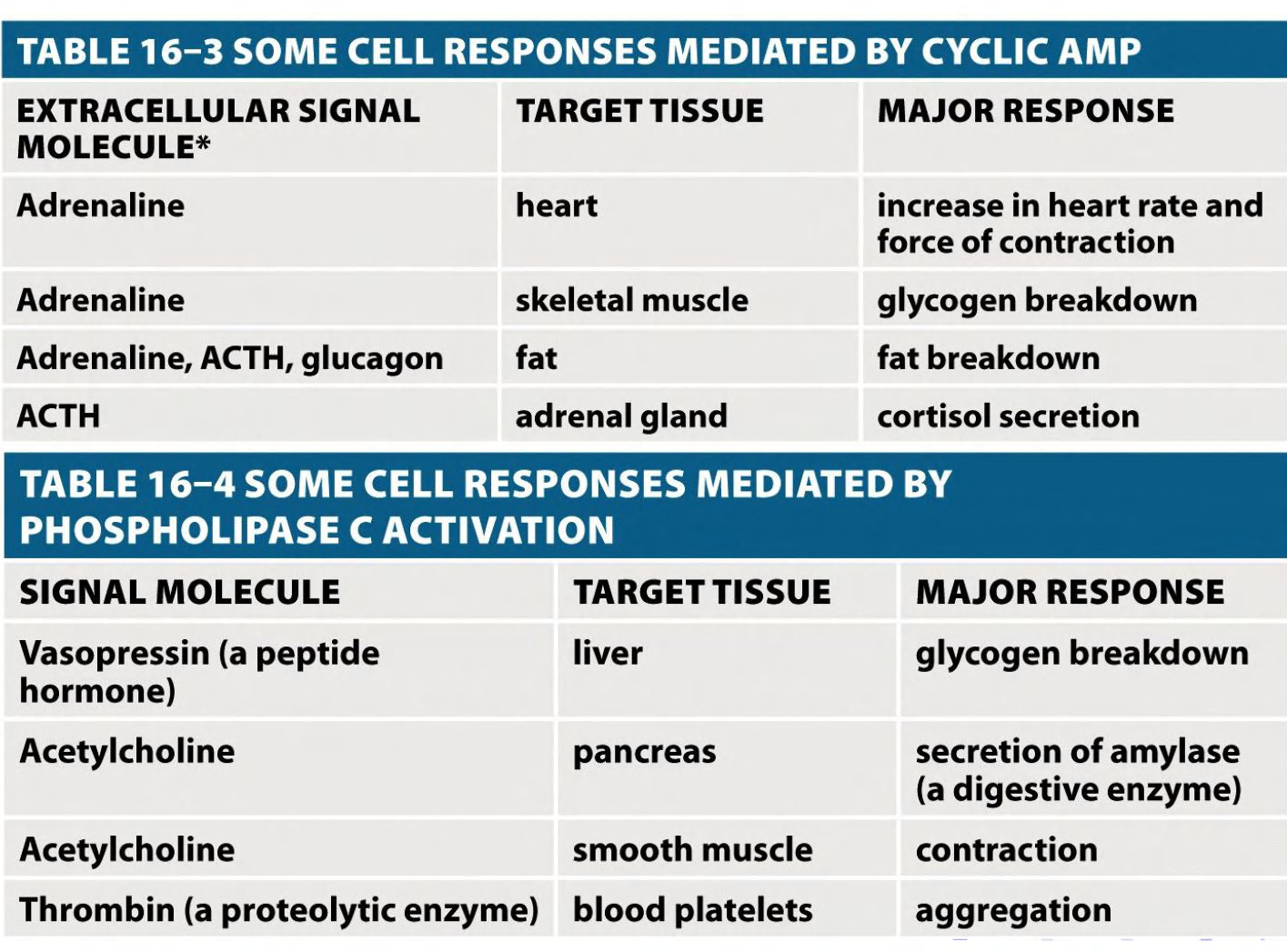
Some G Proteins Regulate the Production of Cyclic AMP
Cyclic AMP (cAMP) acts as a second messenger in some signaling pathways.
Second Messengers and Enzymatic Cascades Amplify Signals
Despite the differences in molecular details, the different intracellular signaling pathways that GPCRs trigger share certain features and obey similar general principles.
- They depend on relay chains of intracellular signaling proteins and second messengers. These relay chains provide numerous opportunities for amplifying the responses to extracellular signals
Any such amplifying cascade of stimulatory signals requires counterbalancing mechanisms(平衡机制) at every step of the cascade to restore the system to its resting state when stimulation ceases.
1. Summary: stimulation of GPCRs
Subunit α binds to GTP/GDP and has GTPase activity
β subunit is bound by α and γ
- β and γ form α functional subunit
Signal facilitate conformational change of receptor
Receptor will activate G protein
- In activated G protein, α subunit binds GTP, other two form β-γ complex
- α and β-γ /or β-γ bind to target protein
- α subunit will finally hydrolyze GTP and deactivate itself plus complex
三、GPCR signaling via different mediators
G-protein may activate either adenylyl cyclase, or phospholipase C
Both are enzymes producing small molecules—second messengers
- Adenylyl cyclase produces cAMP
- Phospholipase C produces inositol triphosphate and diacylglycerol
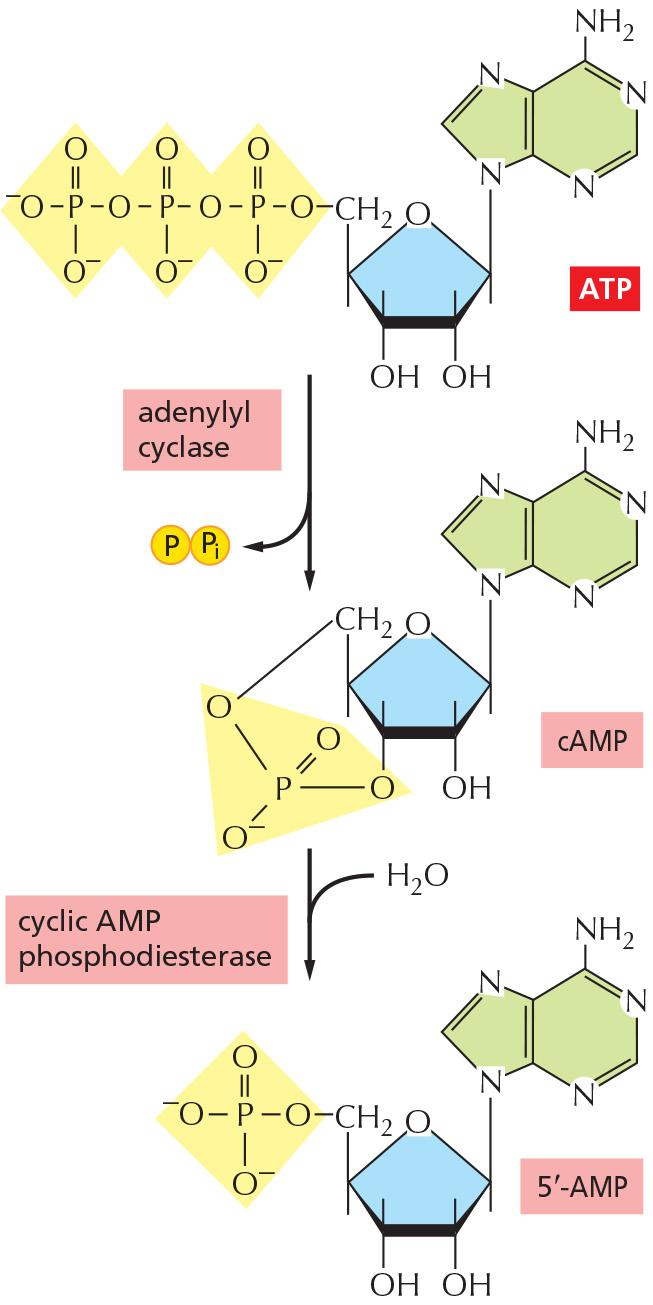
cAMP
Cyclic AMP is synthesized from ATP by an enzyme called adenylyl cyclase, and it is rapidly and continuously destroyed by cyclic AMP phosphodiesterases (Figure 15–25).
- Adenylyl cyclase is a large, multipass transmembrane protein with its catalytic domain on the cytosolic side of the plasma membrane.
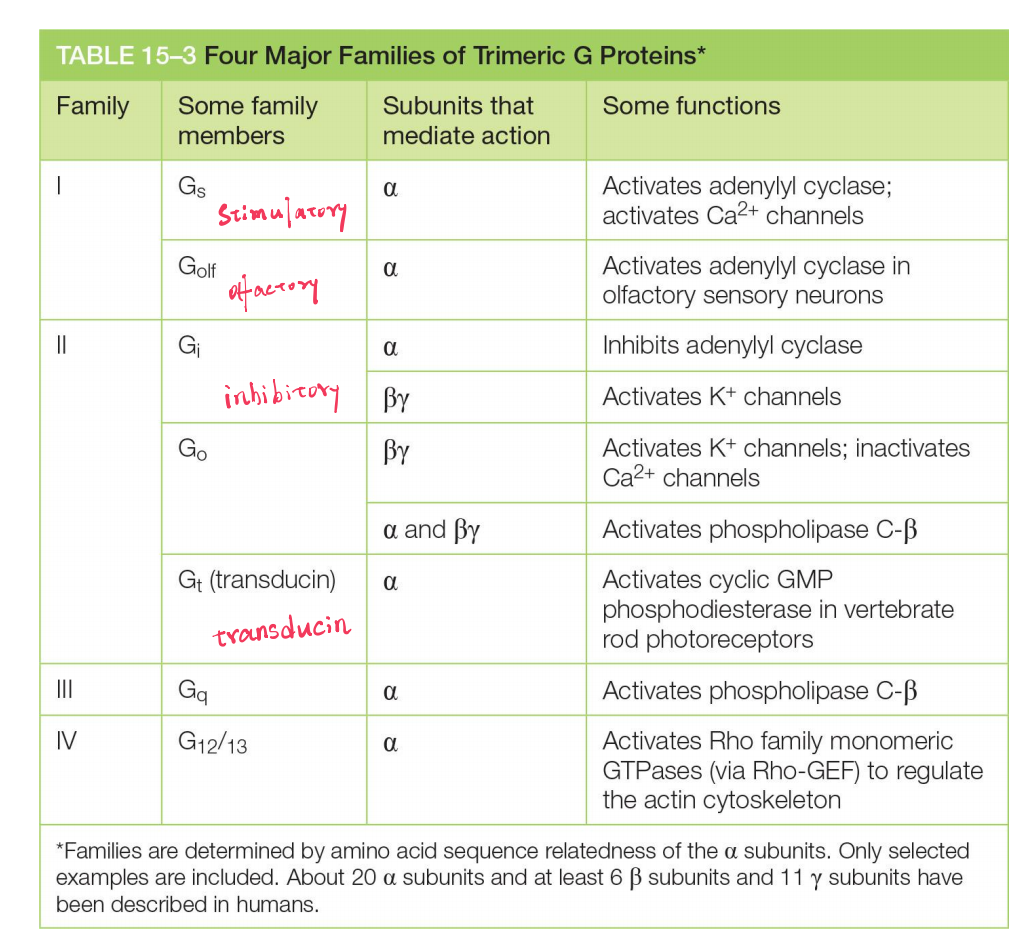
Many extracellular signals work by increasing cAMP concentrations inside the cell. These signals activate GPCRs that are coupled to a stimulatory G protein (G
s)Other extracellular signals, acting through different GPCRs, reduce cAMP levels by activating an inhibitory G protein (G
i)
Two different G-proteins influence cAMP synthesis:
- G
s(stimulatory G-protein) activates adenylyl cyclase - G
i(inhibitory G-protein) inhibits adenylyl cyclase- α-subunits of G
sand Giare target of the cholera toxin(霍乱毒素) - α-subunits of G
iis target of the pertussis toxin(百日咳毒素)
- α-subunits of G
1. Mechanism of cholera toxin: prolonged elevation of cAMP concentrations (Keep Activated)
Cholera toxin stops deactivation of particular G-protein; as a result, water and ions will not be absorbed in gut
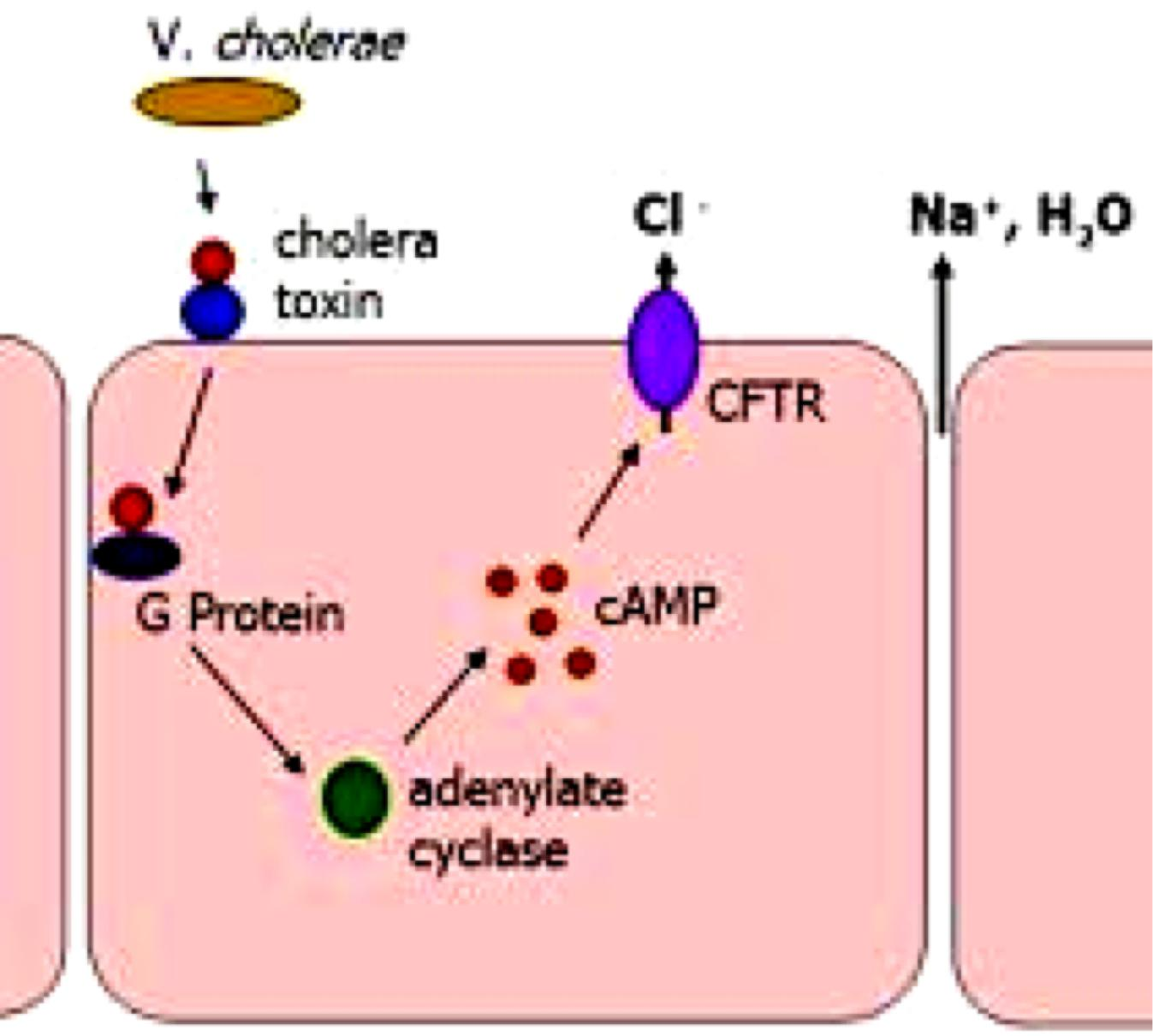
Catalyzes the transfer of ADP ribose from intracellular NAD+ to the $\alpha$ subunit of Gs.
- ADP ribosylation alters the $\alpha$ subunit so that it can no longer hydrolyze its bound GTP.
- It remains in an constitutively active state.
- This indefinitely stimulates the adenylyl cyclase.
The prolonged elevation in cAMP concentration causes influx of Cl- and water from intestinal epithelial cells into the gut, thereby causing the severe diarrhea(腹泻,痢疾) that characterizes cholera.
2. The opposite is true for the mechanism of pertussis toxin: permanent inactivation of G-proteins (Inhibit Inactivated)
Catalyzes ADP ribosylation of $\alpha$ subunit of inhibitory G-protein (Gi), preventing the protein from interacting with the receptor.
Gi is constitutively inactive and is unable to regulate the target proteins.
Pertussis toxin stops activation of Gi, resulting in a cough.
GPCR signaling via cAMP-PKA
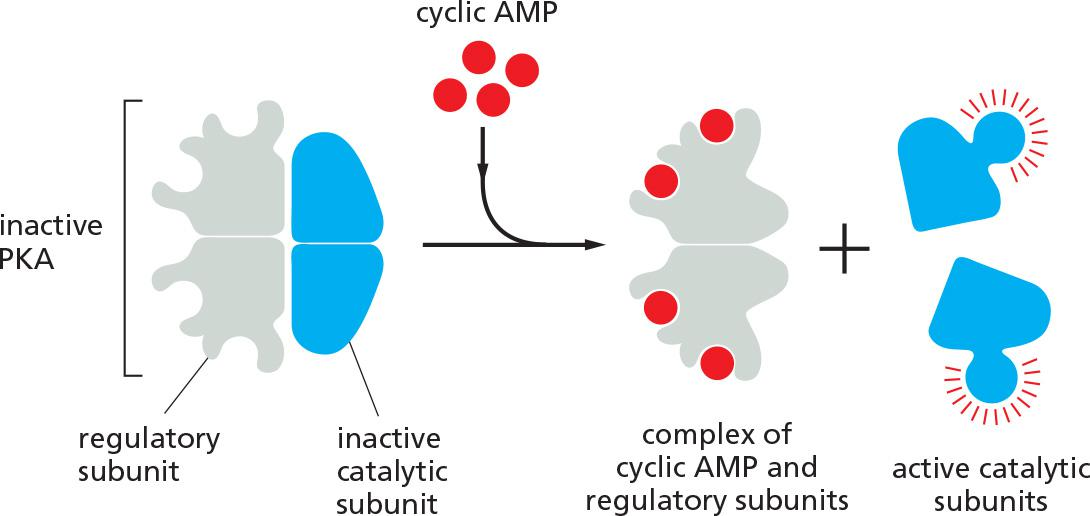
Cyclic-AMP-Dependent Protein Kinase (PKA) Mediates Most of the Effects of Cyclic AMP
In most animal cells, cAMP exerts its effects mainly by activating cyclic-AMP-dependent protein kinase (PKA). This kinase phosphorylates specific serines or threonines on selected target proteins, including intracellular signaling proteins and effector proteins, thereby regulating their activity
- In the inactive state, PKA consists of a complex of two catalytic subunits and two regulatory subunits. The binding of cAMP to the regulatory subunits alters their conformation, causing them to dissociate from the complex. The released catalytic subunits are thereby activated to phosphorylate specific target proteins
- The regulatory subunits of PKA (also called A-kinase) are important for localizing the kinase inside the cell: special A-kinase anchoring proteins (AKAPs) bind both to the regulatory subunits and to a component of the cytoskeleton or a membrane of an organelle, thereby tethering the enzyme complex to a particular subcellular compartment
- An AKAP located around the nucleus of heart muscle cells, for example, binds both PKA and a phosphodiesterase that hydrolyzes cAMP.
PKA (cAMP-dependent protein kinase) mediates most of the effects of cAMP, which can trigger fast and slow responses.
- PKA is a serine/threonine protein kinase. By phosphorylation of substrates (protein modification), PKA mediates GPCR signaling in a fast manner/response.
- PKA also phosphorylates CREB (CRE-binding protein), which then recruits CBP (CREB-binding protein) and activates gene transcription (gene regulation) in a slow manner/response
1. Activated PKA triggers fast responses: (Adrenaline, 肾上腺素)
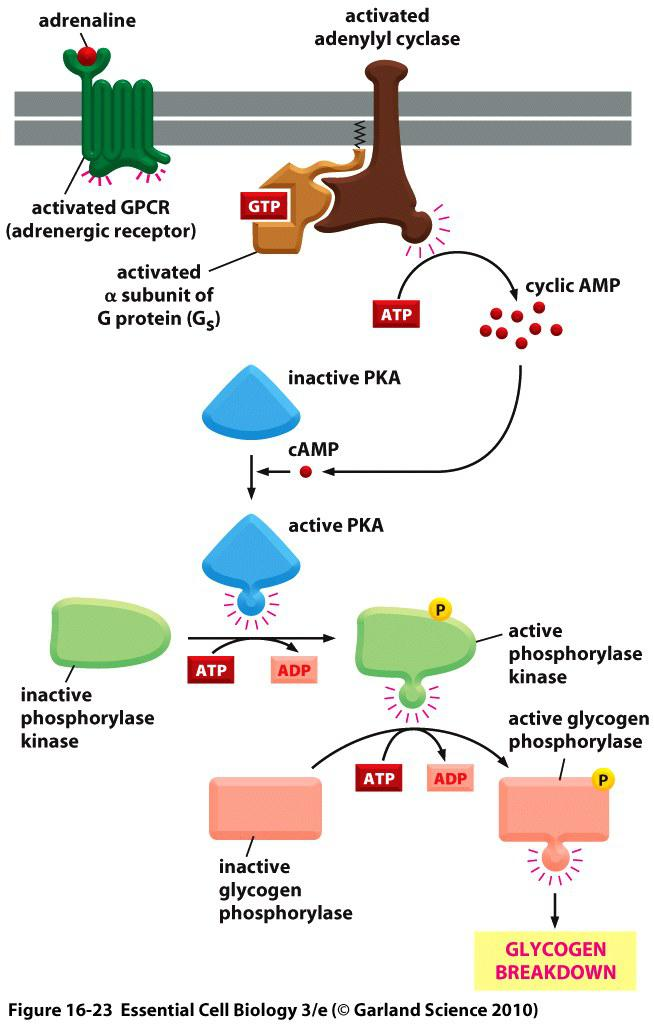
Adrenaline-induced glycogen breakdown
- In skeletal muscle, glycogen breakdown occurs within seconds of adrenaline binding to its receptor (activated by the second messenger (cAMP)
- Receptor activates adenylyl cyclase to produce cAMP
- cAMP activates PKA
- Activated PKA phosphorylates the glycogen phosphorylase kinase
- Glycogen phosphorylase kinase phosphorylates glycogen phosphorylase which in turn triggers glycogen breakdown
2. Activated PKA triggers slow responses (transcription regulation)
In cells that secrete the peptide hormone somatostatin, cAMP activates the gene that encodes this hormone.
The regulatory region of the somatostatin(生长激素抑制素) gene contains a short cis-regulatory sequence, called the cyclic AMP response element (CRE), which is also found in the regulatory region of many other genes activated by cAMP.
A specific transcription regulator called CRE-binding (CREB) protein recognizes this sequence.
- When PKA is activated by cAMP, it phosphorylates CREB on a single serine;
- phosphorylated CREB then recruits a transcriptional coactivator called CREB-binding protein (CBP), which stimulates the transcription of the target genes
Thus, CREB can transform a short cAMP signal into a long-term change in a cell, a process that, in the brain, is thought to play an important part in some forms of learning and memory.
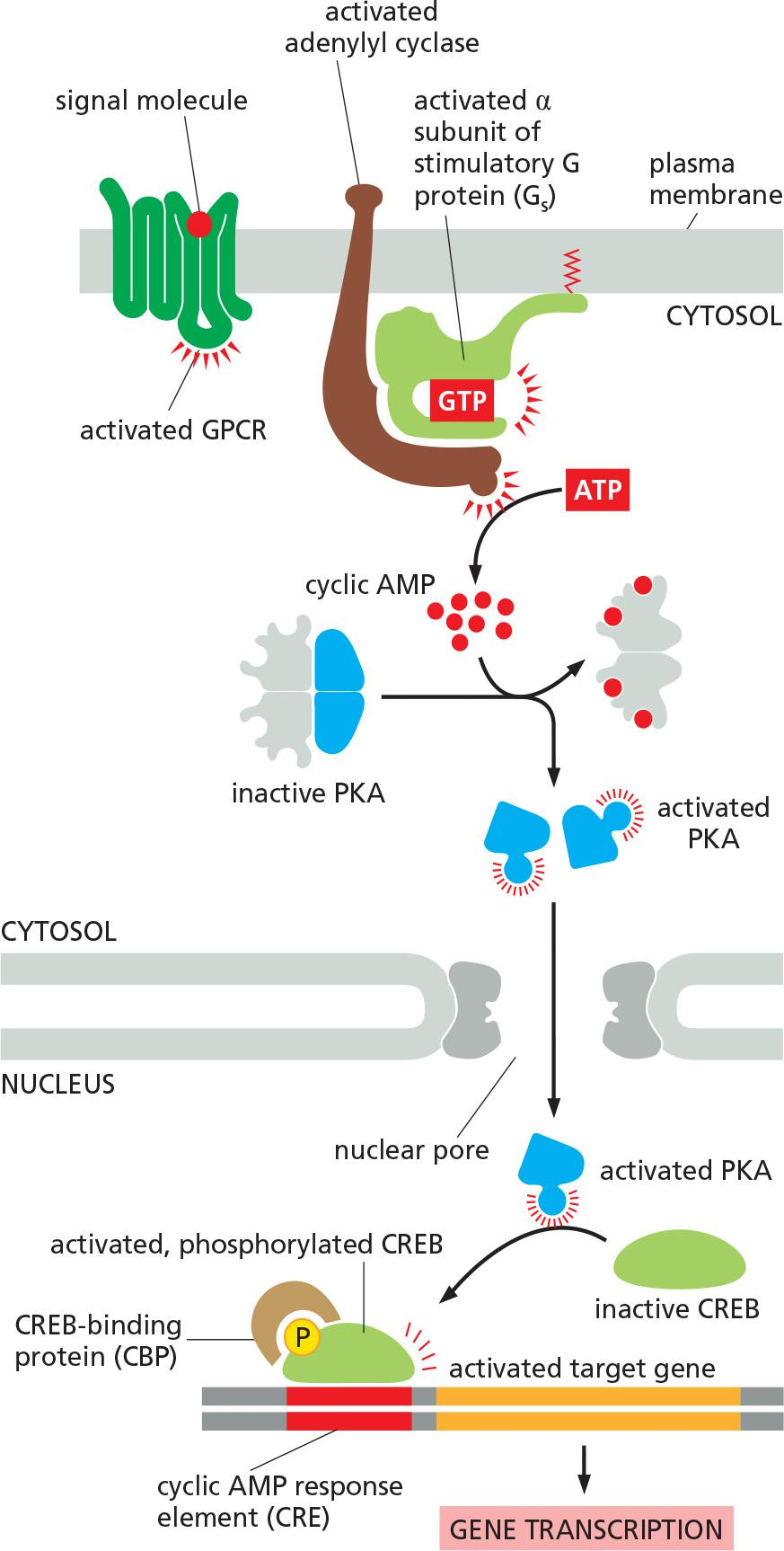
An increase in cAMP level can alter gene transcription!
How a rise in intracellular cyclic AMP concentration can alter gene transcription?
Transcription regulation by PKA:
- activation of PKA by cAMP.
- active PKA moves into the nucleus and phosphorylates CREB (cAMP response element (CRE)-binding protein), which then recruits CBP (CREB-binding protein) and activates gene transcription (gene regulation) in a slow manner/response.
Signaling via inositol phospholipid
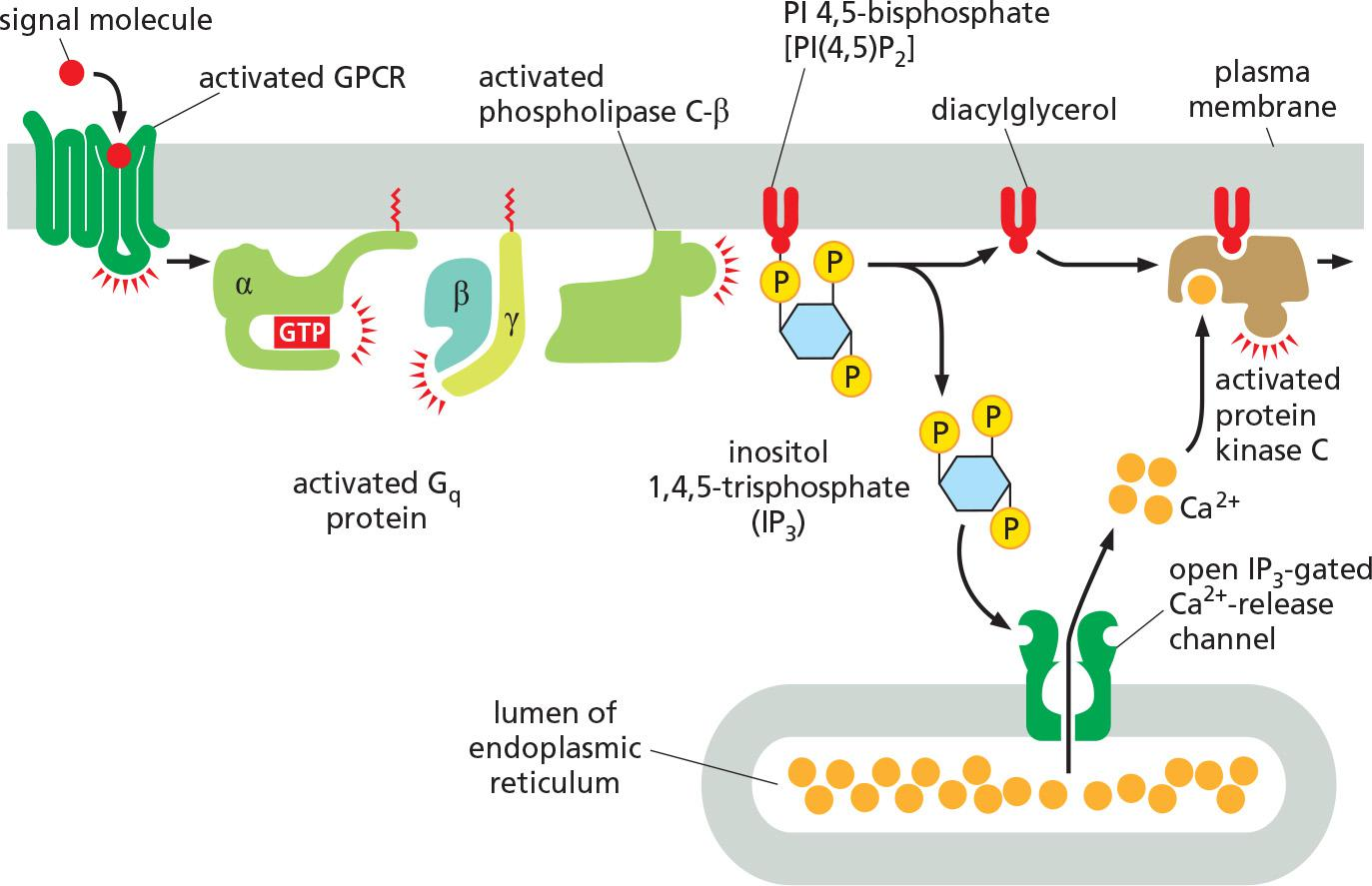
Many GPCRs exert their effects through G proteins that activate the plasma-membrane-bound enzyme phospholipase C-β (PLCβ)
Receptors that activate this inositol phospholipid signaling pathway mainly do so via a G protein called G
q, which activates phospholipase C-β in much the same way that Gsactivates adenylyl cyclase.The activated phospholipase then cleaves the PI(4,5)P2 to generate two products: inositol 1,4,5-trisphosphate (IP
3) and diacylglycerol
- IP
3is a water-soluble molecule that leaves the plasma membrane and diffuses rapidly through the cytosol. When it reaches the endoplasmic reticulum (ER), it binds to and opens IP3-gated Ca2+-release channels (also called IP3receptors) in the ER membrane- At the same time that the IP
3produced by the hydrolysis of PI(4,5)P2 is increasing the concentration of Ca2+ in the cytosol, the other cleavage product of the PI(4,5)P2, diacylglycerol, is exerting different effects. It also acts as a second messenger, but it remains embedded in the plasma membrane, where it has several potential signaling roles. One of its major functions is to activate a protein kinase called protein kinase C (PKC), so named because it is Ca2+-dependent
- Diacylglycerol can be further cleaved to release arachidonic acid(花生四烯酸), which can either act as a signal in its own right or be used in the synthesis of other small lipid signal molecules called eicosanoids(类花生酸)
GPCR-mediated signals described earlier, act primarily through IP
3receptors to stimulate Ca2+ release from intracellular stores in the ER
- The ER membrane also contains a second type of regulated Ca2+ channel called the ryanodine receptor (so called because it is sensitive to the plant alkaloid ryanodine), which opens in response to rising Ca2+ levels and thereby amplifies the Ca2+ signal
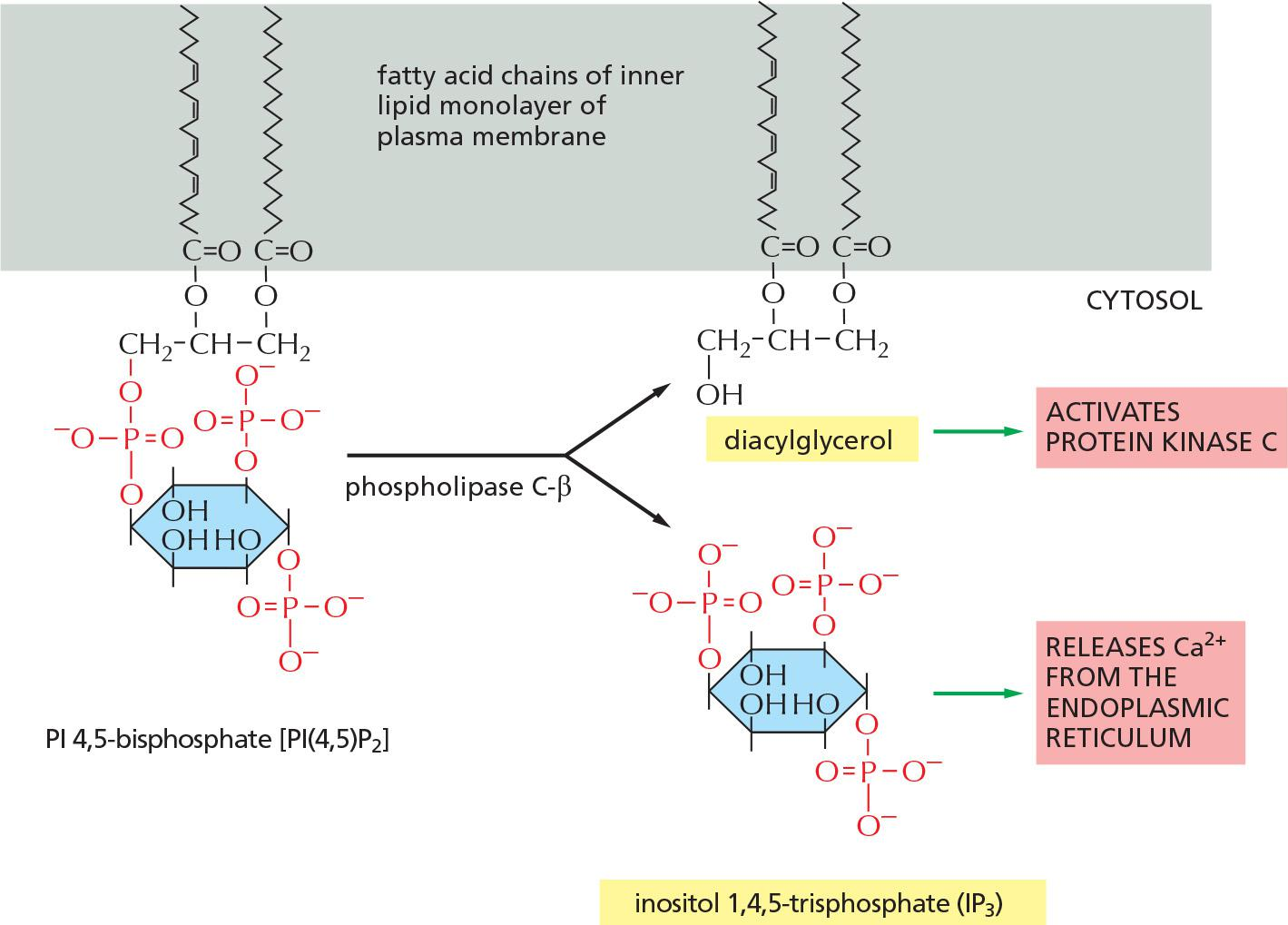
Instead of adenylyl cyclase, some G proteins activate phospholipase C(PLC)-β.
- Phospholipase C-β cleaves inositol
- phospholipid present in the inner layer of membrane.
- Substrates for PLC-β is PI(4,5)P2
- Cleavage of PIP
2results in production of the two second messengers 1,4,5-triphosphate (IP3) and diacylglycerol (DAG).- IP3 activates Ca2+ channels in ER
- DAG activates protein kinase C (Ca2+ is needed)
- Cleavage of PIP
Both Ca2+ and PKC are signals to other proteins
1. Signals that activate phospholipase C-β
Vasopressin, acetylcholine and trombin(加压素,乙酰胆碱和伸缩蛋白) activate this pathway
2. IP3, DAG, Ca2+ and PKC signal
Ca2+ is a frequent intracellular signal which signals egg to divide, muscle cell to contract etc.
Normally, Ca2+ concentration is high both in ER and outer space.
- Free [Ca2+]
out= 1 mM - Free [Ca2+]
in= 0.1 µM
IP3 triggers Ca2+ release from Ca2+ channels (IP3 receptor and ryanodine receptor) in the ER membrane
DAG activates protein kinase C (Ca2+ is needed)
- Together, Ca2+ and DAG activate the protein kinase C (PKC)
Ca2+ can work separately through CaM kinases.
Both Ca2+ and PKC are signals to other proteins
Effects of Ca2+ signaling
- Rise in Ca2+ in fertilized egg cytosol initiates embryonic development (and prevents entry of other sperm cells)
- Muscle contraction
- Release of secretory vesicle
Ca2+ oscillation in response to stimuli as consequence of long delay of negative feedback
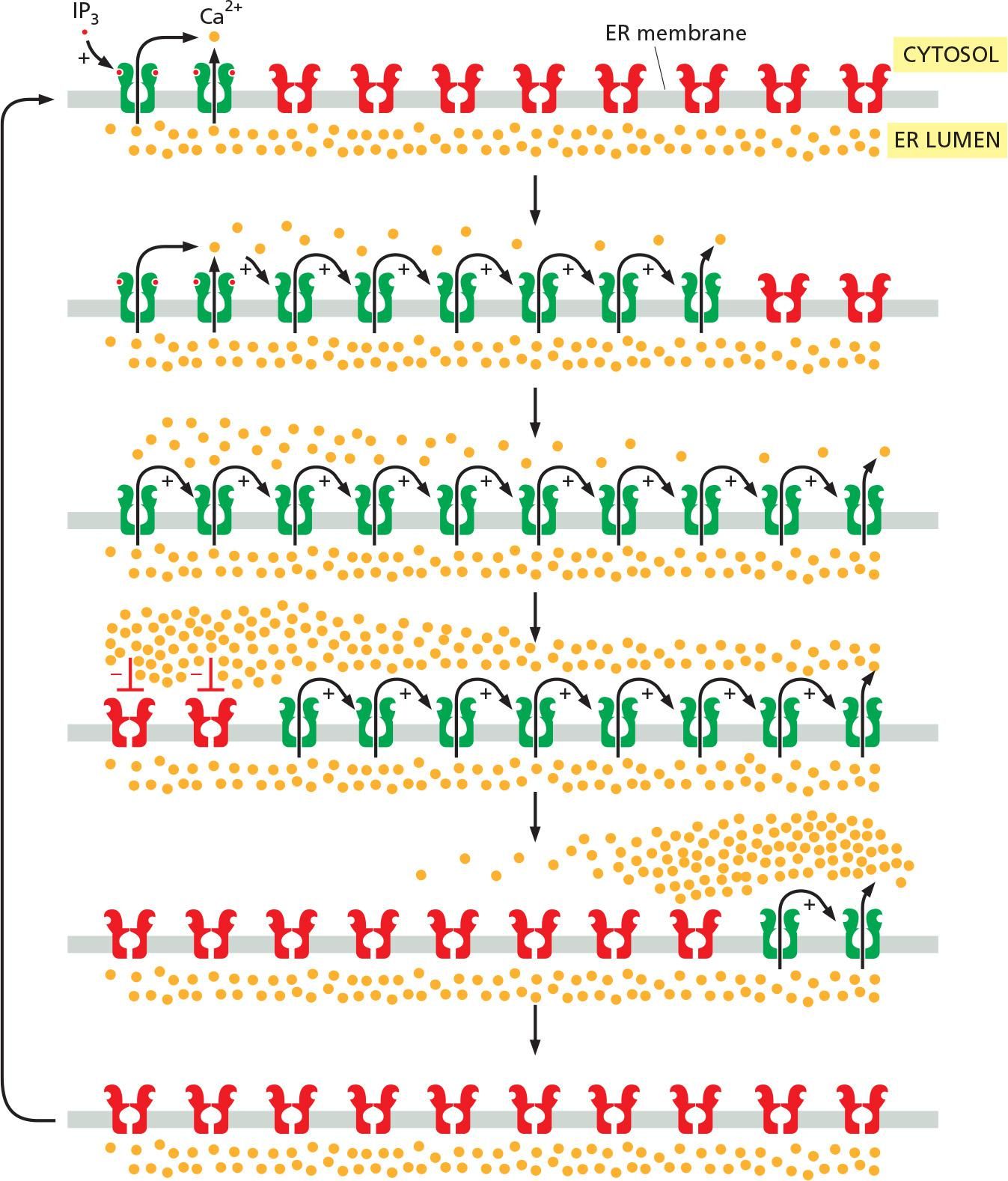
Initial release of Ca2+ from IP3 receptor, which triggers further release from ryanodine receptor (positive feedback)
Another important property of IP
3receptors and ryanodine receptors is that they are inhibited, after some delay, by high Ca2+ concentrations (a form of negative feedback).As in other cases of delayed negative feedback (see Figure 15–18), the result is an oscillation in the Ca2+ concentration. These oscillations persist for as long as receptors are activated at the cell surface, and their frequency reflects the strength of the extracellular stimulus
- The frequency of Ca2+ oscillations can be translated into a frequency-dependent cell response
cells sense the frequency of Ca2+ spikes and change their response accordingly?
- The mechanism presumably depends on Ca2+-sensitive proteins that change their activity as a function of Ca2+-spike frequency.
At high concentrations, Ca2+ inhibits further release (long delay negative feedback), which allow reactivation of Ca2+ release channel, resulting in [Ca2+] oscillation as long as the receptors are activated on the cell surface.
Vasopressin induces Ca2+ oscillation
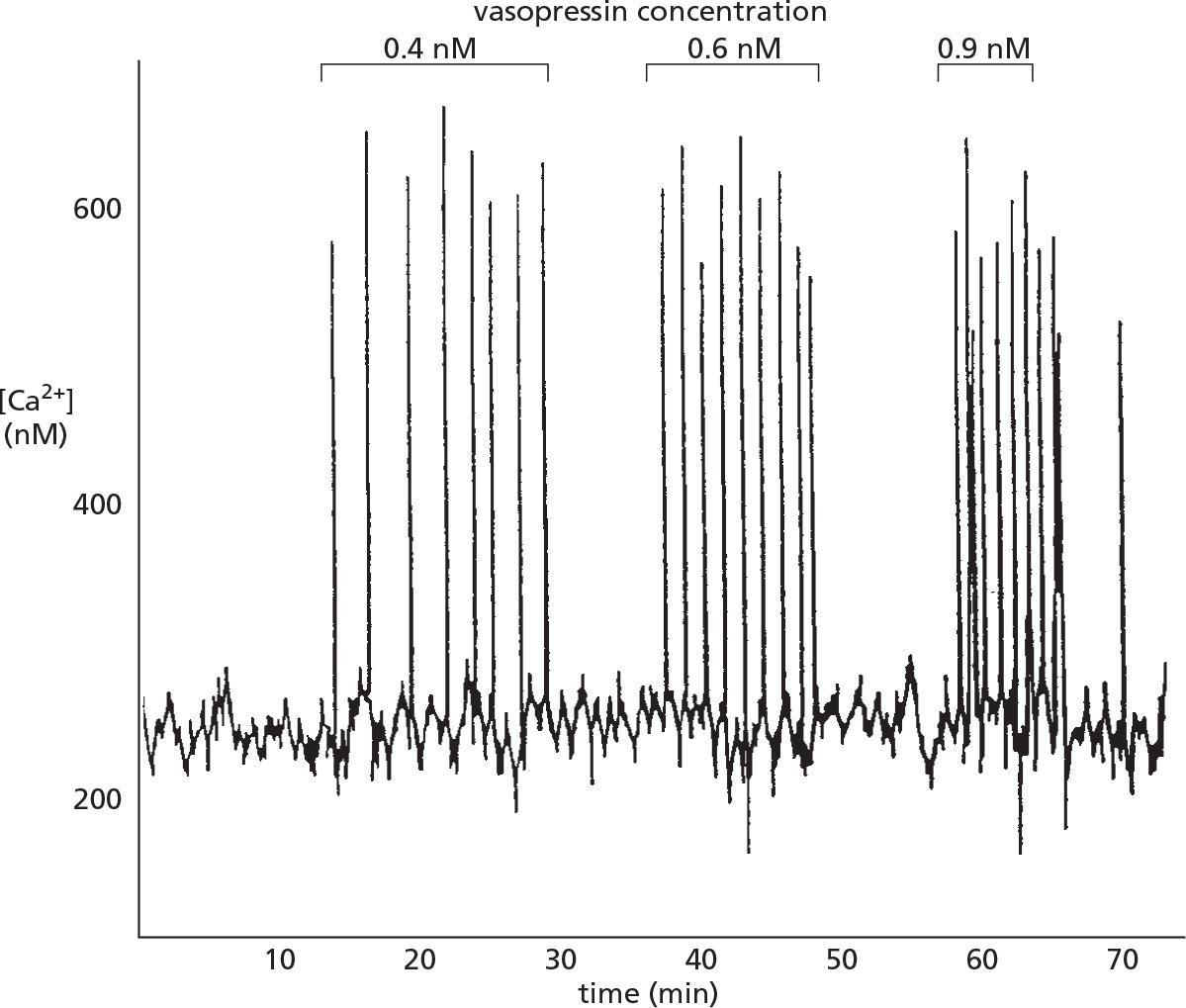
The peptide signal molecule vasopressin activates a GPCR and thereby PLCβ triggers Ca2+ release.
Noted that only the frequency of the Ca2+ spikes increases with increasing concentration of vasopressin but the amplitude of the spikes is not affected.
- The frequency, amplitude, and breadth oscillations can also be modulated by other signaling mechanisms, such as phosphorylation, which influence the Ca2+ sensitivity of Ca2+ channels or affect other components in the signaling system.
Calmodulin
Ca2+/Calmodulin-Dependent Protein Kinases Mediate Many Responses to Ca2+ Signals
Calmodulin functions as a multipurpose intracellular Ca2+ receptor, governing many Ca2+-regulated processes.
- the protein displays a sigmoidal response to increasing concentrations of Ca2+
- Ca2+/calmodulin has no enzymatic activity itself but instead acts by binding to and activating other proteins.
Calmodulin is an abundant Ca2+ responsive protein (1% of total cell protein mass) in all eukaryotes.
- A single polypepetide with 4 Ca2+binding sites.
- It binds to 4 Ca2+ and changes conformation,
(1) Functions
Many effects of Ca2+, however, are more indirect and are mediated by protein phosphorylations catalyzed by a family of protein kinases called Ca2+/calmodulin-dependent kinases (CaM-kinases)
Calmodulin can be permeant regulatory subunit of an enzyme complex
- Through binding to Ca2+, calmodulin can wrap around the target proteins and changes conformation, e.g., Ca2+ pump in the PM
Ca2+/calmodulin-dependent protein kinases (CaM-kinases) are activated by calmodulin and in turn start to phosphorylate other proteins: transcription factors
- Some CaM-kinases are probably responsible for learning and memory.
CaM-kinase II
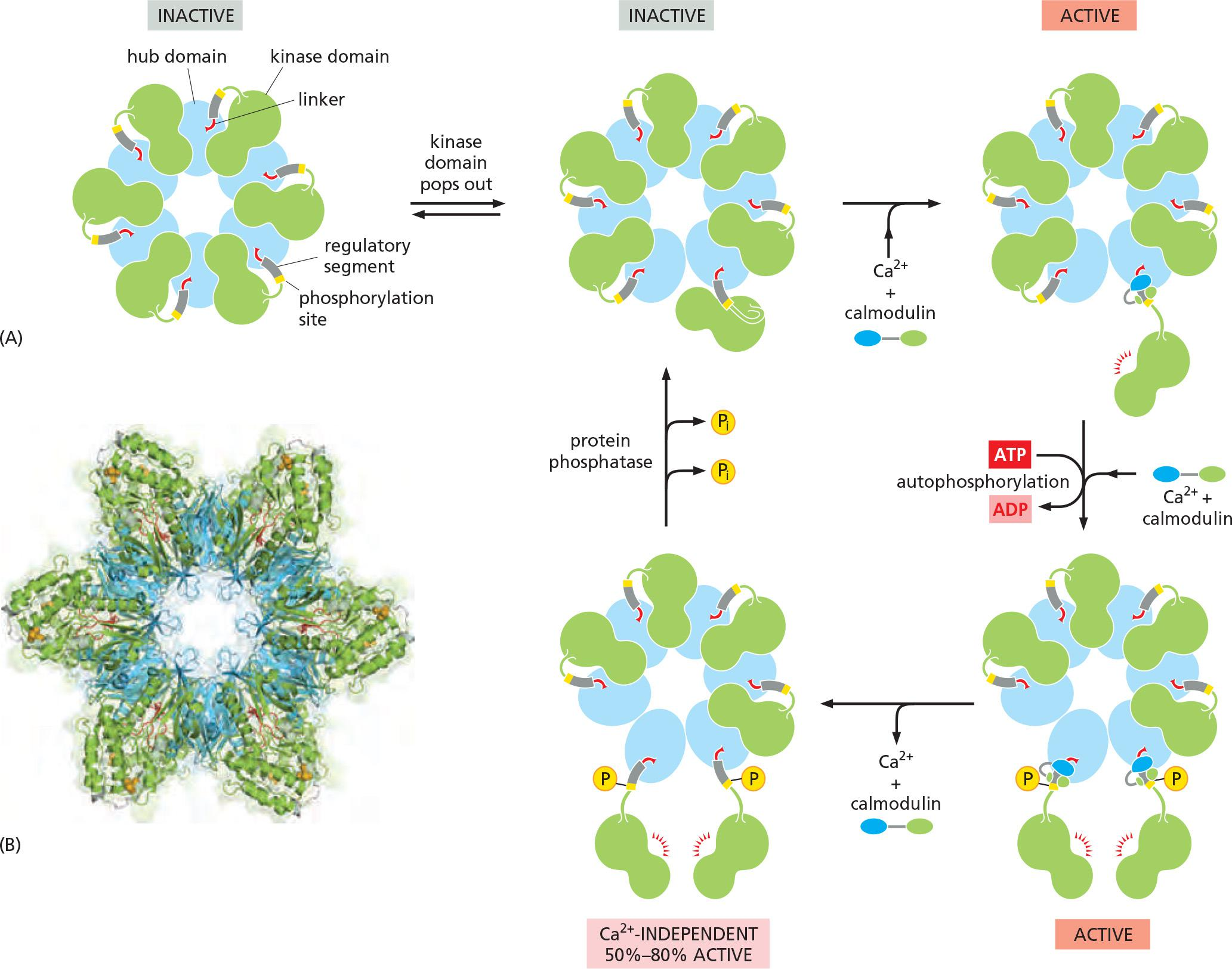
CaM-kinase II, which is found in most animal cells but is especially enriched in the nervous system.
Adjacent kinase subunits can phosphorylate each other (a process called autophosphorylation) when Ca2+/calmodulin activates them (Figure 15–34).
- Once a kinase subunit is autophosphorylated, it remains active even in the absence of Ca2+, thereby prolonging the duration of the kinase activity beyond that of the initial activating Ca2+ signal.
- The enzyme maintains this activity until a protein phosphatase removes the autophosphorylation and shuts the kinase off.
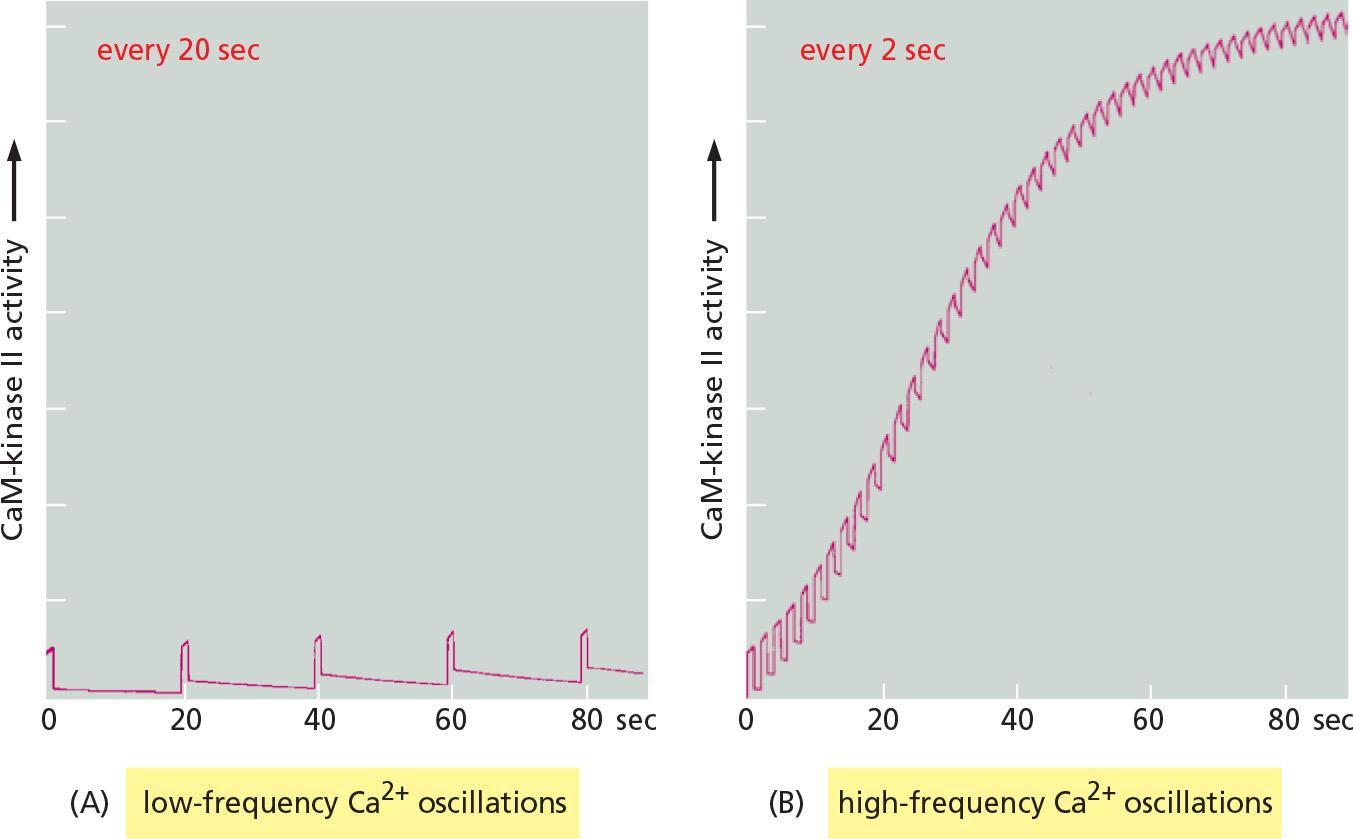
Another remarkable property of CaM-kinase II is that the enzyme can use its intrinsic memory mechanism to decode the frequency of Ca2+ oscillations.
CaM-kinase II is abundant in nervous system (up to 2% protein mass in some region of brain)
- Ca2+/calmodulin –dependent activation (autophosphorylation)
- Ca2+/calmodulin-independent activation even in the absence of Ca2+ until phosphatase-mediated dephosphorylation
CaM-kinase II is a frequency decoder of Ca2+ oscillations
Changes in intracellular Ca2+ levels in a postsynaptic cell as a result of neural activity can lead to long-term changes in the subsequent effectiveness of that synapse (Lecture 8)
Progressive increase in enzyme activity continues until the enzyme is autophosphorylated on all subunits and is therefore maximally activated.
The binding of Ca2+/calmodulin to the enzyme is enhanced by the CaM-kinase II autophosphorylation (an additional form of positive feedback),
Together, a more switch-like response to repeated Ca2+ spikes is generated.
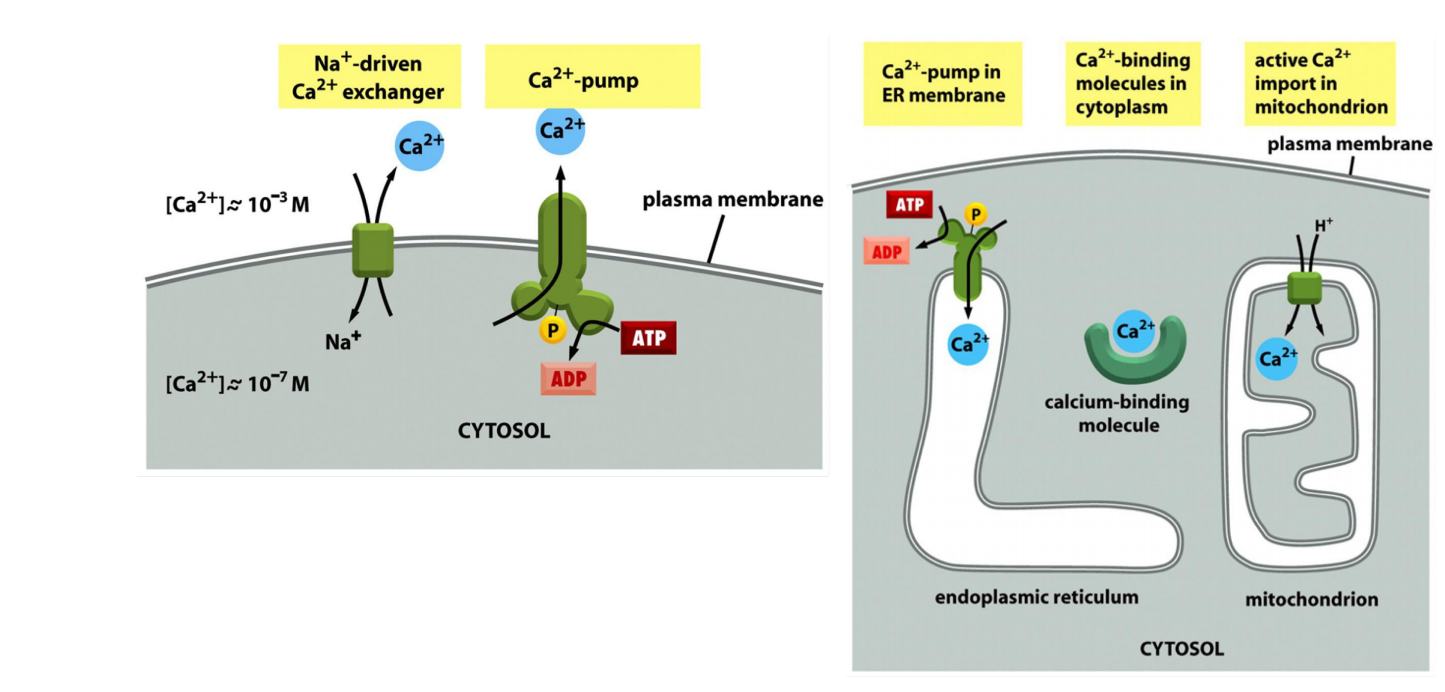
5 ways to keep cytosolic Ca2+ concentrations low:
3. GPCRs for smell and vision
Smell and Vision Depend on GPCRs That Regulate Ion Channels
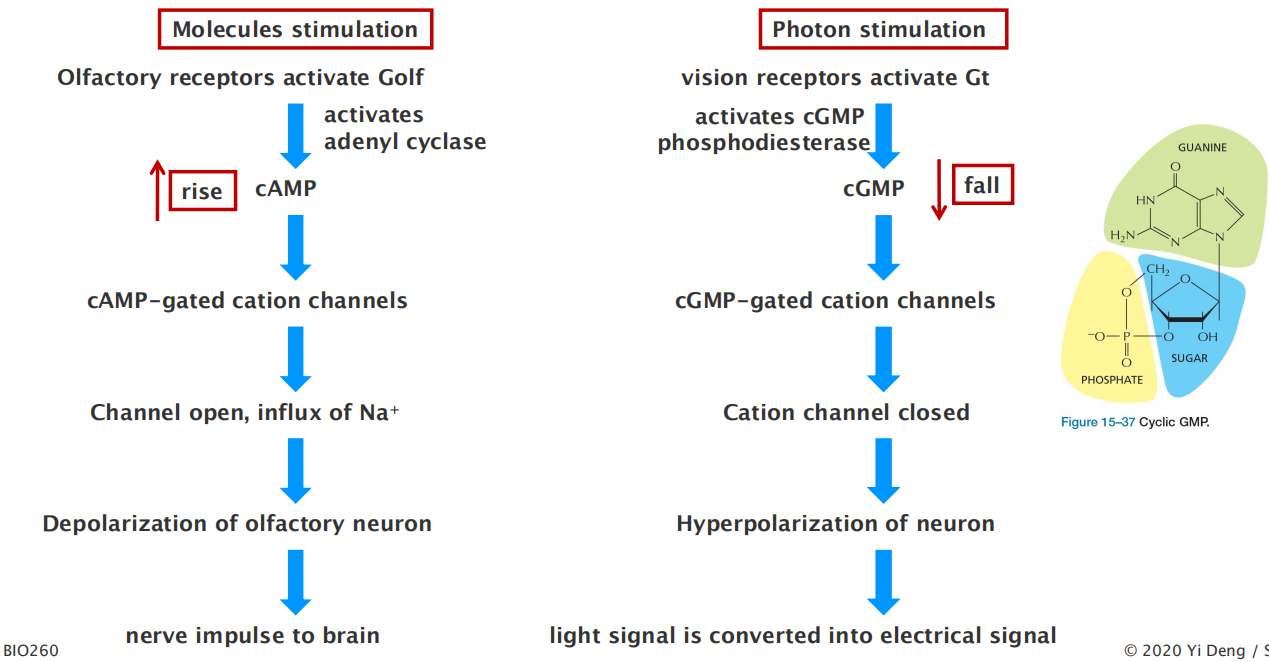
Humans can distinguish more than 10,000 distinct smells, which they detect using specialized olfactory receptor neurons in the lining of the nose. These cells use specific GPCRs called olfactory receptors to recognize odors; the receptors are displayed on the surface of the modified cilia that extend from each cell
- The receptors act through cAMP. When stimulated by odorant (有气味的东西) binding they activate an olfactory-specific G protein (known as G
olf), which in turn activates adenylyl cyclase.The resulting increase in cAMP opens cyclic-AMP-gated cation channels, thereby allowing an influx of Na+, which depolarizes the olfactory receptor neuron and initiates a nerve impulse that travels along its axon to the brain.
- Each olfactory receptor neuron produces only one of these receptors;
Vertebrate vision employs a similarly elaborate, highly sensitive, signal-detection process. Cyclic-nucleotide-gated ion channels are also involved, but the crucial cyclic nucleotide is cyclic GMP (cGMP) (Figure 15–37) rather than cAMP
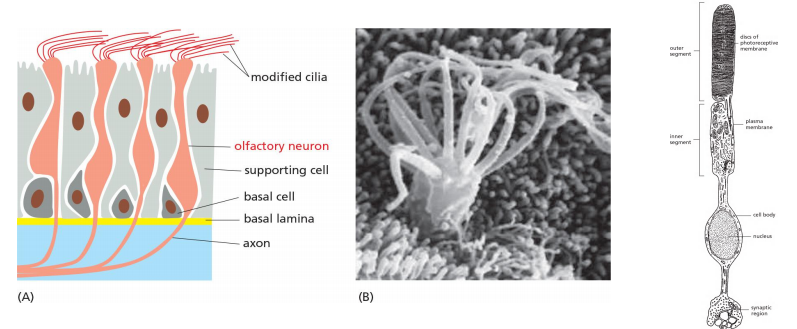
- Over 10,000 smells can be differentiated by ~ 350 distinct receptors for human.
- Rod photoreceptor cell senses black and white; while cone photoreceptor cells sense color
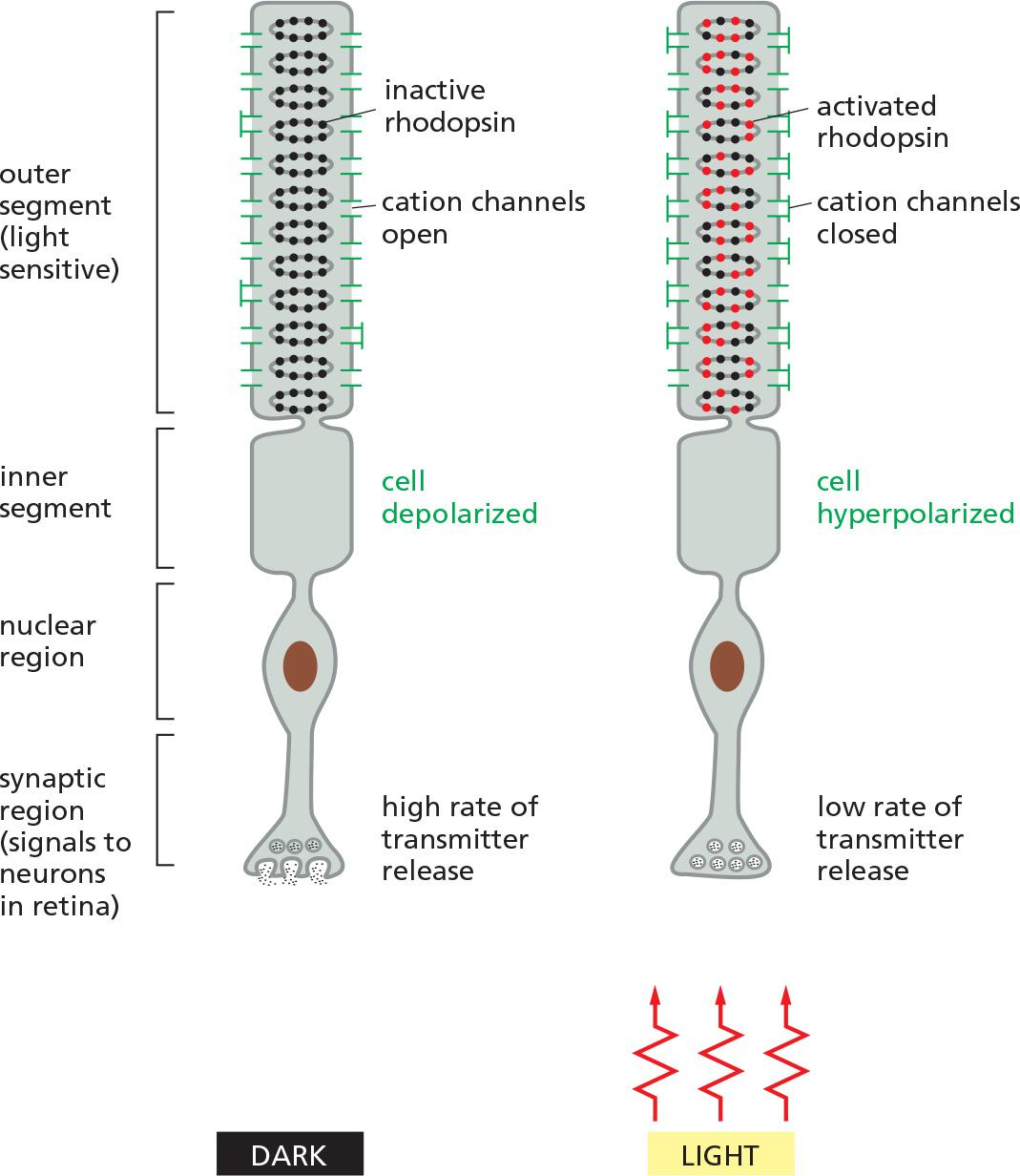
Response of a rod photoreceptor cell to light
The phototransduction apparatus is in the outer segment of the rod, which contains a stack of discs, each formed by a closed sac of membrane that is densely packed with photosensitive rhodopsin molecules.
- The plasma membrane surrounding the outer segment contains cyclic-GMP-gated cation channels.
Rhodopsin is a member of the GPCR family, but the activating extracellular signal is not a molecule but a photon of light.
- The isomerization alters the shape of the retinal, forcing a conformational change in the protein (opsin). The activated rhodopsin molecule then alters the conformation of the G protein transducin (G
t), causing the transducin α subunit to activate cyclic GMP phosphodiesterase.Rods use several negative feedback loops to allow the cells to revert quickly to a resting
- A rhodopsin-specific protein kinase called rhodopsin kinase (RK) phosphorylates the cytosolic tail of activated rhodopsin on multiple serines, partially inhibiting the ability of the rhodopsin to activate transducin.
- An inhibitory protein called arrestin (discussed later) then binds to the phosphorylated rhodopsin, further inhibiting rhodopsin’s activity.
Negative feedback mechanisms do more than just return the rod to its resting state after a transient light flash; they also help the rod to adapt, stepping down the response when the rod is exposed to light continuously.
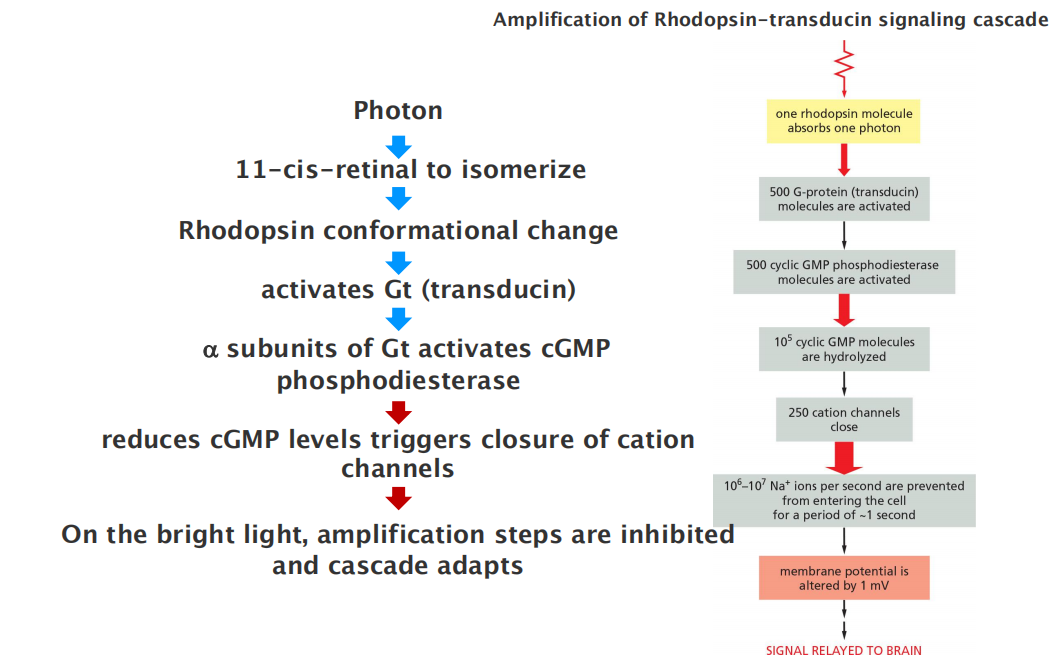
4. Some G proteins directly regulate ion channels
The α subunit of one type of G protein (called G
12), for example, activates a guanine nucleotide exchange factor (GEF) that activates a monomeric GTPase of the Rho family (discussed later and in Chapter 16), which regulates the actin cytoskeleton.In some other cases, G proteins directly activate or inactivate ion channels in the plasma membrane of the target cell, thereby altering the ion permeability
Other G proteins regulate the activity of ion channels less directly, either by stimulating channel phosphorylation (by PKA, PKC, or CaM-kinase, for example) or by causing the production or destruction of cyclic nucleotides that directly activate or inactivate ion channels
Cyclic nucleotide-gated ion channels downstream of GPCRs operate in smell and vision.
5. GPCR signaling via NO gas
Nitric Oxide Is a Gaseous Signaling Mediator That Passes Between Cells
In mammals, one of NO’s many functions is to relax smooth muscle in the walls of blood vessels.
The activated receptor triggers IP
3synthesis and Ca2+ release (see Figure 15–29), leading to stimulation of an enzyme that synthesizes NO
- dissolved NO passes readily across membranes, it diffuses out of the cell where it is produced and into neighboring smooth muscle cells, where it causes muscle relaxation and thereby vessel dilation
NO is made by the deamination of the amino acid arginine, catalyzed by enzymes called NO synthases (NOS)
- The NOS in endothelial cells is called eNOS, while that in nerve and muscle cells is called nNOS.
- Both eNOS and nNOS are stimulated by Ca2+.
- Macrophages, by contrast, make yet another NOS, called inducible NOS (iNOS), that is constitutively active but synthesized only when the cells are activated, usually in response to an infection.
GPCR signaling via NO gas in smooth muscle relaxation in blood vessels
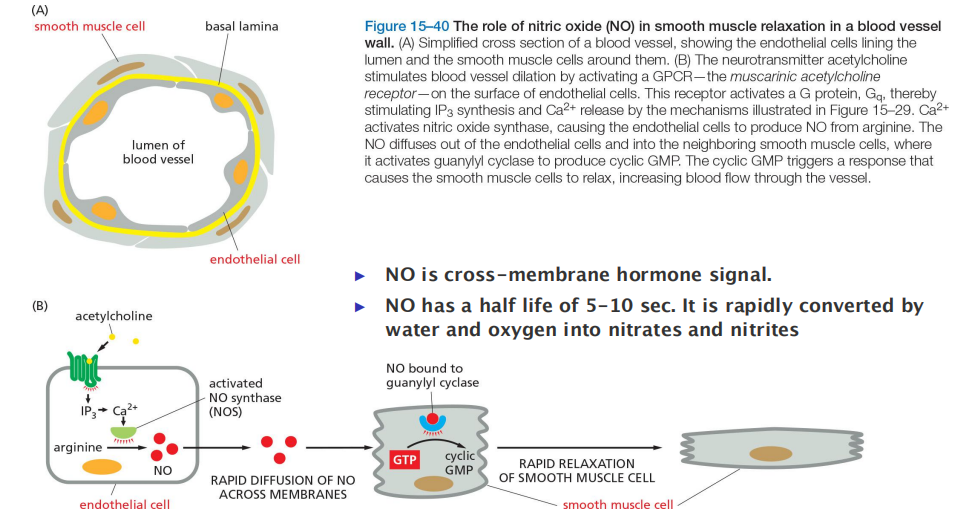
The neurotransmitter acetylcholine activates a GPCR which in turn activates NO synthase in endothelial cell (eNOS) to produce NO.
NO diffuses into smooth muscle cell and activates guanylyl cyclase to produce cyclic GMP (cGMP), which triggers muscle relaxation and thus the dilation of the blood vessel
Smooth and heart muscle cells will relax, blood vessels will dilate
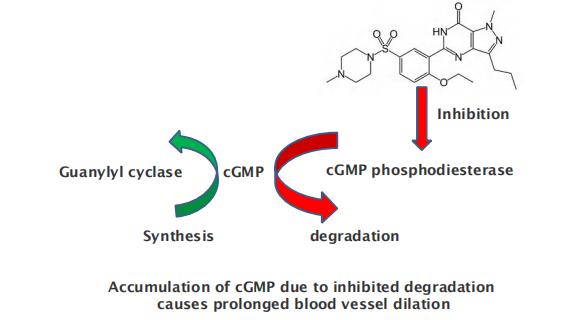
Mechanism of nitroglycerin in treating angina pectoris
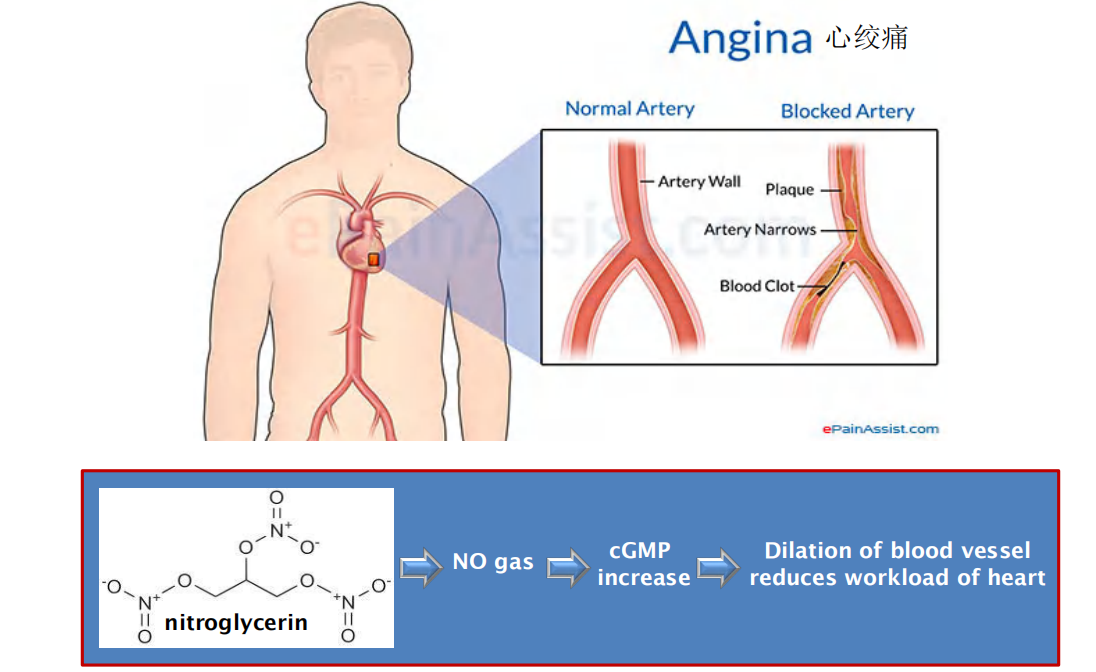
Mechanism of Sildenafil (西地那非) (commercial name Viagra (万艾可))
Viagra drug activates cyclic GMP synthesis and dilation of vessels responsible for erection
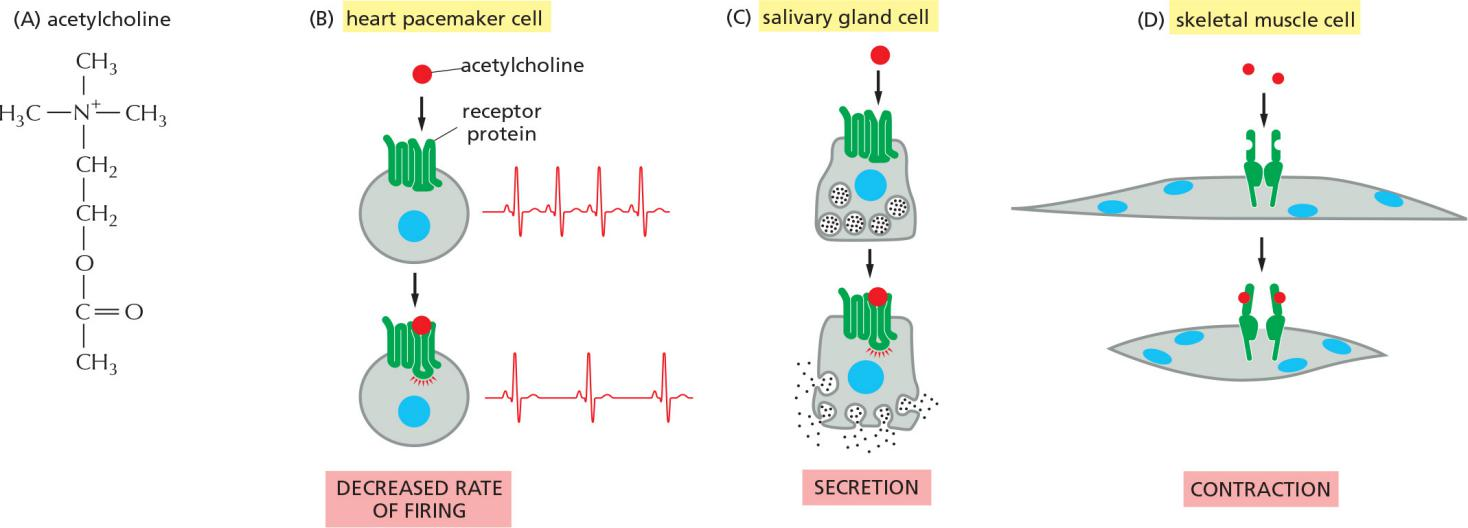
5. GPCRs as ion channels regulators in heart muscle cells
Heart: ion-channel coupled muscarinic acetylcholine receptors
Smooth muscle and nerve: ion-channel-coupled receptors nicotinic acetylcholine receptors
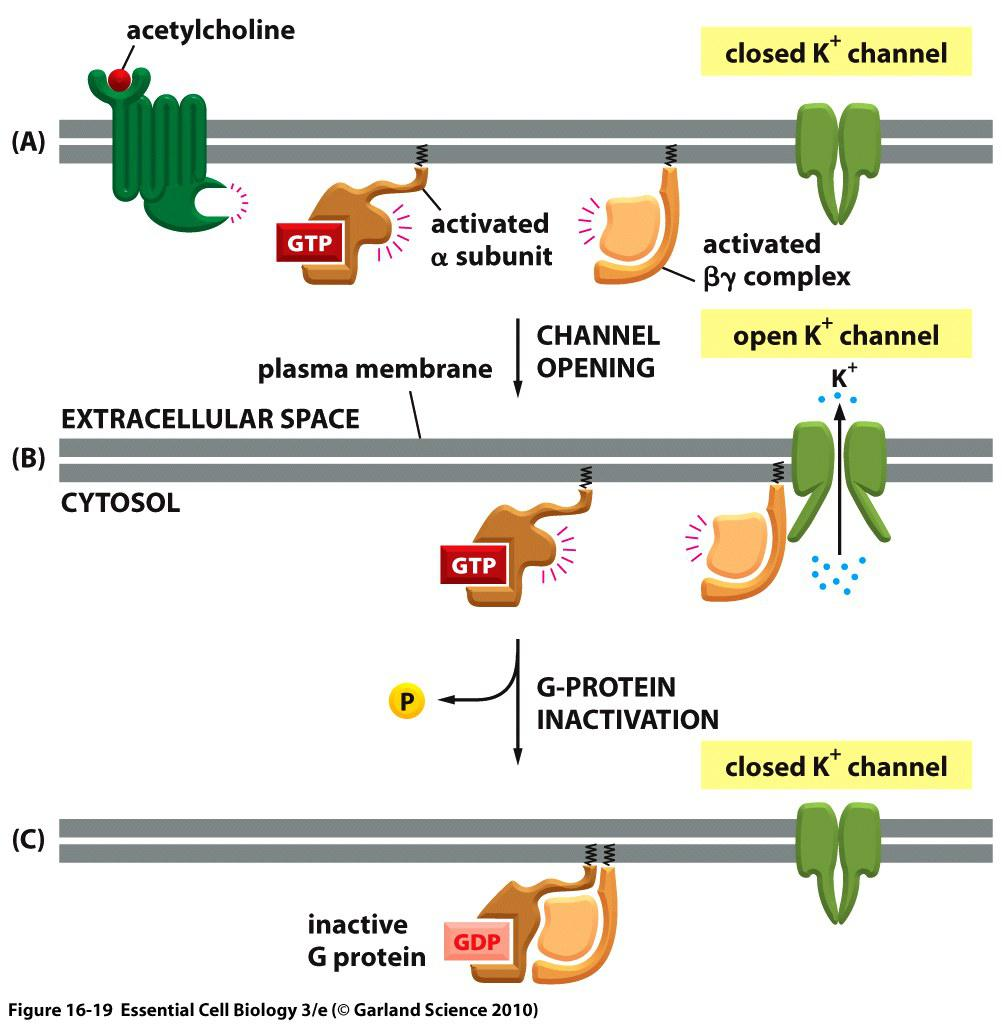
(A) Binding of acetylcholine to its GPCR on the heart cells activates the G-protein, Gi .
(B) The activated $\beta \gamma$ complex directly opens a K+ channel in the plasma membrane, increasing its permeability to K+ and thereby making the membrane harder to activate and slowing the heart rate.
(C) Inactivation of the α-subunit by GTP hydrolysis inactivates the G-protein, the channel closes
五、GPCR desensitization
GPCR Desensitization Depends on Receptor Phosphorylation
when target cells are exposed to a high concentration of a stimulating ligand for a prolonged period, they can become desensitized, or adapted
For GPCRs, there are three general modes of adaptation (see Figure 15–20):
- In receptor sequestration (受体隔离), they are temporarily moved to the interior of the cell (internalized) so that they no longer have access to their ligand.
- In receptor down-regulation (受体下调), they are destroyed in lysosomes after internalization.
- In receptor inactivation (受体去活), they become altered so that they can no longer interact with G proteins
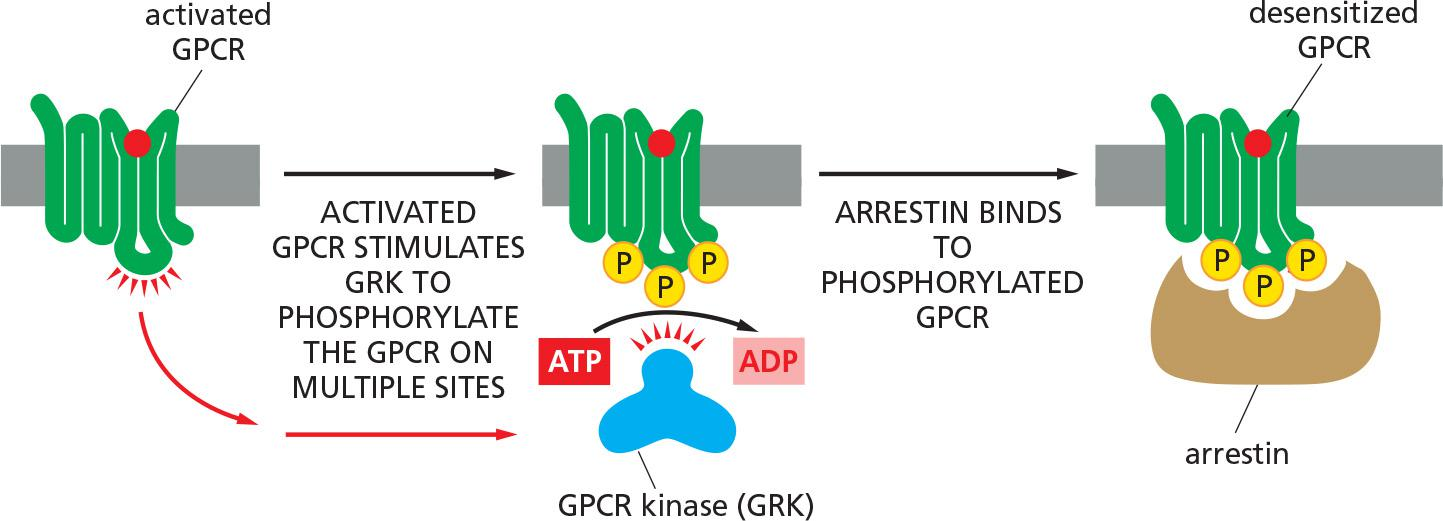
In each case, the desensitization of the GPCRs depends on their phosphorylation by PKA, PKC, or a member of the family of GPCR kinases (GRKs)
- The GRKs phosphorylate multiple serines and threonines on a GPCR, but they do so only after ligand binding has activated the receptor, because it is the activated receptor that allosterically activates the GRK.
- As with rhodopsin, once a receptor has been phosphorylated by a GRK, it binds with high affinity to a member of the arrestin family of proteins. The bound arrestin can contribute to the desensitization process in at least two ways.
- First, it prevents the activated receptor from interacting with G proteins (receptor inactivation).
- Second, it serves as an adaptor protein to help couple the receptor to the clathrin-dependent endocytosis machinery (discussed in Chapter 13), inducing receptor-mediated endocytosis.
Four strategies to terminate/desensitize the GPCR signaling:
- Down-regulation of Receptor
- Receptor sequestration (endocytosis)
- Receptor elimination
- GPCRs are phosphorylated by GPCR kinases, triggering arrestin binding, uncoupling receptors from G proteins and promotes their endocytosis.
- Receptor deactivation
- Deactivation of G protein:
- G-protein $\alpha$-subunit is stimulated by its target protein or RGS to hydrolyse GTP into GDP.
- Elimination of second messenger
- IP
3is dephosphorylated by lipid phosphatase or phosphorylated by lipase kinase. - cAMP/cGMP is hydrolyzed by activated phosphodiesterases.
- Ca2+ is pumped out of cytosol.
- Elimination of mediator
- Phosphorylated protein is dephosphorylated by phosphatases.
GPCR kinases (GRKs) and arrestin-mediated GPCR desensitization
Summary: the GPRC-mediated signaling