L24 Cells to tissues cell-cell adhesion
Two main ways in which animal cells are bound together
Connection is important!
- Allow cells to form distinct communities, i.e. tissues
- Bidirectional communication between interior and exterior of cells.
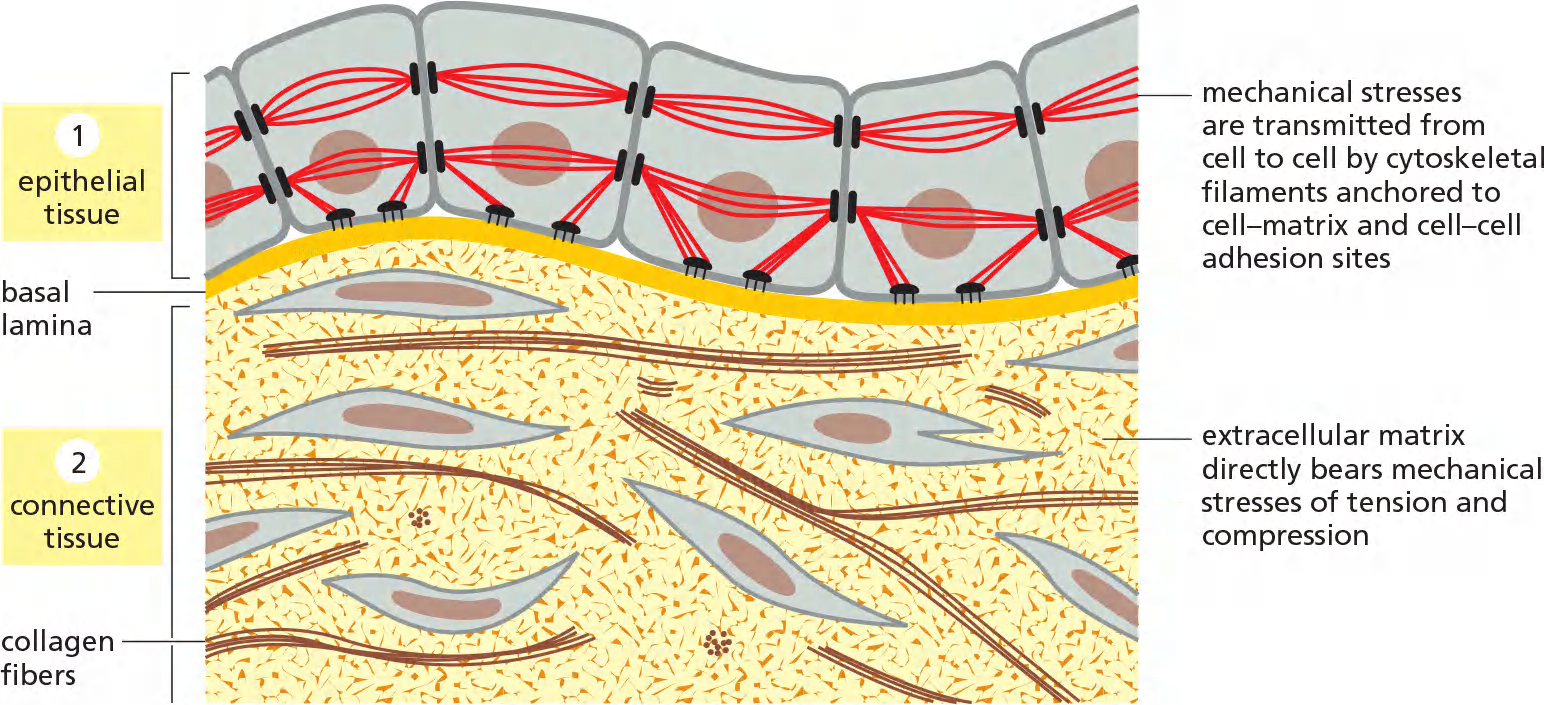
Epithelial tissue (lining sheets):
- cell-cell junctions, cytoskeleton of the cell transmits mechanical stresses
- from cell to cell via cell-cell adhesion sites and to the basal matrix.
Connective tissue (e.g. bones, tendon etc.): Cell-ECM adhesions
- It is the matrix-rather than the cells- that bears most of the mechanical stress to which the tissue is subjected.
Connective tissues, such as bone or tendon, are formed from an extracellular matrix produced by cells that are distributed sparsely in the matrix.
一、Adhesion between cells
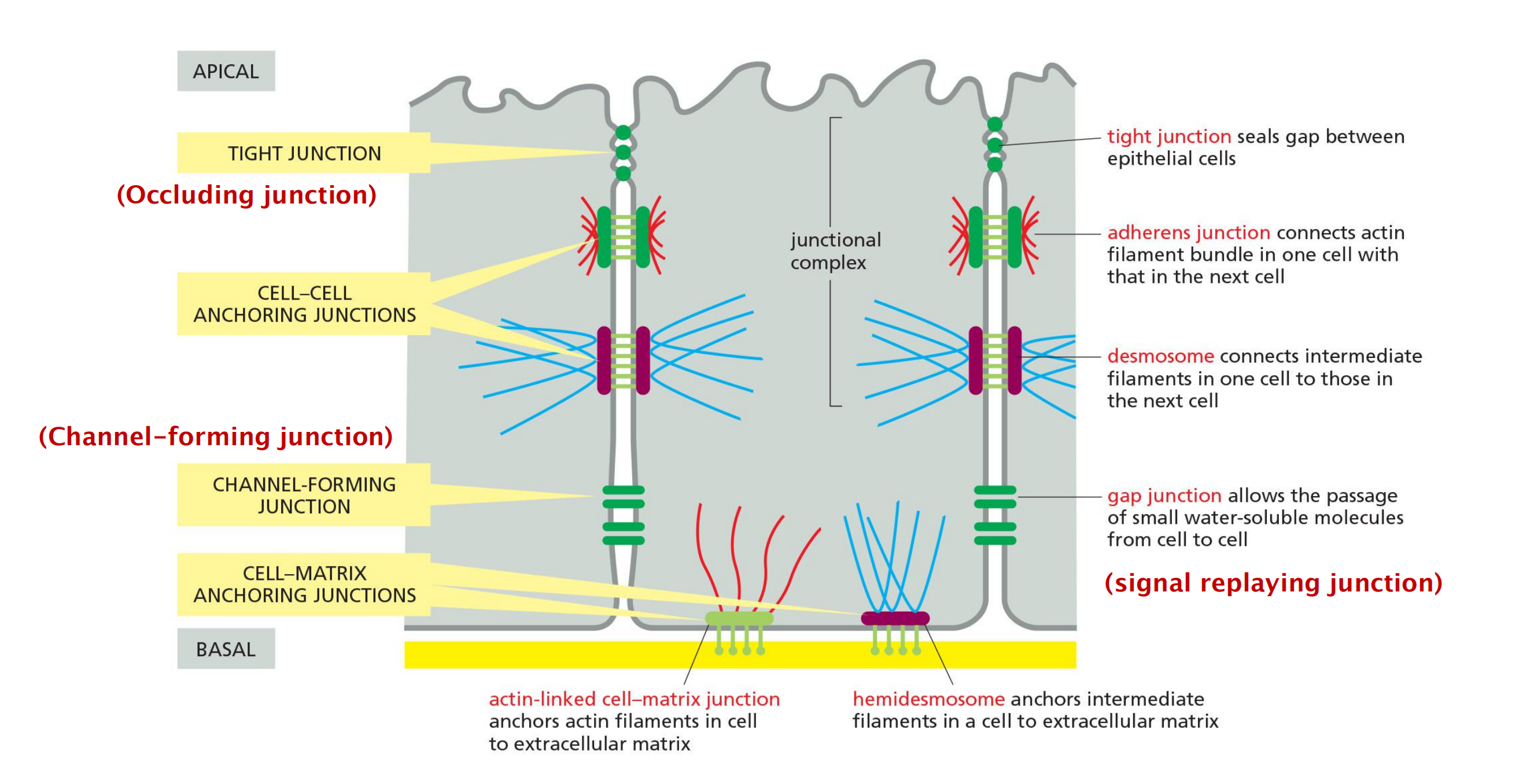
In epithelial tissues, such as the lining of the gut or the epidermal covering of the skin, cells are tightly bound together into sheets called epithelia.
Within the epithelium, cells are attached to each other directly by cell–cell junctions, where cytoskeletal filaments are anchored, transmitting stresses across the interiors of the cells, from adhesion site to adhesion site.
The cytoskeleton of epithelial cells is also linked to the basal lamina through cell–matrix junctions
- These cell–matrix junctions link the cytoskeleton to the matrix, allowing the cells to move through the matrix and monitor changes in its mechanical properties.
- The extracellular matrix is less pronounced, consisting mainly of a thin mat called the basal lamina (or basement membrane) underlying the sheet
Two other types of cell–cell junction are shown in the Figure
- Tight junctions hold the cells closely together near the apex, sealing the gap between the cells and thereby preventing molecules from leaking across the epithelium.
- Near the basal end of the cells are channel-forming junctions, called gap junctions, that create passageways linking the cytoplasms of adjacent cells.
Anchoring junctions
Two types of anchoring junctions(锚接点) link the cytoskeletons of adjacent cells:
- adherens junctions(粘附连接) are anchorage sites for actin filaments;
- desmosomes(桥粒) are anchorage sites for intermediate filaments.
Two additional types of anchoring junctions link the cytoskeleton of the epithelial cells to the basal lamina:
- actin-linked cell–matrix junctions anchor actin filaments to the matrix,
- while hemidesmosomes(半桥粒) anchor intermediate filaments to it.
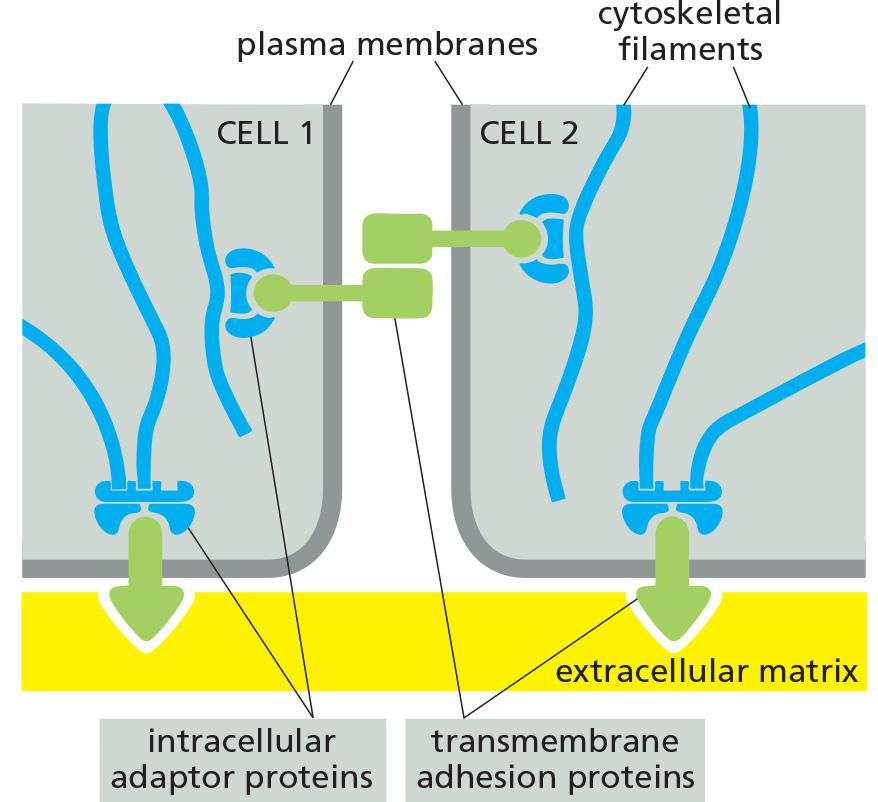
Each of the four major anchoring junction types depends on transmembrane adhesion proteins that span the plasma membrane, with one end linking to the cytoskeleton inside the cell and the other end linking to other structures outside it
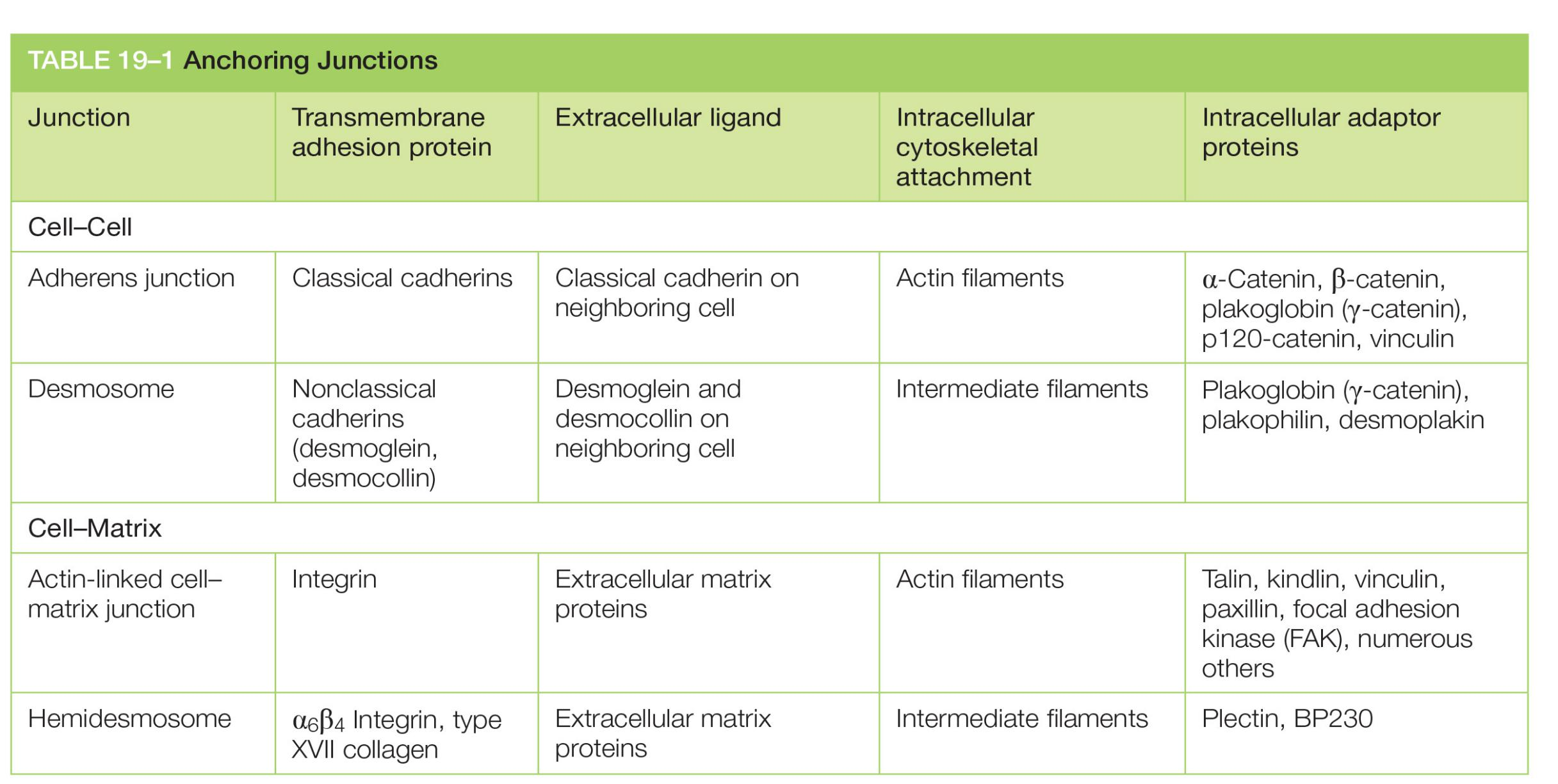
| Junction | Transmembrane adhesion protein | Extracellular ligand | Intracellular cytoskeletal attachment | Intracellular adaptor proteins |
|---|---|---|---|---|
| Adherens junction | Classical cadherins | Classical cadherin on neighboring cell | Actin filaments | α-Catenin, β-catenin, plakoglobin (γ-catenin), p120-catenin, vinculin |
| Desmosome | Nonclassical cadherins (desmoglein, desmocollin) | Desmoglein and desmocollin on neighboring cell | Intermediate filaments | Plakoglobin (γ-catenin), plakophilin, desmoplakin |
| Actin-linked cell–matrix junction | Integrin | Extracellular matrix proteins | Actin filaments | Talin, kindlin, vinculin, paxillin, focal adhesion kinase (FAK), numerous others |
| Hemidesmosome | α6β4 Integrin, type XVII collagen | Extracellular matrix proteins | Intermediate filaments | Plectin, BP230 |
1. Cadherins control selective assortment/recognition of cells
Proteins of the cadherin(钙黏着蛋白) superfamily chiefly mediate attachment of cell to cell
Proteins of the integrin(整联蛋白) superfamily chiefly mediate attachment of cells to matrix.
- There are some exceptions to these rules. Some integrins, for example, mediate cell–cell rather than cell–matrix attachment.
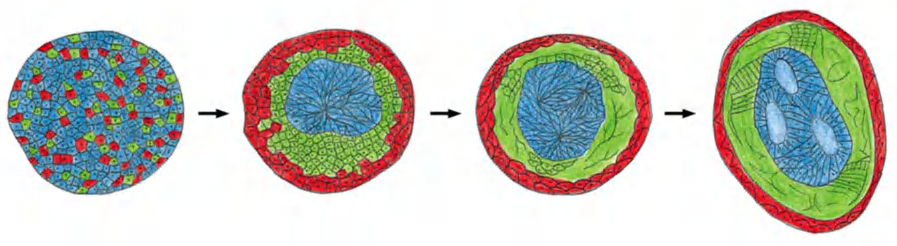
Classical experiment (1950s):
- disaggregation and reaggregation of an early amphibian embryo in vitro.
- The embryo consisting of mesoderm cells, neural plate cells and epidermal cells has been disaggregated and then reaggregated in a random mixture
disaggregated embryo (randomized arrangement of different cell types)
- → “self”-arrangement reminiscent of a normal embryo with a “neural tube” internally, epidermis externally, and mesoderm in between.
2. Cadherins are the adhesion molecule of adhering junctions and desmosomes
- The name is derived from Ca2+ and adherin(粘附素), meaning Ca2+-dependent adhering
- Dissociation of cells from tissue needs EDTA/trypsin(EDTA /胰蛋白酶) (EDTA chelates (螯合) the Ca2+ to deactivate the cadherin-cadherin interaction)
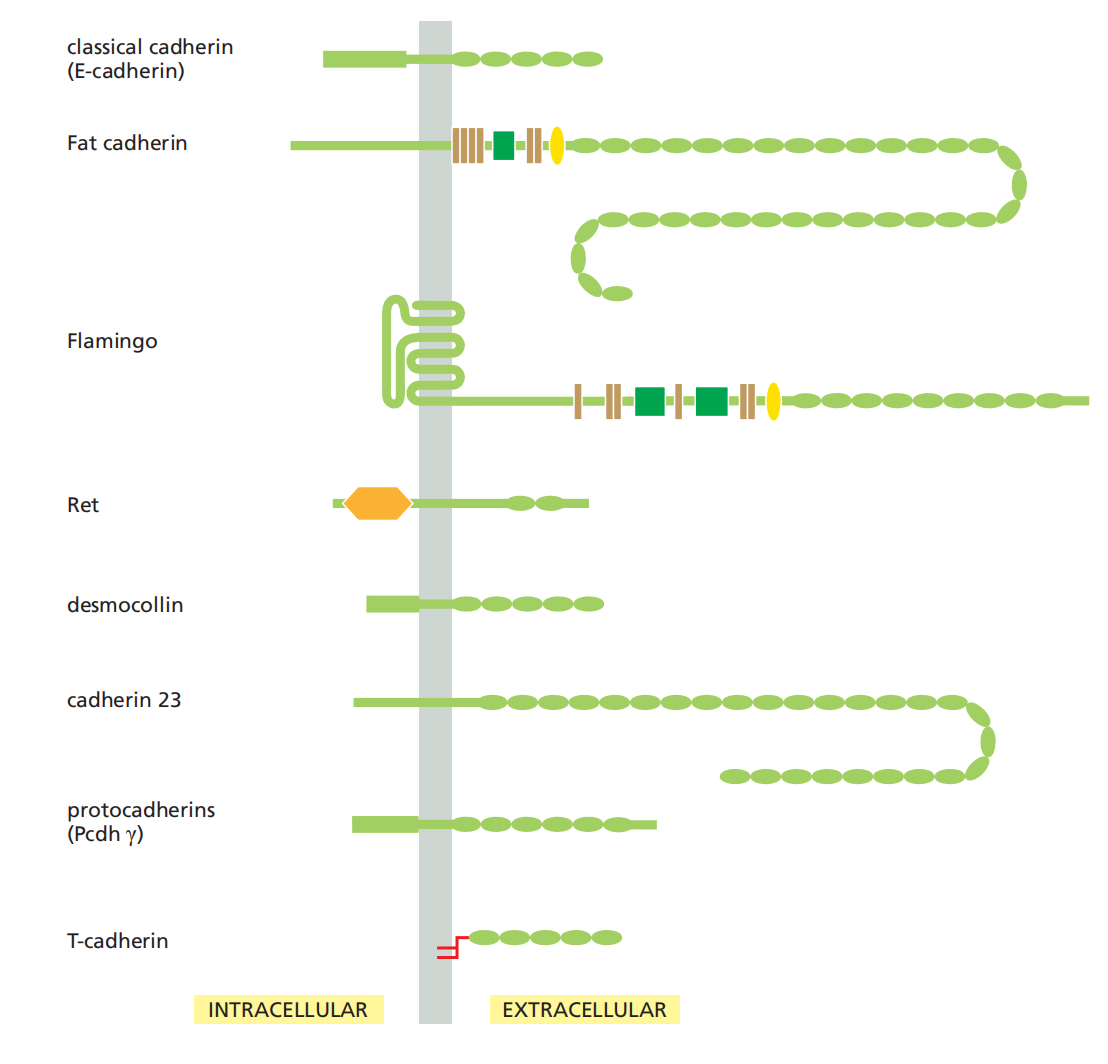
- Classical cadherins and non-classical cadherins. over 180 family members in humans.
- Plants, fungi, bacteria and archaea have no cadherins
Cadherins comprise a large family of proteins
Cadherins Form a Diverse Family of Adhesion Molecules
Cadherins are present in all multicellular animals whose genomes have been analyzed.
- They are also present in the choanoflagellates(鞭毛虫), which can exist either as free-living unicellular organisms or as multicellular colonies and are thought to be representatives of the group of protists from which all animals evolved
Other eukaryotes, including fungi and plants, lack cadherins, and they are also absent from bacteria and archaea.
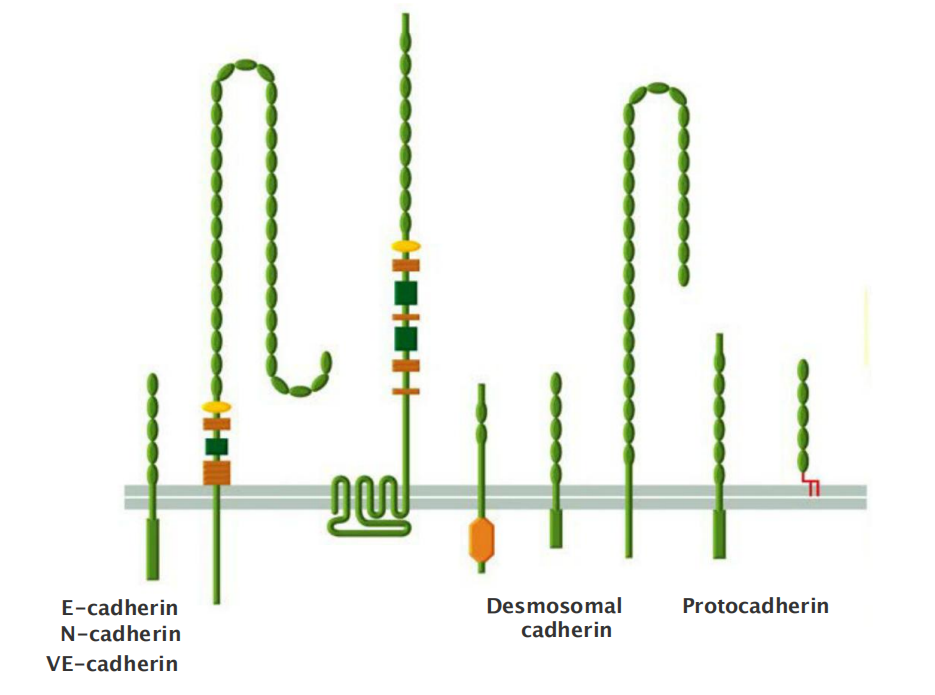
The cadherins take their name from their dependence on Ca2+ ions: removing Ca2+ from the extracellular medium causes adhesions mediated by cadherins to come apart(破碎;崩溃). The first three cadherins to be discovered were named according to the main tissues in which they were found:
- E-cadherin is present on many types of epithelial cells(上皮细胞)
- N-cadherin on nerve, muscle, and lens cells(神经,肌肉和晶状体细胞)
- P-cadherin on cells in the placenta and epidermis(胎盘和表皮)
All are also found in other tissues. These and other classical cadherins are closely related in sequence throughout their extracellular and intracellular domains.
There are also a large number of nonclassical cadherins that are more distantly related in sequence, with more than 50 expressed in the brain alone
- The nonclassical cadherins include proteins with known adhesive function, such as the diverse protocadherins(原钙黏蛋白) found in the brain, and the desmocollins (桥粒胶蛋白) and desmogleins (桥粒糖蛋白) that form desmosomes
Other family members are involved primarily in signaling. Together, the classical and nonclassical cadherin proteins constitute the cadherin superfamily (Figure 19–4), with more than 180 members in humans.
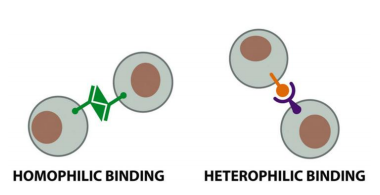
Homophilic adhesion
Cadherins Mediate Homophilic Adhesion
- the binding between cadherins is generally homophilics
- Cadherin molecules of a specific subtype on one cell bind to cadherin molecules of the same or closely related subtype on adjacent cells.
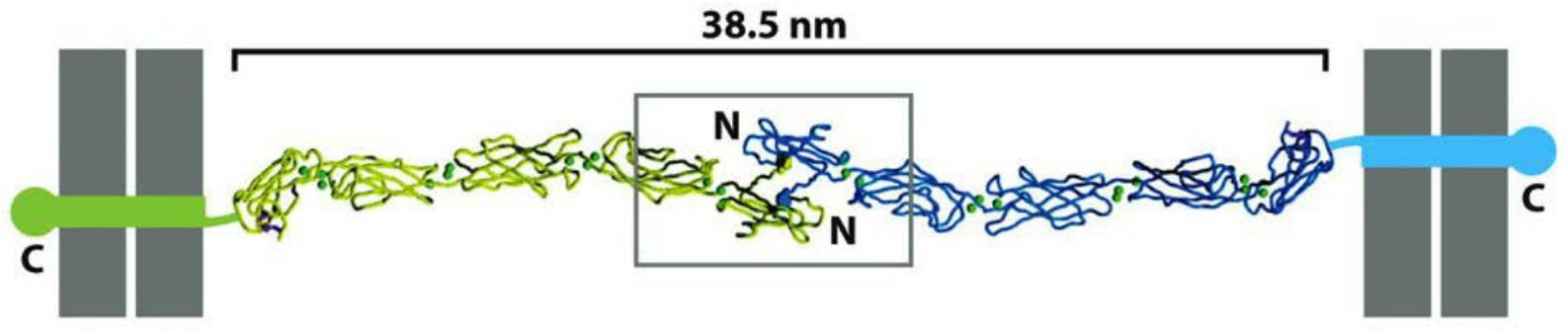
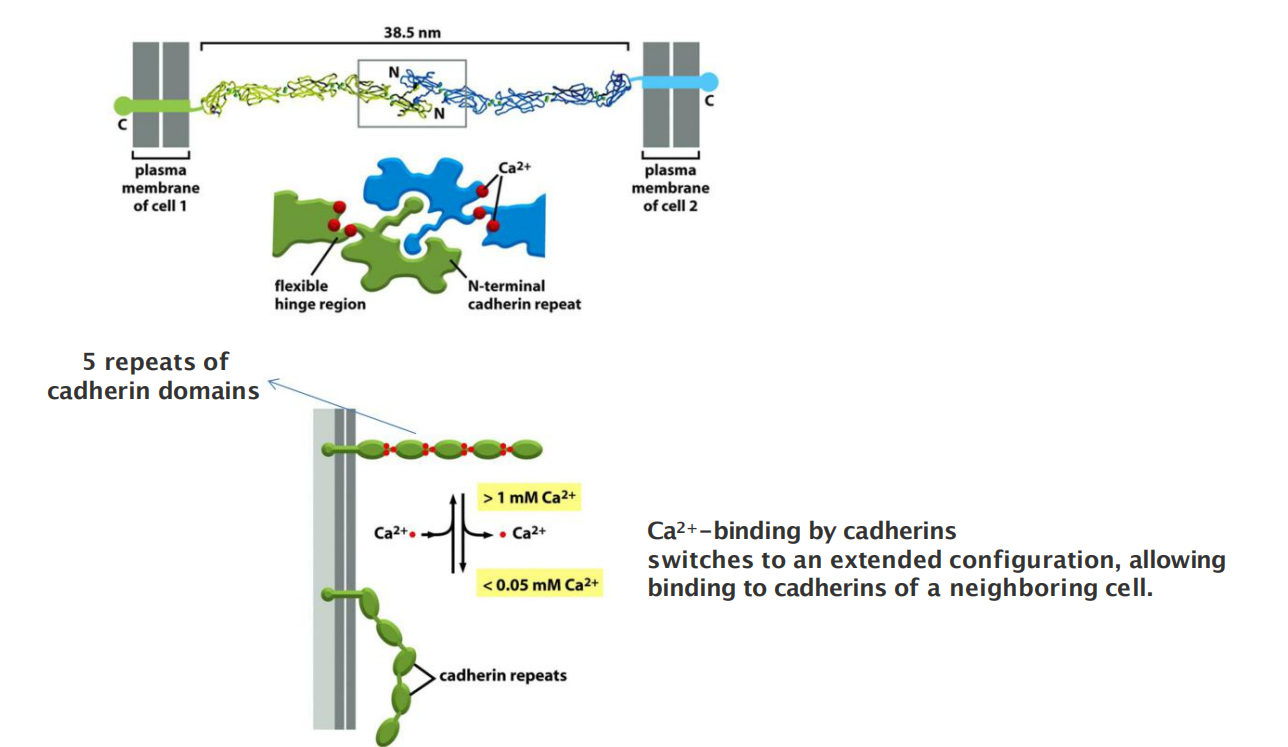
All the members of the superfamily, by definition, have an extracellular portion consisting of several copies of the extracellular cadherin (EC) domain
- Homophilic binding occurs at the N-terminal tips of the cadherin molecules
- Each cadherin domain forms a more-or-less rigid unit, joined to the next cadherin domain by a hinge. Ca2+ ions bind to sites near each hinge and prevent it from flexing, so that the whole string of cadherin domains behaves as a rigid and slightly curved rod.
Cadherins (and most other cell–cell adhesion proteins) typically bind to their partners with relatively low affinity.
Regulatory mechanisms can easily disassemble the junction by separating the molecules sequentially
Homophilic adhesion:
- the same type or similar subtype of molecule binds to each other
- ensures linkage of the same type of cells and make sure that different types of cells remain separated
In general, only the same type of cadherin binds to each other
- ensures linkage of the same type of filaments across cell borders
- ensures linkage of the same type of cells and make sure that different types of cells remain separated
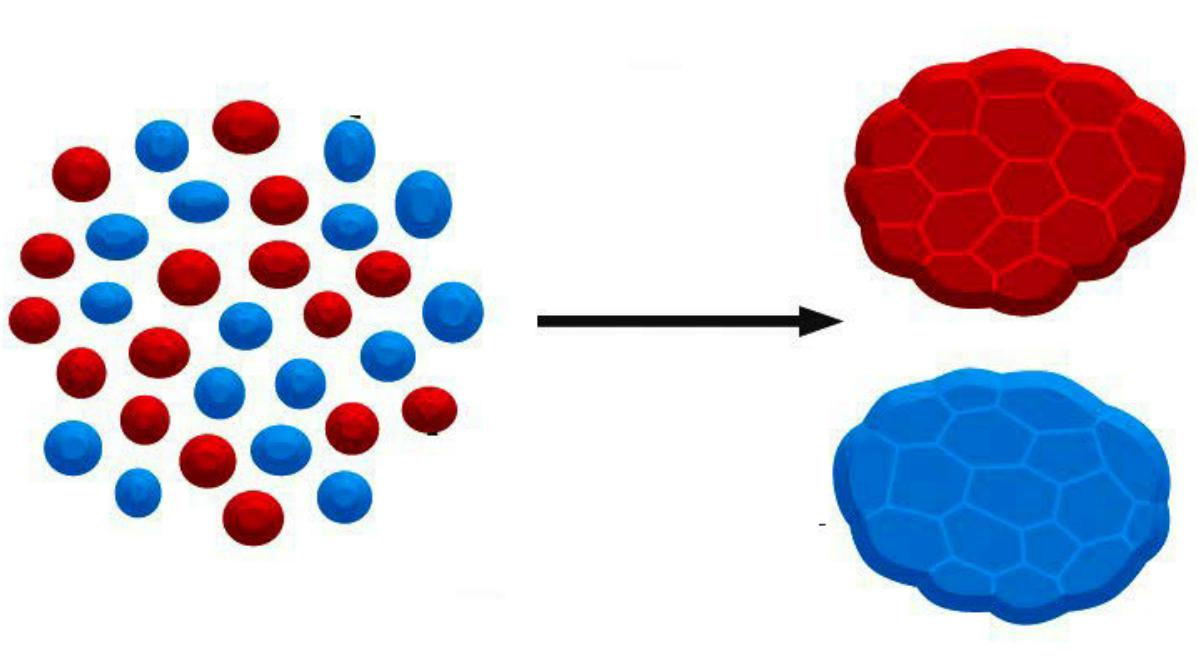
- Cells can be sorted by types and expression level of cadherins
- Qualitative differences in the expression of cadherins play a role in the organization of tissues
Changes in expression of cadherins leads to development of different tissues
Cadherin-Dependent Cell–Cell Adhesion Guides the Organization of Developing Tissues
Cadherins play a crucial part in these cell-sorting processes during development.
Both qualitative and quantitative differences in the expression of cadherins have a role in organizing tissues.
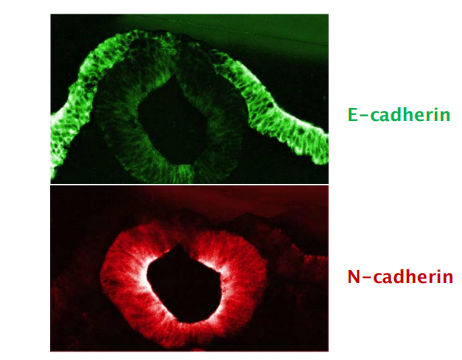
- Changes in expression of cadherins leads to development of different tissues
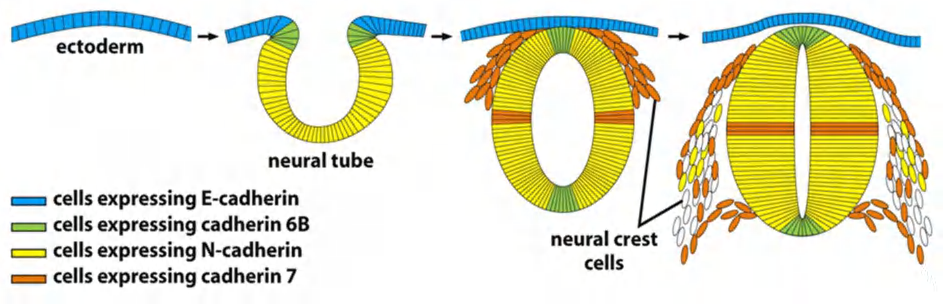
- During embryonic development: cells expressing the same cadherins group together
- The appearance and disappearance of specific cadherins correlate with steps in embryonic development where cells regroup and change their contacts to create new tissue structures.
In the vertebrate embryo changes in cadherin expression are seen when the neural tube forms and pinches off from the overlying ectoderm:
- Neural tube cells lose E-cadherin and acquire other cadherins (N-cadherin) while the cells in the overlying ectoderm continue to express E-cadherin.
- Neural crest cells express cadherin 7, allowing detachment/migration away from the neutral tube but keeps holding them together.
- For ganglion formation (神经节形成), cells switch back to N-cadherin expression.
Cadherin interactions are dependent on calcium
(1) Clustering of cadherins increases strength of interactions between cells
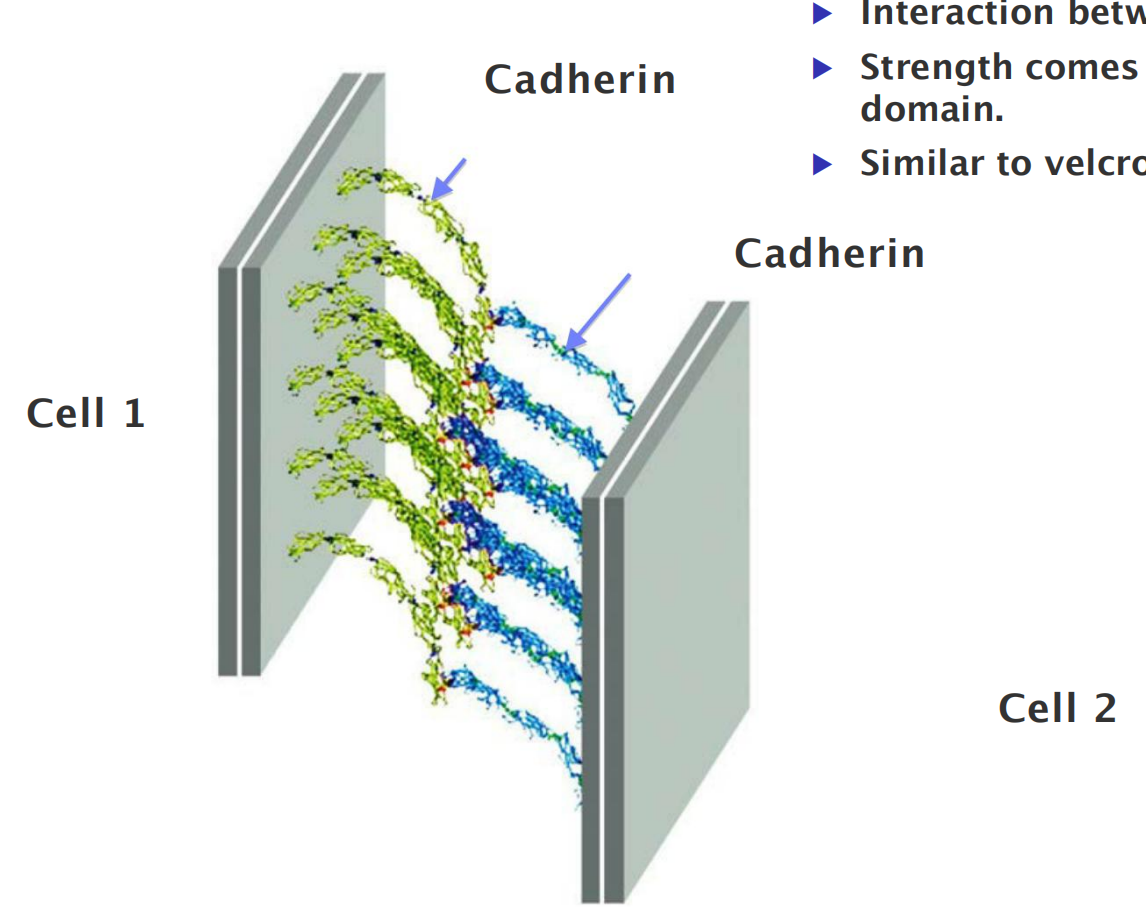
- Interaction between individual cadherins is weak.
- Strength comes from clustering of cadherins in common domain.
- Similar to Velcro (一种尼龙塔扣).
(2) Links to cytoskeleton cluster cadherins in desmosomes and adhering junctions
(3) Catenins(连环素) link cadherins to actin filaments in adherens junctions
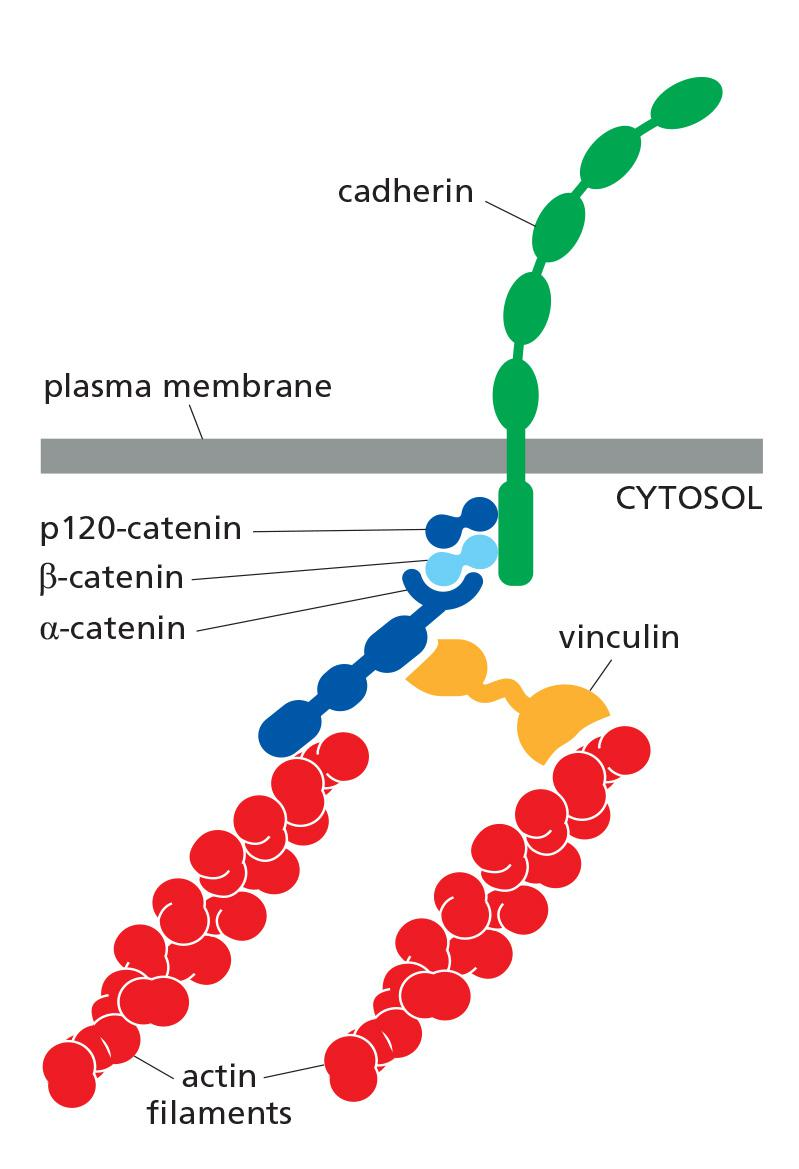
Catenins Link Classical Cadherins to the Actin Cytoskeleton
These cytoskeletal linkages are essential for efficient cell–cell adhesion, as cadherins that lack their cytoplasmic domains cannot stably hold cells together.
The linkage of cadherins to the cytoskeleton is indirect and depends on adaptor proteins that assemble on the cytoplasmic tail of the cadherin
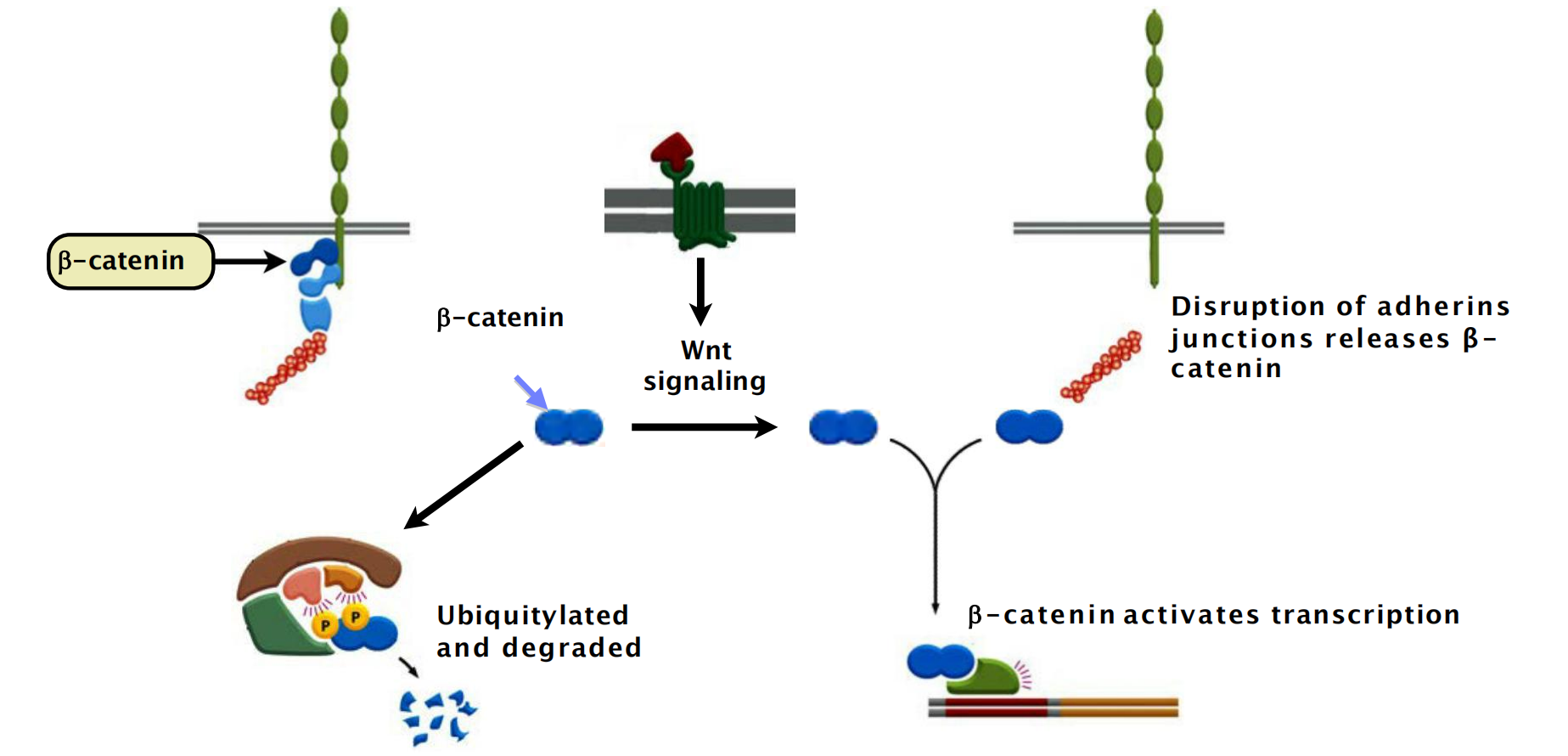
At adherens junctions, the cadherin tail binds two such proteins: β-catenin/ and a distant relative called p120-catenin; a third protein called α-catenin interacts with β-catenin and recruits a variety of other proteins to provide a dynamic linkage to actin filaments
Assembly of an adherent junction
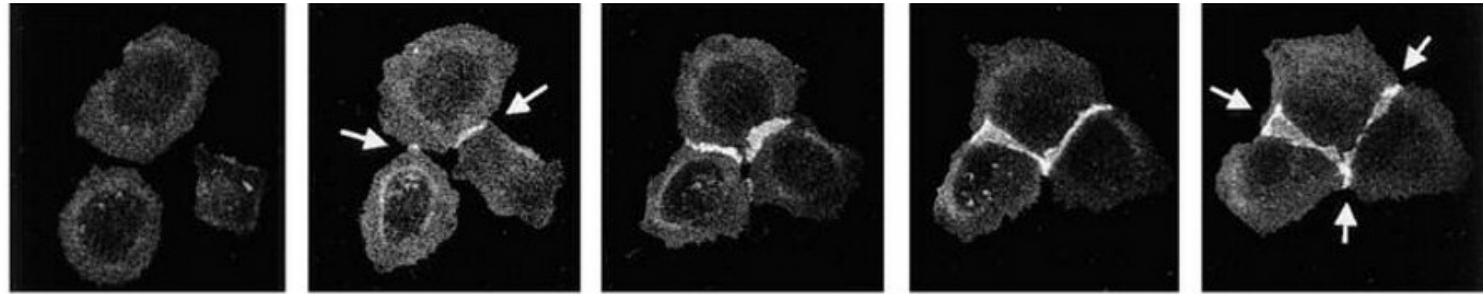
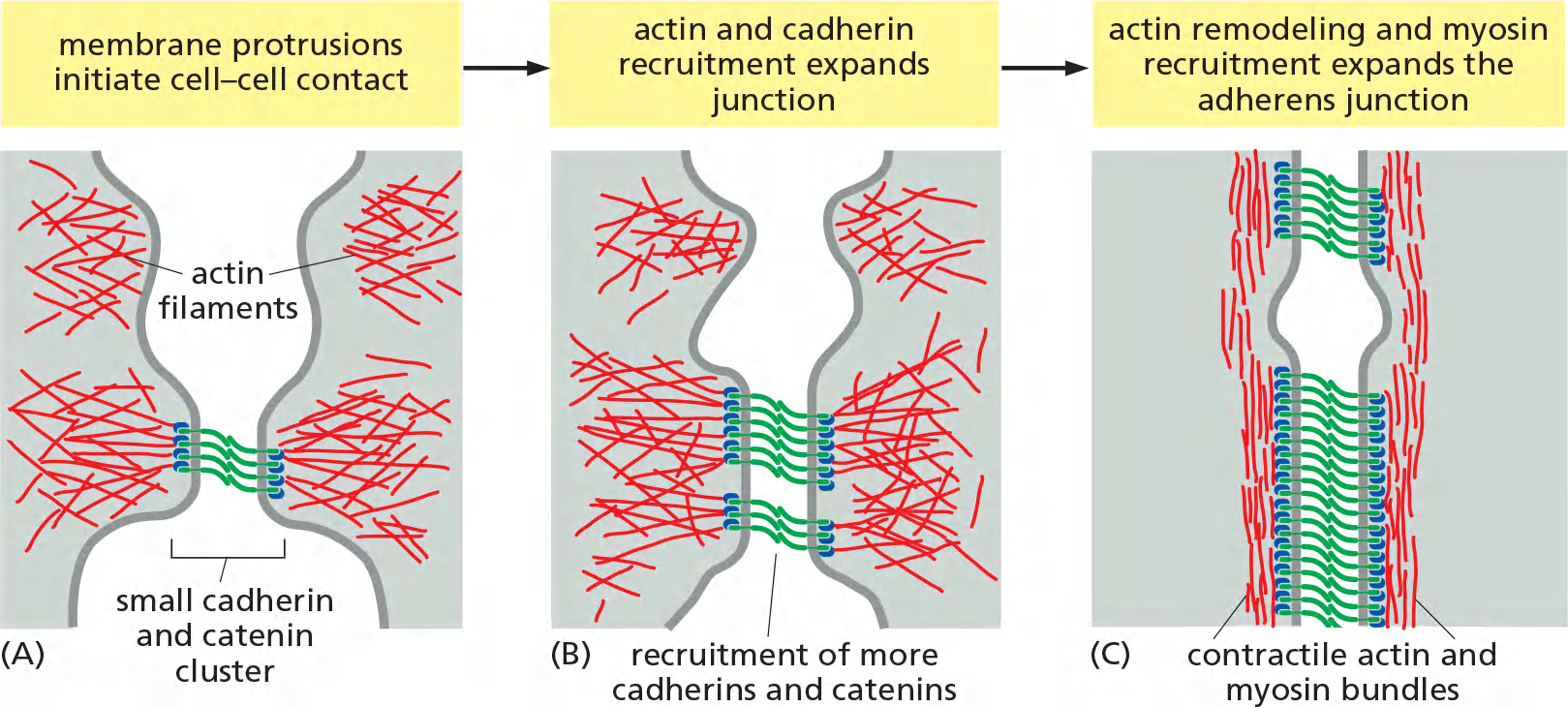
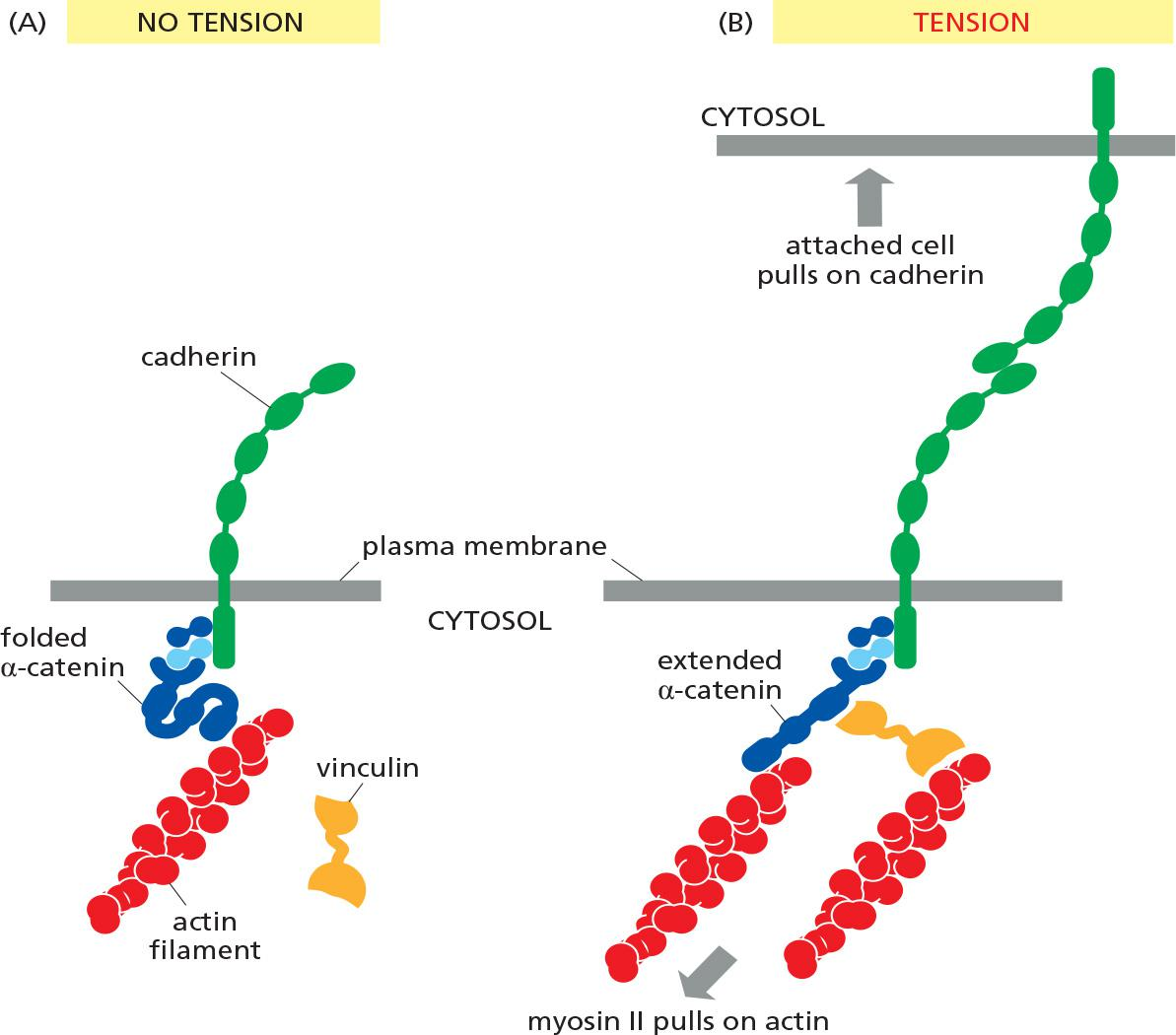
Adherens junctions are not simply passive sites of protein–protein binding but are dynamic tension sensors that regulate their behavior in response to changing mechanical conditions
This ability to transduce a mechanical signal into a change in junctional behavior is an example of mechanotransduction(机械转导). It is also important at cell–matrix junctions.
vinculin (黏着斑蛋白), which promotes the recruitment of more actin to the junction
3. Epithelial–Mesenchymal Transitions Depend on Control of Cadherins
Epithelial–Mesenchymal Transitions(上皮-间质转化) Depend on Control of Cadherins
The assembly of cells into an epithelium is a reversible process. By switching on expression of adhesion molecules, dispersed unattached mesenchymal cells, such as fibroblasts, can come together to form an epithelium
- 细胞组装成上皮是可逆的过程。通过打开粘附分子的表达,分散的未附着的间充质细胞(例如成纤维细胞)可以聚集在一起形成上皮
Such epithelial–mesenchymal transitions play an important part in normal embryonic developments
- the origin of the neural crest is one example
- These transitions depend in part on transcription regulatory proteins called Slug, Snail, and Twist
Epithelial–mesenchymal transitions also occur as pathological events during adult life, in cancer
- Most cancers originate in epithelia, but become dangerously prone to spread—that is, malignant—only when the cancer cells escape from the epithelium of origin and invade other tissues
- Mutations that disrupt the production or function of E-cadherin are often found in cancer cells and are thought to help make them malignant.
4. Mechanotransduction in an adherent junction
Tissue remodeling depends on the coordination of actin-mediated contraction with cell–cell adhesion
Tissue Remodeling (组织重塑) Depends on the Coordination of Actin-Mediated Contraction With Cell–Cell Adhesion
By indirectly linking the actin filaments in one cell to those in its neighbors, they enable the cells in the tissue to use their actin cytoskeletons in a coordinated way.
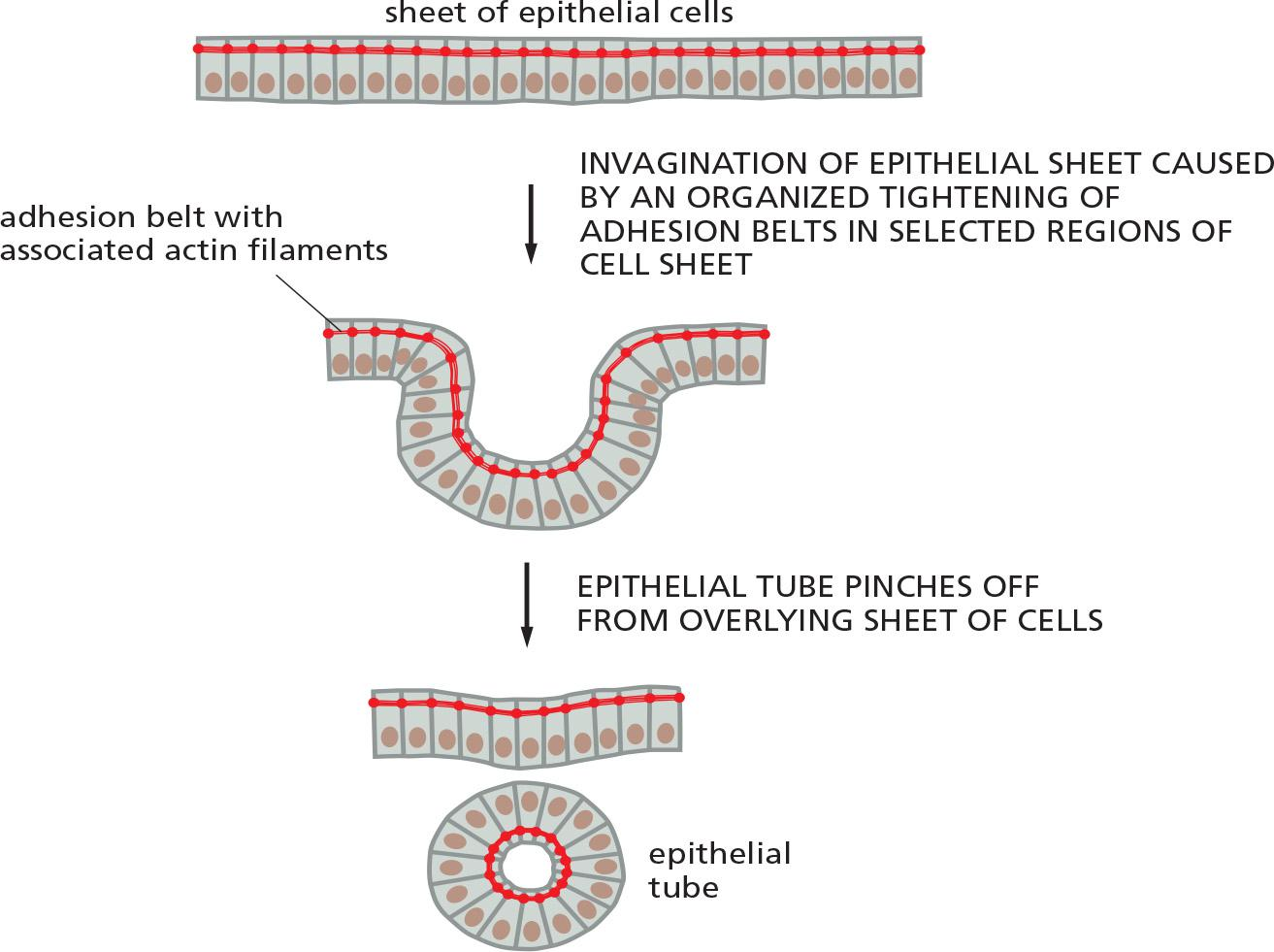
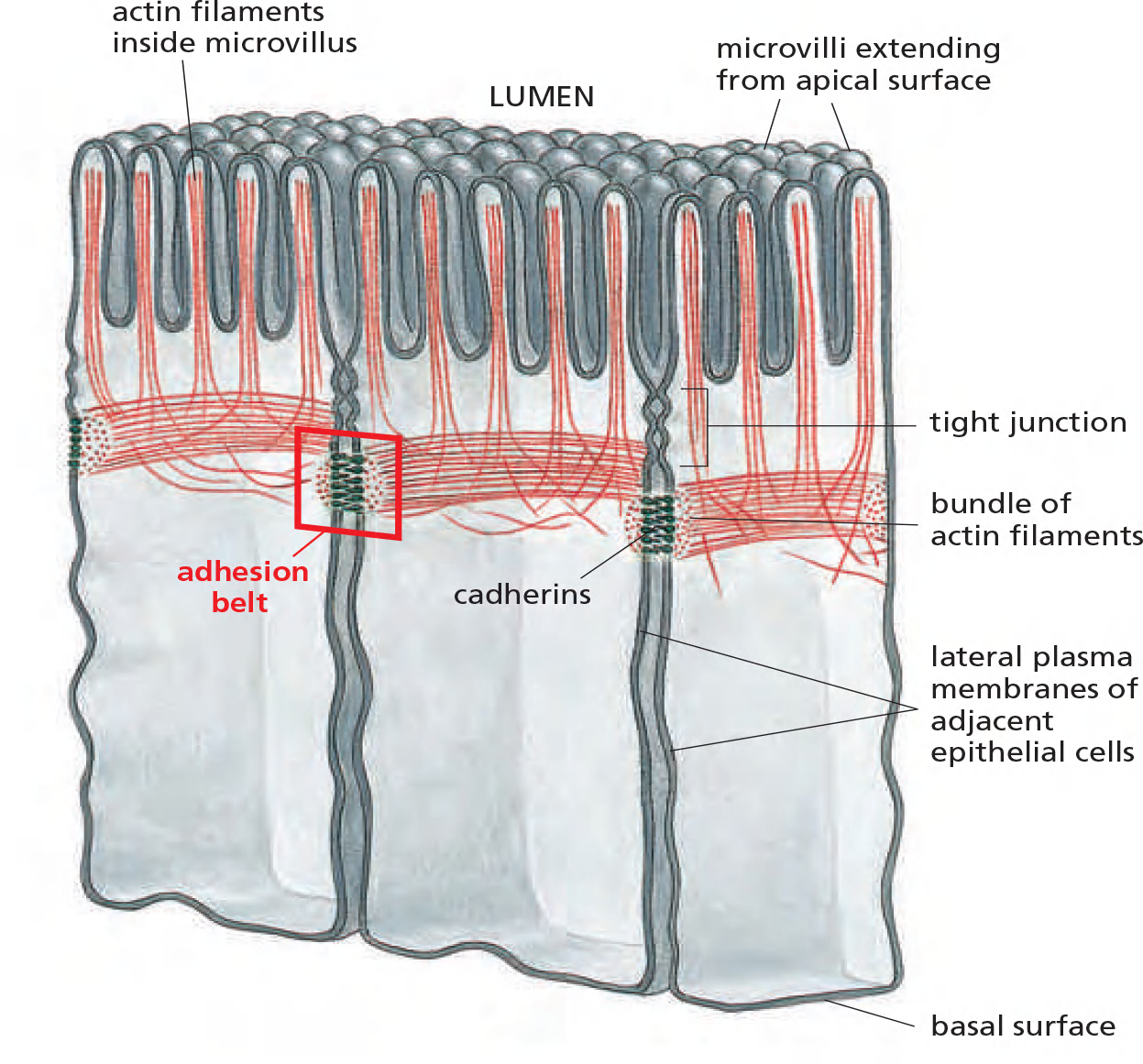
the prototypical examples of adherens junctions occur in epithelia, where they often form a continuous adhesion belt (粘附带) (or zonula adherens (小带粘连)) just beneath the apical face of the epithelium, encircling each of the interacting cells in the sheet
Within each cell, a contractile bundle of actin filaments and myosin II lies adjacent to the adhesion belt, oriented parallel to the plasma membrane and tethered to it by the cadherins and their associated intracellular adaptor proteins.
- The actin–myosin bundles are thus linked, via the cadherins, into an extensive transcellular network.
Myosin motors can cause contraction on the adhesion belt to form epithelial tube
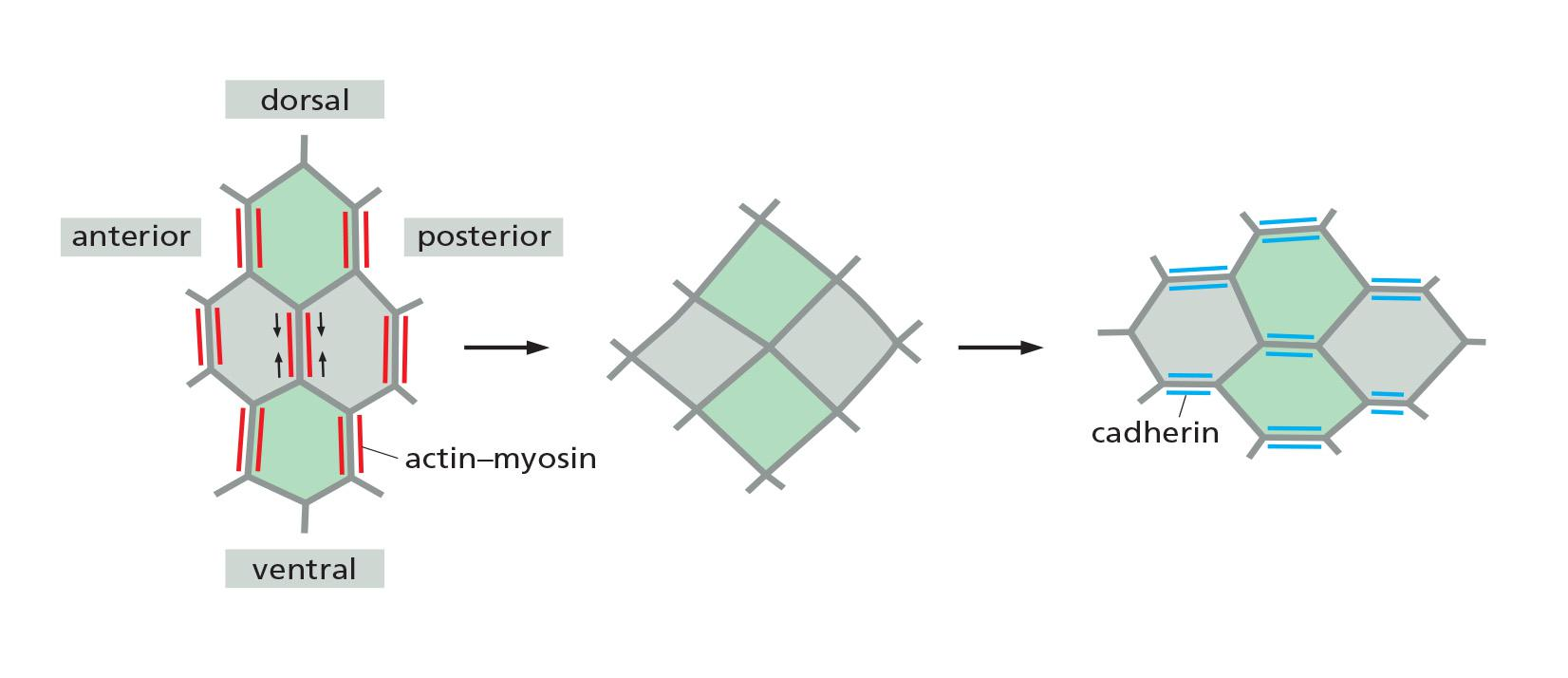
Actin-dependent contraction along specific cell boundaries is coordinated with a loss of specific adherens junctions to allow cells to insert themselves between other cells (a process called intercalation (置闰,插入)), resulting in a longer and narrower epithelium.
The mechanisms underlying the loss of adhesion along specific cell boundaries are not clear, but they depend in part on increased degradation of β-catenin, due to its phosphorylation by a protein kinase that is localized specifically at those boundaries.
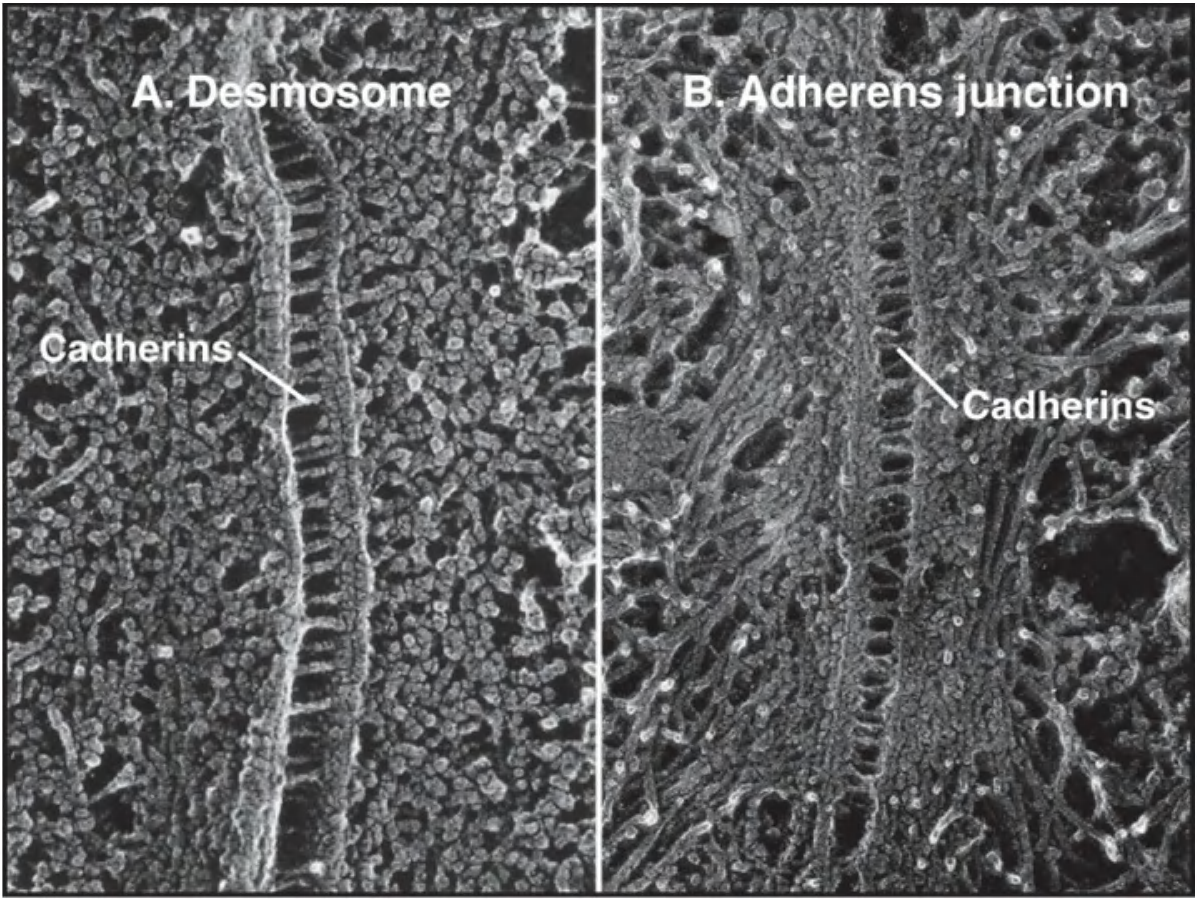
In desmosomes, cadherins are linked to intermediate filaments
Desmosomes are structurally similar to adherens junctions.
- In Desmosomes, cadherins link to intermediate filaments while in adherens junctions, cadherins link to actin filaments.
Desmosomes give cells mechanical strength.
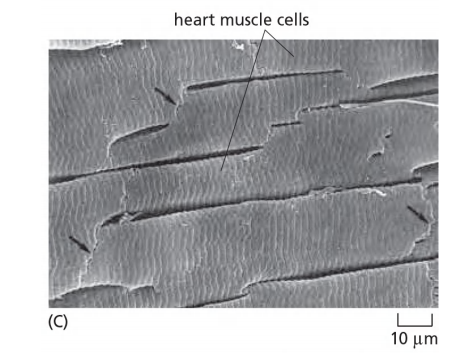
- Desmosomes are particularly plentiful in tissues that are subject to mechanical stress (heart muscle, epithelium).
Desmosomes are not found in Drosophila
5. Desmosome
Desmosomes Give Epithelia Mechanical Strength
They are present in most mature vertebrate epithelia and are particularly plentiful in tissues that are subject to high levels of mechanical stress, such as heart muscle and the epidermis, the epithelium that forms the outer layer of the skin.
The particular type of intermediate filaments attached to the desmosomes depends on the cell type: they are keratin filaments (角蛋白丝) in most epithelial cells, for example, and desmin filaments (结蛋白细丝) in heart muscle cells
- The importance of desmosomes is demonstrated by some forms of the potentially fatal skin disease pemphigus(天疱疮).
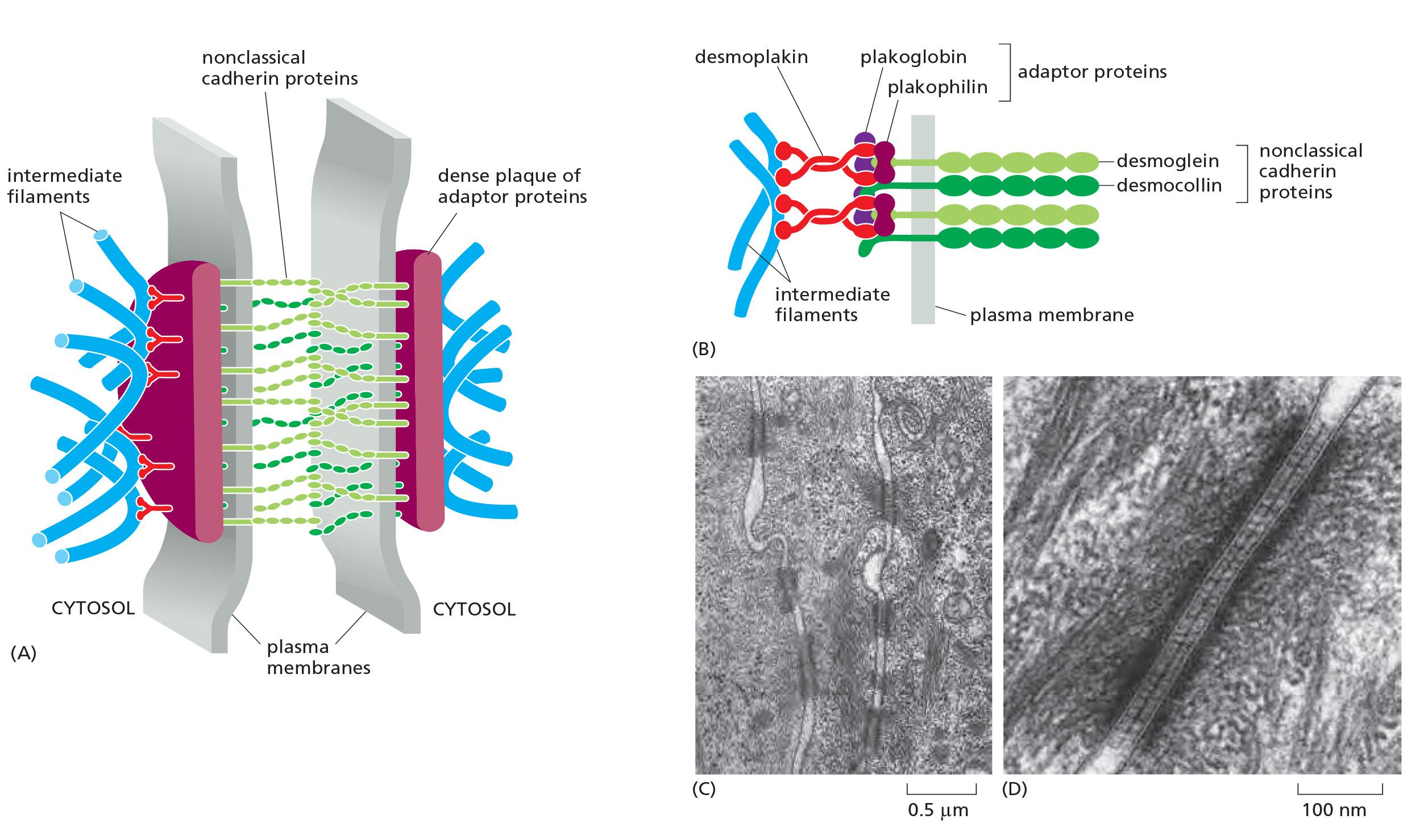
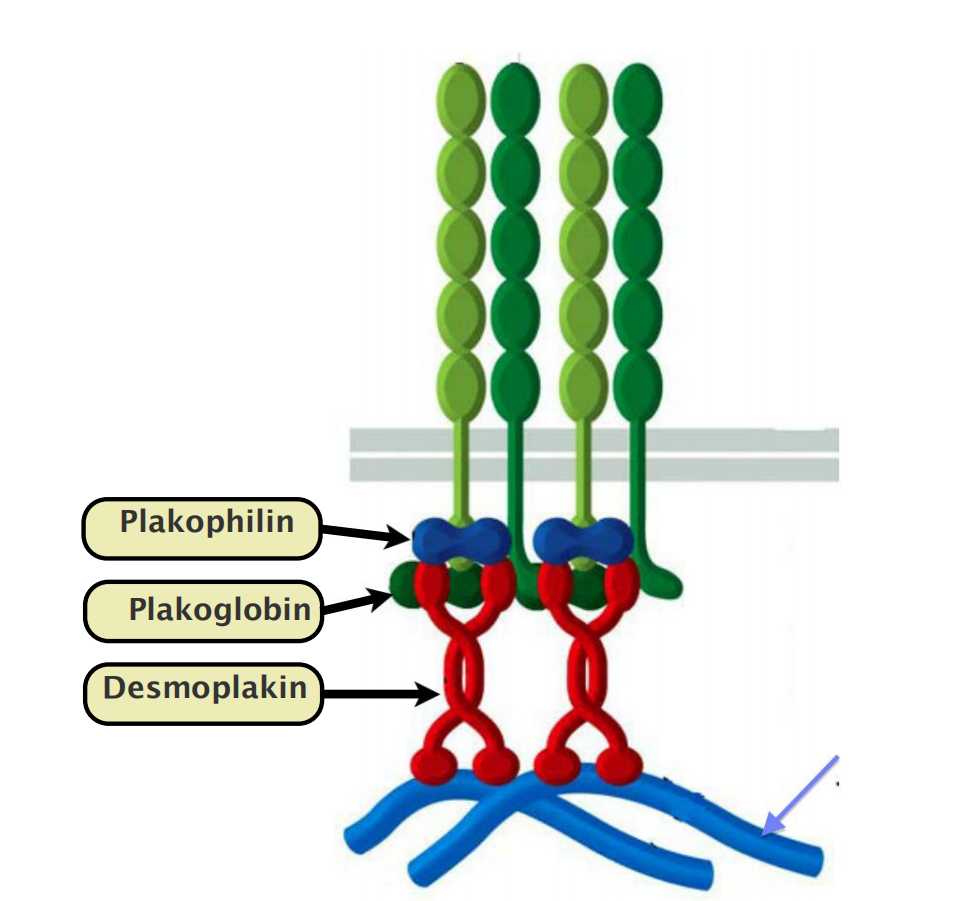
Desmoglein and desmocollin are nonclassical cadherins.
- Their cytoplasmic tails bind plakoglobin(珠蛋白) (γ-catenin) and plakophilin (亲脂蛋白) (a distant relative of p120-catenin), which in turn bind to desmoplakin (去铁素).
Desmoplakin binds to the sides of intermediate filaments, thereby tying the desmosome to these filaments.
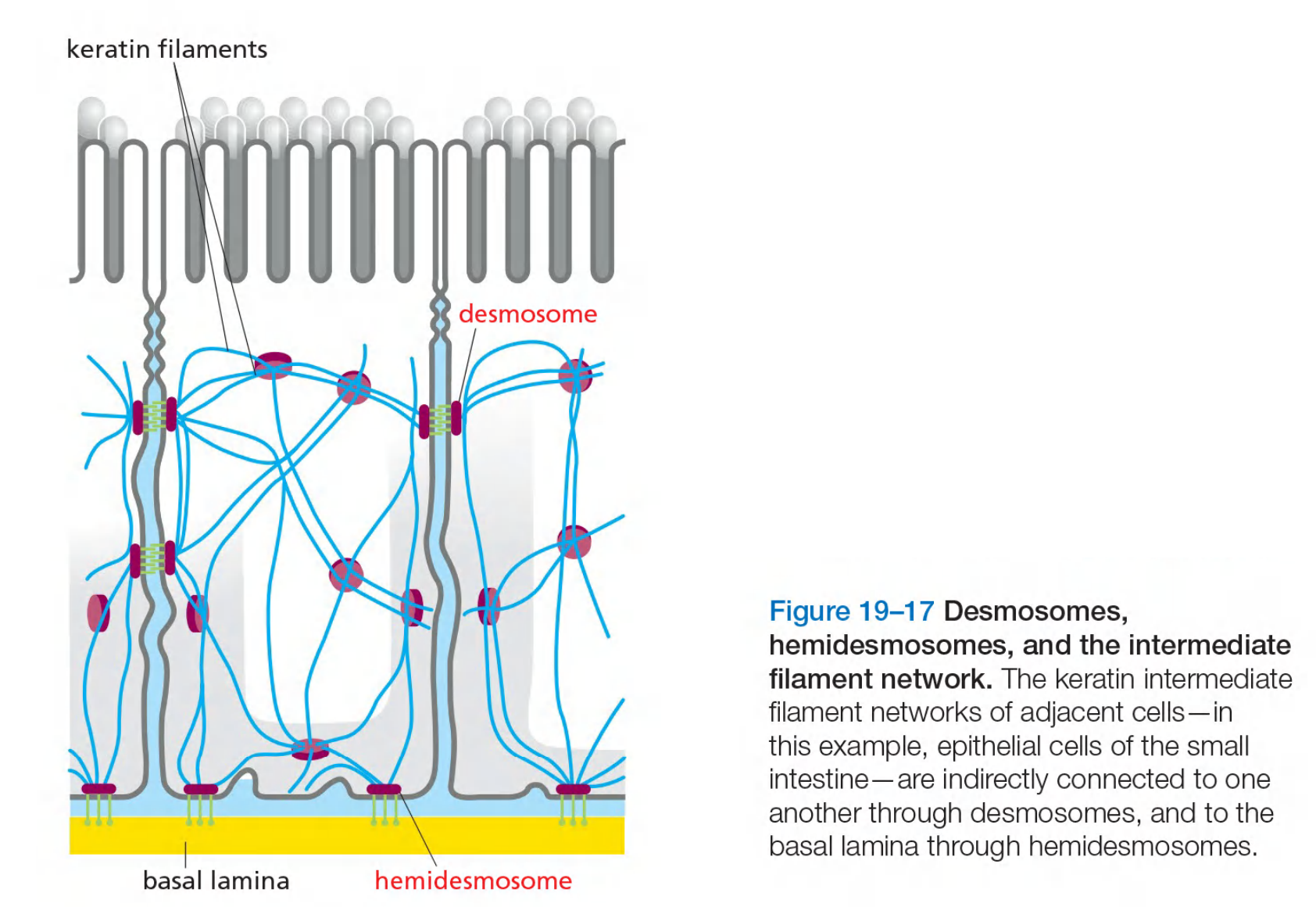
Desmosome, hemidesmosome and intermediate filament network
6. Specialized adhesion: selectin
Selectins Mediate Transient Cell–Cell Adhesions in the Bloodstream
At least three other superfamilies of cell–cell adhesion proteins are important: the integrins, the selectins, and the adhesive immunoglobulin (Ig) superfamily members
- Selectins, like cadherins and integrins, require Ca2+ for their adhesive function; Ig superfamily members do not.
Selectins are cell-surface carbohydrate-binding proteins (lectins(凝集素)) that mediate a variety of transient cell–cell adhesion interactions in the bloodstream
- Their main role, in vertebrates at least, is in governing the traffic of white blood cells into normal lymphoid organs and any inflamed tissues
Each selectin is a transmembrane protein with a conserved lectin domain that binds to a specific oligosaccharide on another cell
There are at least three types:
- L-selectin on white blood cells
- P-selectin on blood platelets and on endothelial cells that have been locally activated by an inflammatory response
- E-selectin on activated endothelial cells. In a lymphoid organ, such as a lymph node or the spleen, the endothelial cells express oligosaccharides that are recognized by L-selectin on lymphocytes, causing the lymphocytes to loiter and become trapped.
Selectins and integrins act in sequence to let white blood cells leave the bloodstream and enter tissues
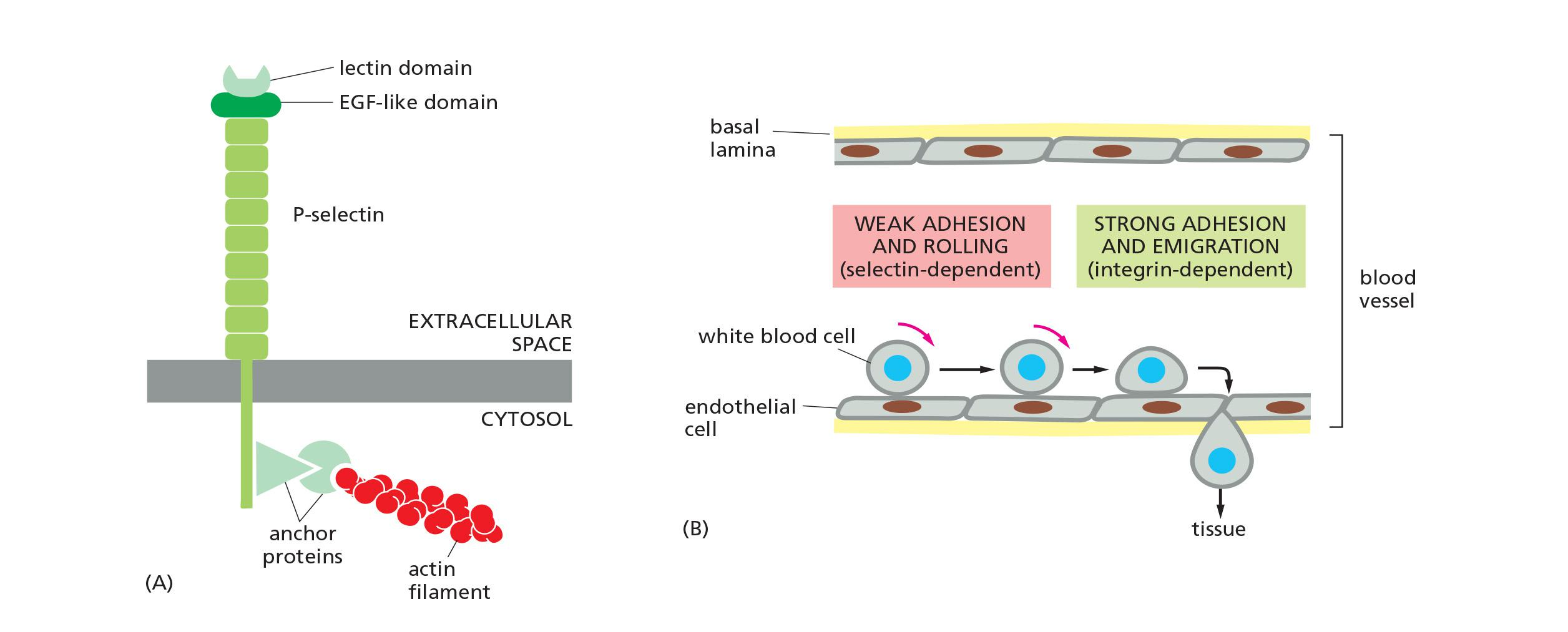
- Selectins are cell-surface carbohydrate-binding proteins (lectins)
- Ca2+-dependent adhesion
- Mediate transient adhesions in the blood stream
- Bind to lectins on other cell-surface proteins
- At least 3 types:
- L-selectin: on white blood cells (leucocytes)
- P-selectin: on platelets and endothelial cells
- E-selectin: on activated endothelial cells selectins binding is weak, they collaborate with integrin to cause migration of white blood cells from the blood stream into tissues.
7. Specialized adhesion: Immunoglobulin superfamily members
Members of the Immunoglobulin Superfamily Mediate Ca2+-Independent Cell–Cell Adhesion
The chief endothelial cell proteins that are recognized by the white blood cell integrins are called ICAMs (intercellular cell adhesion molecules) or VCAMs (vascular cell adhesion molecules).
- They are members of another large and ancient family of cell-surface molecules—the immunoglobulin (Ig) superfamily. These contain one or more extracellular Ig-like domains that are characteristic of antibody molecules.
While ICAMs and VCAMs on endothelial cells both mediate heterophilic binding to integrins, many other Ig superfamily members appear to mediate homophilic binding
- An example is the neural cell adhesion molecule (NCAM), which is expressed by various cell types, including most nerve cells, and can take different forms, generated by alternative splicing of an RNA transcript produced from a single gene
Some forms of NCAM carry an unusually large quantity of sialic acid (with chains containing hundreds of repeating sialic acid units). By virtue of their negative charge, the long polysialic acid chains can interfere with cell adhesion (because like charges repel one another); thus, these forms of NCAM can serve to inhibit adhesion, rather than cause it
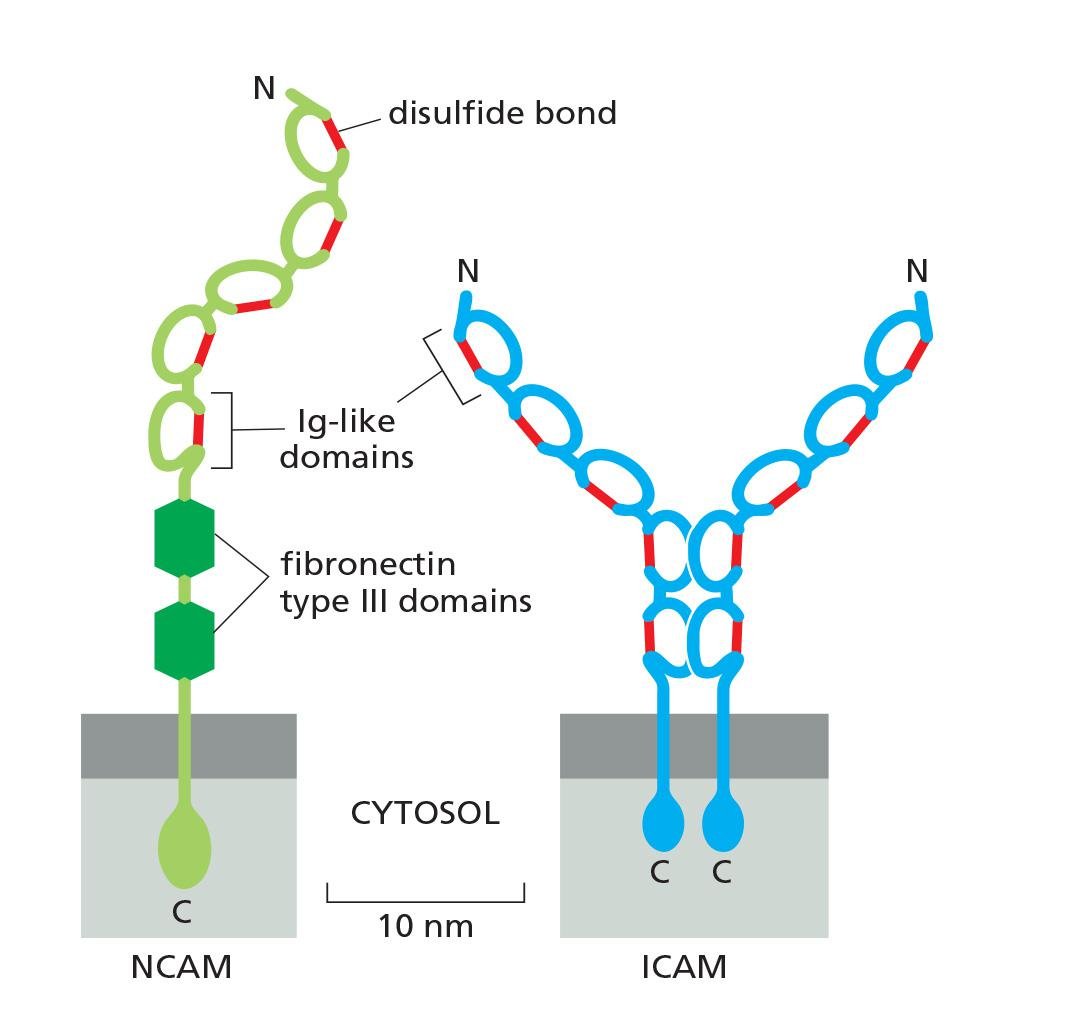
- Ca2+ -independent
- Heavy glycosylation, multiple disulfide bonds
- Bind to integrin
- several major proteins:
- ICAMs (intercellular cell adhesion molecules)
- VCAMs (vascular cell adhesion molecules)
- NCAM (neural cell adhesion molecules)
Although cadherins and Ig superfamily members are frequently expressed on the same cells, the adhesions mediated by cadherins are much stronger, and they are largely responsible for holding cells together, segregating cell collectives into discrete tissues, and maintaining tissue integrity
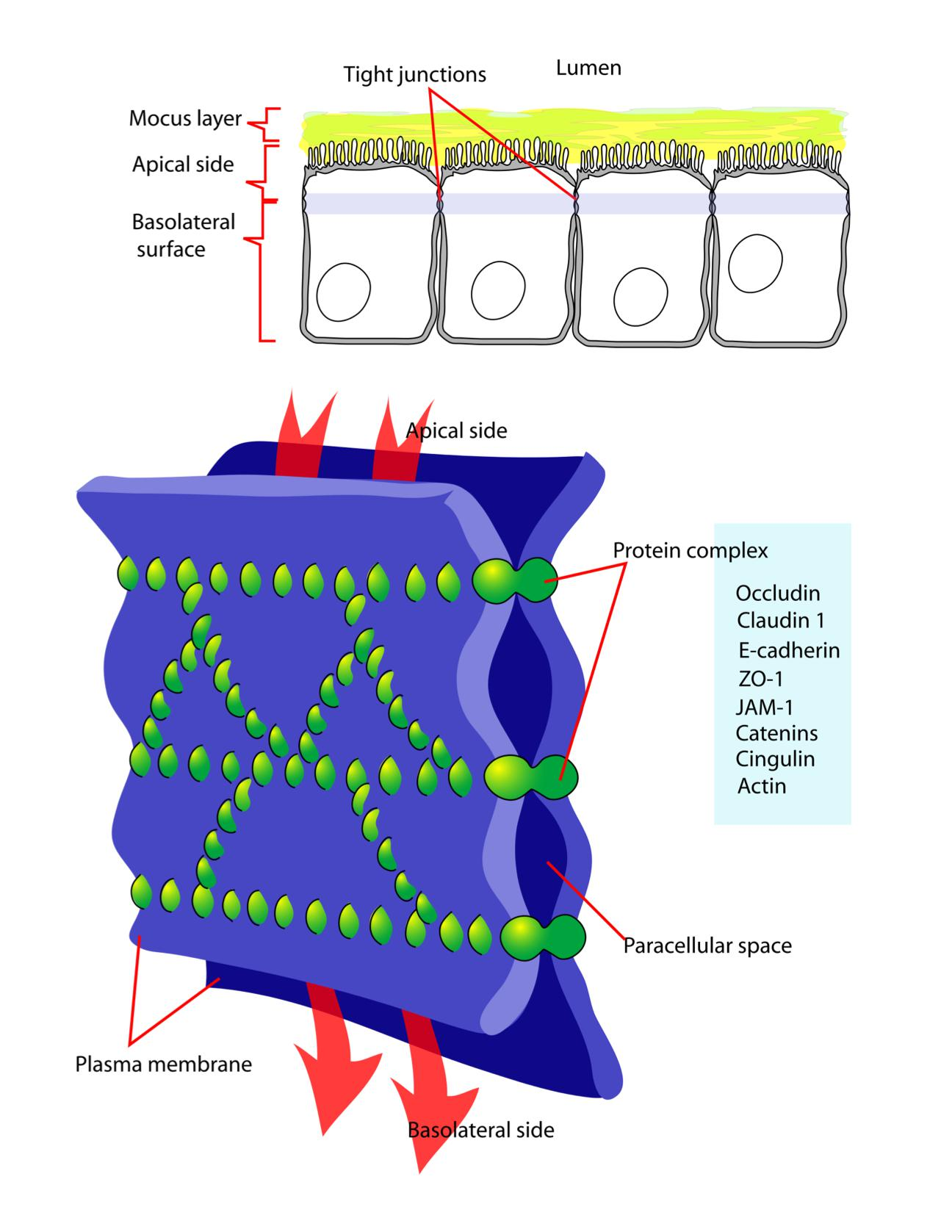
Occluding junctions: tight junctions (zonula occludens)
Tight Junctions Form a Seal Between Cells and a Fence Between Plasma Membrane Domains
Essentially all epithelia are anchored to other tissue on one side—the basal side—and free of such attachment on their opposite side—the apical sides
Correspondingly, all epithelia have at least one function in common: they serve as selective permeability barriers, separating the fluid that permeates the tissue on their basal side from fluid with a different chemical composition on their apical side.
This barrier function requires that the adjacent cells be sealed together by tight junctions, so that molecules cannot leak freely across the cell sheet.
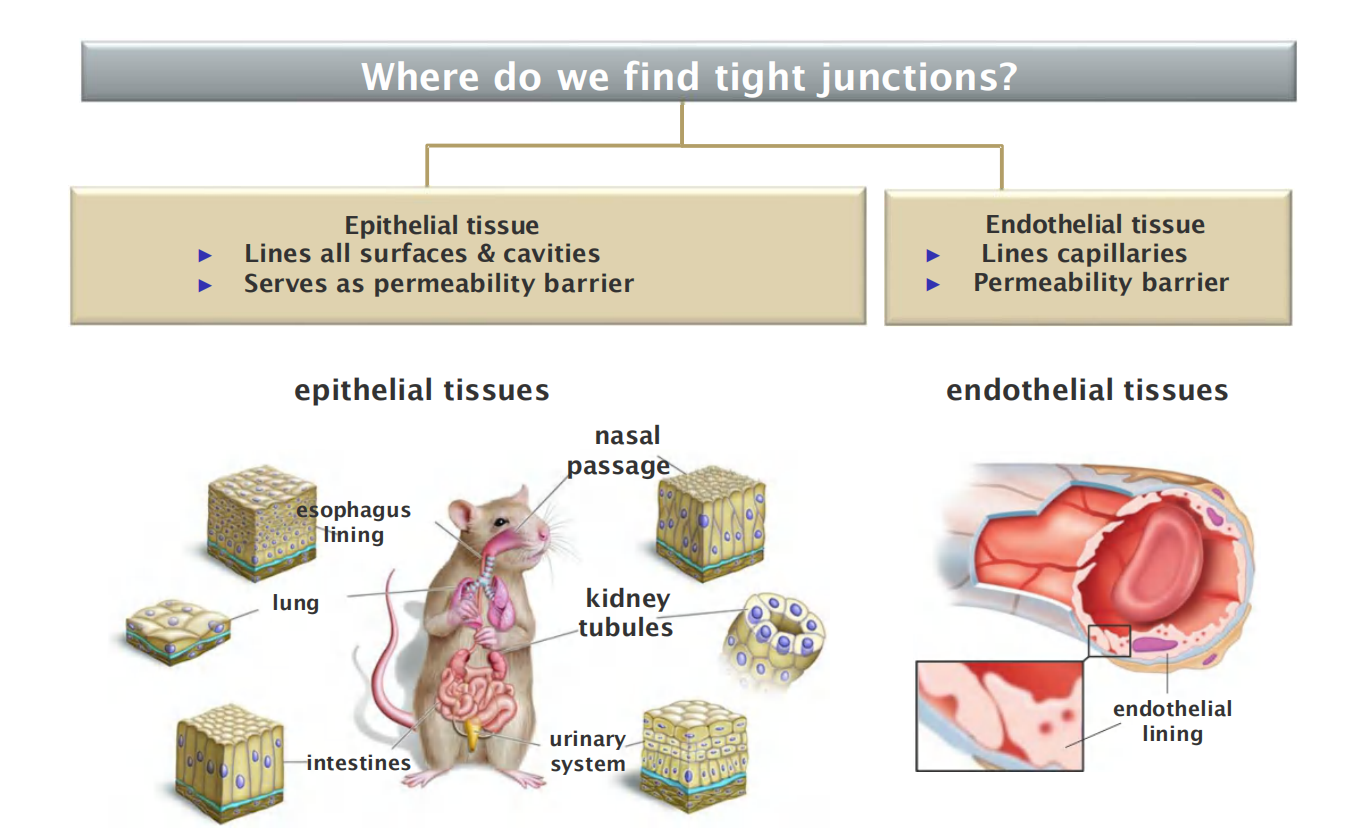
- Epithelial tissue
- Lines all surfaces & cavities
- Serves as permeability barrier
- Endothelial tissue
- Lines capillaries
- Permeability barrier
1. Organization of tight junction
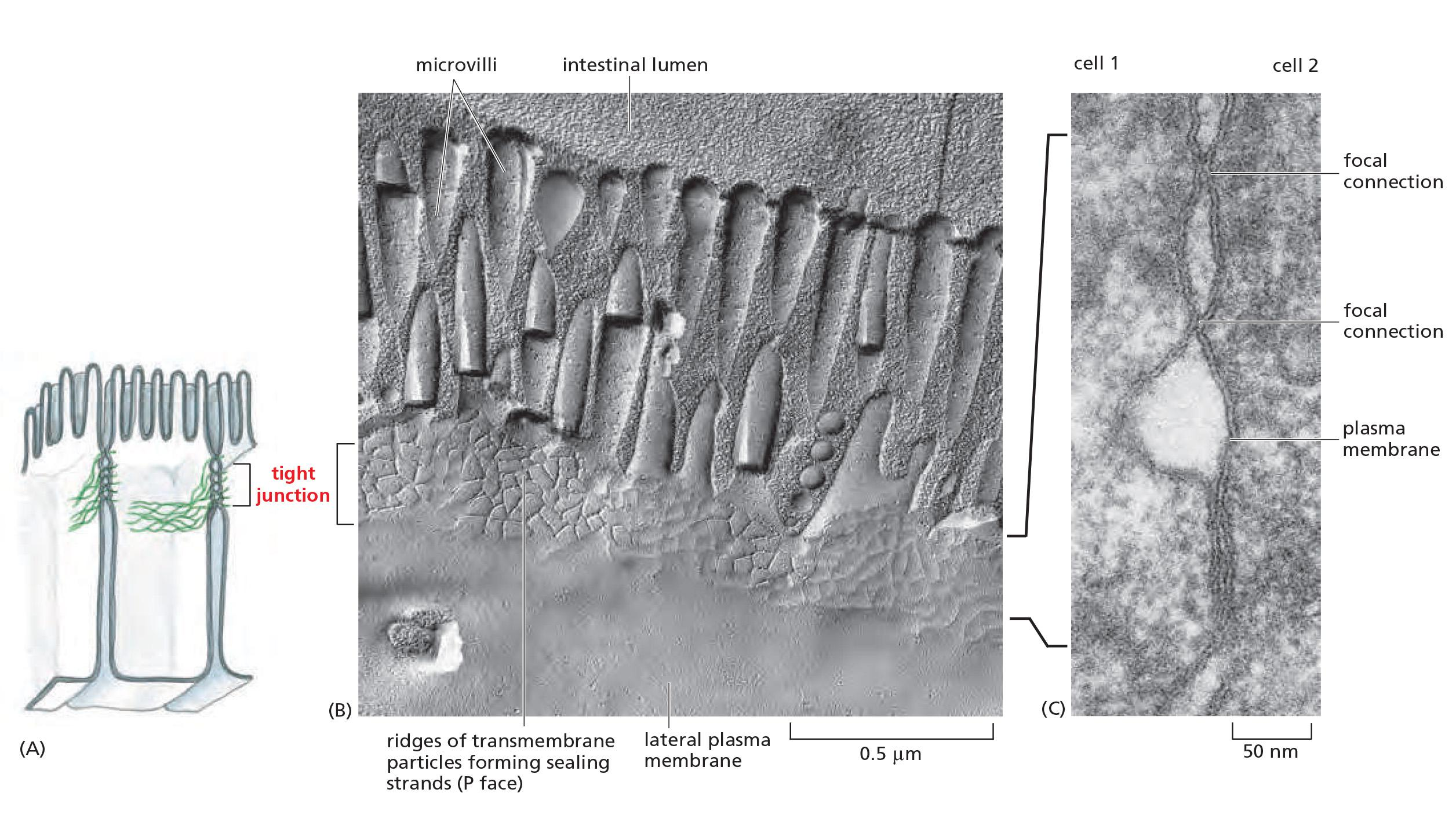
- Tight junctions spot-weld (点焊) the plasma membranes of neighboring cells
2. The function of tight junctions
The epithelium of the small intestine provides a good illustration of tight-junction structure and function
- This epithelium has a simple columnar (柱形的) structure; that is, it consists of a single layer of tall (columnar) cells. These are of several differentiated types, but the majority are absorptive cells, specialized for uptake of nutrients from the internal cavity, or lumen, of the gut.
The absorptive cells have to transport selected nutrients across the epithelium from the lumen into the extracellular fluid on the other side. From there, these nutrients diffuse into small blood vessels to provide nourishment to the organism
- This transcellular transport depends on two sets of transport proteins in the plasma membrane of the absorptive cell.
- One set is confined to the apical surface of the cell (facing the lumen) and actively transports selected molecules into the cell from the gut.
- The other set is confined to the basolateral (基底外侧, basal and lateral) surfaces of the cell, and it allows the same molecules to leave the cell by passive transport into the extracellular fluid on the other side of the epithelium.
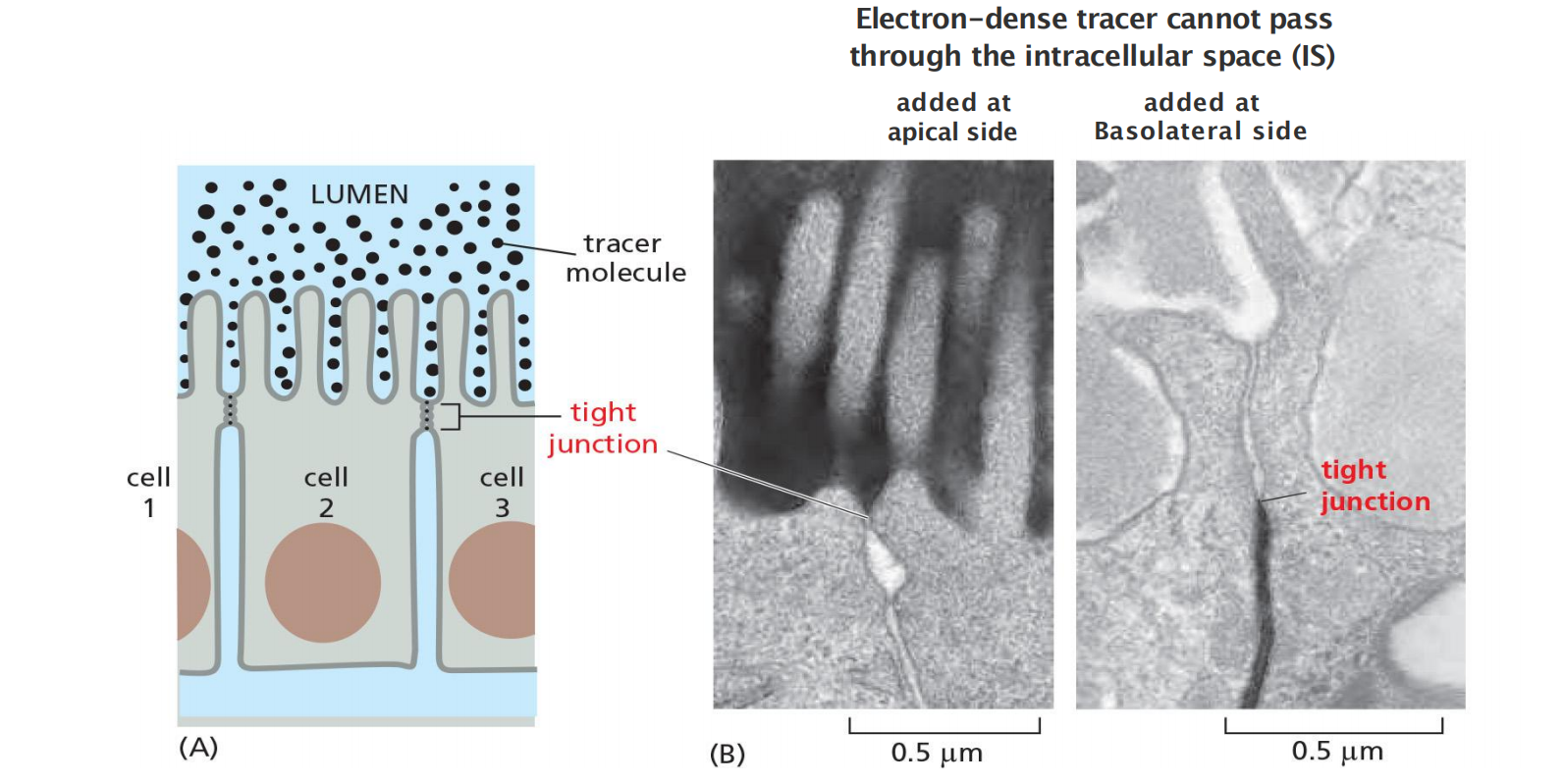
- Tight junctions seal the intracellular space to form a barrier
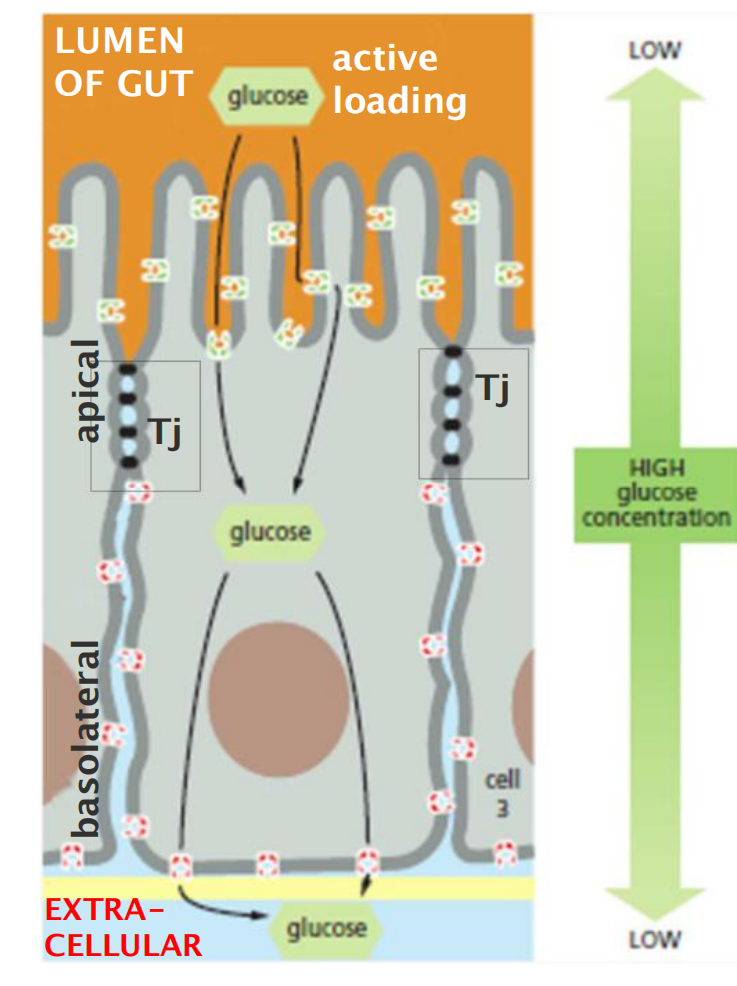
- Tight junctions maintain cell polarity
- This segregation permits a vectorialtransfer (向量转移) of nutrients across the epithelium from the gut lumen to the blood.
- Serve as “fences” to separate domains within the PM”
- Selective for ions, but do not allow macromolecules to pass.
This seal is not absolute, however. Although all tight junctions are impermeable to macromolecules, their permeability to ions and other small molecules varies.
- Tight junctions in the epithelium lining the small intestine, for example, are 10,000 times more permeable to inorganic ions, such as Na+, than the tight junctions in the epithelium lining the urinary bladder
- The movement of ions and other molecules between epithelial cells is called paracellular transport (细胞旁运输), and tissue-specific differences in transport rates generally result from differences in the proteins that form tight junctions.
3. Molecular organization of tight junctions
Tight Junctions Contain Strands of Transmembrane Adhesion Proteins
- When tight junctions are visualized by freeze-fracture electron microscopy, they are seen as a branching network of sealing strands that completely encircles the apical end of each cell in the epithelial sheet
The main transmembrane proteins forming these strands are the claudins (闭合蛋白), which are essential for tight-junction formation and function.
Mice that lack the claudin-1 gene, for example, fail to make tight junctions between the cells in the epidermal layer of the skin
The claudin protein family has many members (24 in humans), and these are expressed in different combinations in different epithelia to confer particular permeability properties on the epithelial sheet
- They are thought to form paracellular pores—selective channels allowing specific ions to cross the tight-junctional barrier, from one extracellular space to another.
Normal tight junctions also contain a second major transmembrane protein called occludin (封端蛋白), which is not essential for the assembly or structure of the tight junction but is important for limiting junctional permeability
A third transmembrane protein, tricellulin, is required to seal cell membranes together and prevent transepithelial leakage at the points where three cells meet.
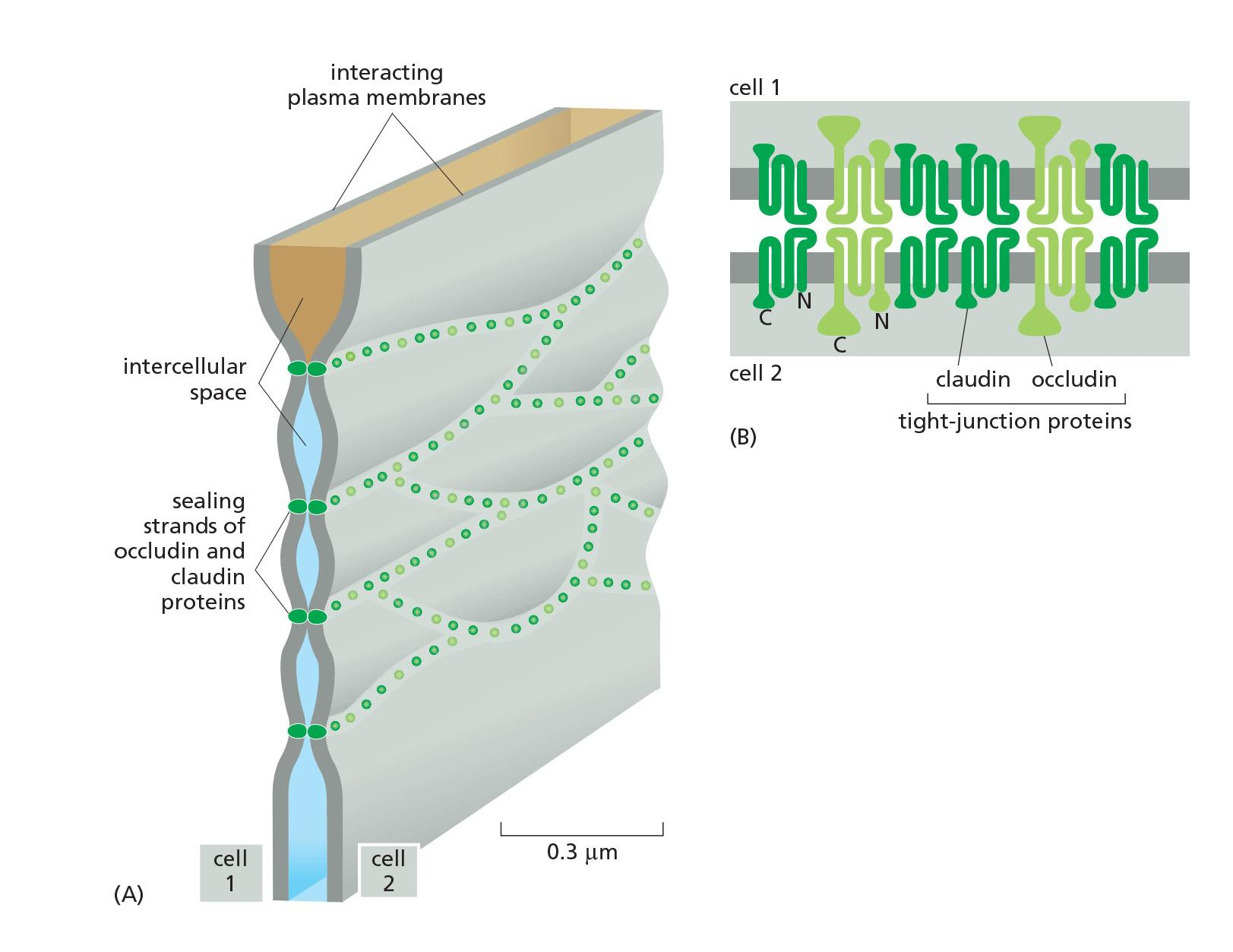
Claudin:
- Loss causes dehydration and death in mice
- Overexpression causes tight junction formation in fibroblasts
Occludin:
- Detailed function unknown
Tricellulin:
- Required to seal membrane
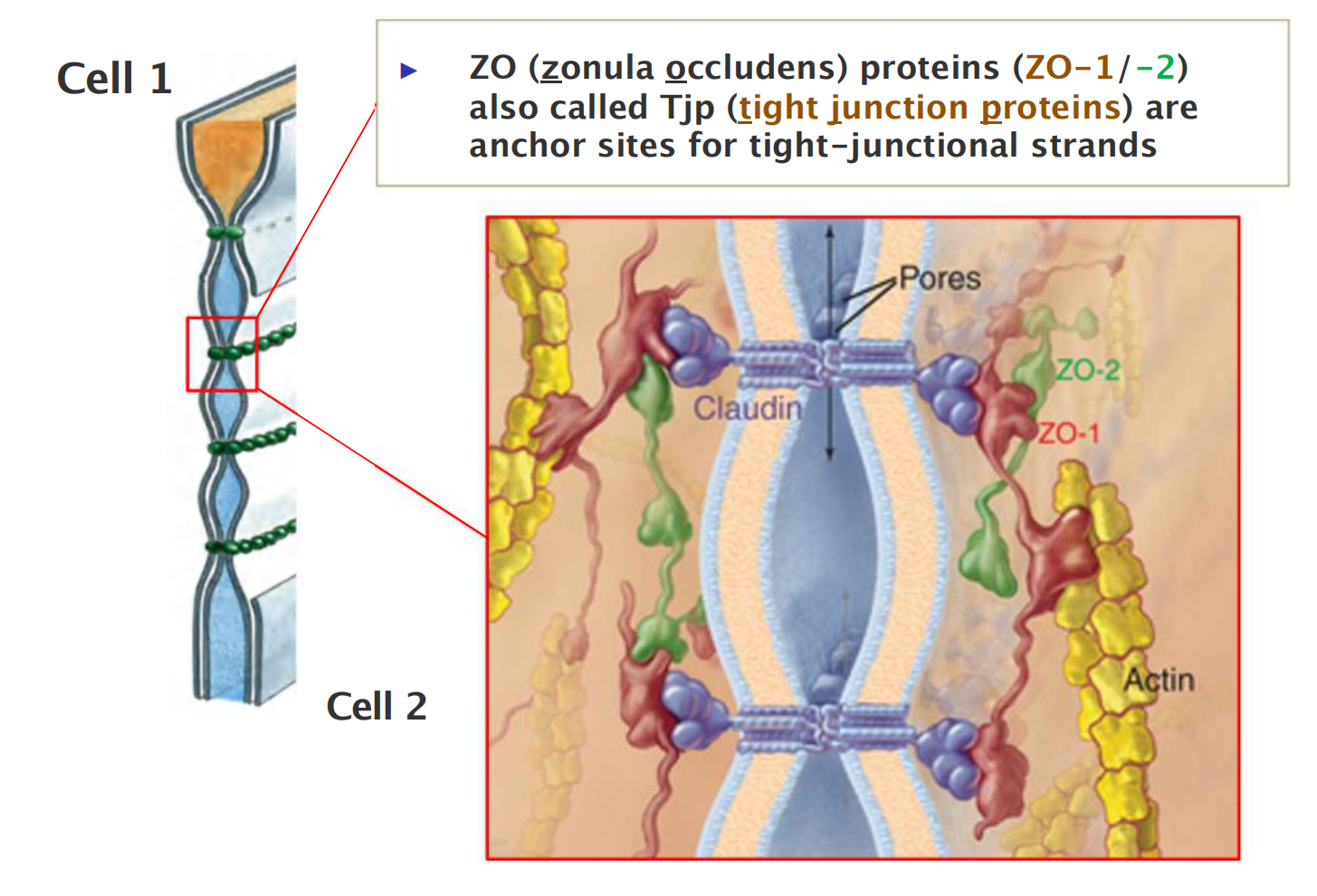
- Scaffold proteins (ZO/Tjp) link claudins to the actin-cytoskeleton
- ZO (zonula occludens) proteins (ZO-1/-2) also called Tjp (tight junction proteins) are anchor sites for tight-junctional strands
Scaffold protein: zonula occludens (ZO)
Scaffold Proteins Organize Junctional Protein Complexes
- Like the cadherin molecules of an adherens junction, the claudins and occludins of a tight junction interact with each other on their extracellular sides to promote junction assembly.
- Also as in adherens junctions, the organization of adhesion proteins in a tight junction depends on additional proteins that bind the cytoplasmic side of the adhesion proteins
The key organizational proteins at tight junctions are the zonula occludens (ZO) proteins.
- The three major members of the ZO family—**ZO-1**, ZO-2, and **ZO-3**—are large scaffold proteins that provide a structural support on which the tight junction is built.
- These intracellular molecules consist of strings of protein-binding domains, typically including several **PDZ domains**—segments about 80 amino acids long that can recognize and bind the C-terminal tails of specific partner proteins
One domain of these scaffold proteins can attach to a claudin protein, while others can attach to occludin or the actin cytoskeleton.
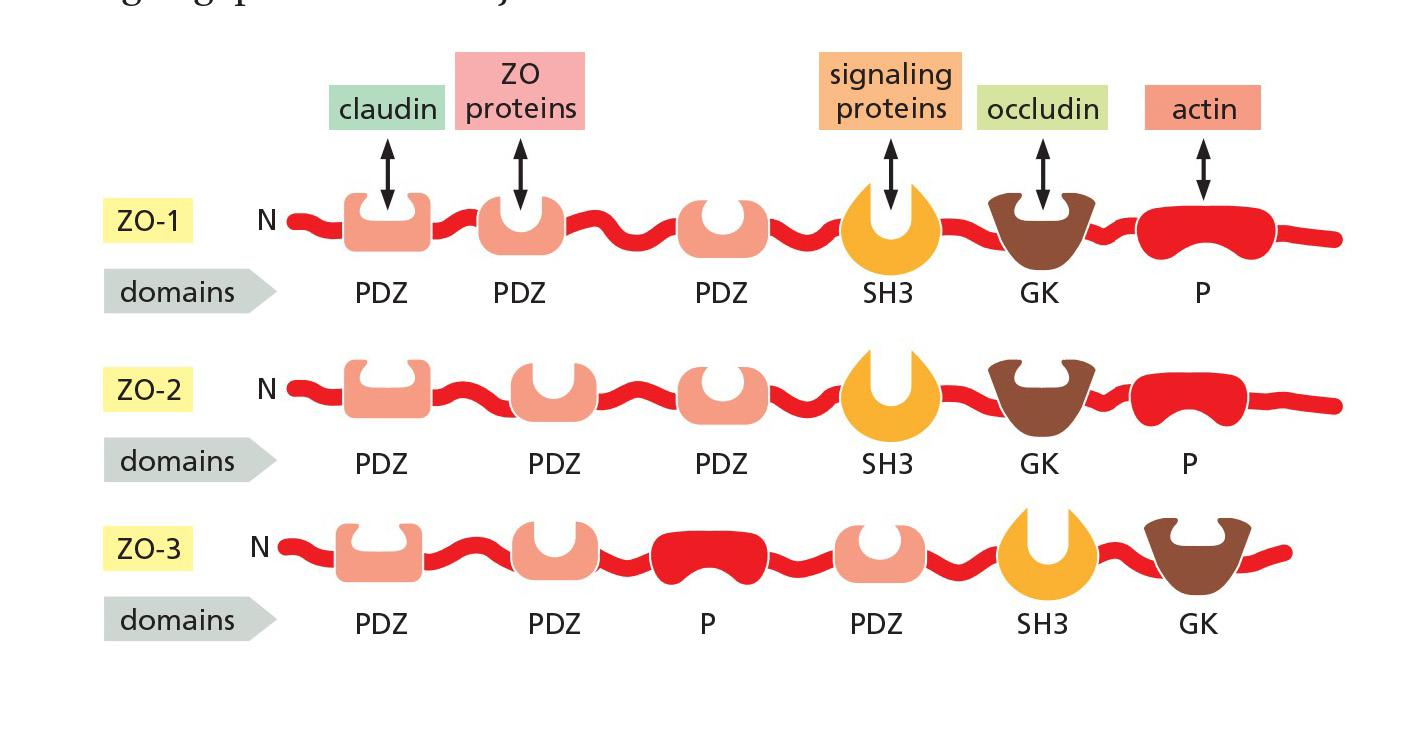
- ZO proteins contains multiple protein-binding domains
- These domains enable the proteins to interact with each other and with numerous other partners to generate a tightly woven protein network that organizes the sealing strands of the tight junction and links them to the actin cytoskeleton.
The tight-junctional network of sealing strands usually lies just apical to adherens and desmosome junctions that bond the cells together mechanically; the whole assembly is called a junctional complex
Tight junction and grass seeds
- Grass seeds normally contain proteins (glutens) which
- have only few of useful amino acids
- inhibit carbohydrate digestion. The latter could be avoided by heating (cooking bread)
- Gliadin (component of glutens) also split into polypeptides which weakens tight cell junctions: this will cause celiac disease. 10% of humans are sensitive to glutens.
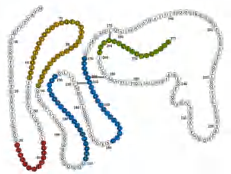
二、Signaling between neighboring cells
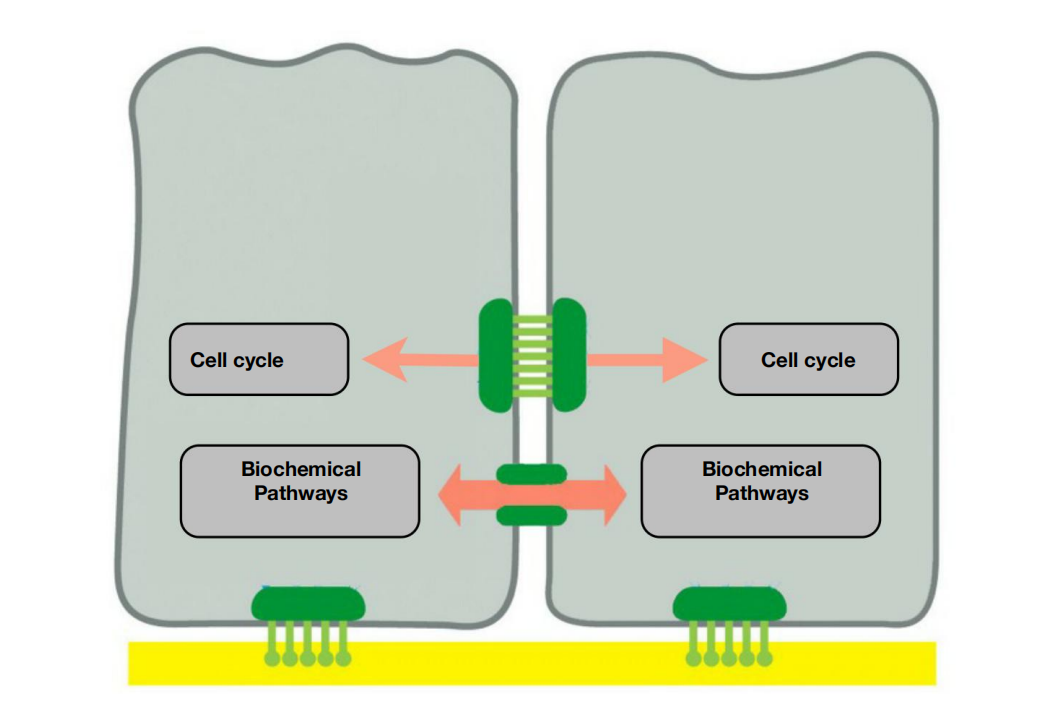
- Cell contacts regulate cell division and coordinate activities between cells
1. β-catenin in adhering junctions is a transcription factor activated by Wnt signaling
2. Neighboring cells communicate directly through gap junctions
Gap Junctions Couple Cells Both Electrically and Metabolically
- Tight junctions block the passageways through the gaps between epithelial cells, preventing extracellular molecules from leaking from one side of an epithelium to the other
- Another type of junctional structure has a radically different function: it bridges gaps between adjacent cells so as to create direct channels from the cytoplasm of one to that of the other. These channels are called gap junctions.

3. Gap junctions allow diffusion of small molecules between neighboring cells
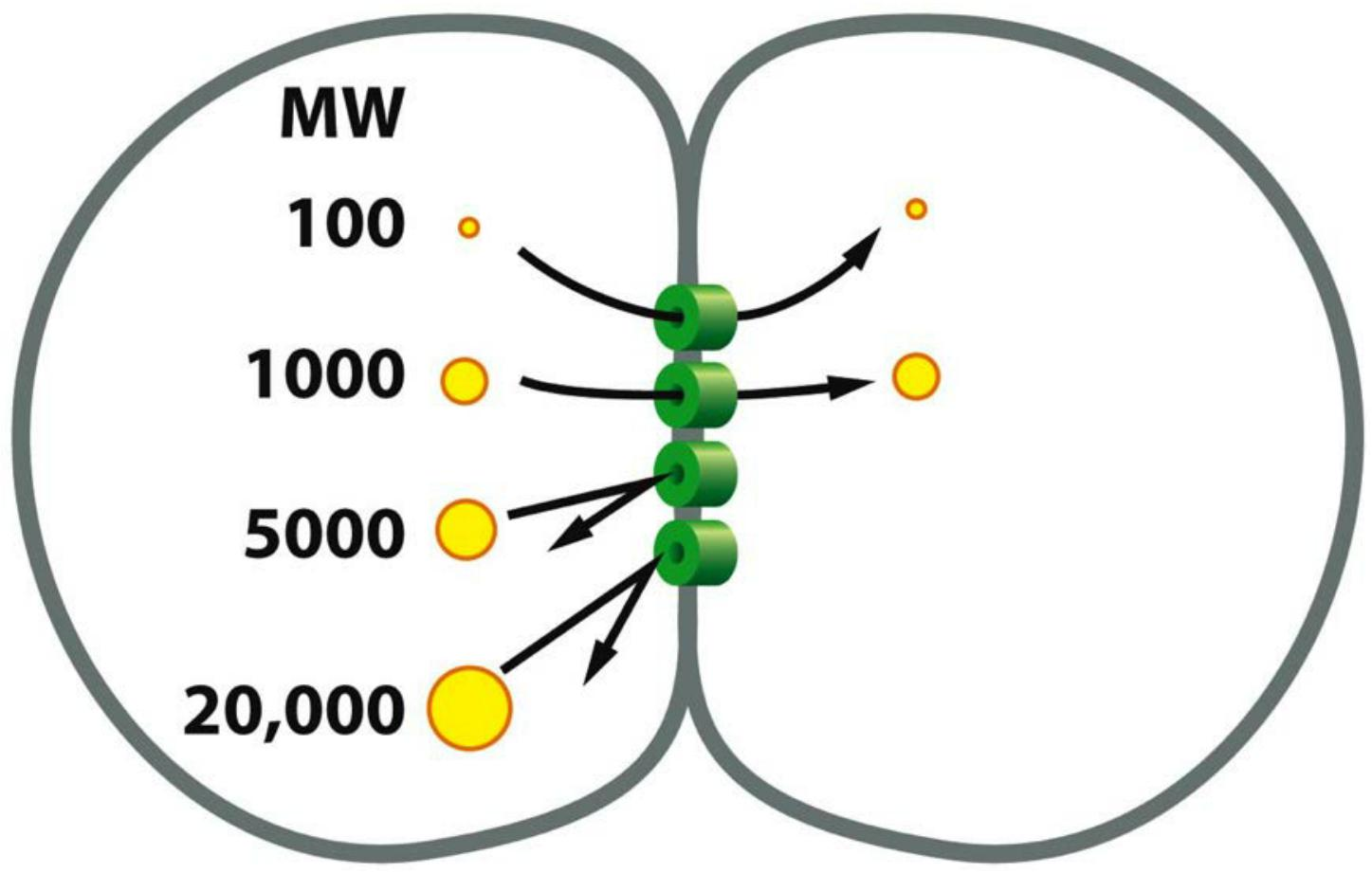
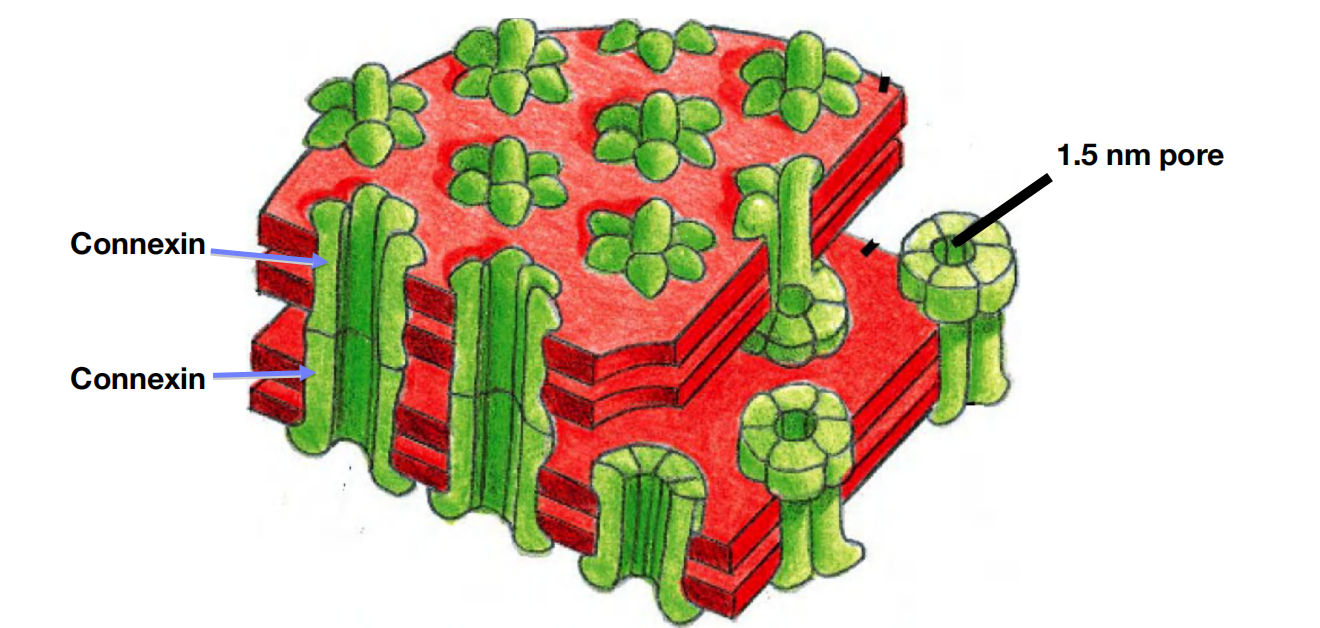
- Connexons are protein assembles which regulate the gap junction transfer.
- Six connexins form one connexon and two connexons build one channel.
Structure of Gap junctions
Each gap junction appears in conventional electron micrographs as a patch where the membranes of two adjacent cells are separated by a uniform narrow gap of about 2–4 nm
The gap is spanned by channel-forming proteins, of which there are two distinct families, called the connexins(连接蛋白) and the innexins.
- Connexins are the predominant gap-junction proteins in vertebrates, with 21 isoforms in humans
- Innexins are found in the gap junctions of invertebrates.
Gap junctions have a pore size of about 1.4 nm, which allows the exchange of inorganic ions and other small water-soluble molecules, but not of macromolecules such as proteins or nucleic acids
This electrical coupling via gap junctions serves an obvious purpose in tissues containing electrically excitable cells: action potentials can spread rapidly from cell to cell, without the delay that occurs at chemical synapses
- In vertebrates, for example, electrical coupling through gap junctions synchronizes the contractions of heart muscle cells as well as those of the smooth muscle cells responsible for the peristaltic movements of the intestine.
In principle, the sharing of small metabolites and ions provides a mechanism for coordinating the activities of individual cells in such tissues and for smoothing out random fluctuations in small-molecule concentrations in different cells.
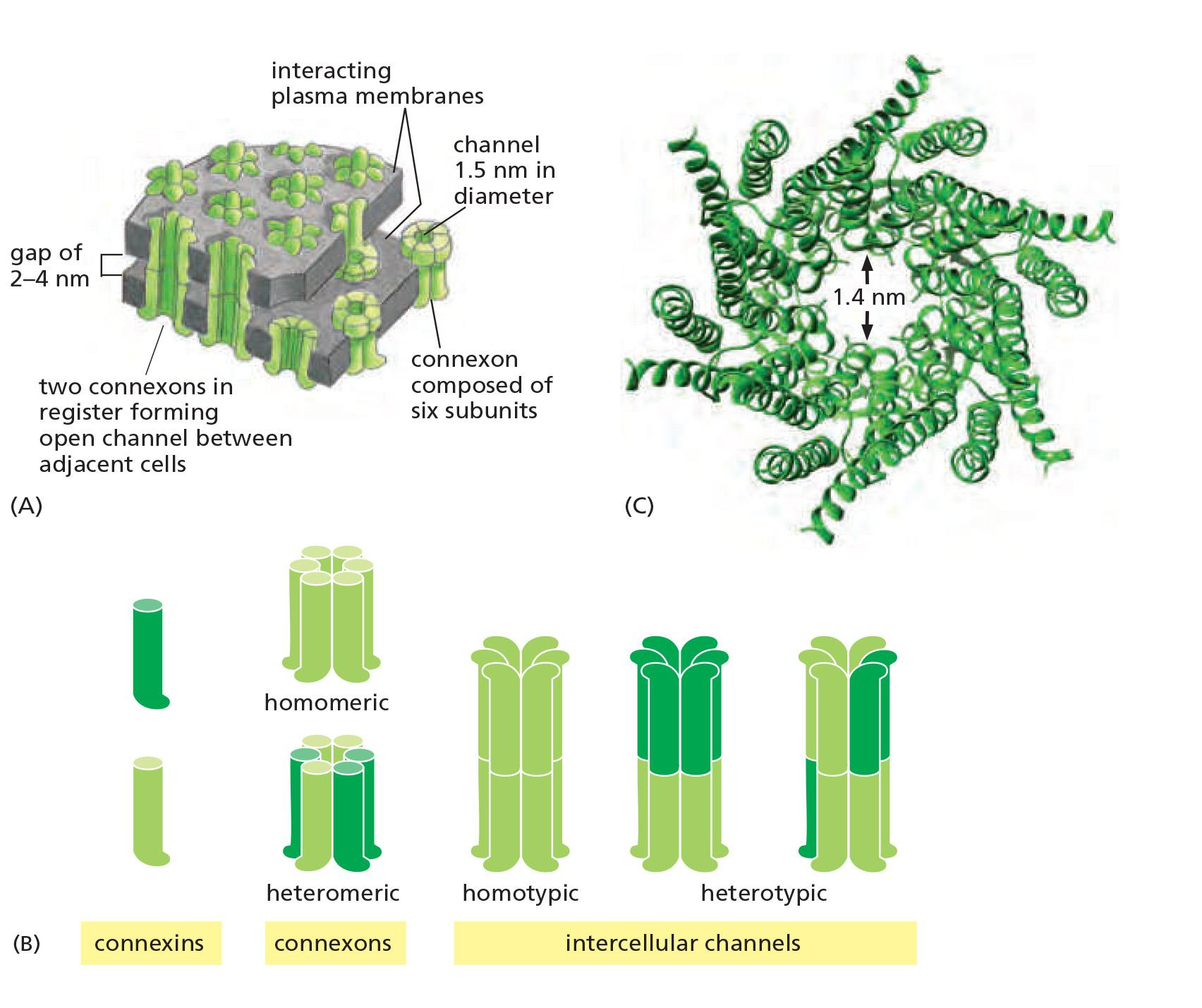
- Six connexons can be homomeric or heteromeric
A Gap-Junction Connexon Is Made of Six Transmembrane Connexin Subunits
- Connexins are four-pass transmembrane proteins, six of which assemble to form a hemichannel, or connexon
A gap junction consists of many such connexon pairs in parallel, forming a sort of molecular sieve.
- Not only does this sieve provide a communication channel between cells, but it also provides a form of cell–cell adhesion that supplements the cadherin and claudin-mediated adhesions we discussed earlier.
Gap junctions in different tissues can have different properties because they are formed from different combinations of connexins, creating channels that differ in permeability and regulation.
- Most cell types express more than one type of connexin, and two different connexin proteins can assemble into a heteromeric connexon
Like conventional ion channels (discussed in Chapter 11), individual gap-junction channels do not remain open all the time; instead, they flip between open and closed states
- These changes are triggered by a variety of stimuli, including the voltage difference between the two connected cells, the membrane potential of each cell, and various chemical properties of the cytoplasm, including the pH and concentration of free Ca2+
Some subtypes of gap junctions can also be regulated by extracellular signals such as neurotransmitters.
1. Gap junctions are dynamic structure
Each gap-junctional plaque is a dynamic structure that can readily assemble, disassemble, or be remodeled and it can contain a cluster of a few to many thousands of connexons
Studies with fluorescently labeled connexins in living cells show that new connexons are continually added around the periphery of an existing junctional plaque, while old connexons are removed from the middle of it and destroyed
- This turnover is rapid: the connexin molecules have a half-life of only a few hours.
- unpaired hemichannels are normally held in a closed conformation,
in some circumstances they can open and serve as channels for the release of small signal molecules.
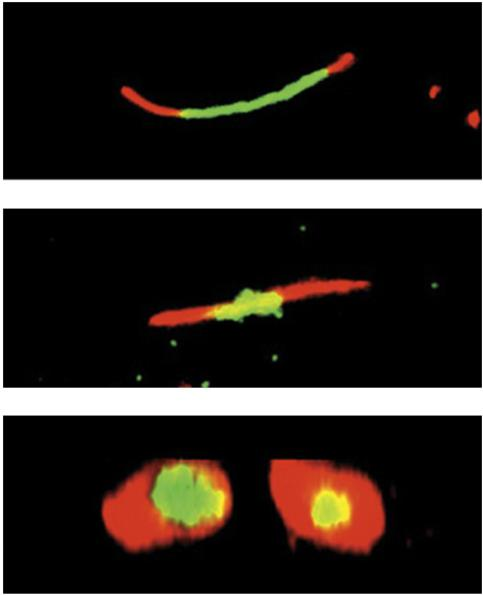
- Assembly and remodeling of gap junctions over 20 hrs
- Experiment (metabolic labeling)
- Cells expressing tetra-cysteine-tagged connexin (connexin-4C) were used to label gap junctions.
- The tag is detected via fluorescent dyes: first with the green dye FlAsH and 4 hrs later with the red dye ReAsH.
- After 8 hrs incubation, most of the “old” green label disappeared and was replaced by newer red connexins.
Regulation of gap-junction communication
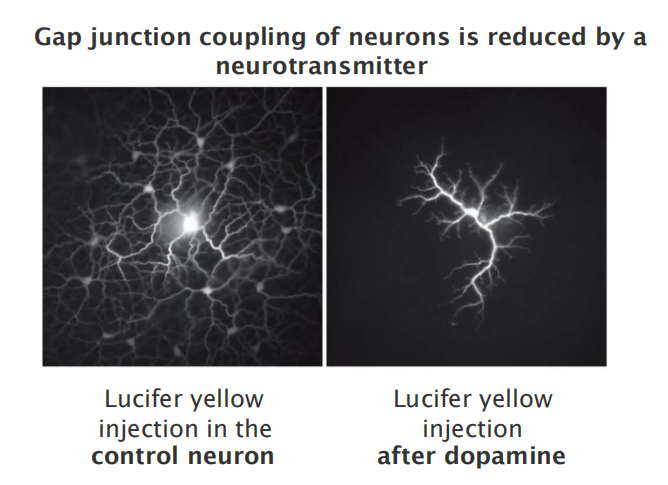
- Gap junctions have open and closed states
- Closure is triggered by lower pH or an increase in Ca2+ levels
- Gap junctions can be regulated by extracellular signals
- Experiment:
- One neuron was injected with a fluorescent dye (lucifer yellow).
- The dye diffuses via gap junctions, revealing all connected cells (left).
- After dopamine treatment, permeability/connectivity between neurons is greatly reduced (right).
1. End-to-end junctions in heart muscle
2. The function of gap junctions
Electrically excitable cells
- rapid spreading of action potentials (no delay by synapses) synchronization of a set of neurons electrical coupling throughgap junctions allows:
- synchronization of heart muscle cells
- synchronization of smooth muscle cells (peristaltic movement of intestine
Other cells:
- sharing of metabolites smoothing-out random fluctuations of metabolites and small molecules coordination of cells in tissues / organs (e.g. liver)
Mutations in connexins are severe:
- deafness (connexin 26)
- cataracts of lenses
- neurodegenerative disease, etc.
Gap junction in plant: plasmodesmata 原生质丝
原生质丝(英语:Plasmodesmata)为植物细胞和部分藻类细胞壁间贯穿细胞壁的特有孔道,可以让相邻细胞的细胞质相互流通。有微小孔道,为细胞间物质运输与信息传递的重要信道,信道中有一连接两细胞内质网的连丝微管,细胞质可经由原生质丝交流及运输,此过程称为共质体运输 (Symplast)。
In Plants, Plasmodesmata Perform Many of the Same Functions as Gap Junctions
The tissues of a plant are organized on different principles from those of an animal.
- This is because plant cells are imprisoned within tough cell walls composed of an extracellular matrix rich in cellulose and other polysaccharides
- Thus, plant cells have only one class of intercellular junctions, plasmodesmata
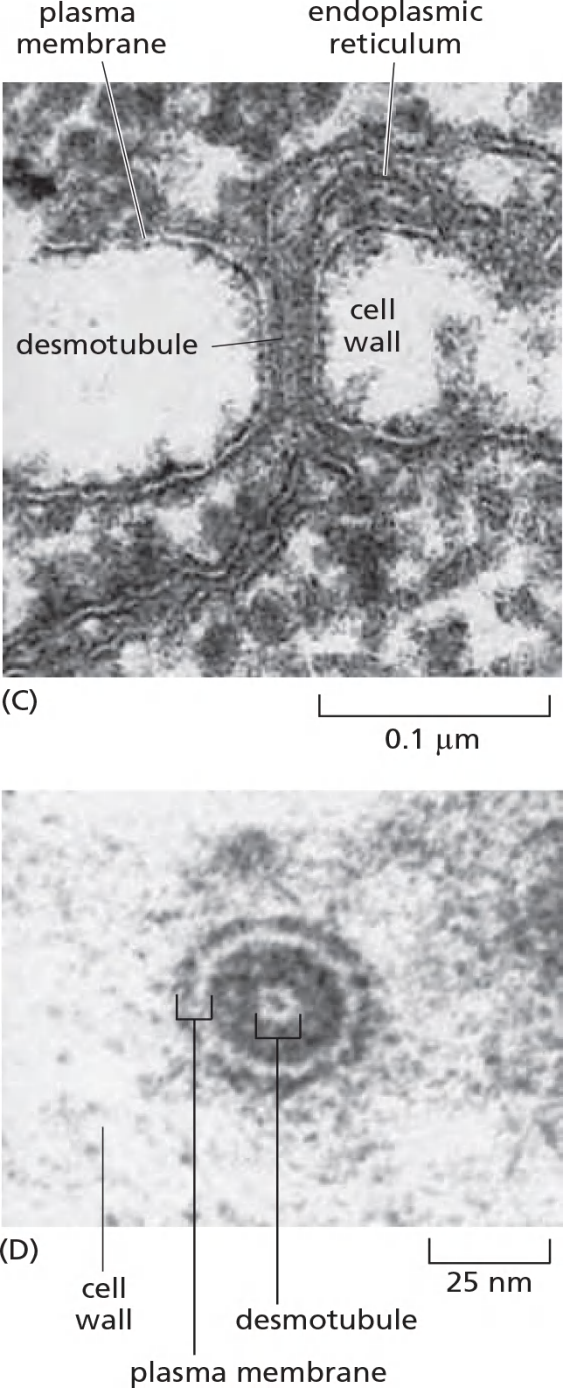
Running through the center of the channel in most plasmodesmata is a narrower cylindrical structure, the desmotubule (连丝微管), which is continuous with elements of the smooth endoplasmic reticulum (ER) in each of the connected cells
They can also be inserted de novo through preexisting cell walls, where they are commonly found in dense clusters called pit fields. When no longer required, plasmodesmata can be removed
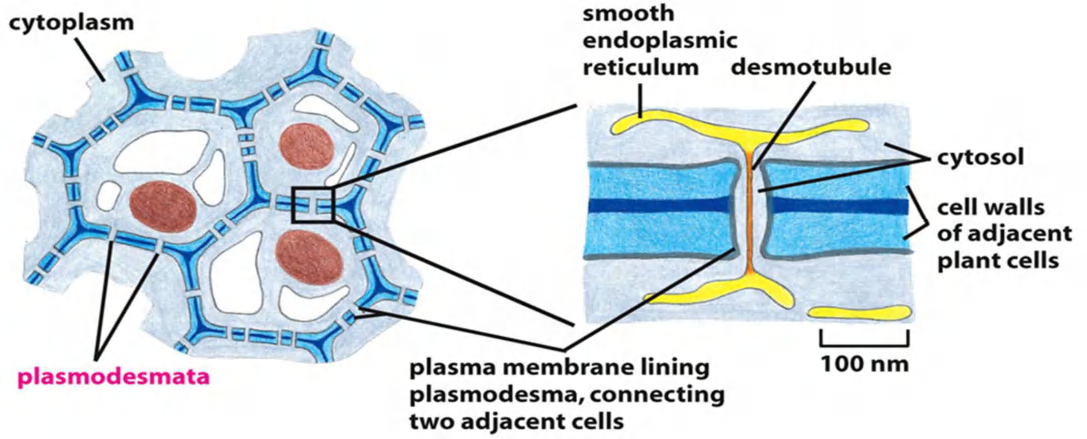
- Connect the cytoplasm of the two adjacent cells, cut-off 800 Da by a rough and cylindrical channel with a diameter of 20-40 nm.
- Connected by desmotubule which is the continuation of the smooth endoplasmic reticulum (ER)
- Certain virus movement proteins spread via plasmodesmata
Signal relayed junctions: synapse
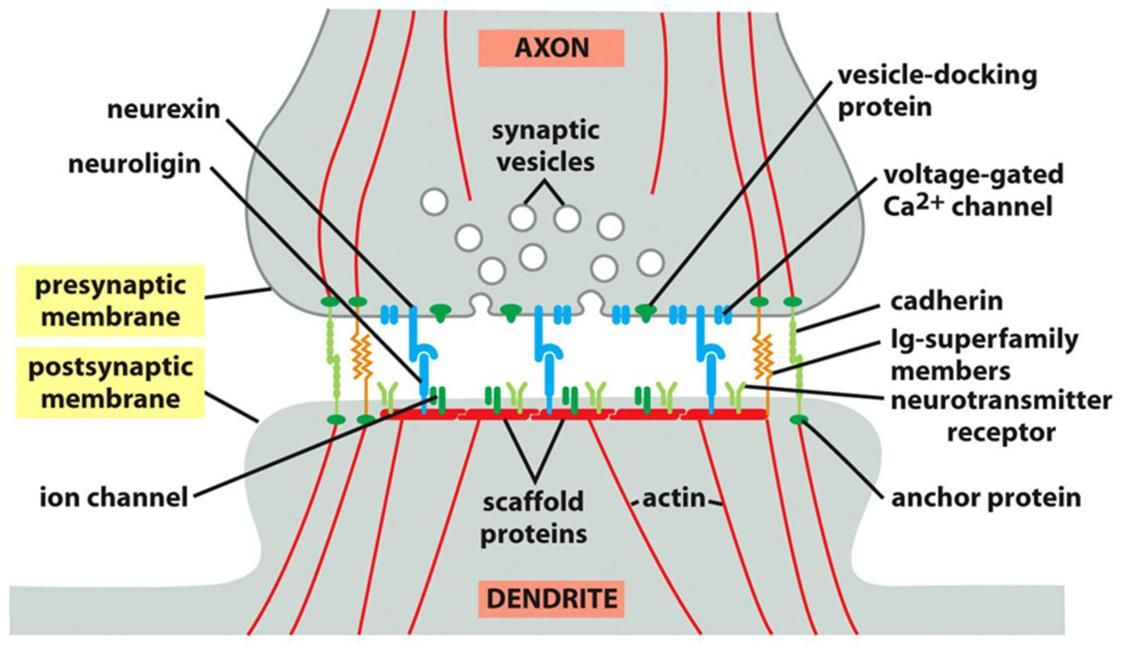
- Many types of adhesion molecules act together to create a synapse.