L08 Lipid Metabolism II Membrane Lipids, Steroids, Isoprenoids, and Eicosanoids
Lipid Metabolism II: Membrane Lipids, Steroids, Isoprenoids, and Eicosanoids (脂质代谢II: 膜脂,类固醇,异戊二烯和花生酸类)
一、Metabolism of Glycerophospholipids
Intracellular synthesis and transport of membrane phospholipids
The most abundant phospholipids are those derived from glycerol.
These glycerophospholipids are found primarily as components of membranes.
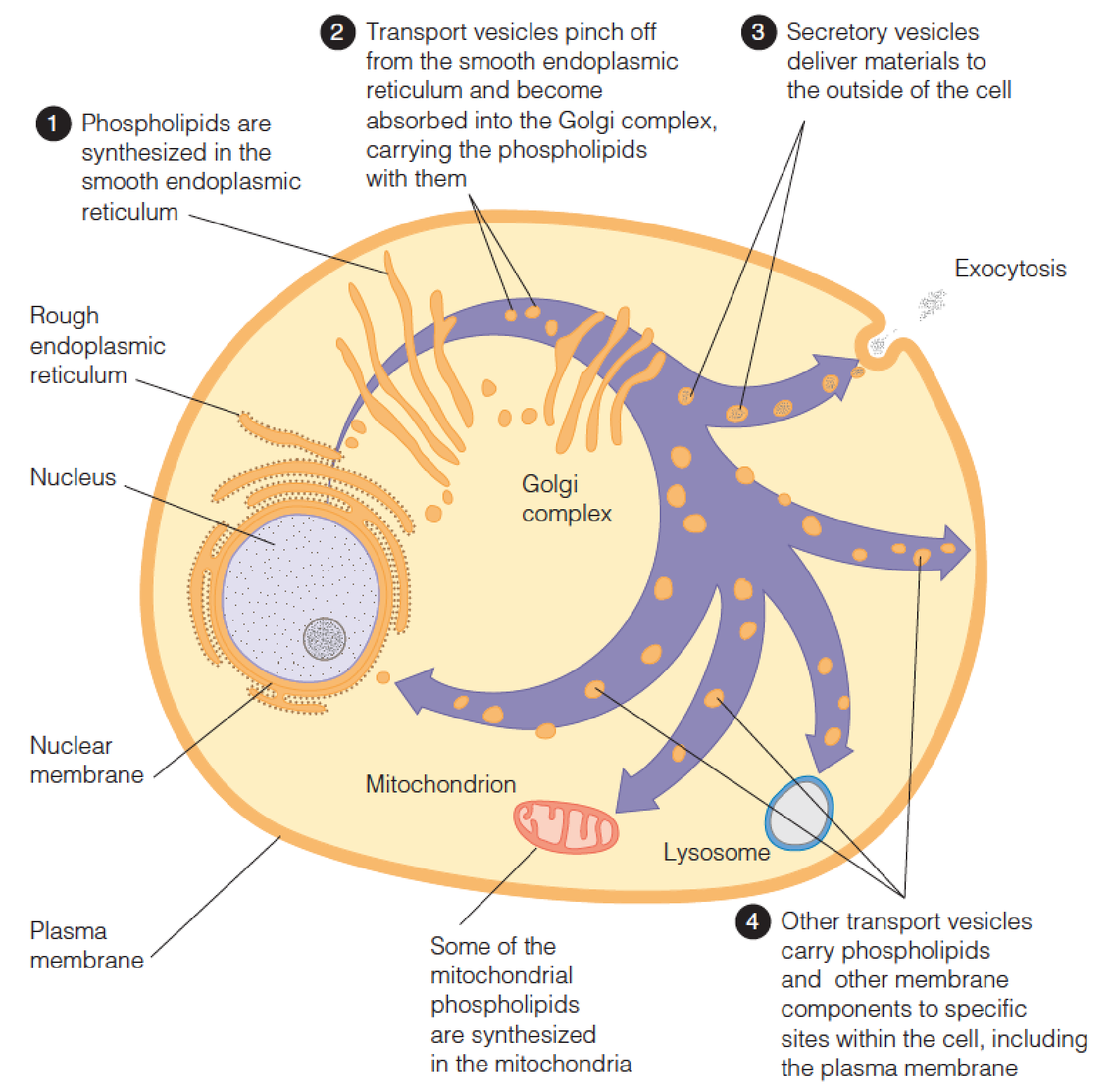
- Lipoprotein: transport lipids
- Blood clotting
- Lung functioning
Pathways in glycerophospholipid biosynthesis
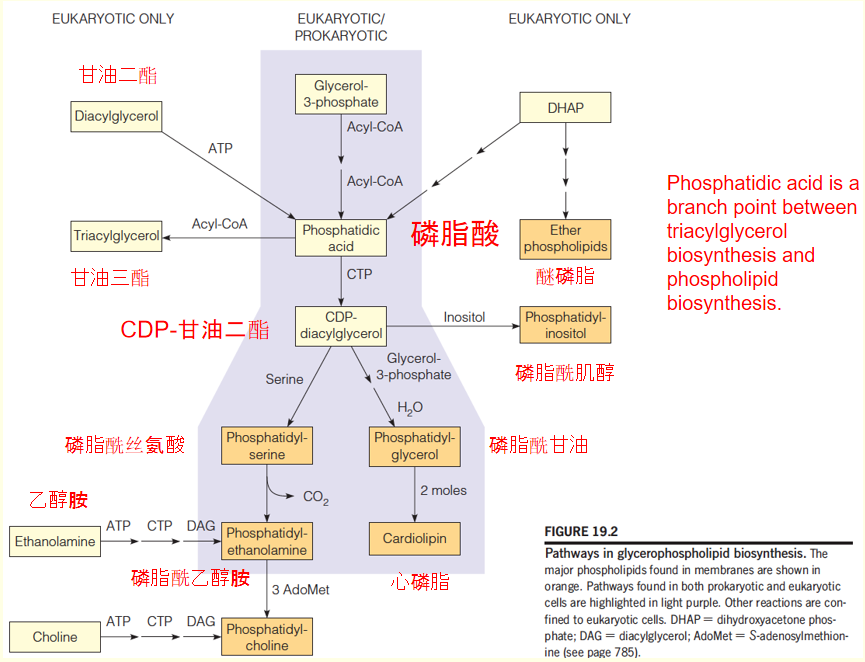
1. Synthesis of phosphatidic acid and CDP-DAG in bacteria
In bacteria, phospholipids: 10% of the dry weight of the cell
- Yet, their only known role is as components of membranes.
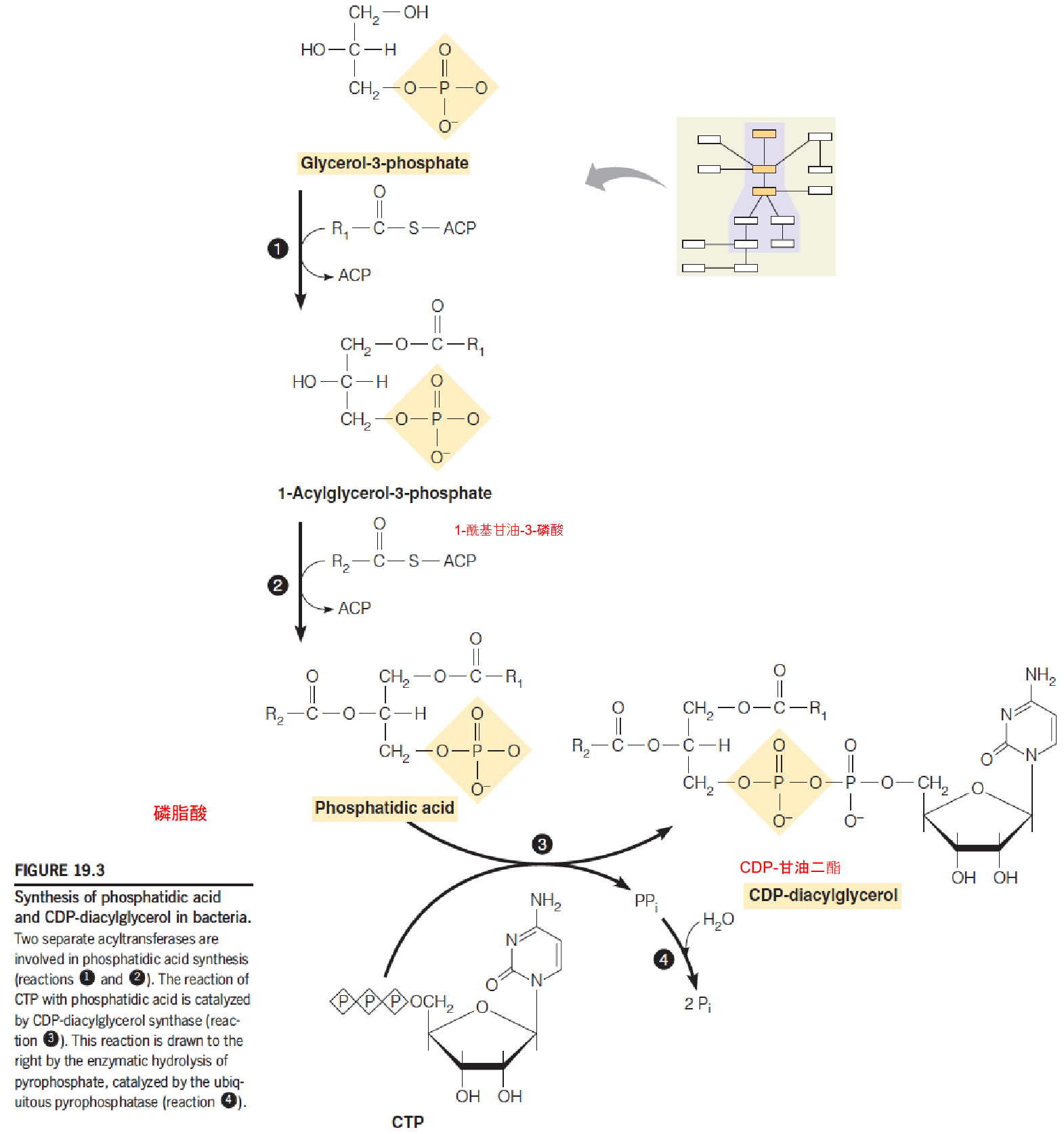
- The energy cofactor: CTP, whose role is similar to that of UTP in polysaccharide synthesis.
- Phosphatidic acid ➔ CDP-DAG (metabolically active) (similar to: G1P ➔ UDP-glucose)
- Phosophatidic acid is synthesized by two successive acylation of glycerol-3-phosphate
- Acyl groups are brone on ACP
2. Synthesis of PS, PE and PG from CDP-DAG in bacteria
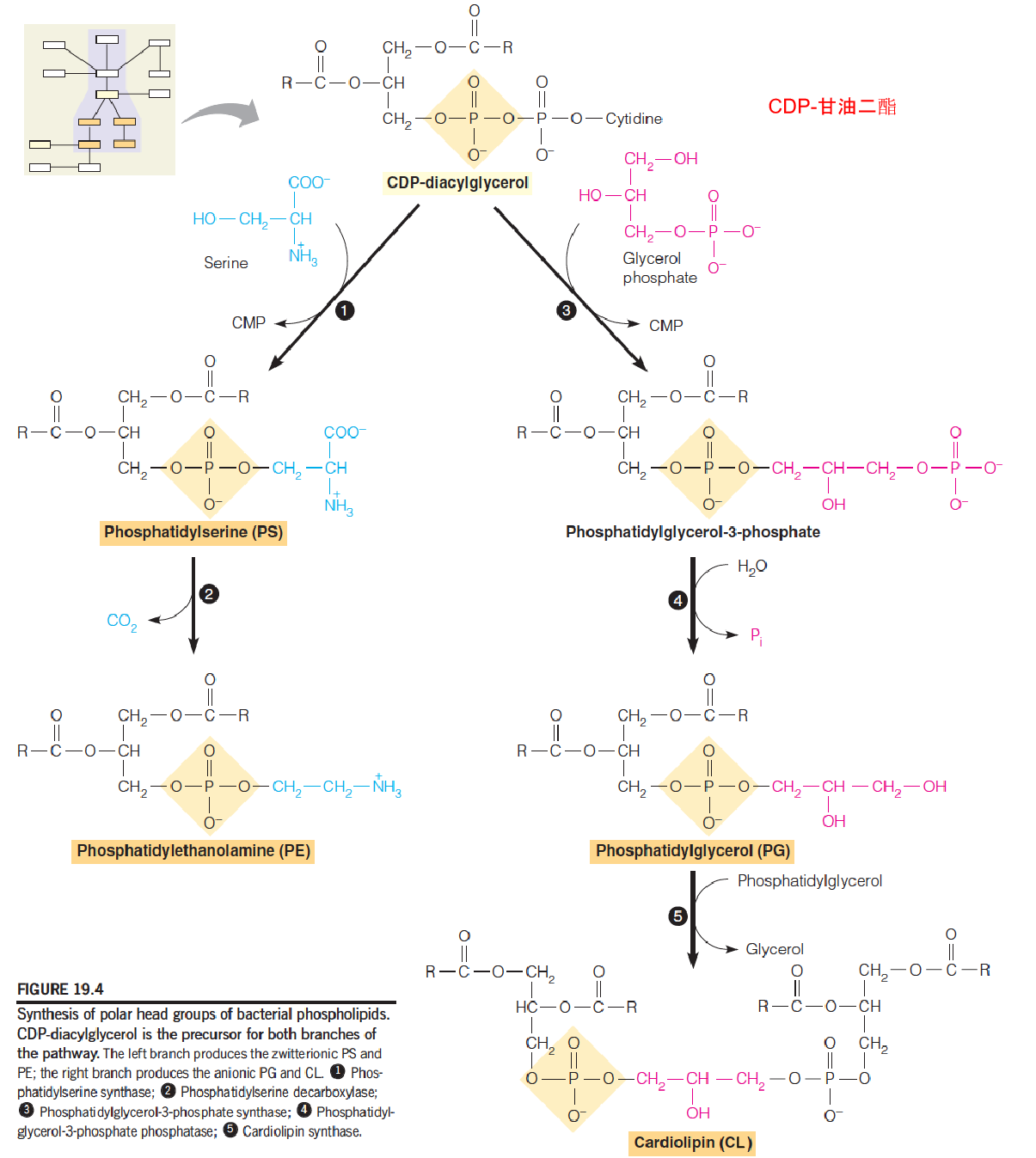
- PS immediately undergoes decarboxylation to PE;
- PS does not accumulate in bacteria
In E.coli, the membrane has 3 phospholipids: phosphatidylethanolamine (PE) (70-80%), phosphatidylglycerol (PG) and cardiolipin (CL).
The fatty acid content of these lipids is also simple, with three dominating: palmitate (16:0,棕榈酸盐(或酯)), palmitoleate (16:1c△9,棕榈油酸酯), cis-vaccenate (18:1c△11)
3. Pathways in glycerolphospholipids biosynthesis in eukaryotic cells
Most eukaryotic cells contain 6 classes of glycerolphospholipids: phosphatidylethanolamine (PE), phosphatydylglycerol (PG), and cardiolipin (CL) found in bacteria; plus phosphatidyserine (PS), phosphatidylcholine (PC), and phosphatidylinositol (PI).
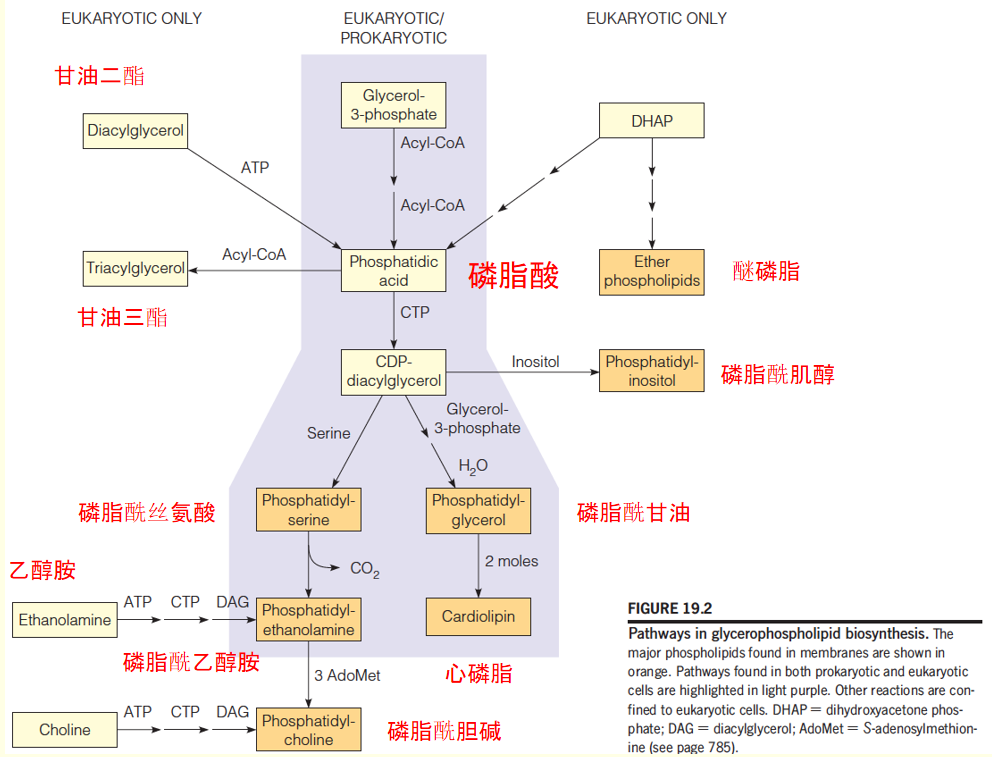
- Phosphatidic acid: major precursor to all six compounds.
- Pathways to PE, PS, PG, and CL are virtually identical to those for bacteria
- In eukaryotic cells, additional pathways that start with the free base-choline(游离碱胆碱) and ethanolamine( 乙醇胺,氨基乙醇,胆胺), respectively, leading to PC and PE.
Three routes to phosphatidic acid in eukaryotic cells
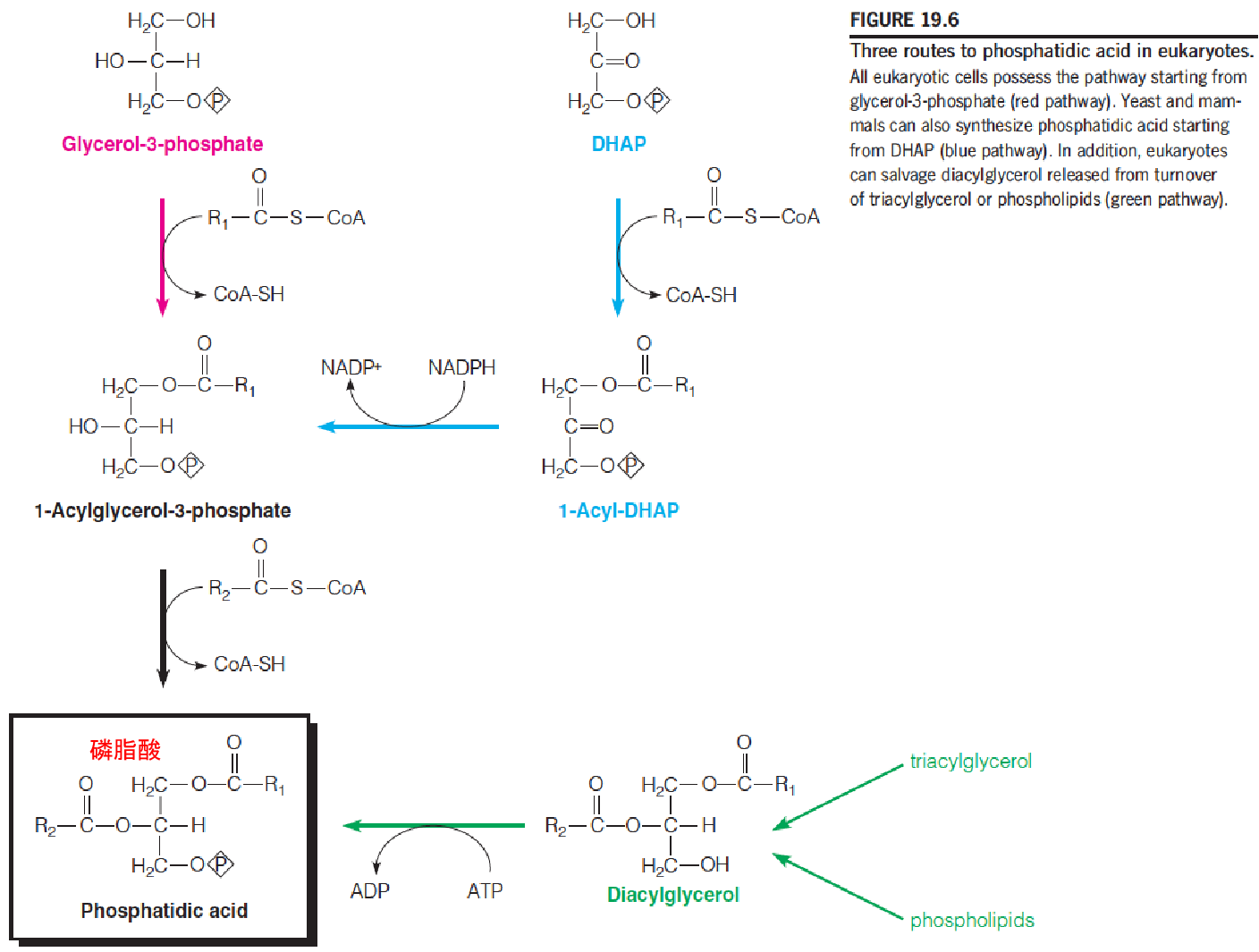
Eukaryotic cells display three biosynthetic routes to phosphatidic acid
- The major pathway, starting with glycerol-3-phosphate(3-磷酸甘油), is similar to that used by bacteria, except that the acyltransferases use acyl-CoAs as substrates instead of acyl-ACPs.
- A second pathway starts with dihydroxyacetone phosphate (DHAP), which accepts a fatty acyl at position 1 from an acyl-CoA, followed by reduction to 1-acylglycerol-3-phosphate and a second acylation.
- The third route simply involves phosphorylation by a specific kinase of DAG.
4. Salvage pathways: Synthesis of PC and PE in mammals
The most abundant phospholipids in most eukaryotic cells are PC and PE.
PC and PE can be synthesized from PS or through alternative pathways that start with free choline(胆碱) or ethanolamine(ethanolamine ) (salvage pathways), respectively.
- Salvage pathways to phospholipids, starting with choline or ethanolamine, are quantitatively important in eukaryotic cells.
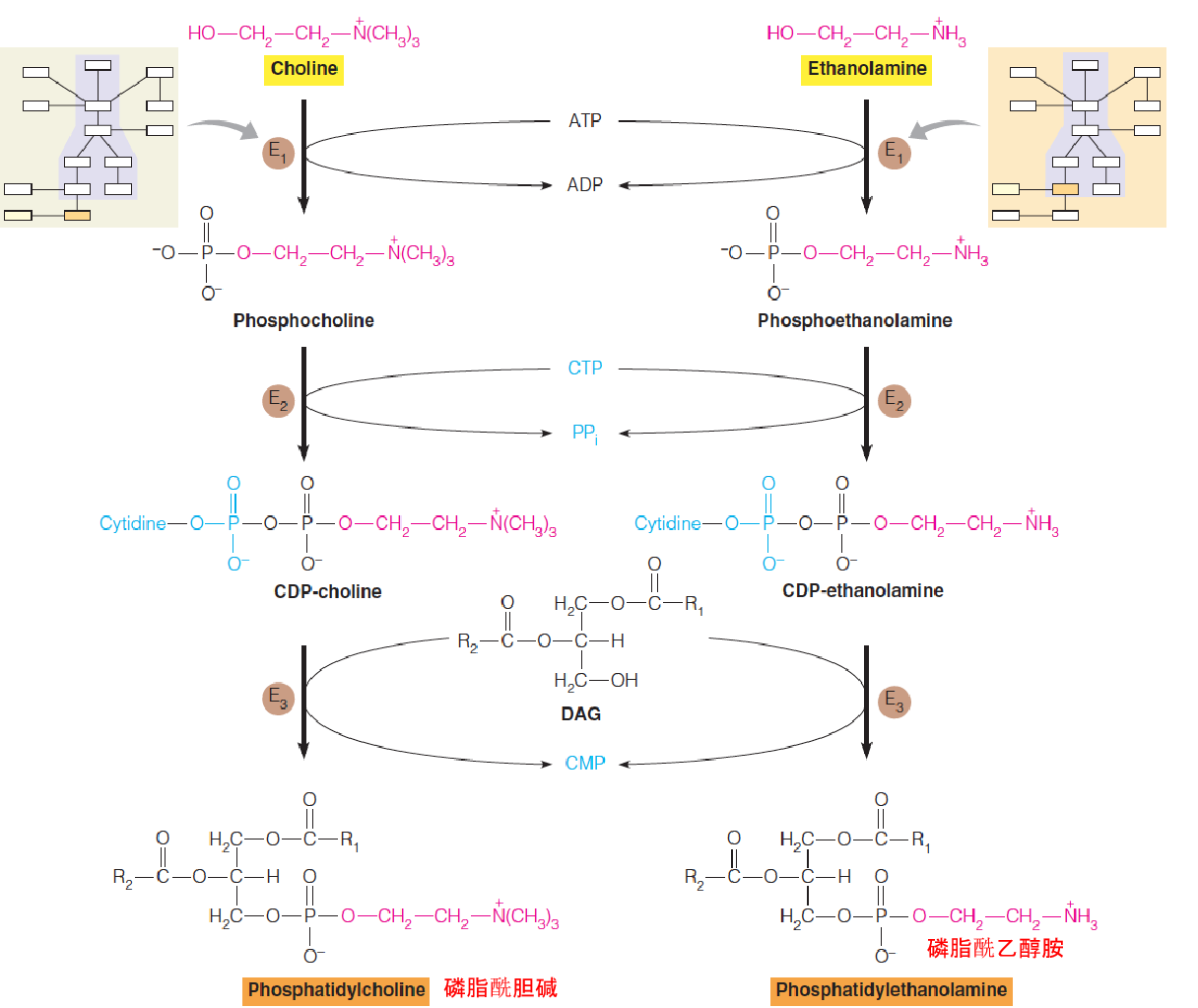
- E1: choline kinase, cytosolic
- E2: CTP:phosphocholine cytidyltransferase, cytosolic and microsomal fractions
- E3: CDP-choline: 1,2-diacylglycerol choline phosphotransferase, a membrane-bound in the endoplasmic reticulum
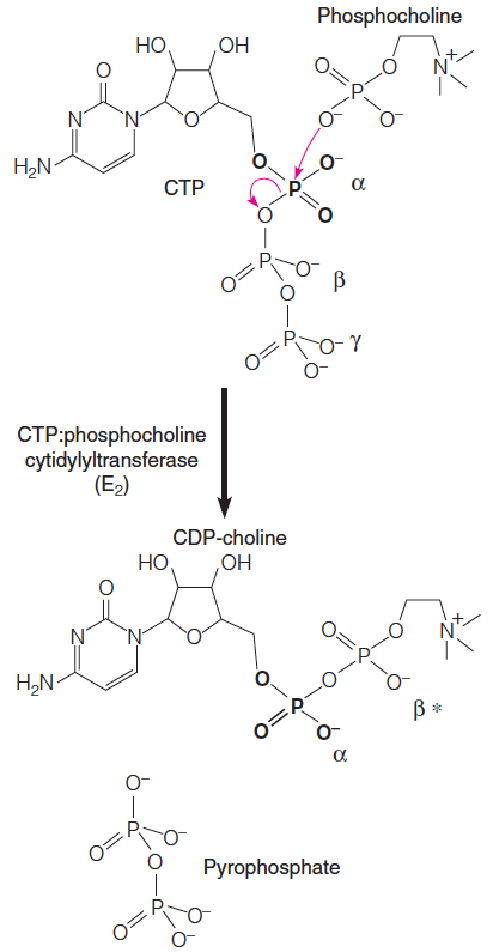
Metabolic activation of phosphocholine
CTP:phosphocholine cytidylyltransferase (胞苷酰转移酶) catalyzes nucleophilic attack at the $\alpha$-phosphate of CTP by a phosphoryl oxygen of phosphocholine, displacing the $\beta$, $\gamma$-phosphates (pyrophosphate) of CTP, forming CDP-choline.
The second pathway to PE and PC begins with the conversion of phosphatidylserine (PS) to PE, which is catalyzed by either of two different enzymes:
Phosphatidylserine decarboxylase (磷脂酰丝氨酸脱羧酶) is a mitochondrial enzyme that, like the corresponding bacterial enzyme, decarboxylates PS to PE.
The second enzyme is a calcium-activated transferase, phosphatidylethanolamine serinetransferase (磷脂酰乙醇胺丝氨酸转移酶), which exchanges free ethanolamine
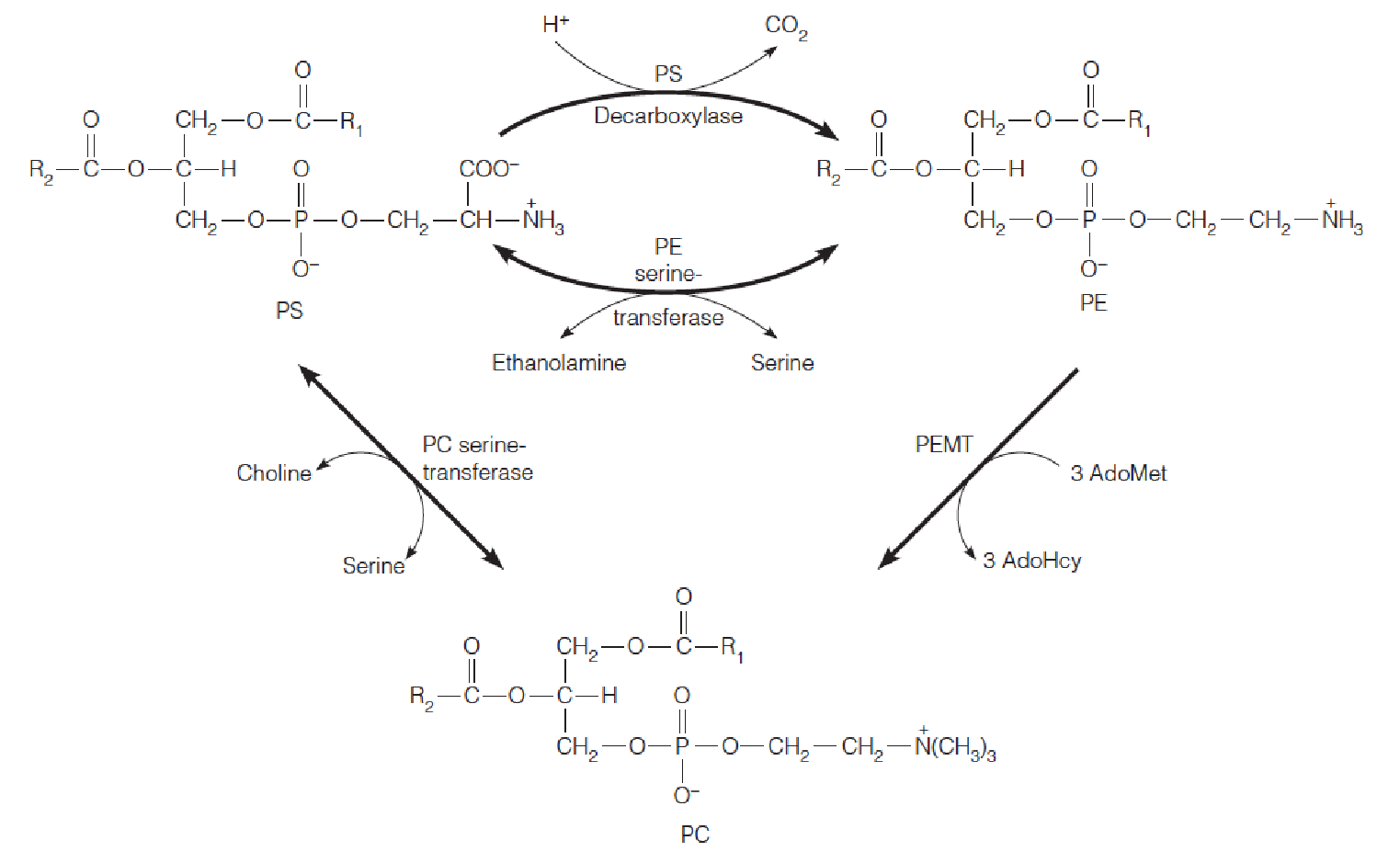
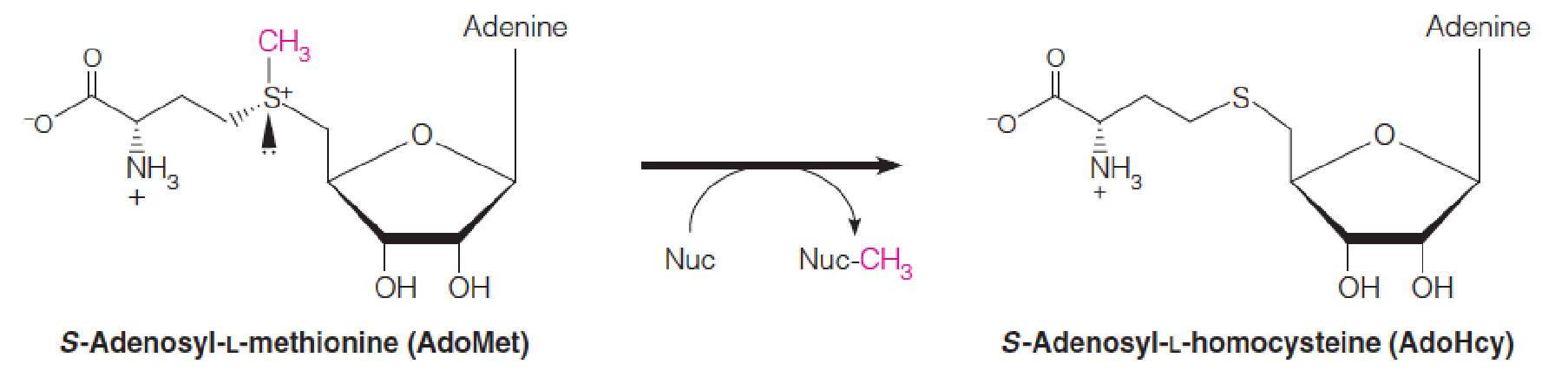
S-Adenosyl-L-methionine (S-腺苷-L-甲硫氨酸) (AdoMet) is the methyl group donor in synthesis of phosphatidylcholine and numerous other methylated metabolites.
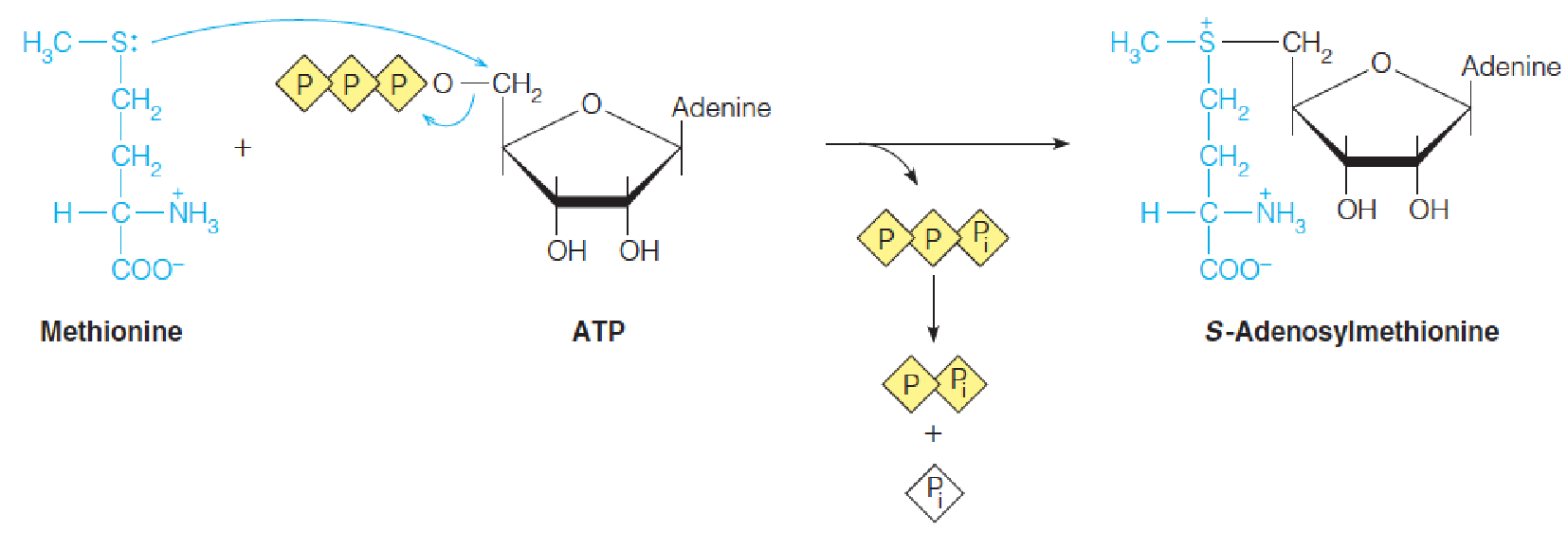
AdoMet is formed from methionine and ATP in an unusual reaction catalyzed by methionine adenosyltransferase (蛋氨酸腺苷转移酶), in which ATP is cleaved to yield inorganic triphosphate (PPPi) plus an adenosyl moiety linked directly via the ribose C5 to the methionine sulfur.
The triphosphate is hydrolyzed by the enzyme to pyrophosphate(PPi) and orthophosphate (Pi), drawing the reaction to completion.
Metabolism of Glycerophospholipids
Fatty acid chains in phospholipids are remodeled through the concerted actions of phospholipases and specific lysophospholipid acyltransferases (溶血磷脂酰基转移酶) to meet the needs of the organism.
Specificities of phospholipases (磷脂酶) A1, A2, C, and D:
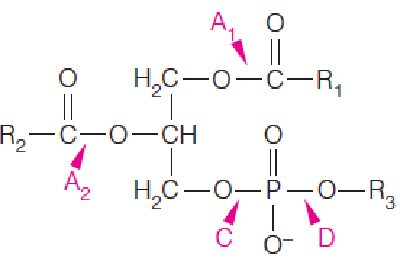
- Phospholipase A2, releases fatty acid from position 2;
- Phospholipase A2: from cobra venom(眼镜蛇毒), causes hemolysis and rupture of red blood cells;
- repair of damaged phospholipids (acyl chains by ROS, reactive oxygen species).
Lung surfactant(肺表面活性剂): 60% dipalmitoylphosphatidylcholine (双棕榈酰磷脂酰胆碱) (a PC in which palmitoyl chains occupy both positions 1 and 2);
- Infants with respiratory distress syndrome: defects in either synthesis or secretion of this substance. Its synthesis is accelerated immediately after birth.
(1) Structure of phospholipase A2
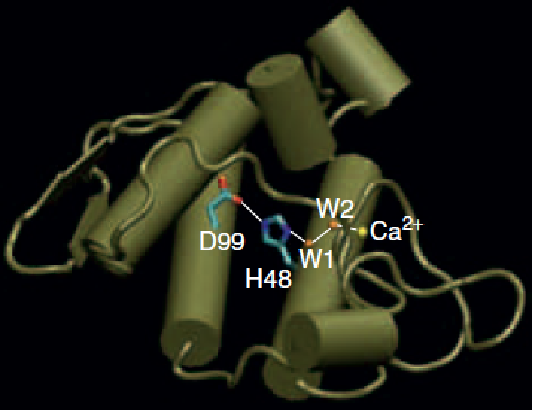
An enzyme that metabolizes membrane phospholipids.
Catabolism of phospholipids at membrane–water interfaces is important both in modification of membrane structure and as a source of second messengers and other regulators.
The crystal structure of the porcine pancreas phospholipase A2 reveals the catalytic triad (D99-H48-water) and the active site Ca2+ ion, liganded to a second water molecule (W2).
(2) Biosynthesis of cardiolipin and phosphoinositides in eukaryotes
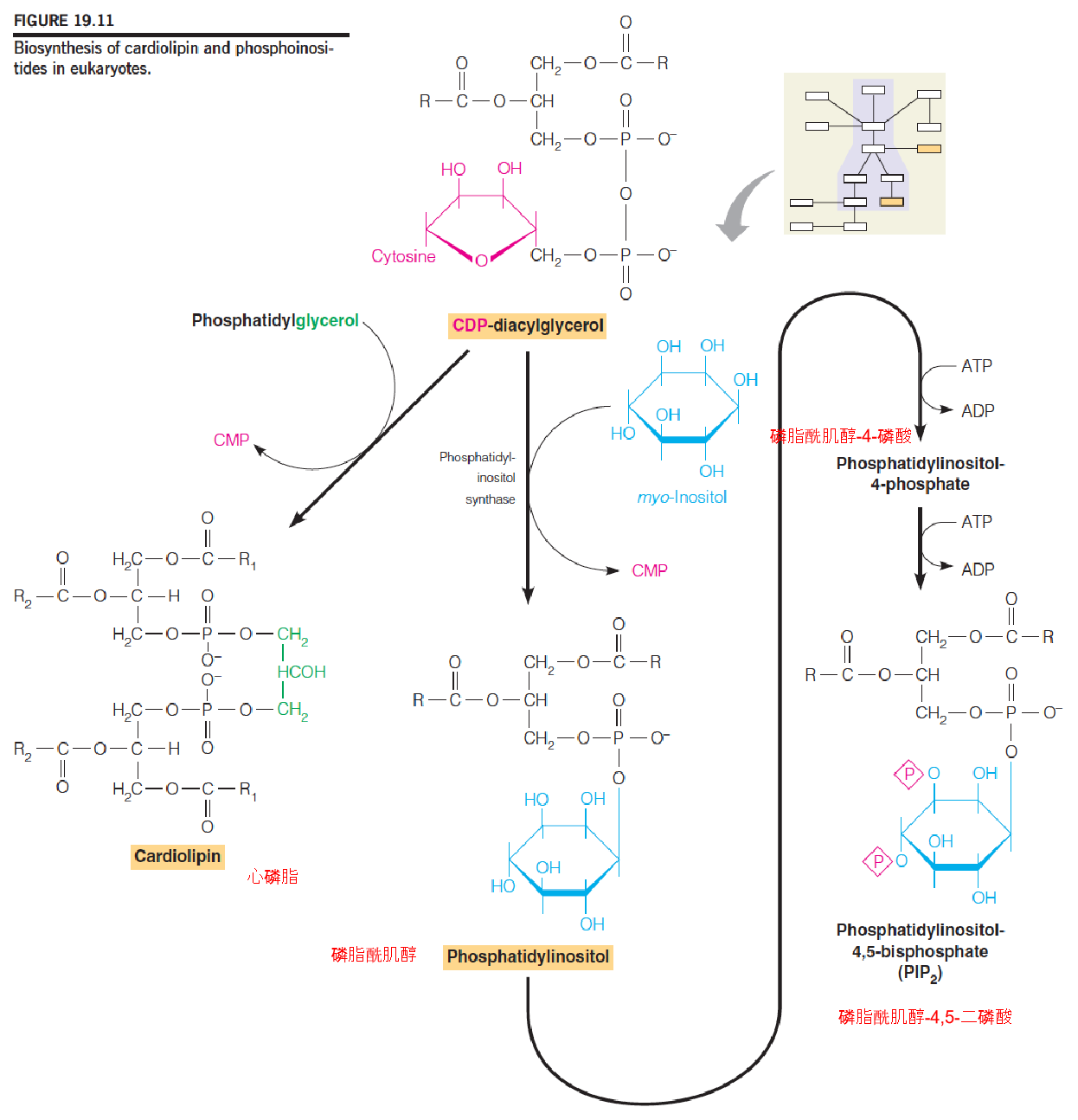
Phosphatidylinositol (磷脂酰肌醇) and its phosphorylated derivatives, collectively termed phosphoinositides (磷酸肌醇), play important roles as precursors of second messengers.
PIP3 plays an important role as a second messenger in transmembrane signaling, the transmission of an extracellular signal to some element of the intracellular metabolic apparatus.
- PIP3 (Phosphatidylinositol (3,4,5)-trisphosphate): 磷脂酰肌醇(3,4,5)-三磷酸
二、Metabolism of Sphingolipids
Nervous tissues;a number of human genetic diseases
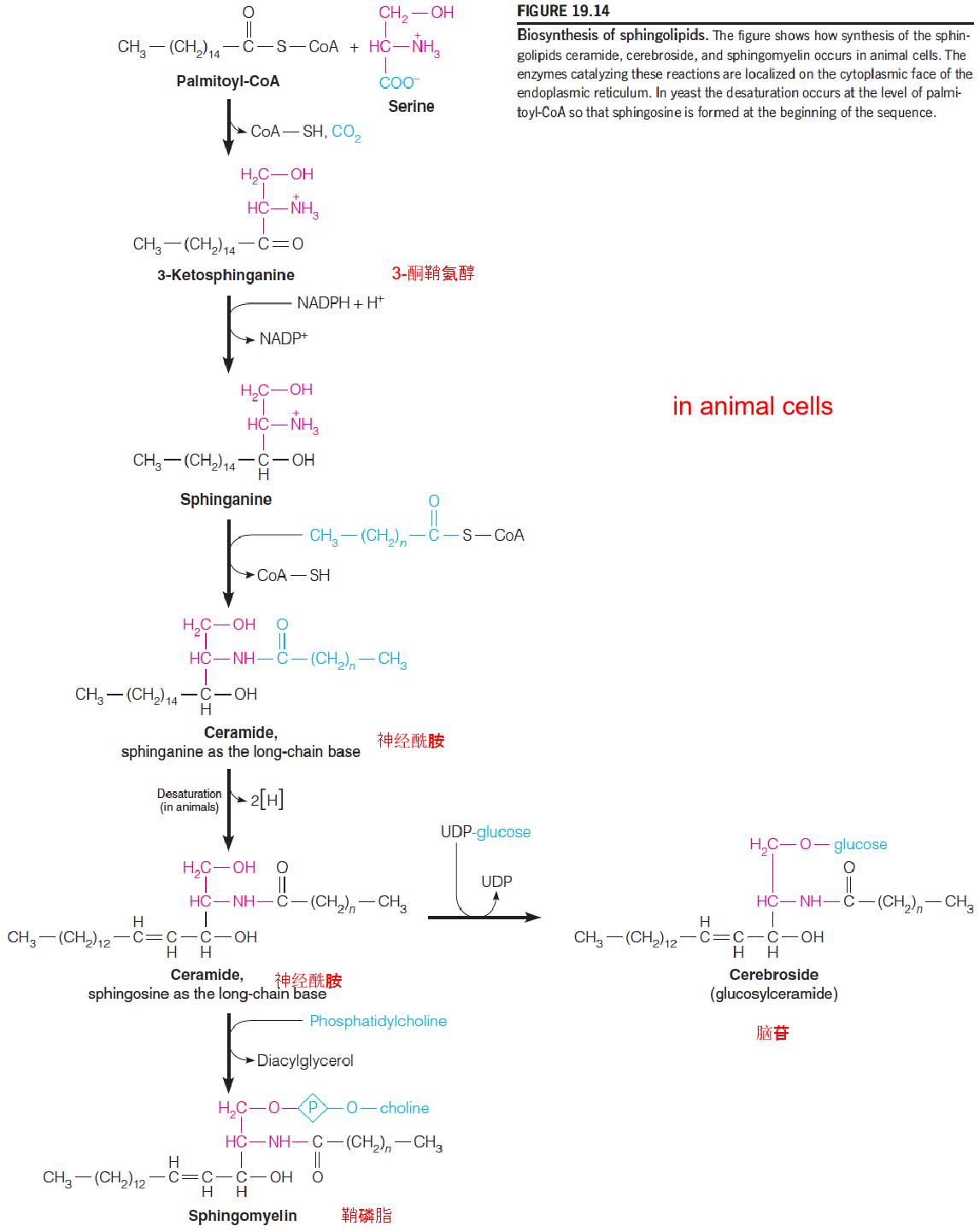
- Sphingolipids: from sphingosine(鞘氨醇) derivative: sphinganine
- Ceramide (神经酰胺)
- Ceramide serves as the precursors to both sphingomyelin and glucosphingolipids
- Cerebroside (脑苷) (glucoceramide)
- Sphingomyelin (鞘磷脂)
Pathways of synthesis of glucosphingolipids (糖鞘脂)
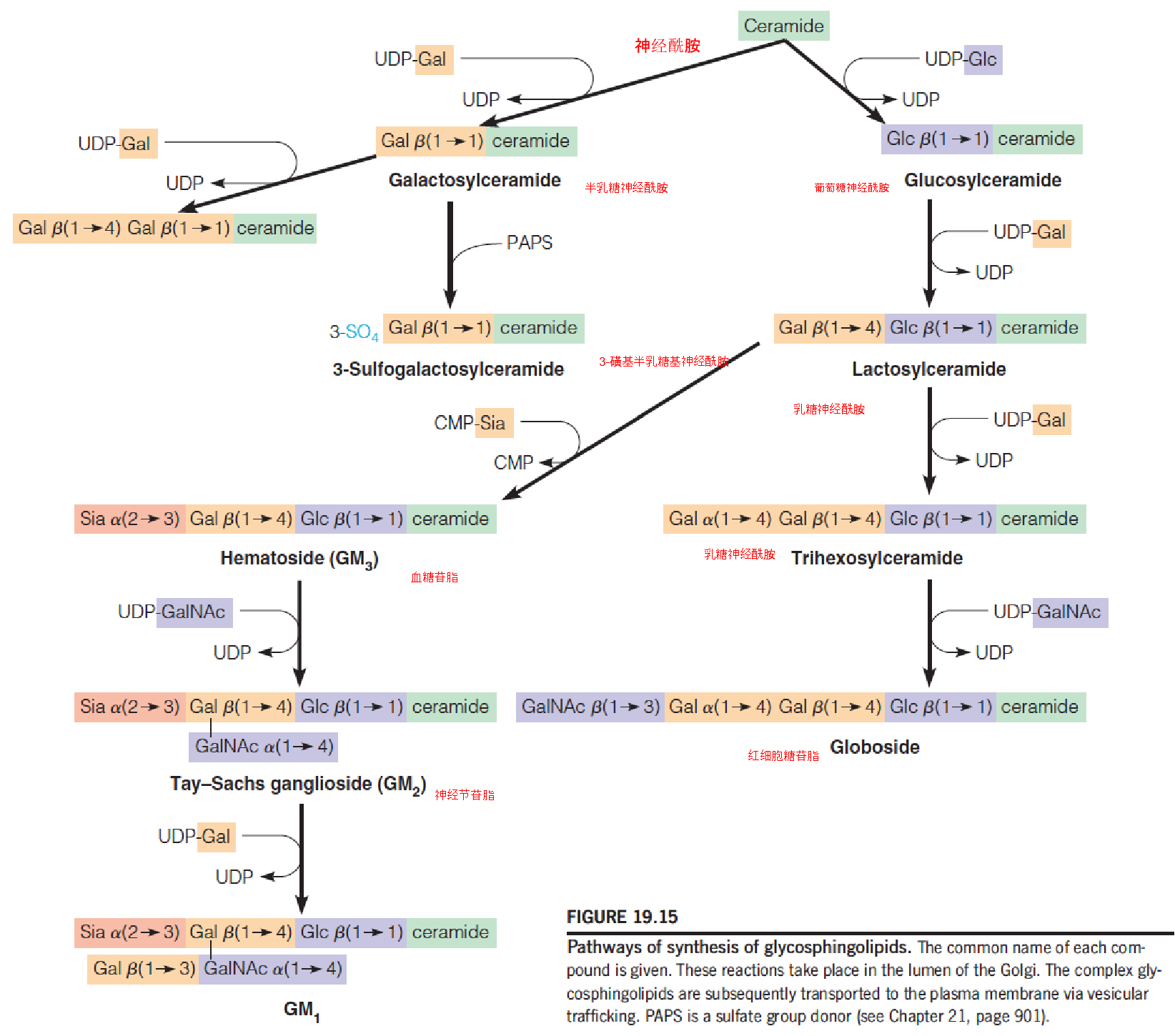
- A stepwise addition of monosaccharide units, using nucleotide-linked sugars as the activated substrates and with ceramide and the initial monosacchride acceptor
- UDP-glucose (UDP-Glc)
- UDP-galactose (UDP-Gal)
- UDP-N-acetylgalactosamine (UDP-GalNAc)
- CMP-N-acetylneuraminic acid (CMP-Sia or CMP-sialic acid)
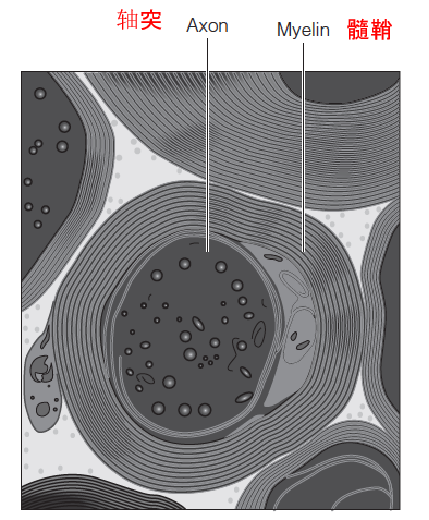
A myelinated axon (髓鞘的轴突) from the spinal cord (脊髓):
Myelin, an insulating layer wrapping about the axon, is rich in sphingomyelin (鞘磷脂).
Lysosomal pathways for degradation of sphingolipids
Sphingolipids degradation in lysosomes(溶酶体)
- A family of hydrolytic enzymes
Sphingolipodoses (also known as lipid storage diseases) (脂质贮积病)
Deficiency of one of the degradative enzymes, with concomitant accumulation within the lysosome of the substrates for the deficient enzyme
Help to establish the degradative pathway
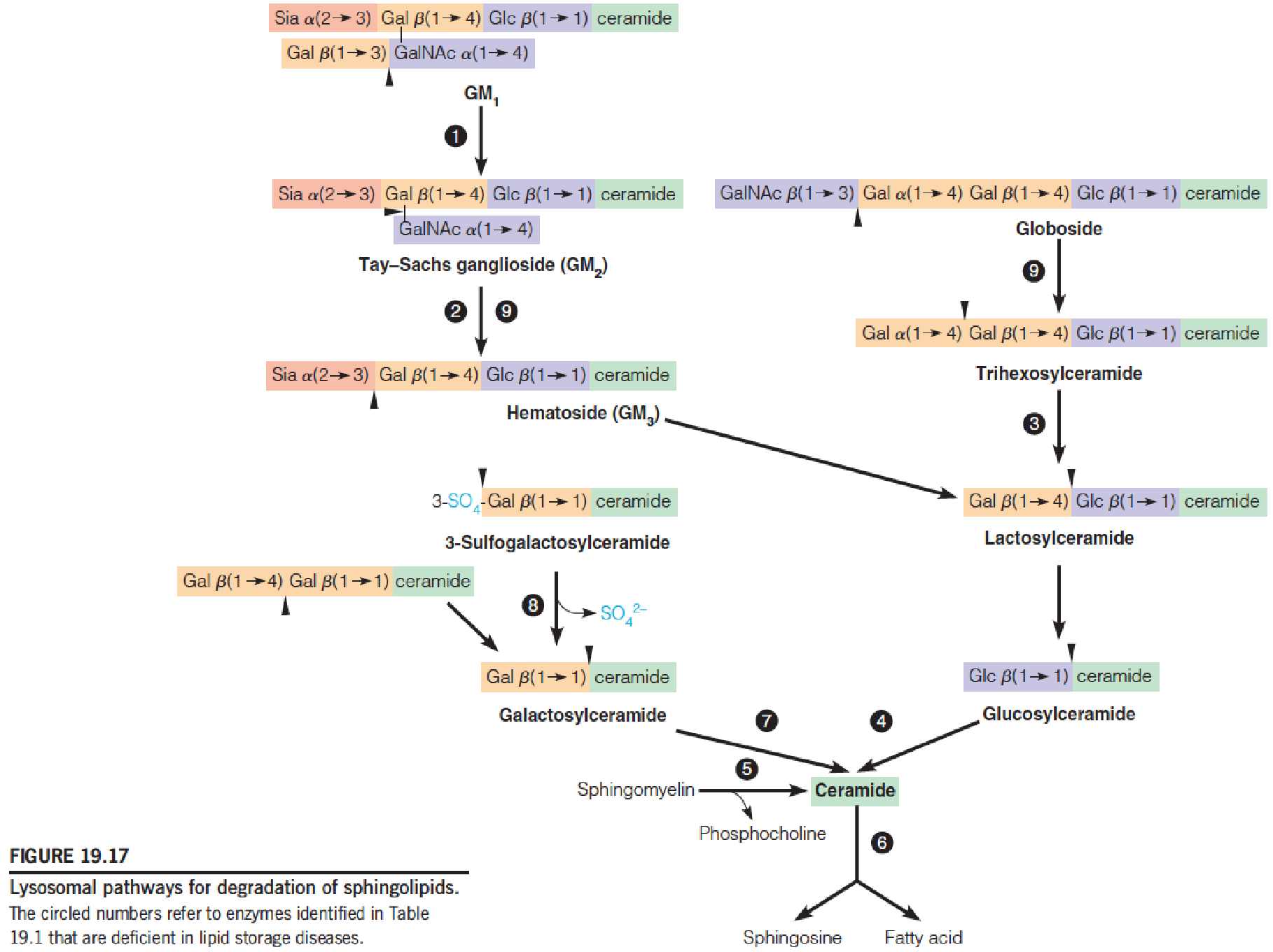
Tay-Sachs disease (1881):
- N-acetylhexosaminidase A deficiency
- Nervous degeneration, mental retardation, blindness, and death, usually by age of four
- American Jews: 1/30 carries the defective gene
- Two heterozygous parents: 25% chance in children
- No cure
- Early diagnosis: conjunction with amniocentesis
三、Steroid Metabolism
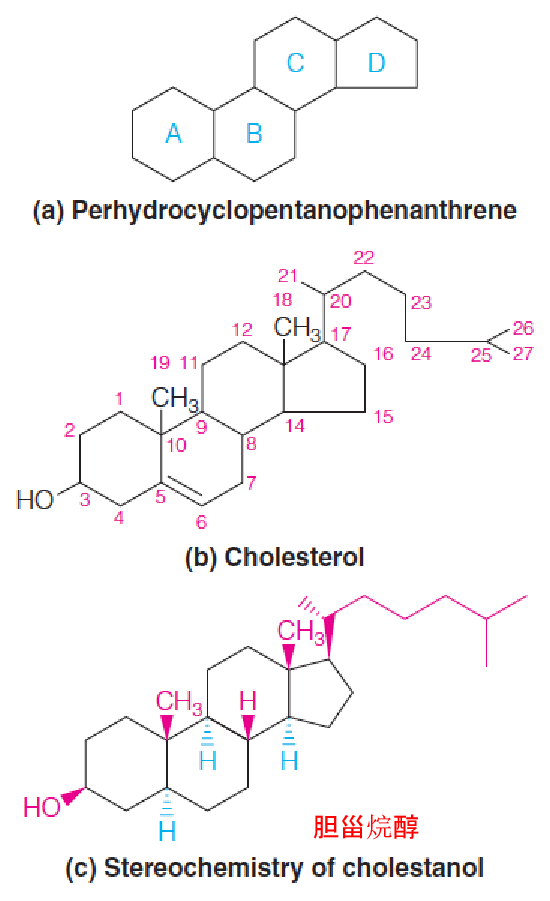
- Ring identification system (a)
- Carbon numbering system (b) used for steroids.
- Structural conventions (c), with cholestanol as the example.
- α-Substituents project below the plane of the steroid ring system (blue dashed wedge), and β-substituents project above that plane (red solid wedge).
- The hydrogens at positions 5, 9, and 14 have the α-configuration, whereas the hydroxyl, the two methyl groups, the hydrogen at C-8, and the aliphatic side chain at C-17 are all β-substituents.
A major component of animal cell membrane (modulation of membrane fluidity)
Precursors: steroid hormones, D3, bile acids
Medical interest: atherosclerosis(动脉粥样硬化) and heart disease
First isolation from gallstones, in 1784
Biosynthesis of Cholesterol
1. Early studies of cholesterol biosynthesis
Cholesterol, the precursor to all steroids, derives all of its carbon atoms from acetate (actually acety-CoA).
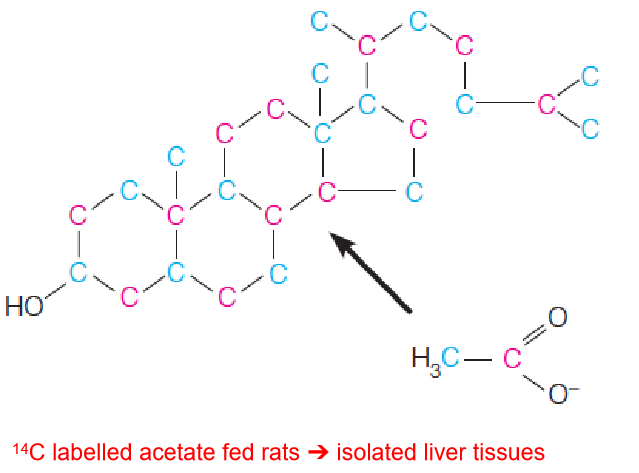
- 14C labelled acetate fed rats ➔ isolated liver tissues
- Each carbon of cholesterol was found to originate from either the methyl carbon (blue) or the carboxyl carbon (red) of acetate (actually, acetyl-CoA)
- Squalene (30C)(鲨烯;三十碳六烯)
- Mevalonic acid (6C)(甲羟戊酸)
- Isopentenyl pyrophosphate (IPP) (5C)(异戊烯基焦磷酸酯)
Three distinct processes were proposed as below:
- Conversion of C2 fragment (acetate) to C6 isoprenoid precursor (mevalonate(甲羟戊酸));
- Conversion of six C6 mevalonates, via activated C5 intermediates (IPP and DPP), to the C30 squalene(鲨烯);
- Cyclization of squalene and its transformation to the C27 cholesterol.
2. Stage 1: Synthesis of Mevalonate from Acetyl-CoA
Biosynthesis of mevalonate and conversion to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DPP):
- The two carbons of the third acetyl group are shown in red.
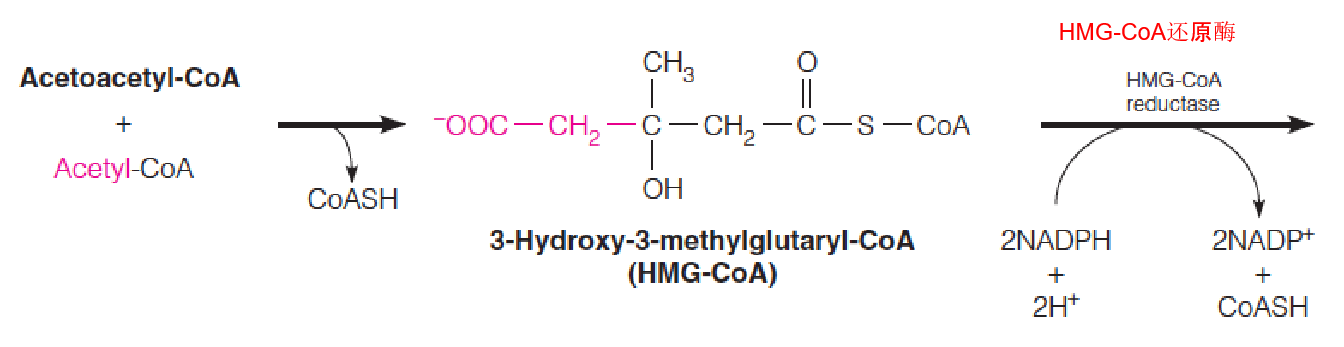
- HMG-CoA is converted into Mevalonate
Is identical to reactions used in ketogenesis, although it occurs in cytosol and the endoplasmic reticulum (rather than in mitochondria)
HMG-CoA reductase, an integral membrane protein in the ER, catalyzes the reduction of HMG-CoA to mevalonate:
- This multistep reaction requires two equivalents of NADPH (4 electrons) to reduce the thioester to an alcohol;
- This is the major step that regulates the overall pathway of cholesterol biosynthesis.
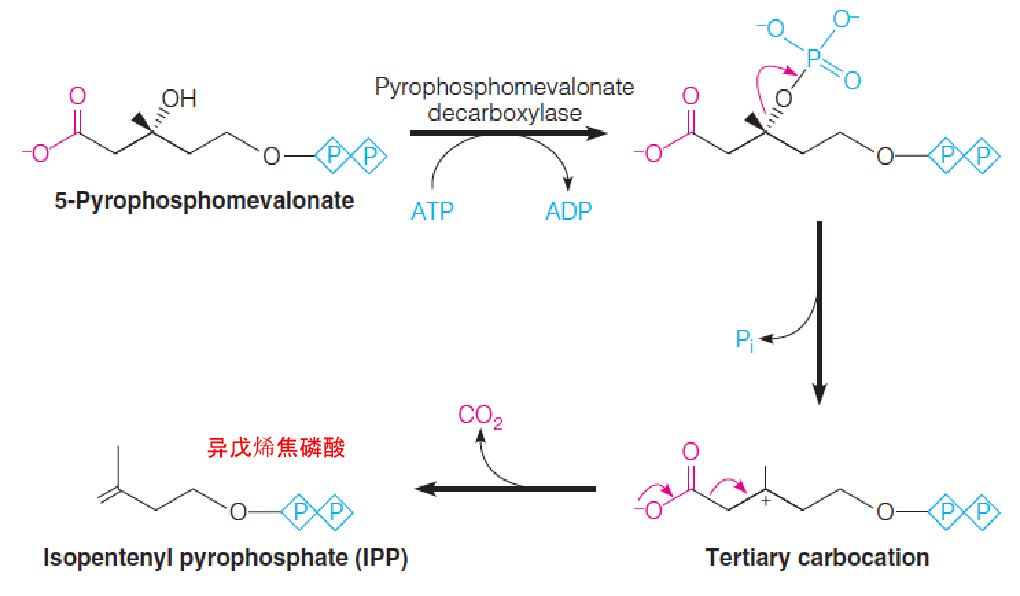
3. Stage 2: Synthesis of FPP from Mevalonate
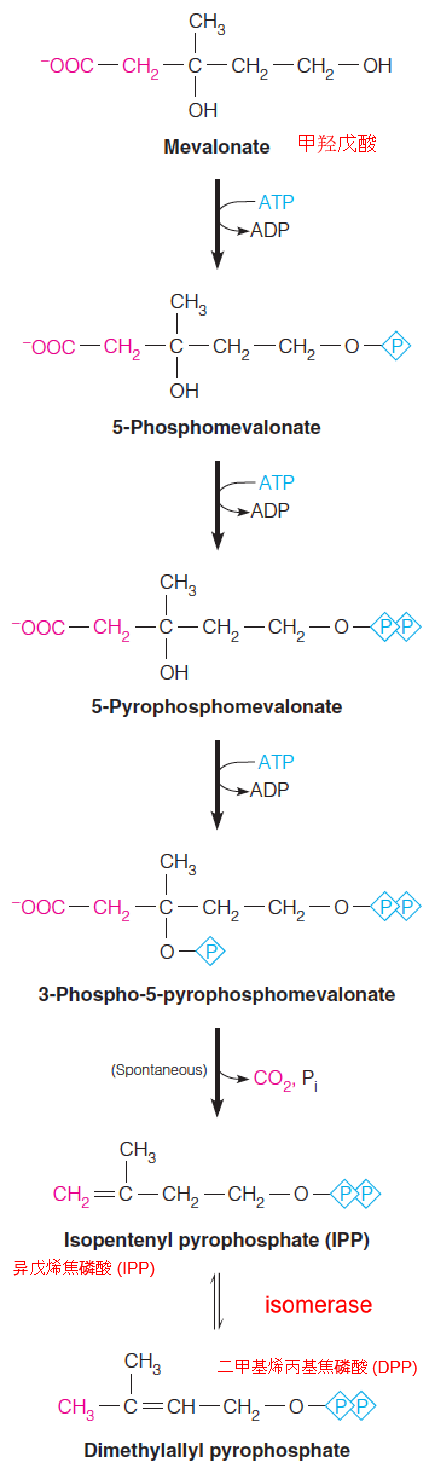
- Mevalonate is converted into IPP/DPP
The next several reactions occur in the cytosol.
First, mevalonate is activated by three successive phosphorylations.
- The first two are simple nucleophilic substitutions on the γ-phosphorous of ATP.
- The third phosphorylation, at position 3, sets the stage for a decarboxylation to give the five-carbon isopentenyl pyrophosphate (IPP)
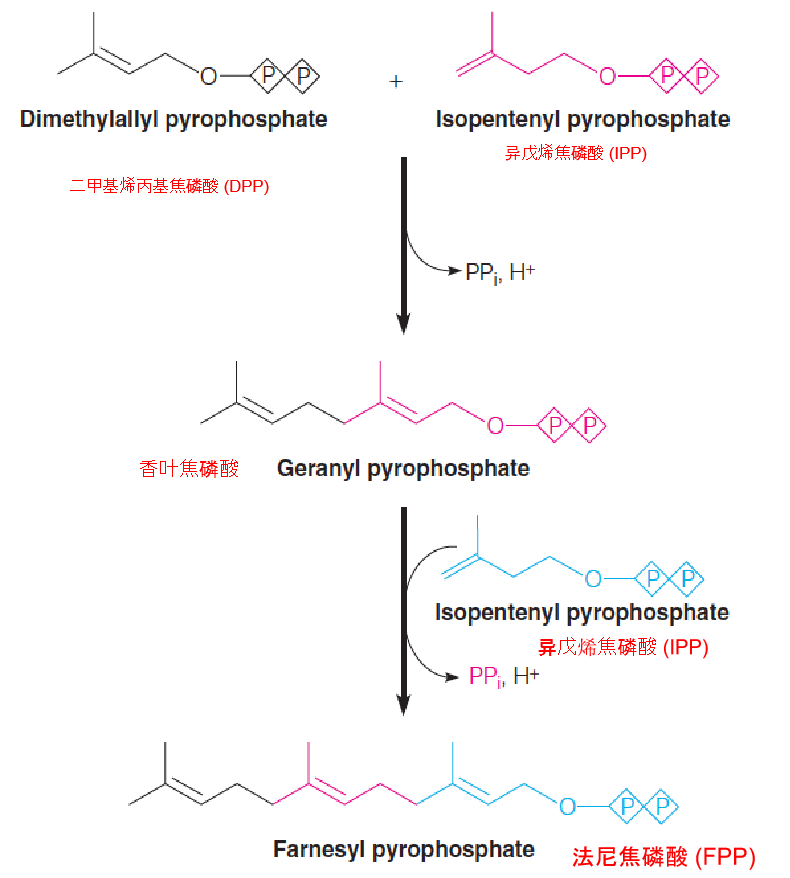
Conversion of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DPP) to farnesyl pyrophosphate (FPP) (C15):
Both of these head-to-tail condensations are catalyzed by the same prenyltransferase (farnesyl pyrophosphate synthase) (异戊二烯基转移酶(法尼焦磷酸合成酶)).
4. Stage 3: Conversion of farnesyl pyrophosphate (FPP) to squalene
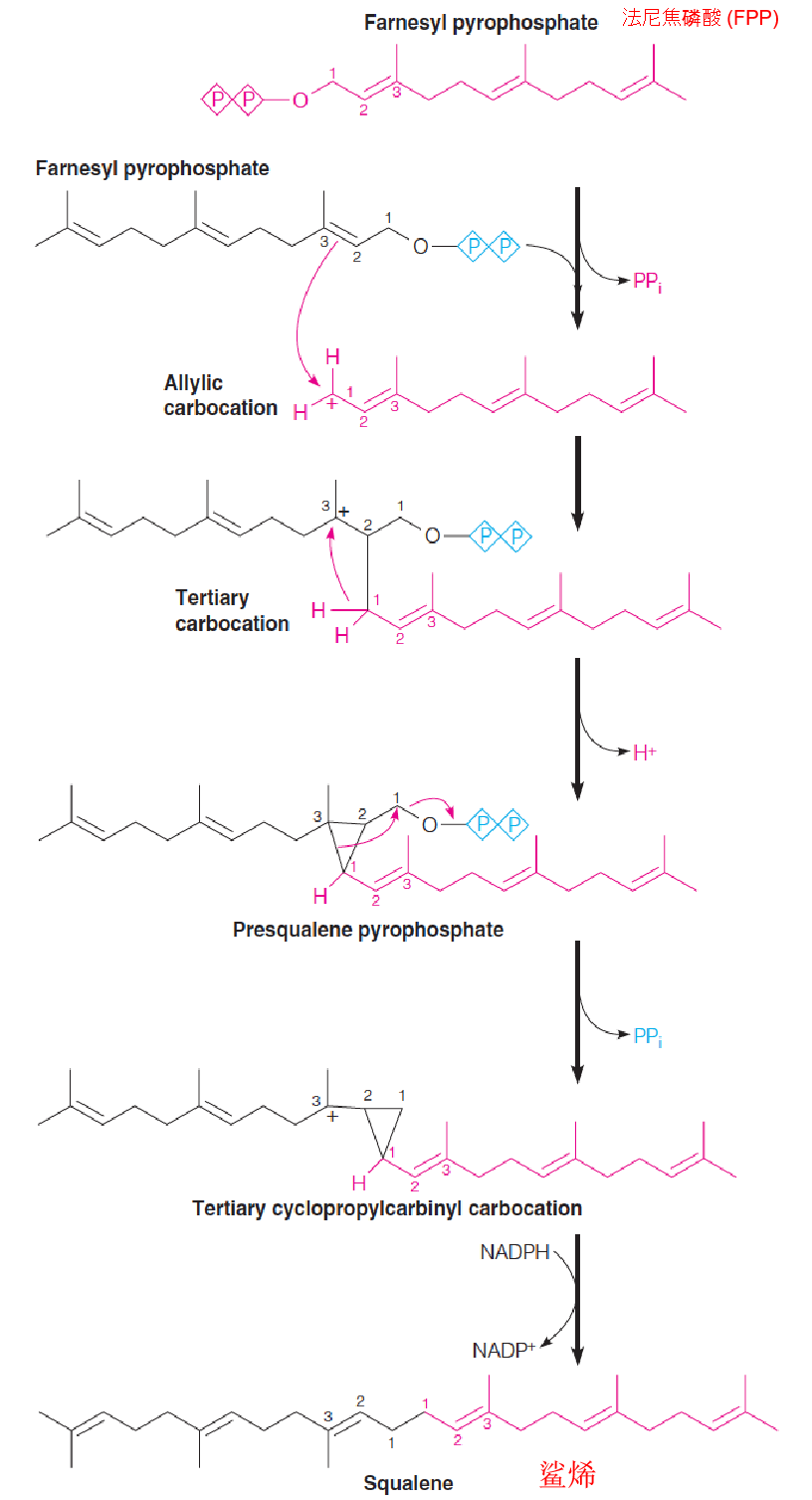
Catalyzed by squalene synthase.
Dissociation of the PPi group on one of the molecules of farnesyl-PPi yields an allylic carbocation.
The double bond nucleophilically (烯丙基) attacks the carbocation (碳正离子), forming a tertiary cation at C3 of the first farnesyl-PPi.
Loss of a proton from C1 gives the activated cyclopropane (环丙烷) intermediate, presqualene-PPi (角鲨烯前体-PPi).
Dissociation of the second pyrophosphate leads to rearrangement and formation of another tertiary carbocation intermediate(叔碳正离子中间体).
Hydride transfer(氢化物转移) from NADPH completes the rearrangement, giving squalene (C30).
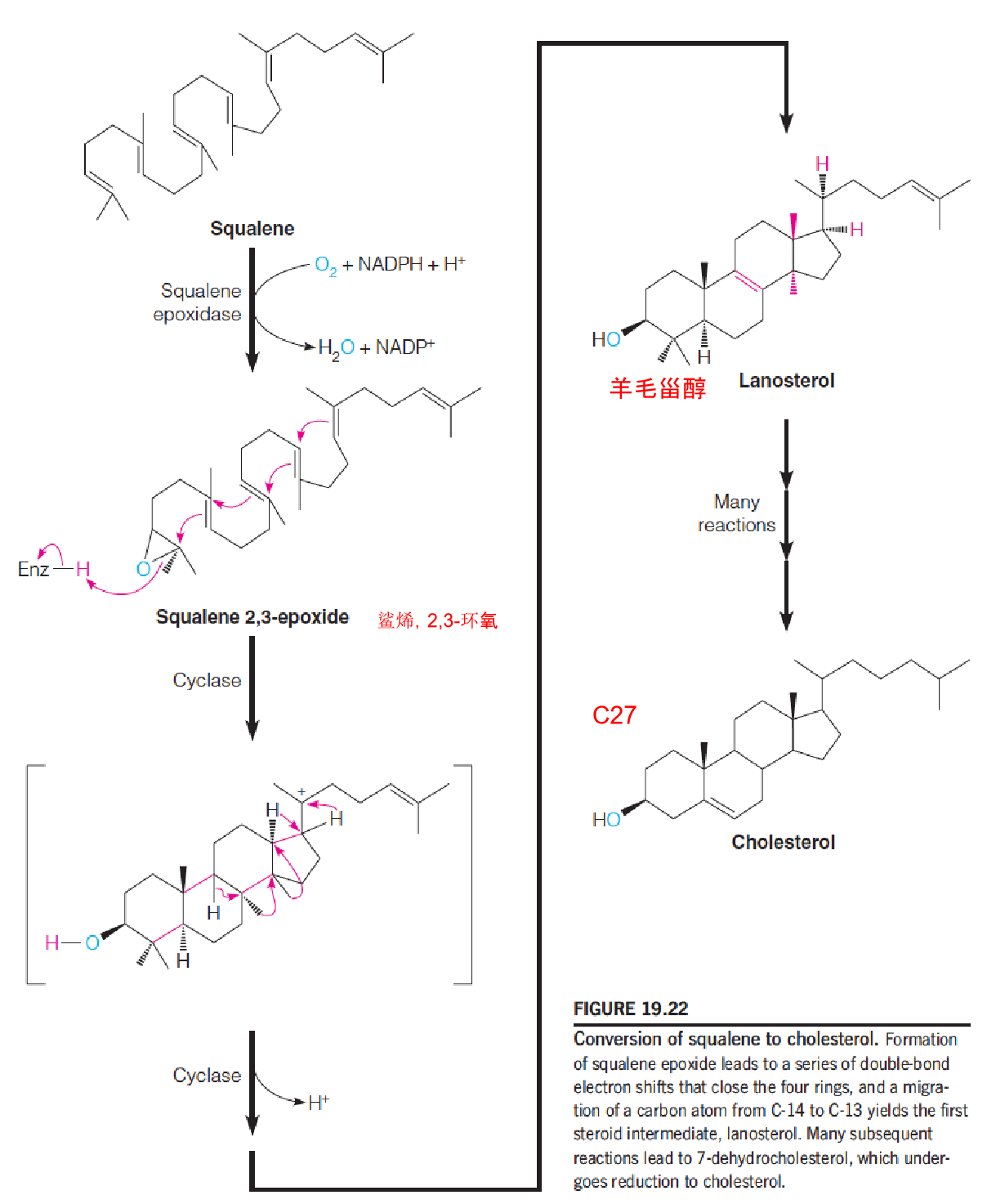
5. Stage 4: Synthesis of Cholesterol from Squalene
These reactions occur in the ER:
Regulation of Steroid Metabolism
Intracellular cholesterol levels are regulated by:
- controlling de novo cholesterol biosynthesis;
- storing excess cholesterol in the form of cholesterol esters;
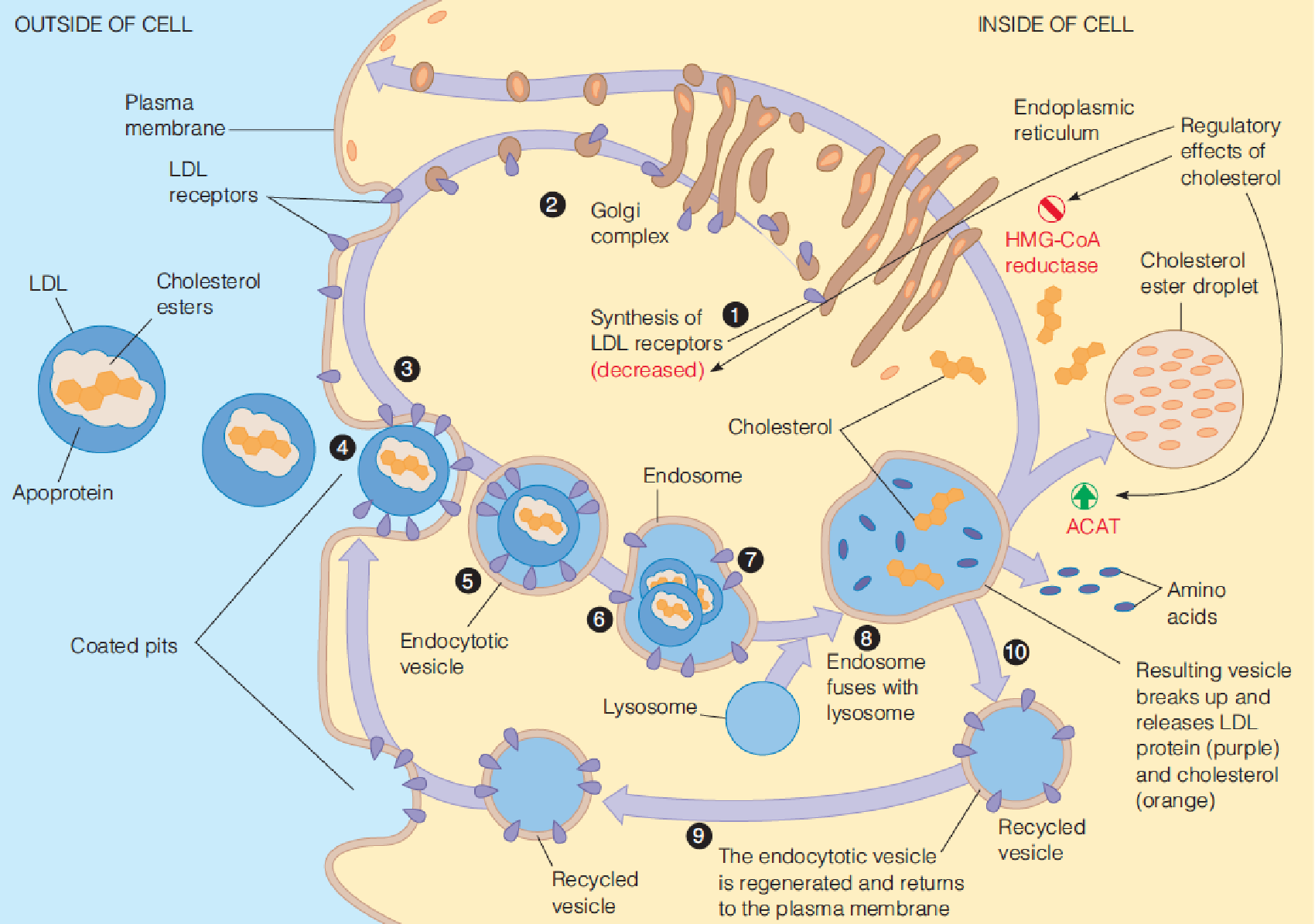
- regulating the synthesis of the LDL receptor, which is responsible for endocytosis of cholesterol from the bloodstream;
- Dietary cholesterol efficiently suppresses the endogenous synthesis of cholesterol;
HMG-CoA reductase represents a major target for regulation of the overall pathway
- HMG-CoA to Mevalonate is the major step in cholesterol biosynthesis
Central players:
- Insigs (insulin-induced growth response genes) 胰岛素诱导的生长反应基因,
- SREBPs (sterol regulatory element binding proteins),
- Scap (SREBP cleavage-activating protein);
All of these proteins are anchored in the ER membrane by multiple transmembrane segments.
1. Insigs control cholesterol synthesis by Degrading HMG-CoA reductase
Insigs control cholesterol synthesis through sterol-induced protein-protein interactions within the ER membrane
Regulation of HMG-CoA reductase by ubiquitin mediated proteolysis (泛素介导的蛋白水解):
- Sterol binding leads to rapid degradation of the enzyme.
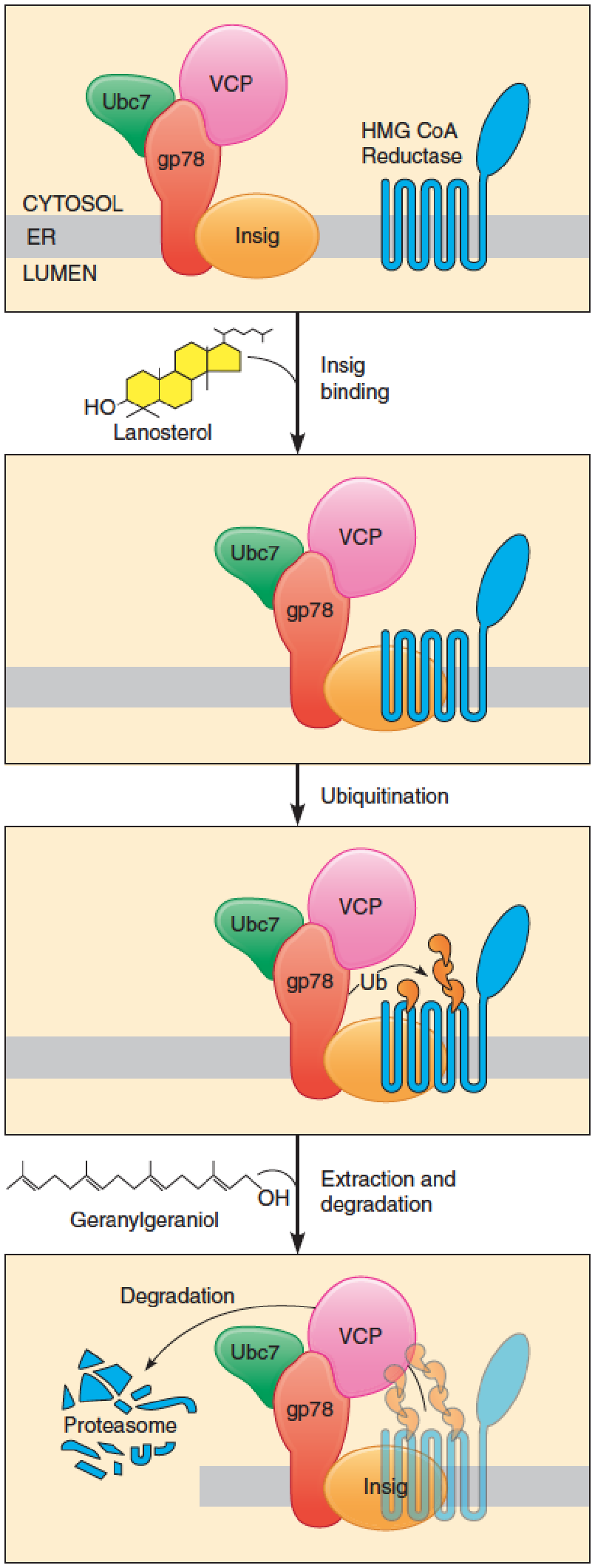
- HMG-CoA reductase: ER membrane;
- N-terminal domain: 8 membrane-spanning segments;
- catalytic C-terminal domain projects into the cytosol
- Sterol binding causes HMG-CoA reductase to bind to a population of Insigs that are associated with a ubiquitination complex comprising gp78, Ubc7, and VCP.
Control of cholesterol biosynthesis at post-transcriptional level
In sterol-depleted cells, this protein is degraded very slowly, with a half-time of more than 12 hours;
When sterols accumulate in the ER membrane, HMG-CoA reductase is rapidly degraded (t1/2 less than 1 hour) by the ubiquitin-proteasome pathway;
Membrane sterols bind to a sterol-sensing domain within the N-terminal membrane domain of the enzyme;
Sterol binding causes HMG-CoA reductase to bind to a population of Insigs that are associated with a ubiquitination complex comprising gp78, Ubc7, and VCP. Gp78 is an E3 ubiquitin ligase, which transfers ubiquitin from the Ubc7, E2 ubiquitin-conjugating enzyme, to the specific lysine residues of the HMG-CoA reductase. This protein-protein interaction, stimulated by sterol binding, thus leads to ubiquitination of the HMG-CoA reductase;
- VCP: an ATPase, extract ubiquitinated reductase from the membrane;
Nonsterol isoprenoid derived from mevalonate, such as geranylgeraniol (香叶基香叶醇) is also required for the extraction and the degradation of ubiquitinated HMG-CoA reductase;
Oxysterols and lanosterol(氧化型胆固醇) but not cholesterol itself regulate the degradation of HMG-CoA.
2. SREBP and SCAP Controls The Cholesterol Synthesis and Uptake
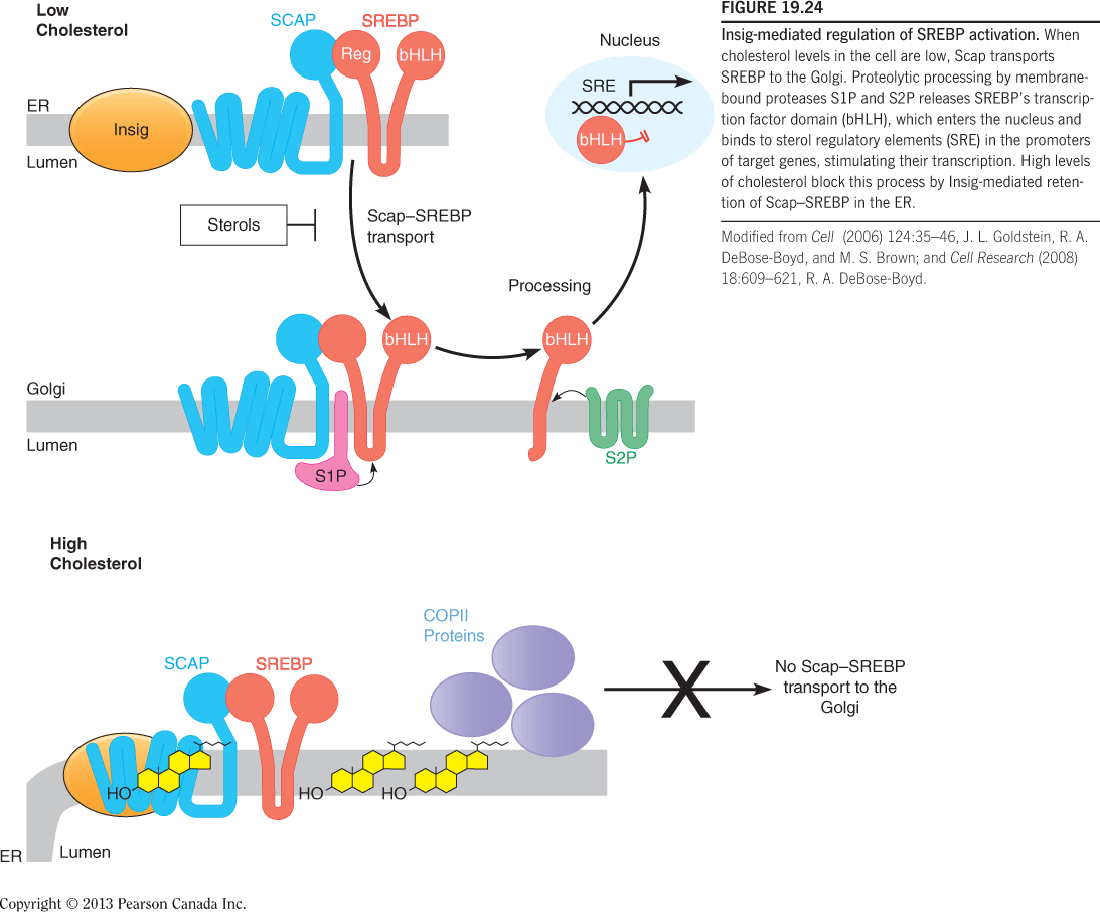
- Sterol Regulatory Element-Binding Proteins (SREBPs)
- When cholesterol levels in the cell are low, Scap transports SREBP to the Golgi.
- Proteolytic processing by membrane-bound proteases S1P and S2P releases SREBP’s transcription factor domain (bHLH), which enters the nucleus and binds to sterol regulatory elements (SRE) in the promoters of target genes, stimulating their transcription.
- High levels of cholesterol block this process by Insig-mediated retention of Scap–SREBP in the ER.
SREBPs are synthesized in the integral membrane in the ER;
When cells are depleted of cholesterol, the SREBPS translocate to the Golgi, where they are proteolytically processed to yield active transcription factors that enter the nucleus;
This process requires Scap, which serves as an escort protein (护送蛋白), and two specific bin the Golgi (S1P and S2P);
When sterols accumulate in the ER membrane (either from the de novo synthesis or from cell uptake), exit of Scap-SERBP complex from the ER is inhibited, blocking the proteolytic activation of SERBPs.
- This causes a decrease in the transcription of SERBP target genes, leading to lower rates of cholesterol synthesis and uptake.
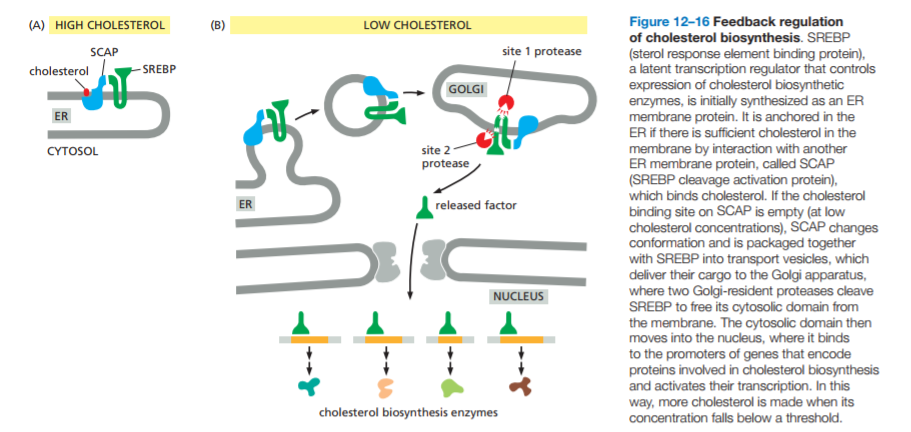
The SREBP pathway is also controlled by membrane sterols and Insigs
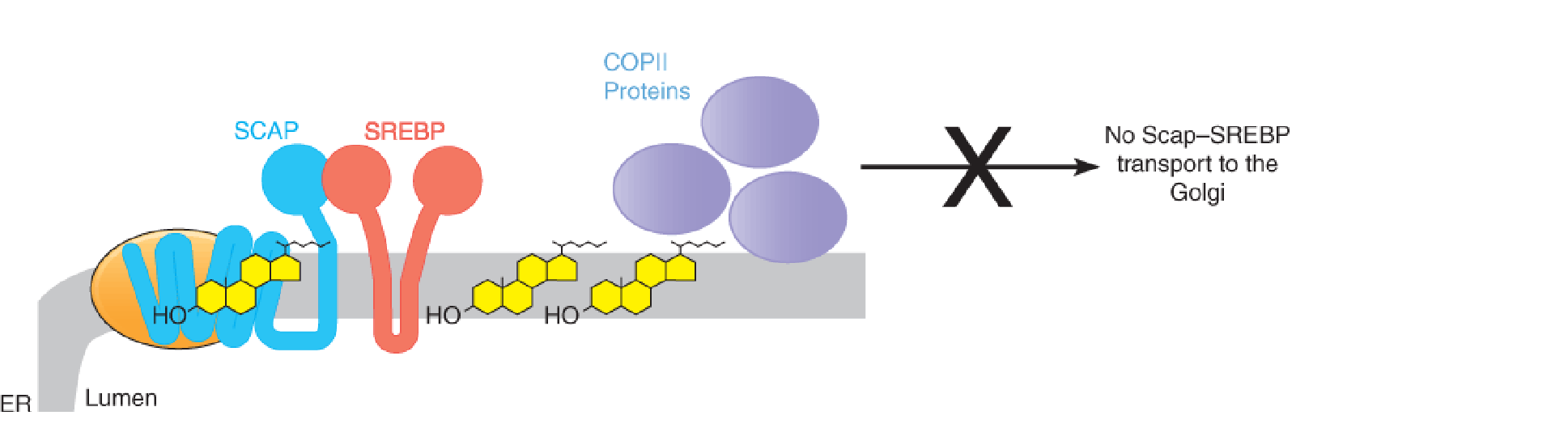
In sterol-replete cells, cholesterol binds to Scap ➔ Scap-Insigs complex ➔ blocks the binding of Scap to COPII proteins ➔ blocks the translocation of cargo molecules (Scap-SREBP) from the ER to Golgi ➔ blocks proteolytic processing of SREBP (release of the bHLH)
Regulation at the transcriptional level: SREBPs and Scap;
- SREBPS are transcription factors that bind to the promoter of genes required to produce cholesterol, including the HMG-CoA reductase;
Conversion of acetyl-CoA to cholesterol requires at least 20 enzymes, all of whose genes are activated by SREBP binding;
SREBP also activates the transcription of the LDL receptor gene, which mediates uptake of dietary cholesterol.
3. Summary
Insig proteins integrate these transcriptional and post-transcriptional regulatory mechanisms. In both processes, sterol binding to a sterol-sensing domain (in HMG-CoA reductase or Scap) stimulates Insig binding.
- For HMG-CoA reductase, Insig binding means ubiquitination and degradation.
- For Scap, Insig binding turns off a transcription activation pathway.
Together, these mechanisms ensure fine control over cellular cholesterol metabolism.
Finally, in some tissues, HMG-CoA reductase is subject to short-term regulation by reversible phosphorylation/dephosphorylation;
- Phosphorylation by AMP-activated protein kinase (AMPK) inactivates HMG-CoA reductase (Cholesterol biosynthesis is particularly expensive pathway: requiring 36 moles of ATP and 16 moles of NADPH per mole of cholesterol; thus, under conditions of low energy charge, activated AMPK switches off cholesterol synthesis by inhibiting HMG-CoA reductase);
- Dephosphorylation by type 2A protein phosphatase activates HMG-CoA reductase
How do HMG-CoA reductase inhibitors reduce the serum cholesterol level?
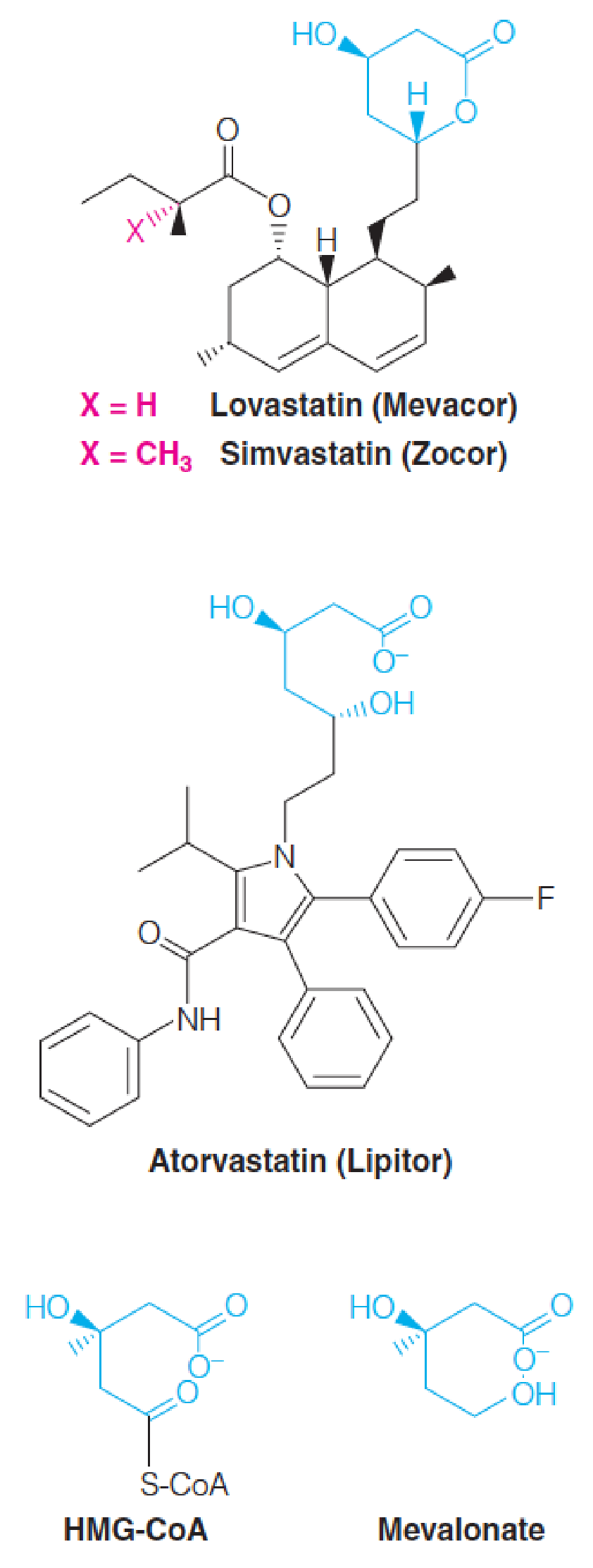
Once the rate-limiting role of HMG-CoA reductase in cholesterol biosynthesis was understood, specific inhibitors were sought to lower blood cholesterol levels.
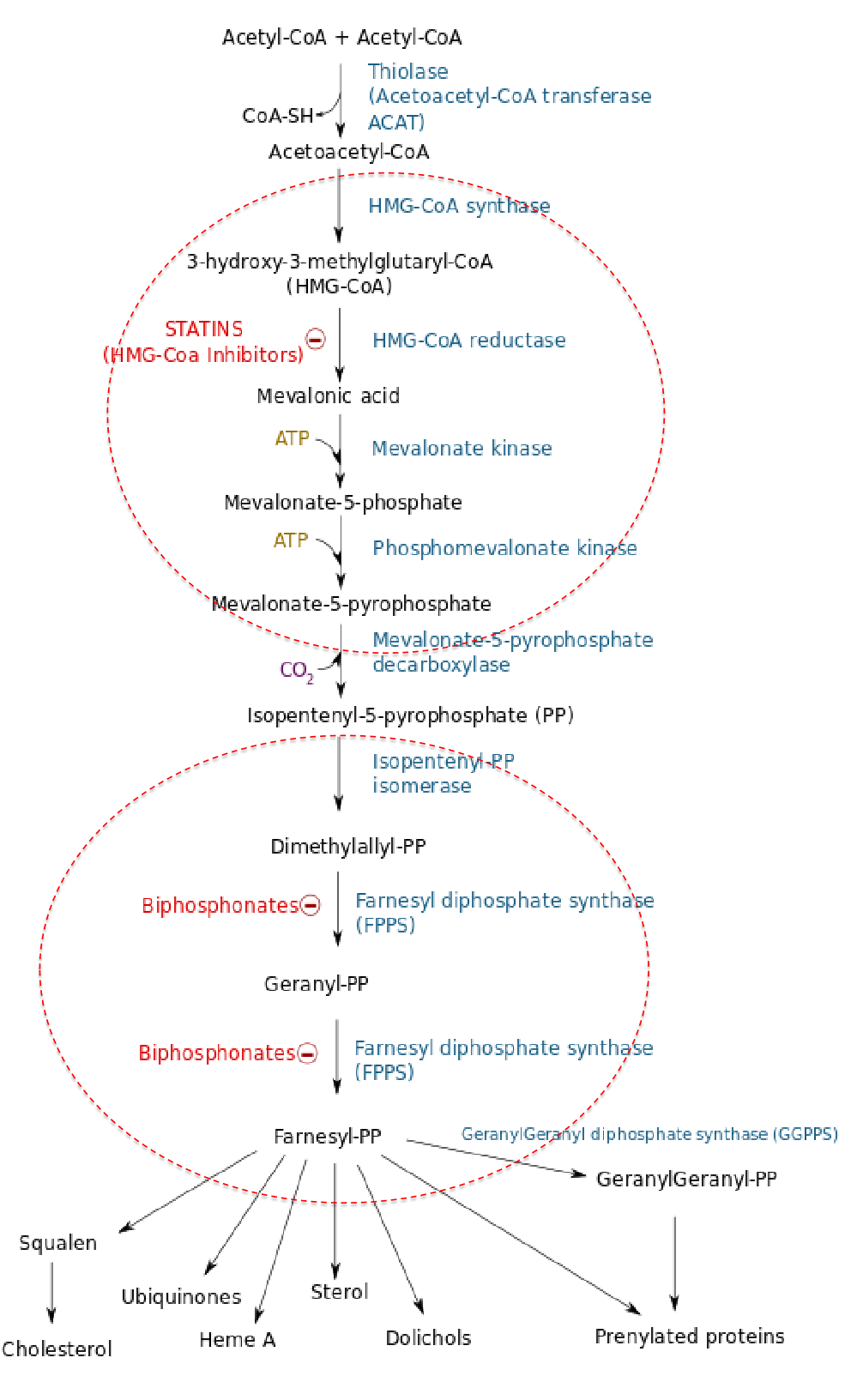
The compounds discovered are collectively called statins. (他汀类药物)
- They act by competitively inhibiting HMG-CoA reductase.
- Each statin carries a mevalonate-like moiety (blue), explaining the competitive nature of their activity.
- Inhibition of HMG-CoA reductase depresses de novo cholesterol biosynthesis and, hence, intracellular cholesterol levels.
- This in turn leads to increased production of LDL receptors, allowing more rapid clearance of extracellular cholesterol from the blood, thus lowering blood cholesterol levels.
Lovastatin (洛伐他汀) and simvastatin (辛伐他汀) are fungal polyketides (聚酮化合物) and atorvastatin (Lipitor®,阿托伐他汀(立普妥)) is synthetic.
STATINS: HMG-CoA reductase inhibitor
Bile Acids
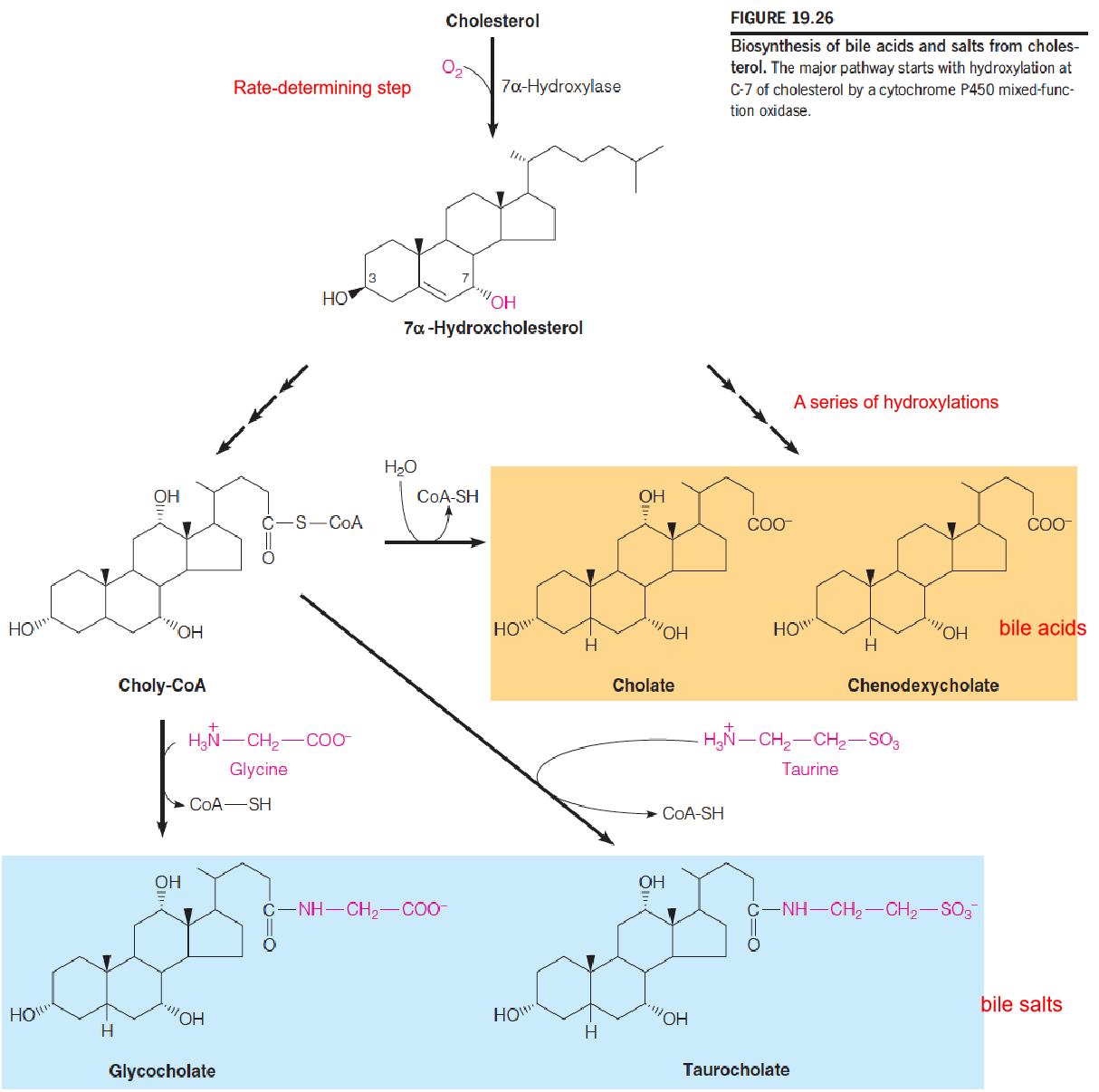
In Chapter 17, bile acids: detergent properties and emulsify dietary lipids in the intestine and thereby promotes fat digestion and absorption.
- They are secreted from the liver, stored in the gallbladder, and passed through the bile duct(胆管) and into the intestine
Biosynthesis of bile acids represents the major metabolic fate of cholesterol, accounting for approximately 90% of cholesterol catabolism in the normal human adult. By contrast, steroid hormone accounts for only about 50 mg of cholesterol metabolized per day.
Although about 400-500 mg of bile acids are synthesized daily much more than this is secreted into the intestine. Most of the bile acids secreted into the upper intestine(上肠) are absorbed in the lower small intestine and returned to the liver for reuse, through the portal blood(门脉血).
- This process, which handles 20-30 g of bile acids per day, is called the enterohepatic circulation (肠肝循环).
- Daily elimination of bile acids in the feces amounts to just 0.5 g/day or less, which is compensated for by synthesis in the liver.
The most abundant bile acids in humans are cholic acid and chenodeoxycholic acid (胆酸和鹅去氧胆酸).
- These are usually conjugated in amide linkage with the amino acids glycine or taurine(牛磺酸;氨基乙磺酸), giving compounds called bile salts.
- The cholic acid conjugates with glycine and taurine are called glycocholate and taurocholate (甘氨胆酸和牛磺胆酸盐), respectively.
- Another bile acid, deoxycholate(脱氧胆酸盐), is abundant in the bile of some other mammals. It is widely used as laboratory reagent, to solubilize membrane proteins
Biosynthesis of bile acids and salts from cholesterol
Steroid Hormones
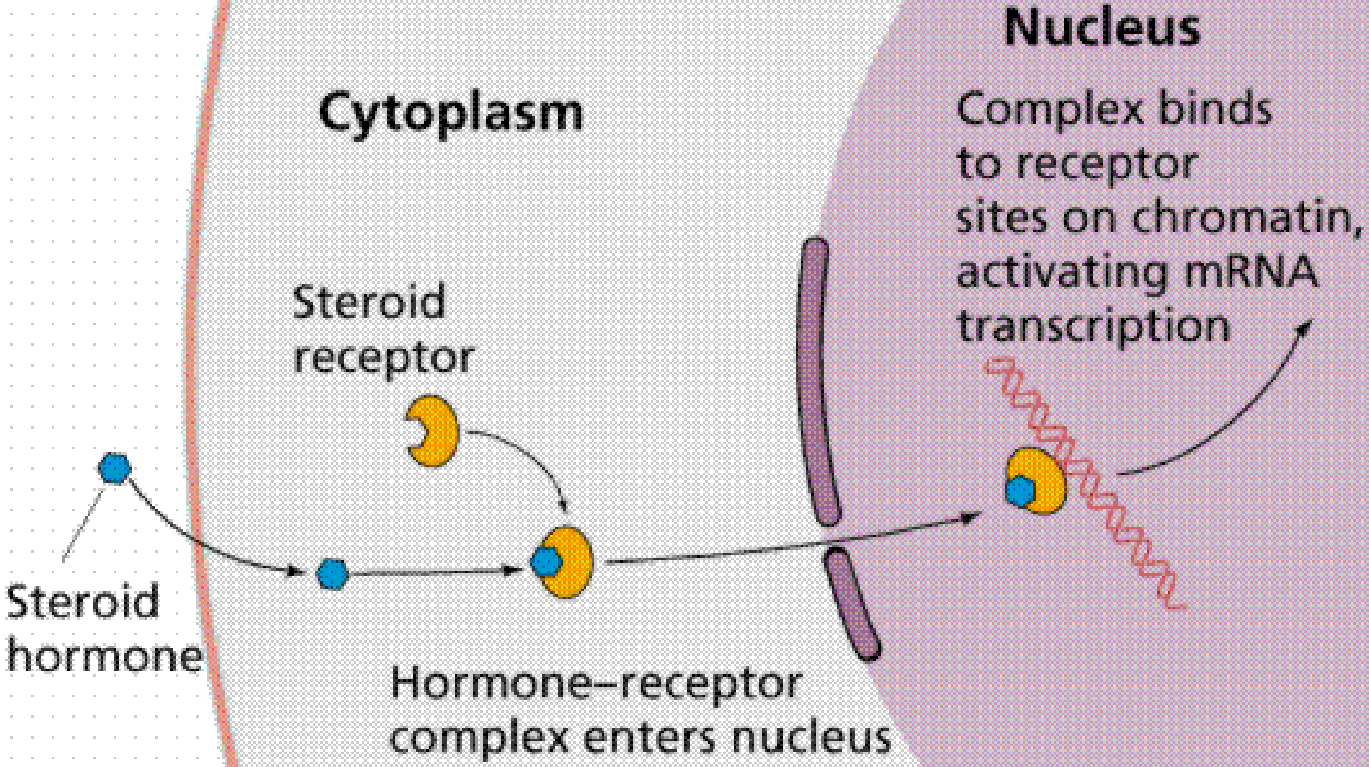
Cholesterol is the biosynthetic source of all steroid hormones;
In this Chapter, we introduce the biosynthetic pathways to steroid hormones; their actions are discussed in Chapter 23;
In general hormone control metabolism at the gene level;
They interact with intracellular protein receptors;
The hormone-receptor complexes bind to specific sites in the genome and affect transcription of neighboring genes.
Five major classes of steroid hormone
- the progestins (progesterone,孕激素(黄体酮)), which regulates events during pregnancy;
- the glucocorticoids (cortisol and cortisterone, 糖皮质激素(皮质醇和皮质酮)), which promote gluconeogenesis and, in pharmacological doses, suppress inflammation reactions;
- the mineralocorticoids (aldosterone, 盐皮质激素(醛固酮)), which regulate ion balance by promoting reabsorption of K+, Na+, Cl-, and HCO3- in the kidney;
- the androgens (androstenedione and testosterone, 雄激素(雄烯二酮和睾酮)), which promote male sexual development and maintain male sex characteristics;
- the estrogens (estrone and estradiol, 雌激素(雌酮和雌二醇)), or female sex hormone, which support female characteristics.
Biosynthesis of steroid hormones
A general feature of steroid hormones is that they are not stored for release after synthesis; Therefore, the level of a circulating hormone is controlled primarily by its rate of synthesis, which is often controlled ultimately by signals from the brain;
Corticotropin releasing hormone (CRH, 促肾上腺皮质激素释放激素) is released from cells in the hypothalamus(下丘脑) in response to central nervous system inputs.
- CRH stimulates release from the pituitary gland (垂体) of corticotropin(促肾上腺皮质激素), or adrenocorticotropic hormone (ACTH (促肾上腺皮质激素)), which in turn stimulates the synthesis of glucocorticoids (糖皮质激素) in adrenal cortex(肾上腺皮质).
- Glucocorticoids inhibits CRH release.
Hydrolysis of cholesterol esters and uptake of cholesterol from cellular stores (ER and plasma membrane) into mitochondria of cells in the target organs;
Translocation of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane (IMM) is the rate-limiting step in the production of all steroids, and requires at least two proteins:
- steroidogenic acute regulatory (StAR) protein
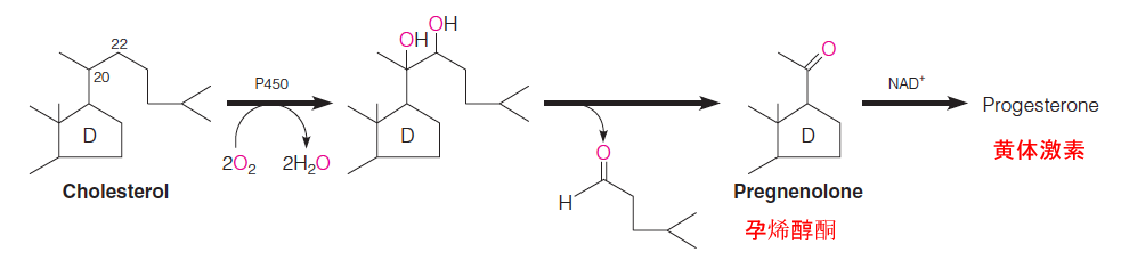
- cytochrome P450 called cholesterol side chain cleavage enzyme(胆固醇侧链裂解酶) hydroxylates the side chain of cholesterol at C-20 and C-22 and cleaves it, to yield pregnenolone (促肾上腺皮质激素), the precursor to all other steroid hormones;
Prognenolone then moves to the ER, where its conversion to all of the other steroid hormones takes place.
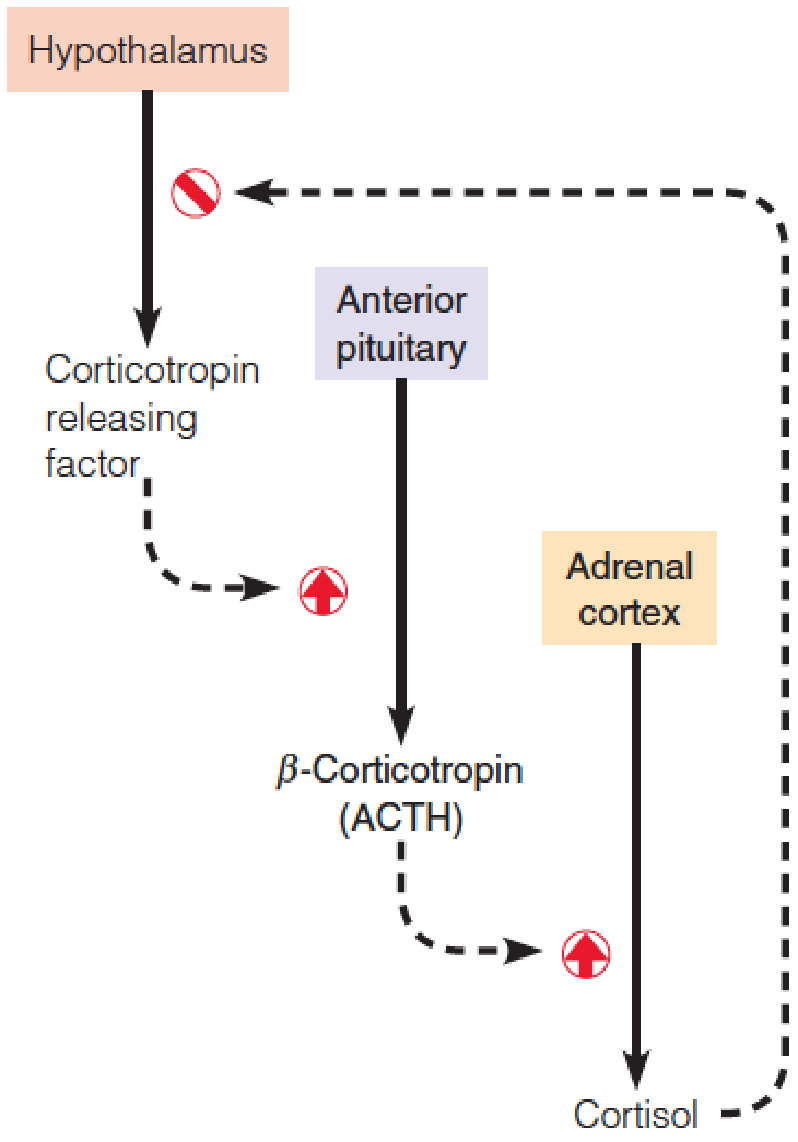
1. Hierarchical(分等级的) Nature of Hormonal Control
An example of feedback regulation of a hormone:
Corticotropin releasing hormone (CRH, 促肾上腺皮质激素释放因子) stimulates the release of β-corticotropin (ACTH) from the anterior pituitary.
ACTH stimulates the adrenal cortex to release cortisol (皮质醇), which feeds back on the hypothalamus to inhibit further release of CRF.
Pregnenolone (孕烯醇酮) is an intermediate en route from cholesterol to all other known steroid compounds.
2. Biosynthetic routes from pregnenolone to other steroid hormones
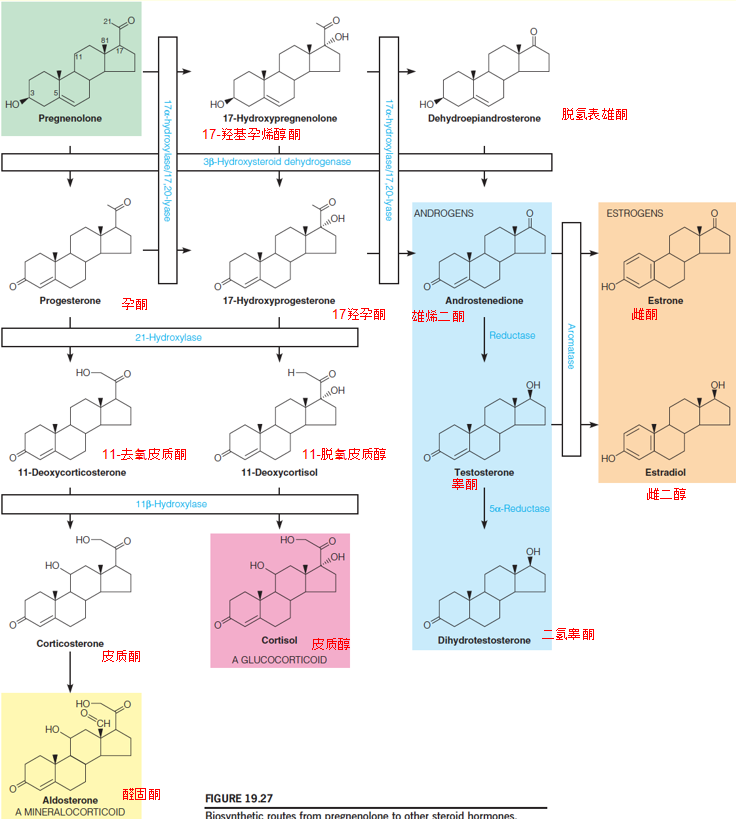
A series of hydroxylation, catalyzed by microsomal cytochrome P450 mixed function oxidase;
The first of these, catalyzed by 17-α-hydroxylase, is rate-determining and hence plays the major role in controlling the overall pathway
Human enzymes deficiencies have been described for all of the above enzymes;
A deficiency of the 21-hydroxylase ➔ synthesis of glucocorticoids(糖皮质激素) and mineralocorticorids(盐皮质激素)↓ ,progesterone and 17-hydroxyprogesterone ↑ ➔ overproduction of testosterone in the adrenal glands ↑ ➔ virilization (masculinization) (男性化) of females;
At the same time, cortisol ↓ ➔ interferes with a feedback loop of CRH and ACTH ➔ ACTH secretion ↑ ➔ the adrenals to grow (hyperplasia) ➔ synthesize steroids ↑ testosterone ↑ ➔ virilization (masculinization) (男性化) of females;
A deficiency of the 11β-hydroxylase also causes adrenal hyperplasia(肾上腺增生) and is characterized by accumulation of the mineralocorticoid 11-deoxycorticosterone, causing hypertension(高血压), and the glucocorticoid 11-deoxycortisol;
A deficiency of the 5α-reductase decreases androgen(男性荷尔蒙;雄性激素) levels and leads to feminization(女性化) of males;
Fortunately, these and other steroid abnormalities can be treated with hormone replacement therapy, if detected early enough in life.
四、Other Isoprenoid Compounds
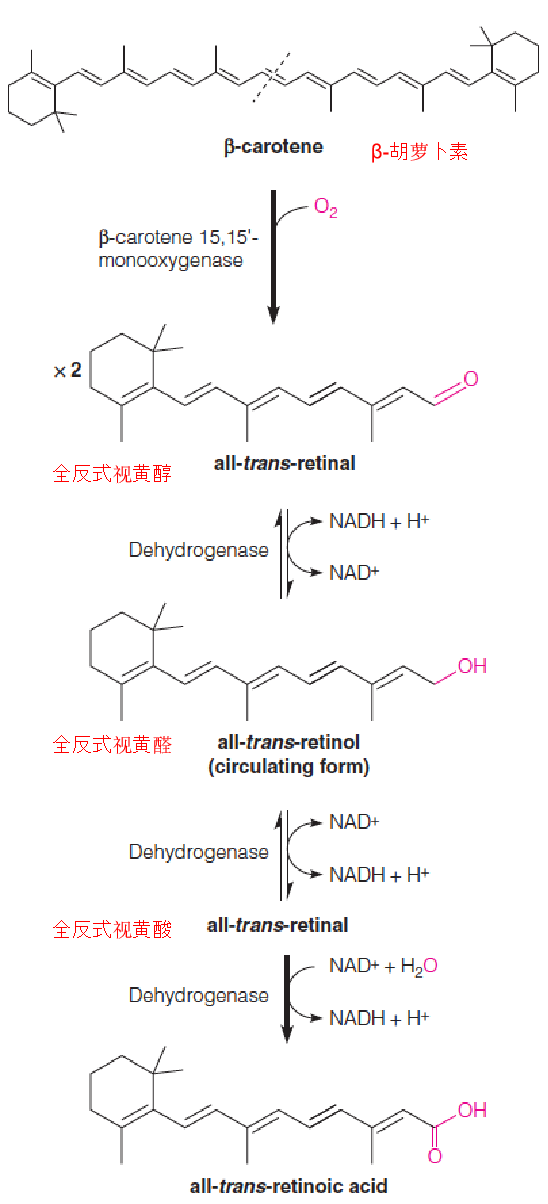
There are three active forms of vitamin A: all-trans-retinol, -retinal, and -retinoic acid (全反式视黄醇,视黄醛和视黄酸). Collectively, these are referred to as retinoids (维甲酸).
The vitamin can be either consumed in the diet as esterified retinol(酯化视黄醇), or biosynthesized from β-carotene (β-胡萝卜素), a plant isoprenoid especially abundant in carrots.
β-carotene is cleaved in the intestine by a monooxygenase to form two molecules of all-trans-retinal (retinaldehyde (视黄醛)), which are then reduced to retinol.
All-trans-retinol is the form which circulates in the blood, which has the highest biological activity.
Schematic drawing of a rod cell (视杆细胞)
- The outer segment is a stack of membranous disks, which contain the photoreceptive pigments.
- This segment is connected by a thin cilium to the inner segment, which contains the cell nucleus, cytosol, and synaptic body (突触体).
- The potential change produced in the outer segment travels to the synaptic body and is transmitted to one or more of the neurons of the retina (视网膜).
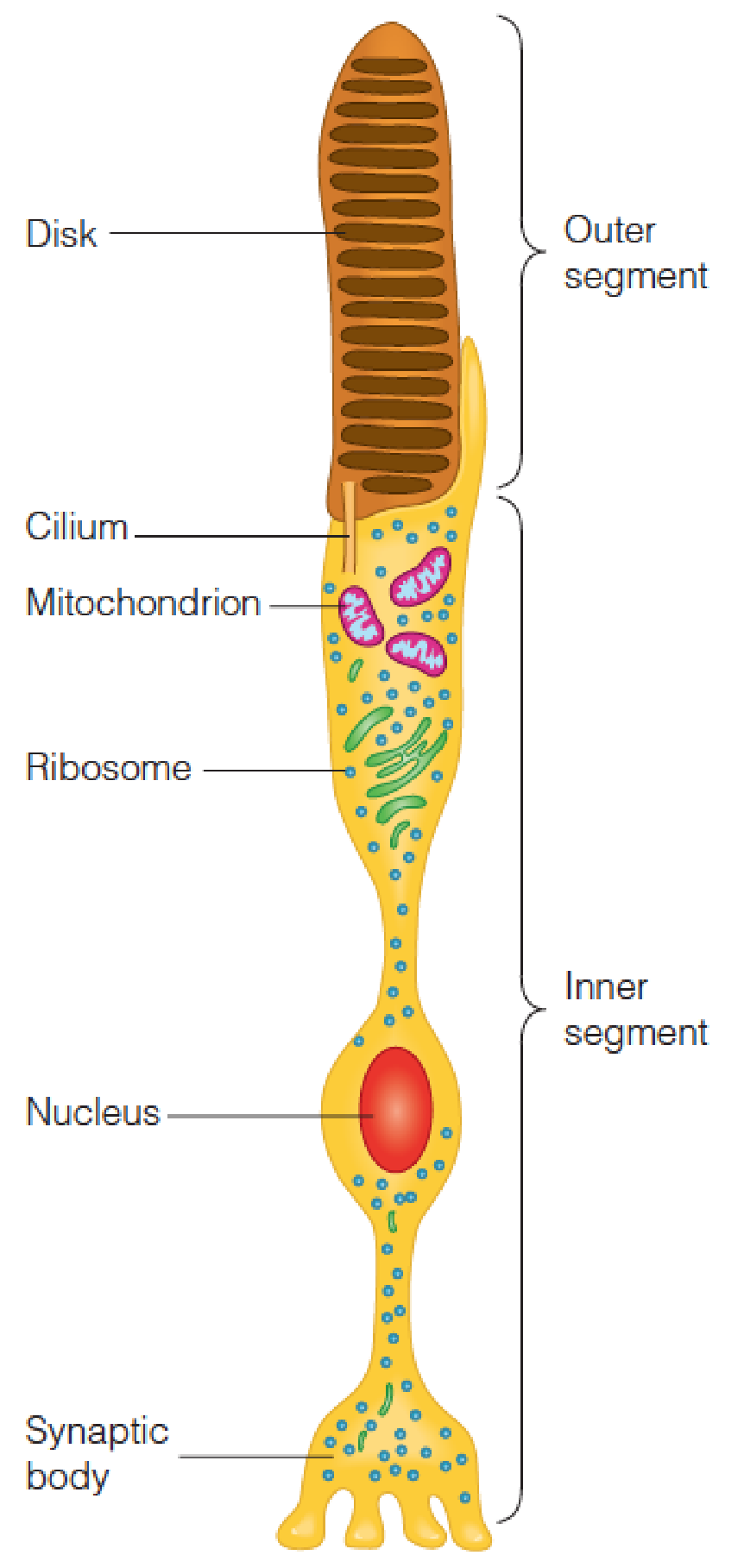
- Rod cells of the retina, the cell primarily responsible for low-light vision, with relatively little color detection(视网膜的杆状细胞,主要负责弱光视力,颜色检测相对较少)
1. The chemical changes in photoreception(感光)
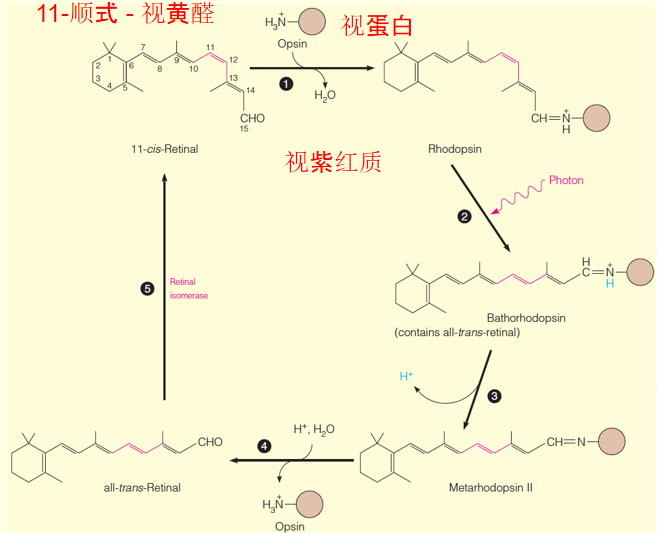
11-cis-retinal and opsin(视蛋白) in a rod cell (视杆细胞) combine to form rhodopsin.
Absorption of a photon of light leads to the chemical changes shown in steps 2 and 3.
Step 3 involves at least 3 distinct conformational changes that occur in ~1 ms.
Metarhodopsin II is the species that activates transducin (转导素) (not shown) to initiate the visual cascade.
After about 1 second, metarhodopsin II dissociates into all-trans-retinal, which isomerizes as the cycle begins again.
Structures of other important isoprenoids (异戊二烯)
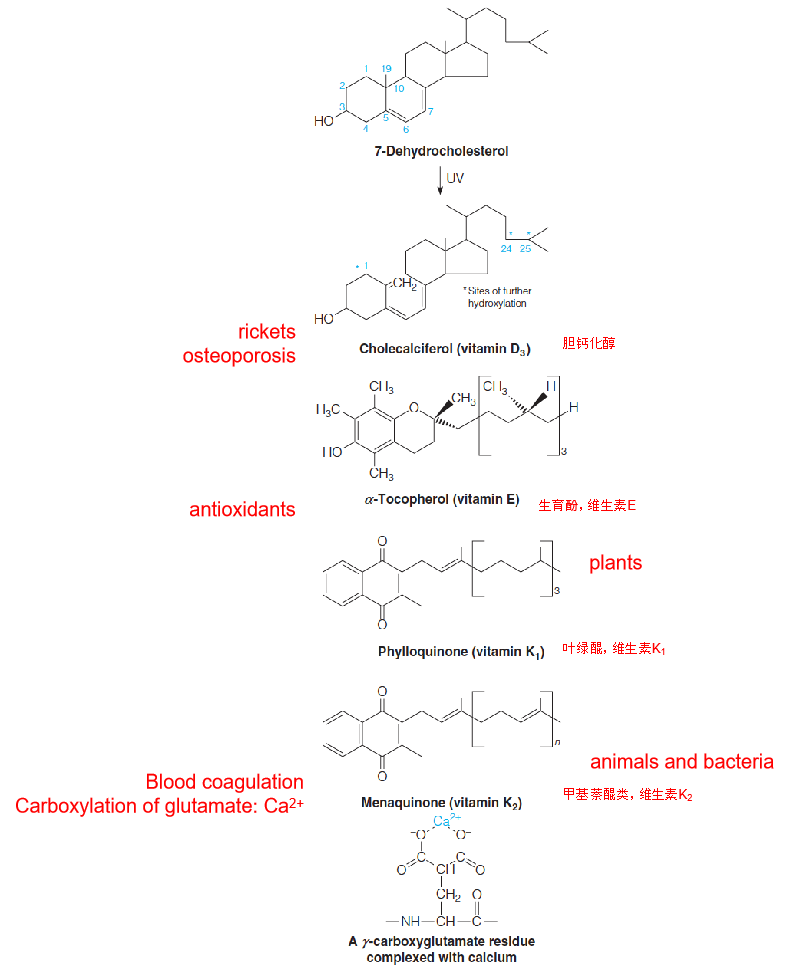
五、Eicosanoids: Prostaglandins, Thromboxanes, and Leukotrienes
- 花生酸类:前列腺素,血栓素,和白三烯
Potent physiological properties, low levels in tissues, rapid turnover, and common metabolic origin;
They are called eicosanoids because of their common origin from C20 polyunsaturated fatty acids, the eicosaenoic acid, particularly arachidonic acid;
Act locally, near their sites of synthesis;
Catabolized extremely rapidly.
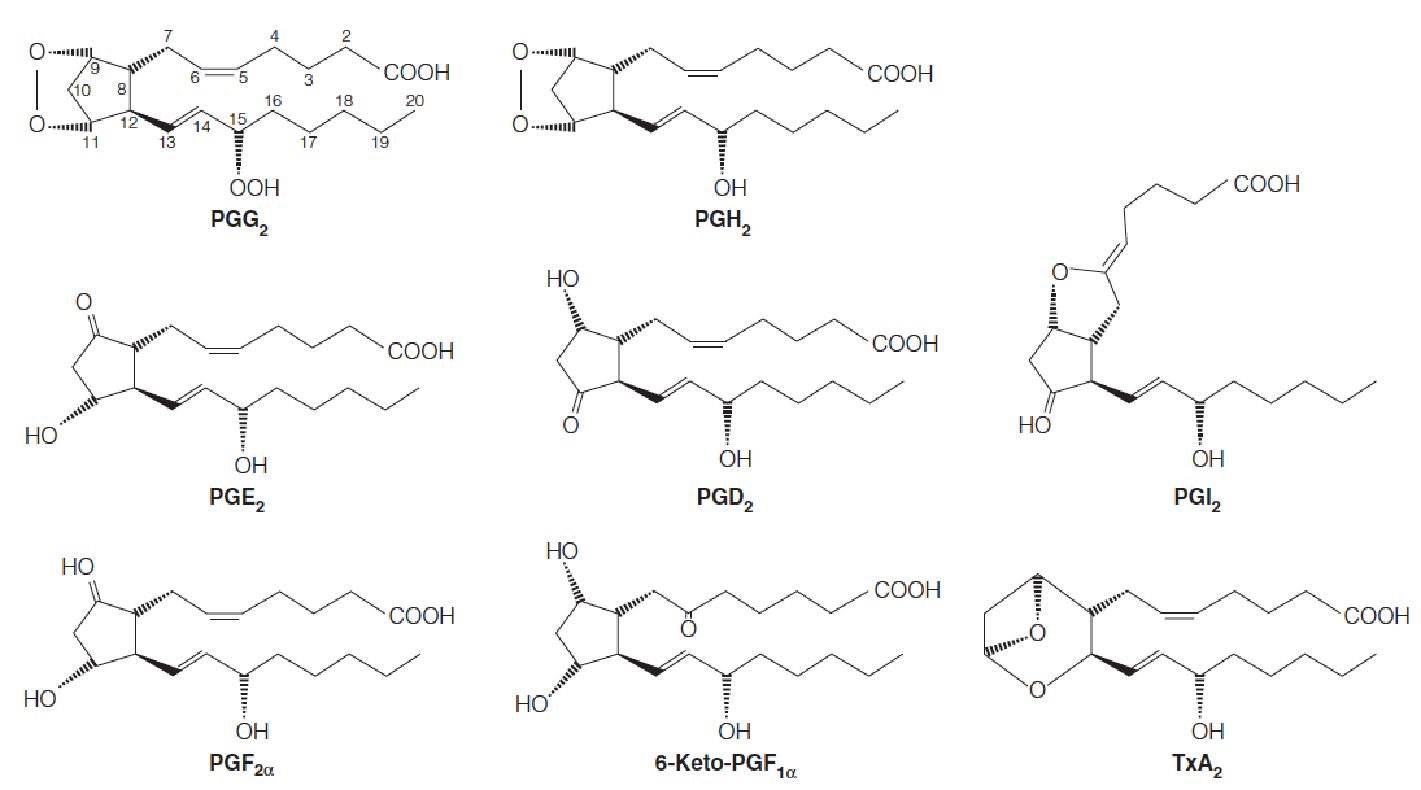
Structures of the major prostaglandins and thromboxane A2
The most abundant prostaglandins are those of the 2-series are shown.
They are derived from arachidonic acid (花生四烯), as is thromboxane A2.
Numbering of carbons begins with the carboxyl group as shown for the structure of PGG2.
In 1930s, lipid extracts of human semen, on injection into animals, stimulated smooth muscle contraction or relaxation and affected the blood pressure;
Because of their origin in the prostate gland …prostaglandins
1950s, first structure elucidation; 1960s, biosynthesis; 1970s, the thromboxanes and leukotrienes were discovered;
Later, widely expressed in many tissues;
Aspirin and other nonsteroidal anti-inflammation drugs (NSAIDs) inhibit PG biosynthesis
Summary of biosynthetic routes to the major prostaglandins and thromboxane A2
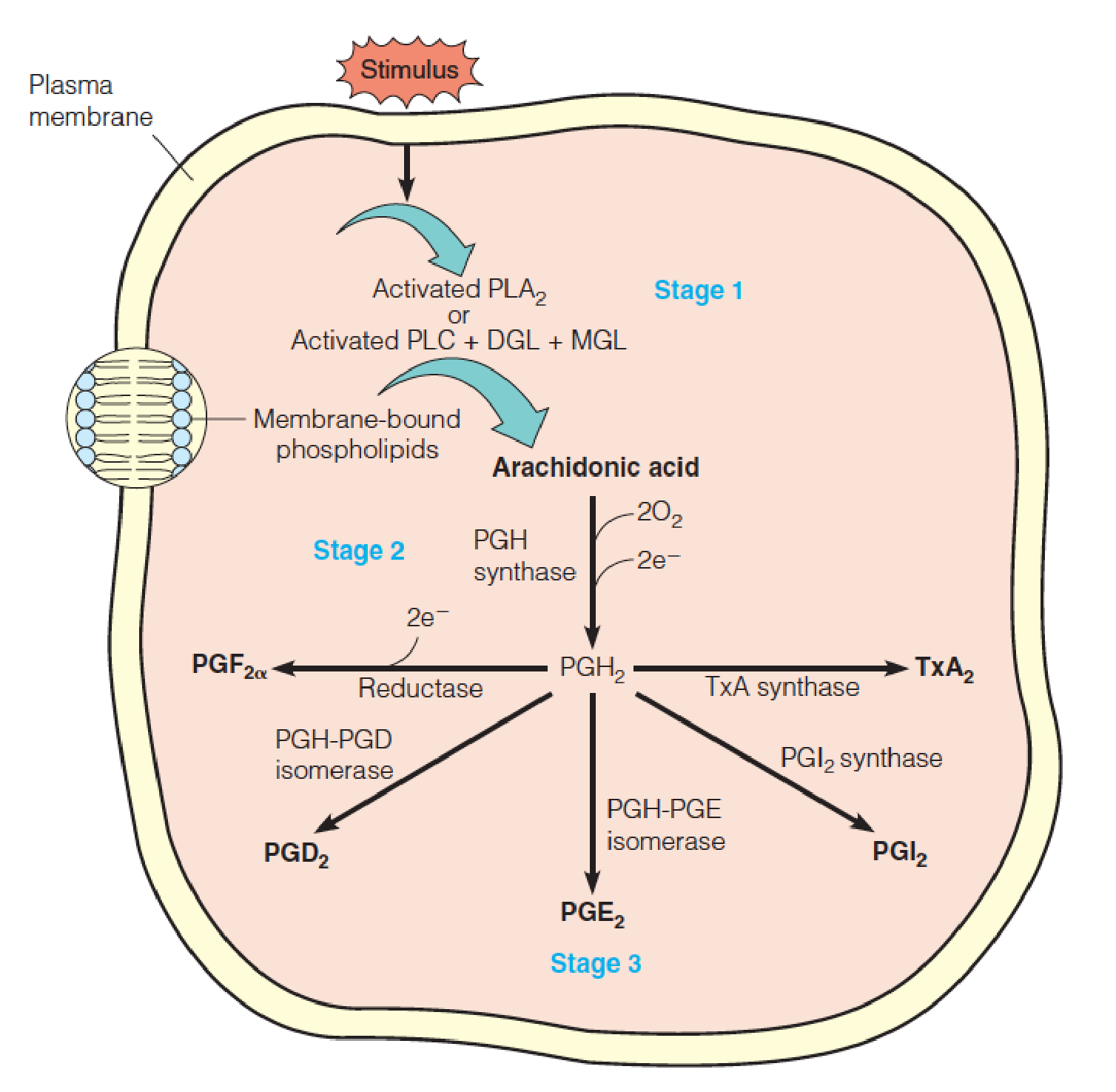
- PLA2, phospholipase A2,磷脂酶A2
- PLC, phospholipase C,磷脂酶C
- DGL, diacylglycerol lipase,二酰基甘油脂酶
- MGL, monoacylglycerol lipase,单酰基甘油脂酶
- Release of arachidonic acid(花生四烯酸) from membrane phospholipids;
- Oxygenation of arachidonate to yield PGH2, a precursor to other PGs;
- Depending on the enzymes present in the cell, the conversion of PGH to other PGs or TxA2.
Release of arachidonic acid from membrane phospholipids in stage 1: bradykinin (缓激肽) or epinephrine or by proteins such as thrombin (凝血酶);
- Bee stings: melittin promotes arachidonate release, which causes local inflammation;
Free arachidonate is acted on, in stage 2, by PGH synthase, a bifunctional endoplasmic membrane with 2 activities in a single heme-containing polypeptide chain. The first, a cyclooxygenase…. The second activity, a peroxidase (过氧化物酶),… to give PGH2;
- Mammalian cells contain two distinct forms of PGH synthase, called PGHS-1 and PGHS-2 (or COX-1 and COX-2, where COX stands for cyclooxygenase(环加氧酶)).
- COX-1 is constitutively expressed and responsible for the physiological production of PGs
- COX-2 is induced by cytokines, mitogens, endotoxins in inflammatory cells and is responsible for the elevated production of PGs during inflammation;
- Both forms are inhibited by aspirin(阿司匹林). Aspirin acetylates a specific serine residue, which in turn blocks access of the fatty acid substrate to the cyclooxygenase active site;
- The anti-inflammatory and analgesic properties of aspirin derive from the inhibition of COX-2.
- However, the inhibition of COX-1 has undesirable side effects on the gastrointestinal system(肠胃系统;消化系统), including ulceration(溃疡);
- Another widely used NSAID (nonsteroidal anti-inflammatory drugs), ibuprofen(布洛芬,异丁苯丙酸), acts somewhat more specifically upon COX-2 but is a less effective inhibitor than aspirin;
- Highly selective COX-2 inhibitors such as Vioxx and Celebrex, introduced in 1990s: recent studies, associated with increased risk of heart attack and stroke (Vioxx was withdrawn from the market in 2004).
- The anti-inflammatory and analgesic properties of aspirin derive from the inhibition of COX-2.
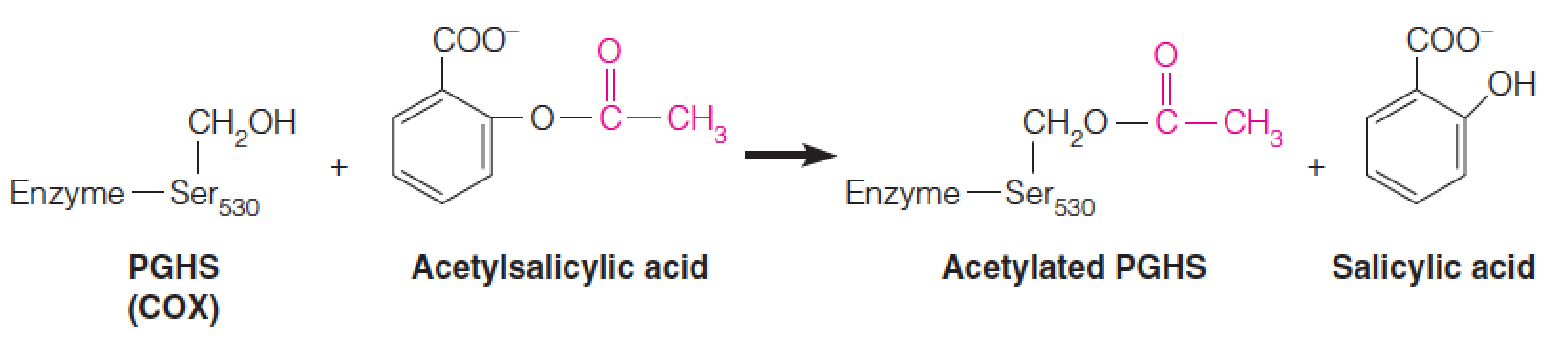
1. Mechanism of Aspirin
COX-1 is constitutively expressed in most tissues, and is responsible for the physiological production of prostaglandins (前列腺素类).
COX-2 is induced by cytokines, mitogens, and endotoxins in inflammatory cells and is responsible for the elevated production of prostaglandins during inflammation.
- Both isoforms are covalently modified, and hence inactivated, by reaction with aspirin (acetylsalicylic acid). (乙酰水杨酸)
As shown, aspirin acetylates a specific serine residue, which in turn blocks access of the fatty acid substrate to the cyclooxygenase (环氧化酶) active site.
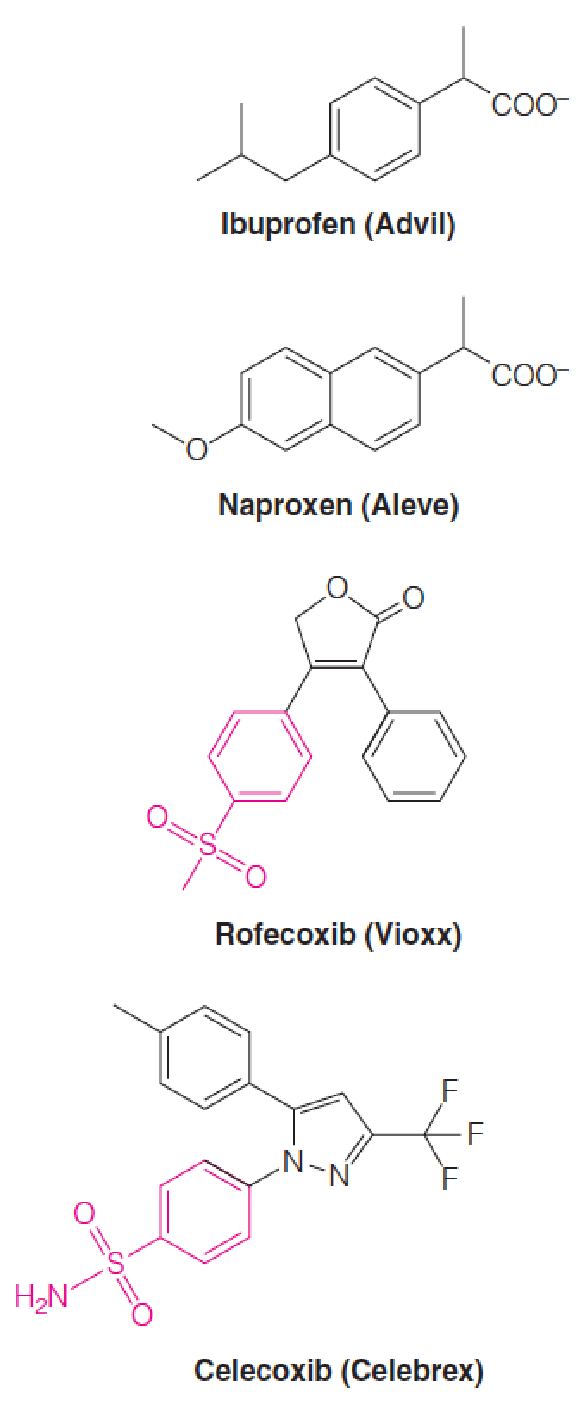
2. Mechanism of Nonsteroidal anti-inflammatory drugs (NSAIDs)
Ibuprofen (布洛芬) and naproxen (萘普生) are examples of nonselective COX Inhibitors.
Rofecoxib (罗非昔布) (Vioxx®) and celecoxib (塞来昔布) (Celebrex®) are selective COX-2 inhibitors.
- The phenylsulphonamide moieties, which contribute to their selectivity, are highlighted in red.
Cyclooxygenase (COX) (环氧化酶) is an enzyme that is responsible for formation of prostanoids, including prostaglandins, prostacyclin and thromboxane.
Biosynthesis of leukotrienes
Leukotriene C was originally discovered in the class of white blood cells called polymorphonuclear leukocytes (PMNs,多形核白细胞) and was named after the source (leukocytes) and the triene (三烯) structure.
- It is a potent muscle contractant that is involved in the pathogenesis of asthma (哮喘), through constriction of the small airways in the lung.
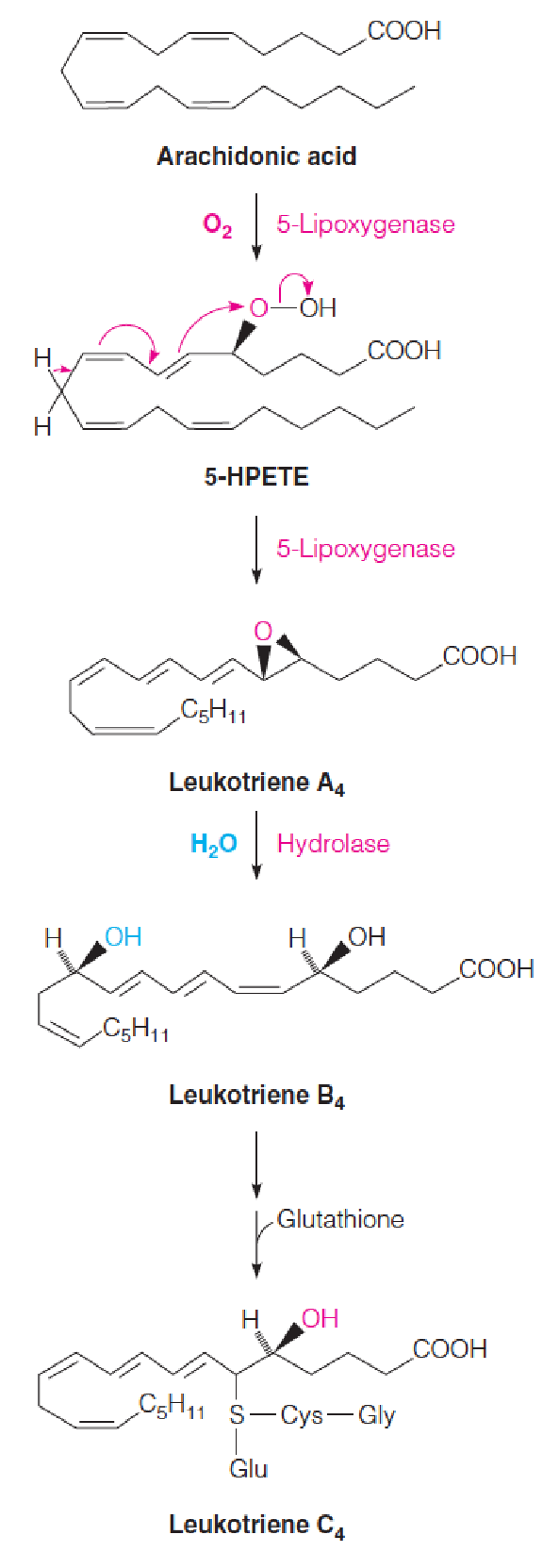
- Arachidonic acid 5- hydroperoxide (5-hydroperoxyeicosatetraenoic acid, 5-HPETE)
LTs are formed from the initial attack on arachidonate (花生四烯酸) of a lipoxygenase (脂氧合酶), which adds O2 to C-5, giving 5-HPETE.
- A dehydration to give the epoxide coupled with isomerization of double bonds gives leukotriene A4.
- Hydrolysis of the epoxide ring (环氧环) yields leukotriene B4.
- Transfer of the thiol group of glutathione yields leukotriene C4.
- Subsequent modifications of the peptide chain yield related compounds, leukotrienes D and E.