L02 Weak Interactions
The Nature of Noncovalent Bonds
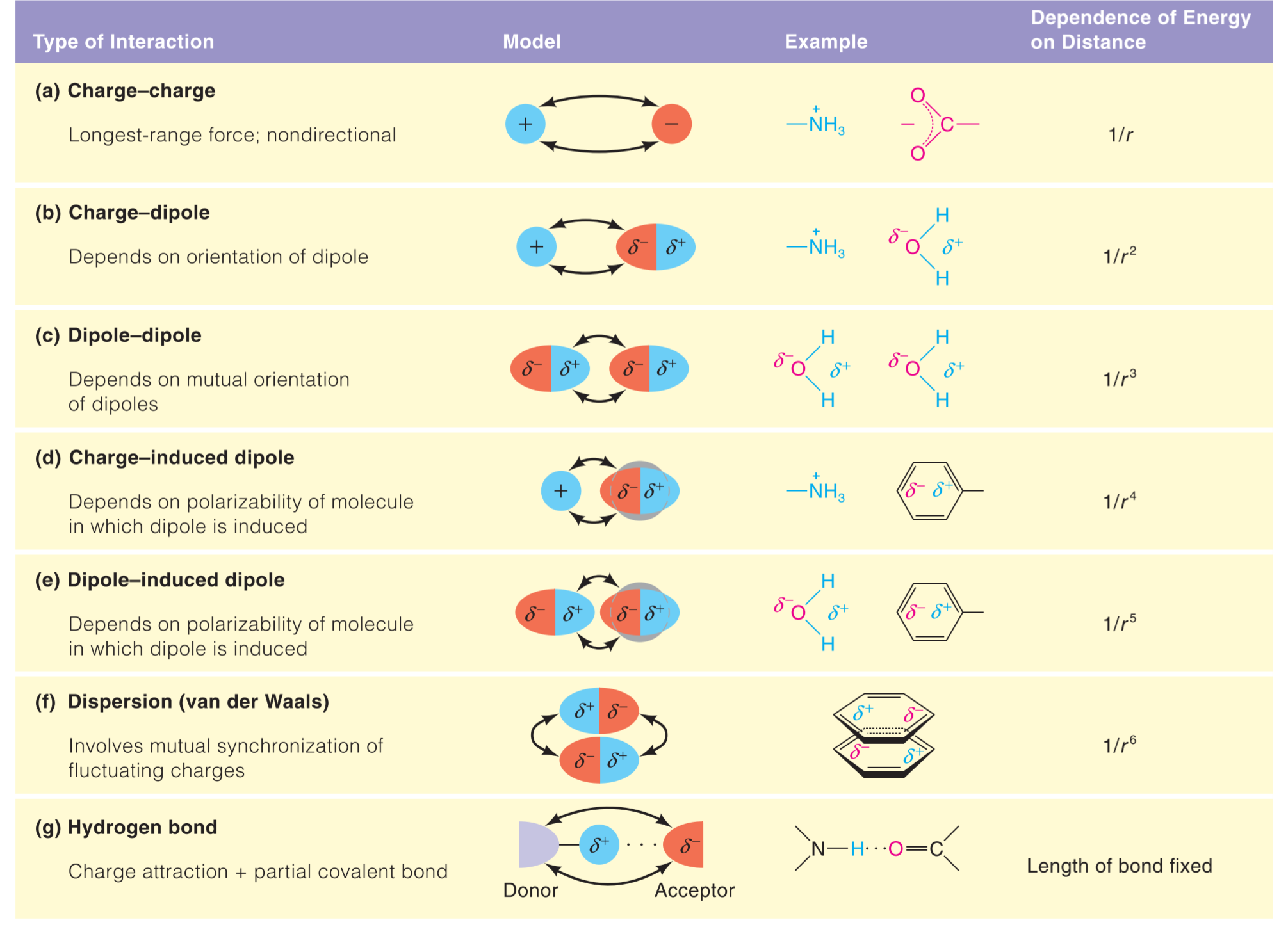
Charge–Charge Interactions
在真空中,两个电荷之间的作用力可以使用Coulomb’s law:
$$
F = k \frac{q_1 q_2}{r^2}
$$
In the c.g.s. (centimeter–gram–second) system, with charges in electrostatic units, k is unity.
we use the SI, or international, system of units. Here $$q_1$$ and $$q_2$$ are in coulombs (C), r is in meters (m), and k = 1/(4$\pi \varepsilon_0$).
The quantity $\varepsilon_0$ is the permittivity of a vacuum and has the value 8.85 10^-12^ J^-1^C^2^m^-1^, where J is the energy unit joules. F is in newtons (N).
但是细胞中并不是真空,所以需要加上一个dielectric constant ($\varepsilon$):
$$
F = k \frac{q_1 q_2}{\varepsilon r^2}
$$
Energy ofinteraction (E):
$$
E = k \frac{q_1 q_2}{r}
$$
Permanent and Induced Dipole Interactions
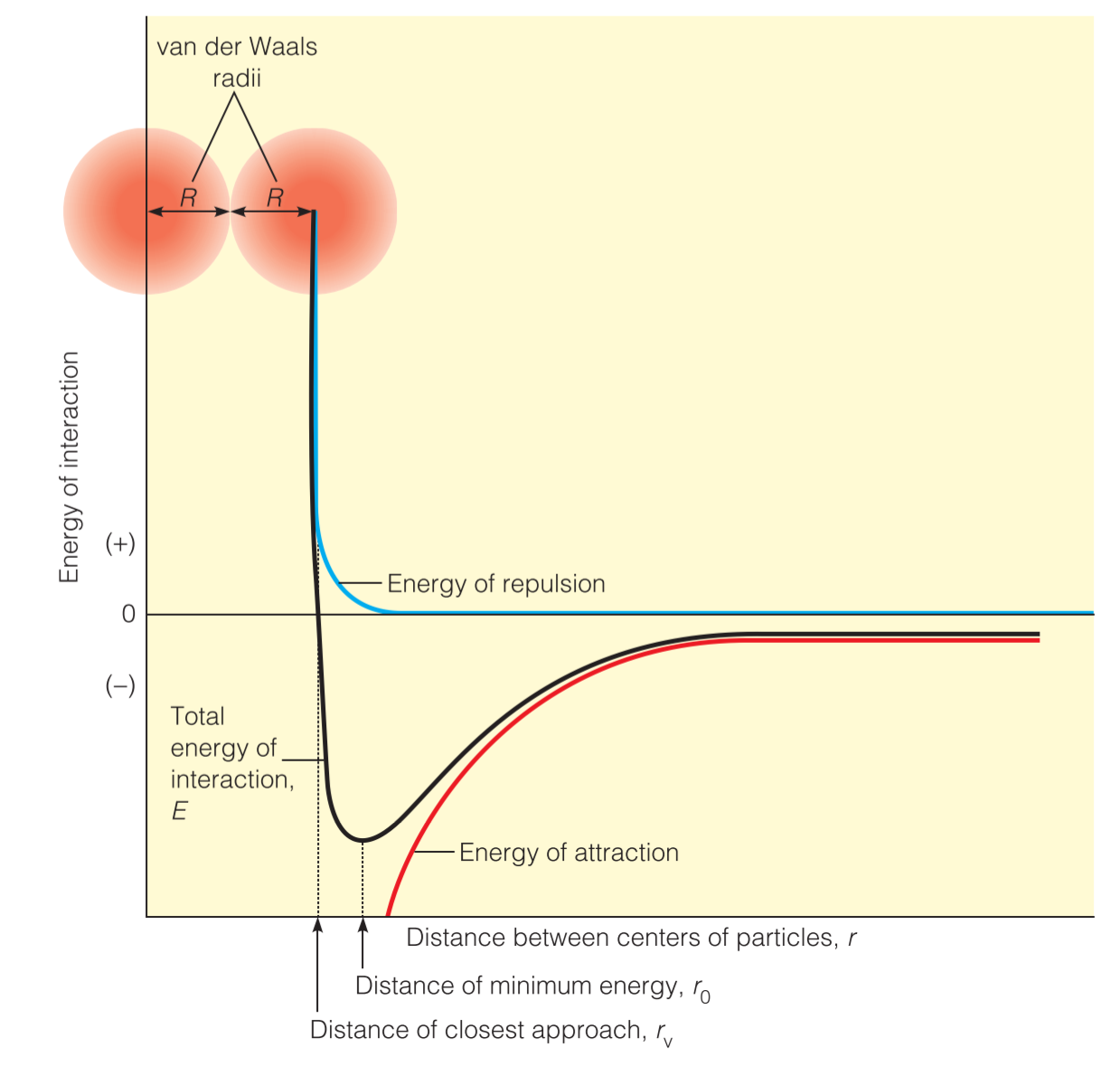
Molecular Repulsion at Extremely Close Approach: The van der Waals Radius
The van der Waals radius, rw, of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom.
$$
F 与 \frac{1}{r^6}正比
$$
Hydrogen Bonds
Hydrogen bonds具有方向(Highly directional),细胞因此形成ordered structure, 并且许多生物大分子的结构比如蛋白质,Dna 等,都依靠H-bond来进行stabilize
H-bond is partially colvalent
- 1/10 - 1/20 binding energy of covalent bonds
Hydrophilic And Hydrophobic
water:
cells themselves are about 70%-95% water
The unique properties of water
- Expansion upon freezing $\rarr$ Lower density of ice
- Ability to moderate temperature $\rarr$ High HV
- Cohesive behavior
- High viscosity(粘度)
- high surface tension
- Versatility as a solvent
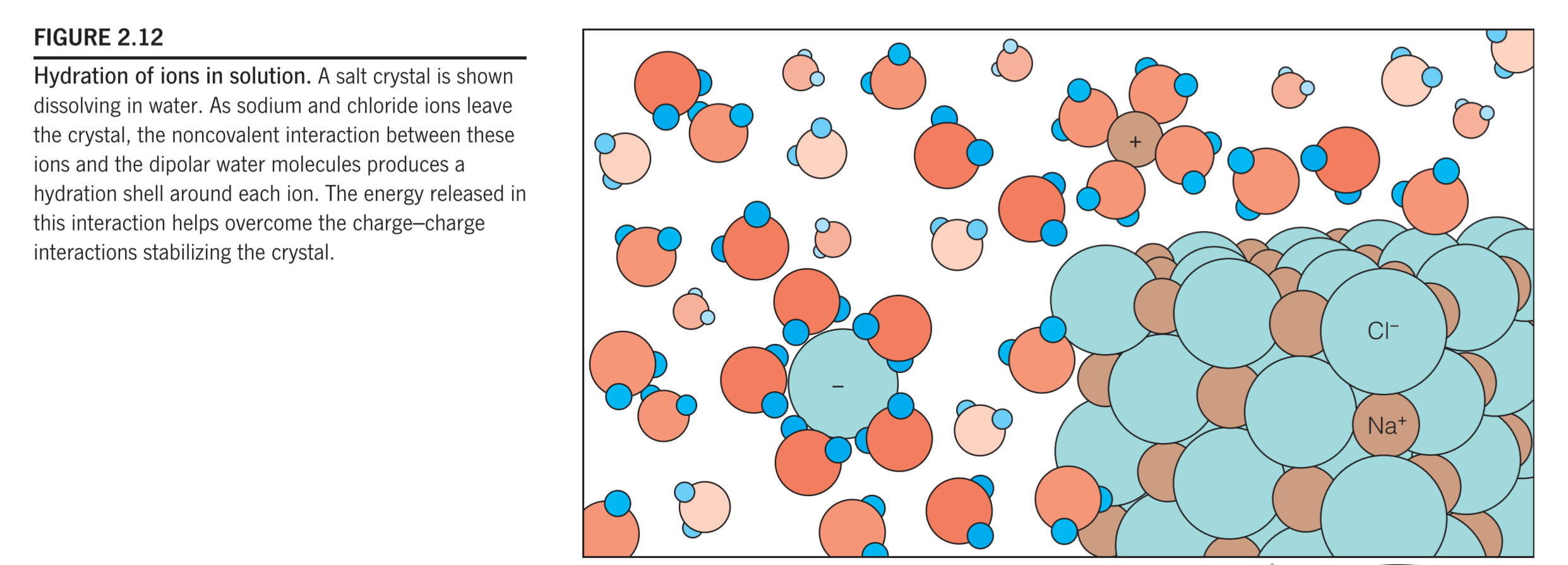
Hydrophilic Substances
The interactions of the negative ends of the water dipoles with cations and the positive ends with anions in aqueous solution cause the ions to become hydrated(水化作用), that is, surrounded by shells of water molecules called hydration shells(水化壳)
Hydrophobic Substances
Without hydrogen bonds
Thermodynamically unfavorable
- Hydrophobic 物质在水中溶解的时候,并不会像亲水物质一样形成一个水化壳,而是形成 ice-like clathrate structures, or “cages” about nonpolar molecules.
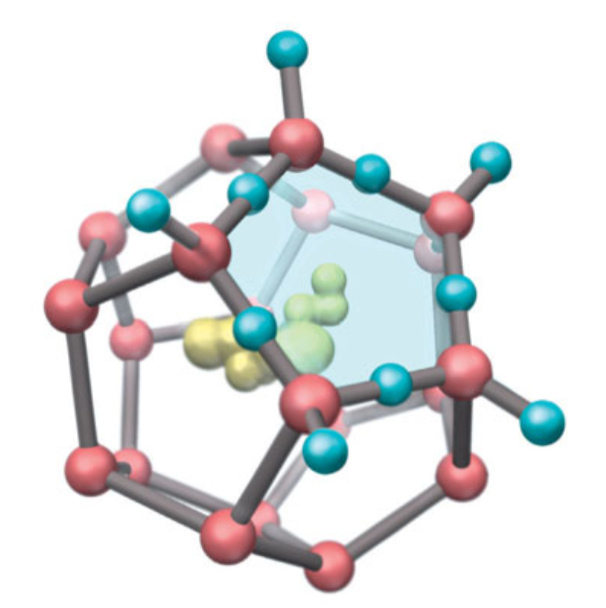
This ordering of water molecules, which extends well beyond the cage, corresponds to a decrease in the entropy, or randomness, of the mixture. 这种结构增大了水分子的“表面积”,更加稳定,使得混合物的熵值减少。
但是hydrophobic effect倾向于使得整个系统的熵值增加:Decreased ordered water molecules and thereby increase entropy of the system.(水分子释放后更加自由,系统的熵值增加)
Decreasing entropy is unfavorable thermodynamically; thus, the dissolving of a hydrophobic substance in water is entropically unfavorable.
Surrounding two hydrophobic molecules with two separate cages requires more ordering of water in clathrates
than surrounding both hydrophobes within a single cage.
Thus, the hydrophobic molecules tend to aggregate because doing so releases some water molecules from the clathrates, thereby increasing the entropy of the system. This phenomenon, called the hydrophobic effect(疏水效应,又称疏水性效应,属于非极性分子的一种性质,会使这些分子在水溶液中具有自我聚集(self-associate)的特性).
Hydrophobic Interactions
- The forces that hold the nonpolar regions of the molecules together
- Proton folding
- Lipid bilayer assembly
Acid and Bases
- An acid is any substance that increases the H^+^ concentration of a solution
A base is any substance that reduces the H^+^ concentration of a solution
pH
Most biological fluids have pH values in the range of 6~8
根据具体目标溶液的pH选择buffer
Macroions
Definition
Large polyelectrolytes such as nucleic acids and polyampholytes(聚电解质) such as proteins are classed together as macroions
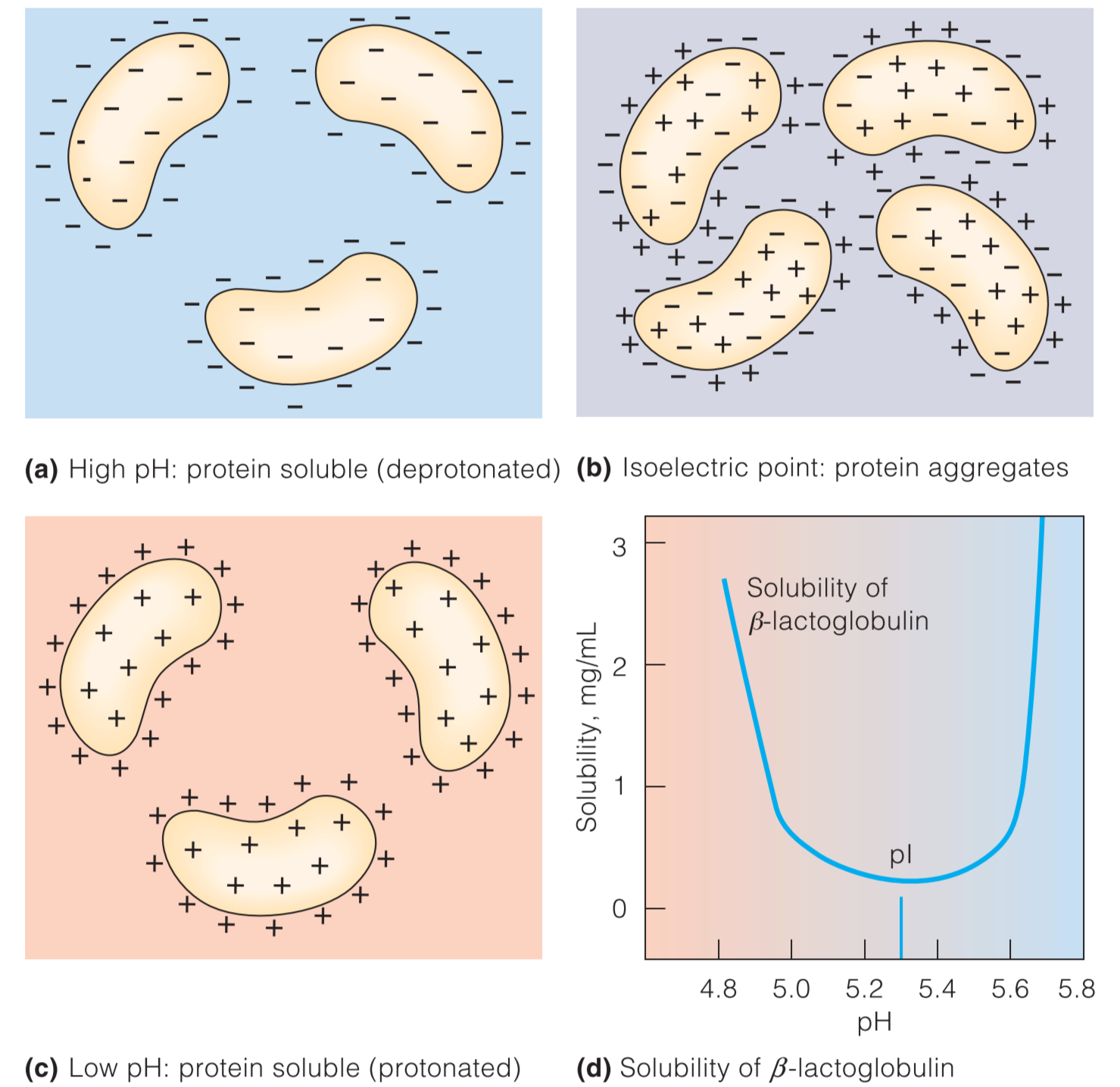
Macroions and PH
由于Macroions可以被看做net charge,所以其对其它带电粒子具有吸引或排斥的作用。正式因为这个性质,DNA分子倾向于彼此分离,并且倾向于与带正电的蛋白质结合
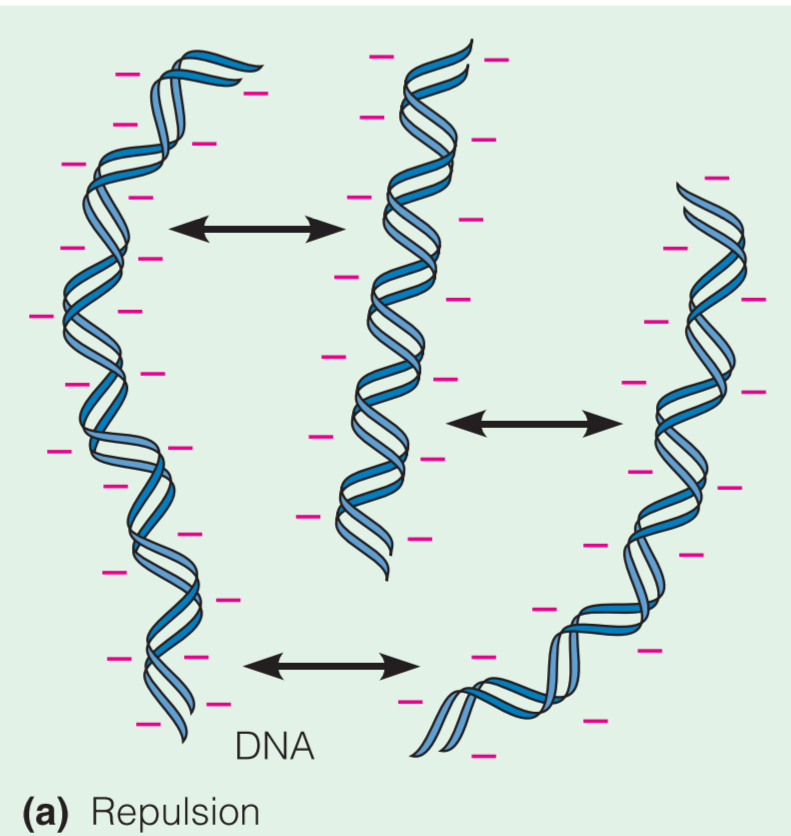
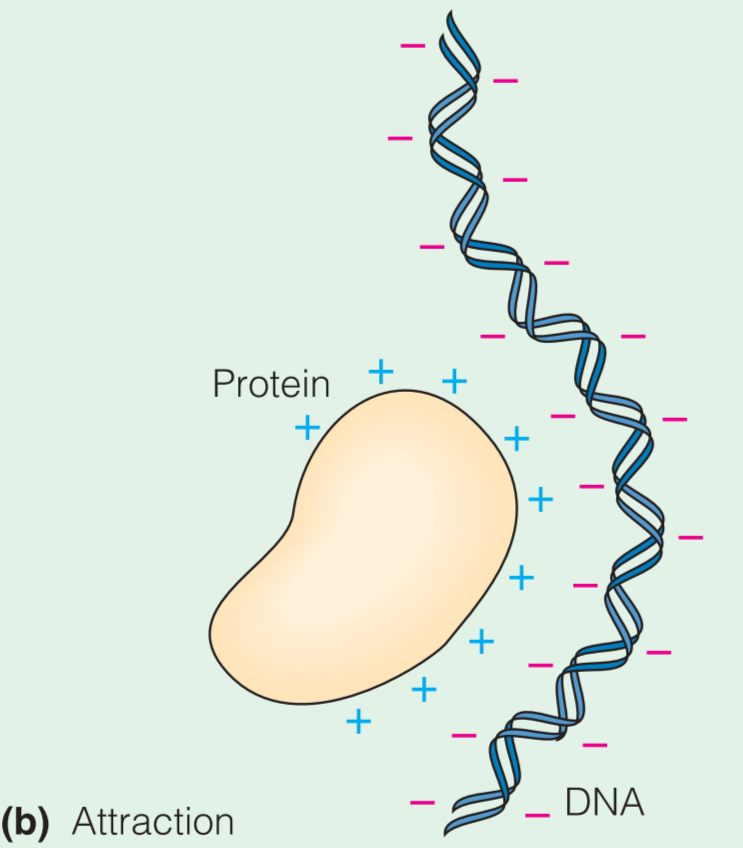
On the other hand, if positively and negatively charged macromolecules are mixed, electrostatic attraction makes them tend to associate with one another
Macroions and ionic strength
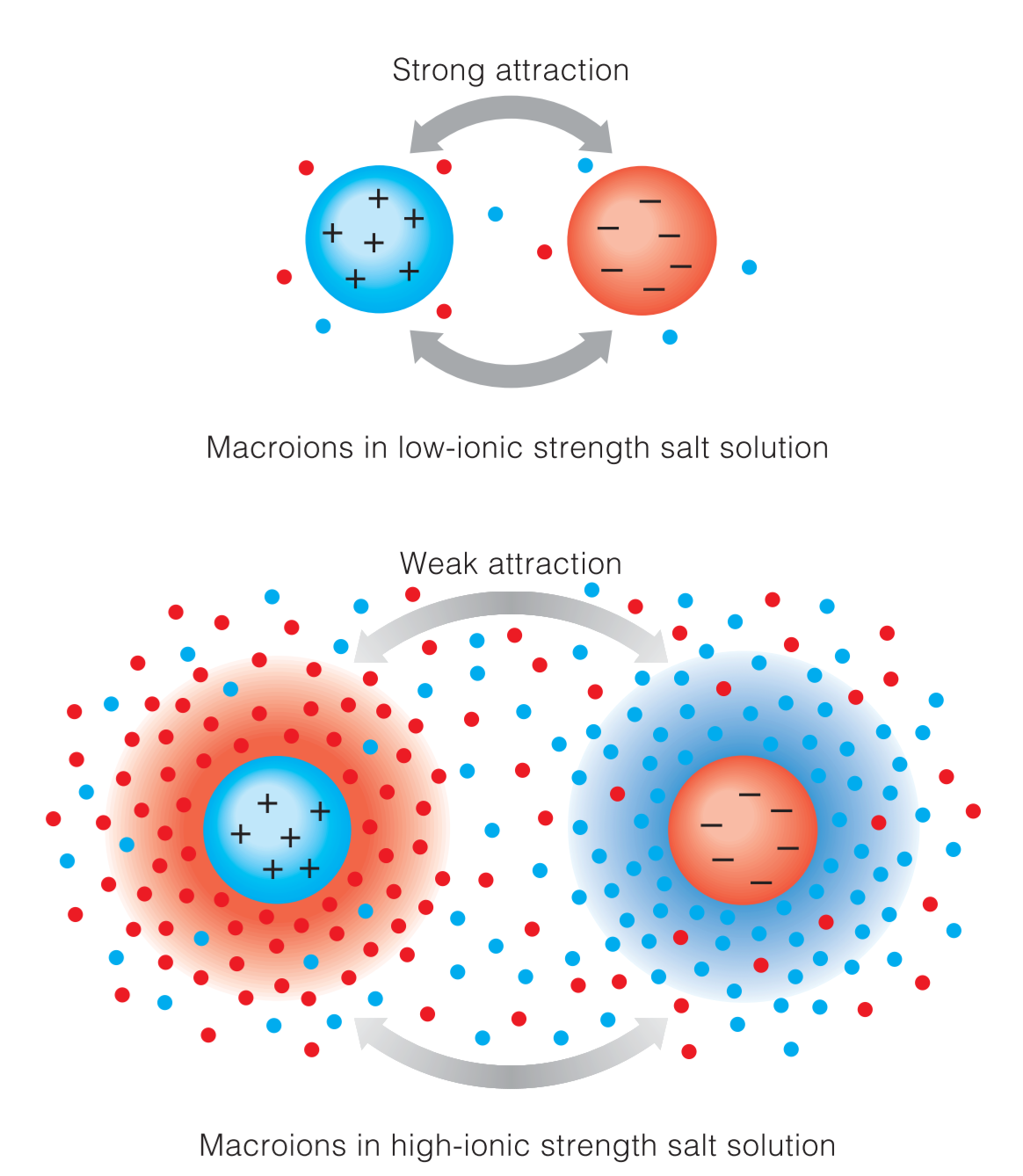
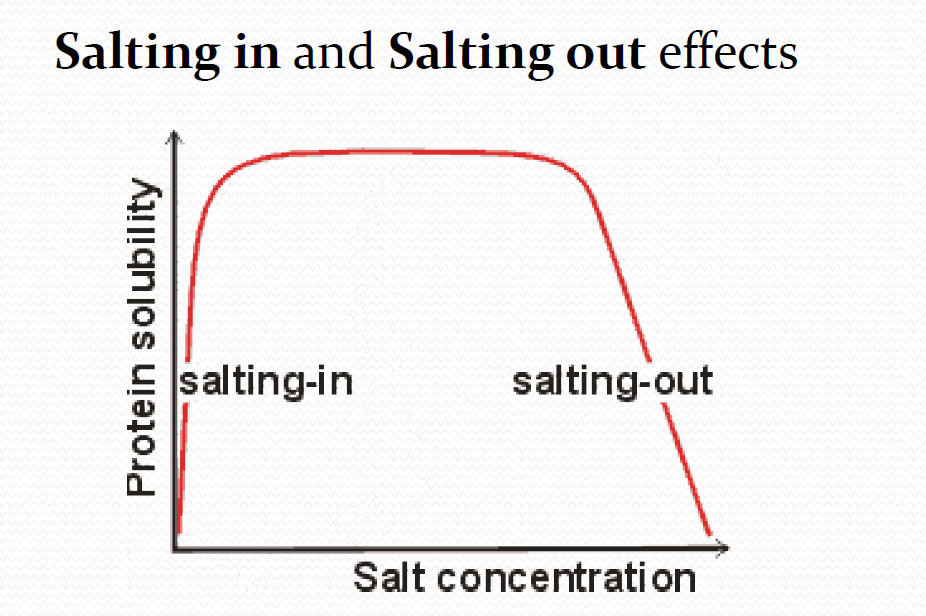
1. Salting in
在起始点因为macroion本身的带电性,不同离子间正负点部分相互吸引,使得macroion之间aggregate。逐渐加入salt之后,salt在macroion周围形成counterion atmosphere,减弱了macroion之间的相互吸引作用,使得macroions它们更倾向于进行水合作用,提高了solubility,因此形成salting in effect。
2. Salting out
当salt concentration增高到一定浓度的时候,salt ions就会抢夺hydration shells,加上hrdyophobic effect,使得macroions重新聚集。
In very concentrated salt solutions, much of the water that would normally solvate and help solubilize the protein molecule is bound up in the hydration shells of the numerous salt ions, preventing sufficient hydration of the protein.
Van der Waals interactions
Disperision forces
Major stabilization forces for biomolecular structures
Between any of two atoms if they are close enough
- When outer electron orbits overlapped
The fluctuated distribution of electronic charge in a molecular
Stacking forces
Stacking forces are strong van der waals interactions